Abstract
The beneficial effects of cortical activation for functional recovery after ischemic stroke have been well described. However, little is known about the role of early sensory stimulation, i.e. stimulation during first 6 h after stroke onset even during acute treatment. In recent years, various preclinical studies reported significant effects of acute sensory stimulation that range from entire neuroprotection to increased infarct volumes by 30–50%. Systematic knowledge about the effect of acute sensory stimulation on stroke outcome is highly relevant as stroke patients are subject to uncontrolled sensory stimulation during transport, acute treatment, and critical care. This article discusses the current stage of knowledge about acute sensory stimulation and provides directions for future experimental and clinical trials.
Keywords: Collateral reperfusion, cortical activation, ischemic stroke, sensory stimulation, stroke models
Introduction
The role of sensory stimulation during the acute phase of ischemic stroke is fundamentally different from its putative role for long-term recovery. The latter evokes a complex process of neuroplasticity and finally aims to improve functional recovery but not primarily to protect brain tissue from ischemia.1 In contrast, sensory stimulation within the time window for acute stroke therapy might change critical parameters for stroke size and neuronal survival including regional cerebral blood flow or regional oxygen demand.2,3
This direct effect of sensory stimulation on acute ischemia is presently thought to be determined by three factors: (i) activation of neuronal tissue during ischemia, (ii) vascular response to neuronal activity during ischemia; and finally (iii) the balance of demand and supply of oxygen and nutrients. For the healthy brain, the interaction among these factors is well understood. Neuronal activity evokes a regional vasodilation according to a process of neurovascular coupling that delivers an excess of oxygen and nutrients (up to 200%).4,5 To our current knowledge, neuronal activity and neurovascular coupling are severely impaired during ischemia.6 Residual blood flow levels below approximately 0.1 ml/mg/min (approx. 20% of resting state) quickly lead to depolarization of neurons in the core region and flow levels below 0.18 ml/mg/min (approx. 30% of resting state) silence neuronal activity in the penumbra region of ischemic stroke for up to several hours.7–9 Additionally, vascular autoregulation and neurovascular response in wide parts of the lesional hemisphere are impaired or lost in reaction to ischemia.6,10,11
Until recently, these findings limited the interest in sensory stimulation acutely after stroke. However, recent publications point towards a more complex interaction between neuronal activation and the vasculature during ischemia.2,12–14 Acute stimulation of the ischemic core has repeatedly been reported to protect the brain of rats entirely by an increased blood flow through anastomoses between the territories of cerebral arteries.3,12–14 On the other hand, neuronal activation in the peri-infarct zone has been shown to lower oxygen levels thereby causing waves of depolarization and vasoconstriction in mice.2 Via this mechanism, cerebral blood flow decreases and infarct volume increases by around 50%.2 Here, we provide the context for these seemingly contradictory findings and explain the critical role of experimental models and stimulation paradigm. Further, we discuss the design of potential clinical trials that need to coordinate sensory stimulation with acute stroke treatment and critical care.
Sensory stimulation as a potential therapy for ischemic stroke
The role of acute sensory stimulation delivered to the affected limbs or whiskers after stroke gained attention when Ron Frostig’s group from UC Irvine reported a dramatic therapeutic effect.3 Mild sensory stimulation of the whisker(s) protected Sprague Dawley rats entirely from neuronal damage and functional loss after occlusion of the middle cerebral artery (MCAO).12,13 These findings have been independently repeated and also been demonstrated in aged Fisher 344 rats and with several anesthesia protocols.13,14 Interestingly, the critical factors that determined the protective effect of sensory stimulation were not the length of stimulation—10 min of condensed stimulation were sufficient for total protection—but the timing and number of whiskers stimulated.3,15 Sensory stimulation during the first 2–3 h after MCAO was protective which overlaps with the time window of thrombolysis therapy and thrombectomy.12,15,16 On the contrary, stimulation 3 h after stroke onset or later was even detrimental by markedly increasing infarct volume and worsening functional outcome.12,15 We will discuss the potential risk of early sensory stimulation after stroke later in this article.
As mentioned above, it is critical to understand how neuronal activity and vasoreactivity change under ischemic conditions in order to explain the effects of early sensory stimulation. When exposed to ischemia, neuronal activity is suppressed; evoked responses vanish first and below a certain threshold also spontaneous activity is undetectable. However, the specific thresholds of cerebral blood flow that lead to neuronal silencing change over time. The commonly cited blood flow levels of approximately 30% of baseline for neuronal silencing and 20% for depolarization and irreversible damage have been determined several hours after stroke.17 It has been shown that thresholds for neuronal silencing substantially increase within the first 2 h of ischemia to more than twofold.18 This goes along with the concept of stroke progression: prolonged ischemia continuously shifts the infarct border by functional and finally membrane failure of peri-infarct neuronal tissue.19
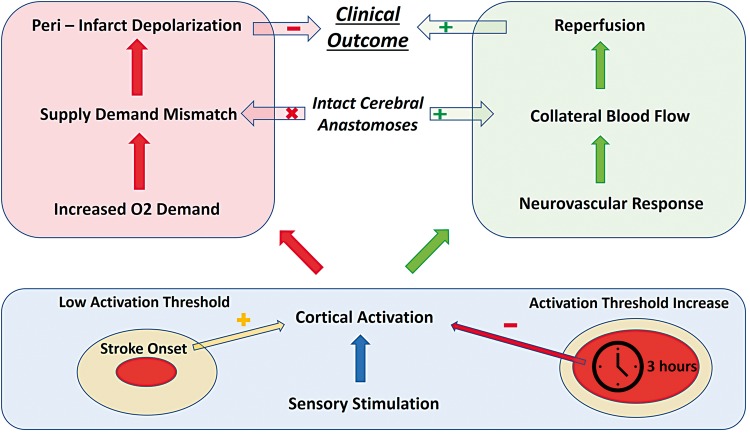
The Frostig group reported residual blood flow levels of 26–28% of baseline in the S1 whisker cortex after MCAO according to Laser Speckle Imaging.3 These blood flow levels are in the critical range where neuronal function might be preserved transiently before persistent ischemia shifts the threshold for neuronal silencing above these levels. Therefore, the early timing of sensory stimulation is a possible explanation why spontaneous neuronal activity could be recorded even during ischemia and evoked responses recovered gradually during stimulation (Figure 1). Noteworthy, the whisker barrel has a cortical representation that overlaps with almost the entire stroke territory after distal MCAO including peri-infarct zones outside the depolarized stroke core.20 Thus, most likely a combination of timing, when neurons are moderately impaired but still able to function and recover, and spatial relation to the blood flow deficit, within the stroke territory but at least partwise outside the core zone of depolarization, determines the effect of sensory stimulation on cortical activity (Figure 1).
Figure 1.
The role of sensory stimulation during the acute phase of ischemic stroke. The cerebral blood flow threshold for neuronal activation increases with time elapsed after arterial occlusion. Therefore, early sensory stimulation is more likely to activate viable cortical areas than delayed stimulation, e.g. 3 h after stroke onset (blue box). Cortical activation increases the regional oxygen demand and might lead to supply–demand mismatch in case of insufficient collateral flow. Supply–demand mismatch transients may in turn trigger peri-infarct depolarizations that worsen stroke outcome (red box). Conversely, cortical activation induced by early stimulation leads to neurovascular coupling that enhances collateral flow through an efficient system of cerebral anastomoses. Enhanced collateral flow is the supposed mechanism that mediates reperfusion and improves clinical outcome in the concept of early sensory stimulation-induced neuroprotection (green box).
Even if neuronal activity can still be evoked under ischemic conditions, the vascular response is critical for its protective effect (Figure 1). Focal ischemia itself leads to regional vasodilation via release of metabolic factors and consecutive activation of shear stress receptors in pre-capillary arterioles.21 According to the early stimulation paradigm, neurovascular coupling is supposed to enhance the vasodilating reaction to brain ischemia.5 This is the most controversial part of the sensory stimulation theory and there have been several studies reporting absence or even inversion of neurovascular coupling under ischemic conditions.22 More research is needed to fully understand the interaction of cortical activity, local metabolic factors, and the vascular response. For these upcoming studies, it is again critical to standardize the timing of stimulation because prolonged ischemia does not only impair neuronal but also vascular function.
However, based on the assumption that neurovascular coupling is still relatively preserved under ischemic conditions, the mechanism underlying residual perfusion of the ischemic territory despite MCAO is dependent on increased collateral blood flow. The Frostig group described this collateral blood flow to be the critical limitation of neuroprotection upon sensory stimulation.23 Ligation of anastomoses completely abolished the beneficial effect of stimulation after stroke.23 Thus, in addition to the stimulation paradigm, the vascular anatomy and especially efficient cortical anastomoses between the cerebral arteries also determine the protective potential of sensory stimulation (Figure 1).
Limitations and risks of early sensory stimulation
When discussing the potential risks of acute somatosensory stimulation, one needs to be aware that extensive sensory stimulation is abundant for acute stroke patients during routine transport, treatment, and critical care. Therefore, the role of sensory stimulation in acute stroke therapy needs to be investigated broadly and for a wide range of patient groups.
The potential risk of early sensory stimulation arises from increased metabolic demand during neuronal activation. The protection paradigm is based on the critical assumption that neuronal activity leads to enhanced vasodilation in the stroke territory thereby redirecting blood flow through anastomoses from neighboring cerebral arteries. What are the consequences of evoked neuronal activity when these reperfusion mechanisms are impaired? It has been demonstrated in a mouse study that transient sensory stimulation of peri-infarct tissue at a blood flow range of 27–35% of baseline reduces local oxygen levels significantly.2 Moreover, these stimulation attempts caused peri-infarct depolarizations (PIDs) 60% of the time.2 PIDs are waves of neuronal depolarization that swipe over wide parts of the peri-infarct cortex and cause transient vasoconstriction.24,25 They are recognized as a principal mechanism of infarct progression and led to an increase in infarct volume by about 30% in stimulated animals.2,24 These data seemingly contradict the protective effect of early sensory stimulation. Interestingly, the Frostig group reported in a recent publication that the stimulation paradigm protecting rats from ischemic damage did not lead to neuroprotection in mice.26 These findings again point to the critical role of vascular anatomy. Just 10% of C57/BL6 mice have a complete circle of Willis which significantly reduces the possibility of reperfusion through anastomoses.27 A similar experimental study in rats could not find a neuroprotective effect of stimulation and observed an increased number of PIDs when occluding the common carotid artery (CCA) in addition to the MCA.28 Note that ligation of the CCA limits collateral blood flow by decreasing arterial inflow into the circle of Willis. Taken together, it seems that a reduced number and function of connecting vessels between cerebral arteries hinder or even abolish the neuroprotective effect of sensory stimulation. When additional blood supply cannot match the increased demand of neuronal activation, this mismatch can finally worsen prevailing hypoxia and contribute to infarct growth via PIDs (Figure 1).
In this context, it is worth discussing the role of neurovascular coupling as the local reaction to neuronal activation. The BOLD (Blood Oxygenation Level Dependent) response to cortical activation is diminished in stroke patients but anatomically still related to the expected S1 representation of the stimulated area.29 A clinical study of acute stroke patients who underwent median nerve stimulation revealed heterogeneous blood flow responses.30 In some patients, neurovascular coupling was still intact, while in others, a neurovascular response could not be observed.30 Noteworthy, intact neurovascular coupling was predictive for better clinical stroke outcome.30 This variability of the local effect of neuronal activity adds another level of complexity to the prerequisites for putatively neuroprotective sensory stimulation.
In summary, functional cortical anastomoses and preserved neurovascular coupling in the activated stroke region (i) appear critical for the neuroprotective effect of sensory stimulation and (ii) protect peri-infarct regions from supply–demand mismatch that otherwise might worsen tissue outcome (Figure 1).
Defining the borderline between benefits and risks of sensory stimulation
The above review of recent experimental research revealed that early sensory stimulation can have a significant beneficial or detrimental impact on stroke outcome. More deeply, the intensity, timing, and location of sensory stimulation as well as vascular anatomy and function seem to determine the direction and size of this effect. The exact conditions and thresholds defining beneficial and safe stimulation, however, remain unclear. Unlike other treatment options, sensory stimulation is inevitable and abundant in all clinical scenarios and for all subgroups of stroke patients. This constellation demands expedited clinical translation and therefore a redirection of experimental efforts. Most of the studies discussed above were designed for a maximum effect of stimulation to better understand beneficial or detrimental mechanisms. Future designs, however, will need to better simulate clinical settings and patient characteristics while at the same time minimizing experimental artifacts. In this section, we will describe appropriate stroke models to discern potentially therapeutic from harmful stimulation.
With regard to experimental stroke models, systemic physiology and anesthesia are critical sources of artifacts. As explained above, marginal changes in local oxygen delivery and blood flow levels as small as 5–10% during stimulation can have major differential consequences on tissue outcome, ranging from protective collateral reperfusion to detrimental triggering of PIDs or no significant effect because of neuronal silencing. Therefore, the arterial partial pressure of oxygen needs to be maintained in a range around 100 ± 20 mm Hg with oxygen fraction in the breathing air not exceeding 30%. Excessively higher oxygen supplies lead to a stage of normobaric hyperoxia that prevents PIDs and significantly increases cerebral blood flow.2 Regarding the impact of anesthesia, the Frostig group has published several studies using nembutal, isoflurane, or no anesthesia at all during sensory stimulation.23,31,32 Undiminished neuroprotection was observed under all anesthetic regimens. The only anesthetic drug reported to interfere with sensory stimulation so far was ketamine, which prevented PIDs and should therefore be avoided in future experiments.2
Beyond these basic considerations, systematically varying the stroke model employed should be used to define the limits of safe stimulation. The crucial role of collateral flow and stroke location has already been discussed. These variables can be addressed with specific alterations of MCAO technique. All neuroprotection studies used a rat model of MCAO with direct surgical access to the MCA. This approach limits impending ischemia almost entirely to the somatosensory cortex and allows arterial flow into proximal branches of the MCA.2 Moreover, rats have a marked circle of Willis that delivers ample collateral flow.27 Under these conditions, the entire stroke territory could be reperfused which prevented the formation of unstable peri-infarct zones. However, this setting does not represent clinical stroke cases with extracortical contribution. Therefore, it needs to be clarified whether sensory stimulation can also have neuroprotective effects in the presence of ischemic damage in subcortical areas. More precisely, the size, location, and hypoxic time of an ischemic lesion that precludes beneficial effects should be determined. An appropriate stroke model for this purpose is filament MCAO which occludes the origin of the MCA and can be combined with ipsilesional CCA ligation.33 Therefore, ischemic damage reaches striatal levels and collateral flow is significantly reduced.33 The exact timing of beneficial stimulation is to be determined in this setting. Ideally, the physiological state limiting neuroprotective effects can be correlated to radiological patterns in MRI and CT which could serve as thresholds for safe stimulation.
Vascular anatomy and function have also been determined as key factors for stimulation therapy. Hence, one objective of future translational studies will be to better define anatomical and functional profiles that facilitate, or conversely diminish or abolish neuroprotection. This will require systematically varying the research animals used in terms of species, genetic modifications, and age. Lay et al. already published that stimulation in aged rats leads to an undiminished reperfusion.13 Additionally, obese, diabetic, and hypertensive rats should be tested as models for vascular dysfunction mimicking the diversity of human phenotypes. A variety of genetic and induced models of obesity and diabetes are available, including Zucker rats with mutated leptin receptor and high-fat diet protocols for various genetic backgrounds.34 Spontaneously hypertensive rats can be used as a model for chronic hypertension which has been shown to reduce collateral flow from the leptomeningeal network.35,36
With respect to vascular anatomy, marked differences between animal species have been reported. We have already discussed that mice tend to have less collateral flow than rats and have not been protected by stimulation thus far.2,27 In future experiments, additional animal species need to be included, especially large animals like sheep and non-human primates. For all studies, angiography is recommended to define specific anatomical preconditions for safe stimulation. In a subset of animals for each species, perfusion with carbon black suspension will allow for precise mapping of neurovascular collaterals and their impact on microvascular reperfusion.37 Absolute blood flow measurement using 14C-iodoantipyrine technique could further determine the exact contribution of different neurovascular networks to successful reperfusion.38 In this context, the role of leptomeningeal arteries as a critical source of collateral flow should be investigated. The filling state of the middle meningeal artery can be monitored continuously with laser speckle flowmetry. Finally, more basic research is needed to understand cellular mechanisms of neuroprotection. We have discussed above the critical role of preserved neurovascular coupling. The interaction between activated neurons, astrocytes, and endothelial cells at different time points and levels of ischemia is a key factor to understand the role of sensory stimulation in acute stroke.
The overreaching goal of all pre-clinical studies is to define specific radiological thresholds and pre-existing conditions that preclude stroke patients from safe and beneficial sensory stimulation. For these subgroups at risk, the opposite scenario needs to be investigated next, i.e. the therapeutic potential of reduced stimulation.
Can sensory stimulation be combined with revascularization therapy?
The current treatment options for acute ischemic stroke are substantial but still limited. Thrombolysis therapy and more recently thrombectomy have demonstrated their potential to improve stroke outcome via early revascularization of occluded artery segments.16,39 We already discussed that the critical time window for sensory stimulation overlaps with the time window for lysis and thrombectomy.16,23,39 Therefore, possible interactions need to be discussed as well as the timing of treatment initiation.
Intravenous thrombolysis is initiated as soon as an ischemic stroke has been diagnosed with CT or MRI imaging and no contraindications apply.16 Cerebrovascular imaging is the most critical factor that determines the time from emergency call until the treatment starts. For sensory stimulation, which based on rat studies may be efficacious and safe only if started very early after stroke onset, MRI or CT imaging is most likely necessary to locate the ischemic territory and map the cerebral anastomoses. In an effort to utilize an ultra-early time window of 1 h after symptom onset, Stroke Emergency Mobile units (STEMOs) are being tested as ambulances with a mobile CT scanner which allows initiating the i.v. thrombolysis even before hospital admission.40 With specific perfusion thresholds from pre-clinical studies, sensory stimulation could be initiated in STEMOs as well. In clinical trials, earlier treatment initiation in STEMOs was correlated to modest improvement of short-term functional outcome and survival, but not all trials could confirm significant benefits.40–42 In combination with sensory stimulation, however, STEMOs might increase their potential to protect ischemic lesions from irreversible damage. Whether performed in STEMO or immediately after hospital admission, safety criteria for sensory stimulation need to be framed as an easy and quantifiable score specified for MRI (diffusion, perfusion, angiogram) as well as for CT imaging (perfusion, angiogram) to avoid delay of stimulation onset or—even worse—the initiation of i.v. thrombolysis or thrombectomy.
In addition to logistical aspects, the combination of different protection mechanisms needs to be discussed. Once the occluded artery segment is recanalized, the hemodynamic situation changes radically. Blood flow levels markedly increase so that neuronal activation is no longer needed to recruit blood flow through anastomoses.43 Enhanced blood flow might even increase the risk of reperfusion injury. Therefore, terminating sensory stimulation treatment after recanalization seems to be reasonable which can be timed precisely in case of thrombectomy but might be more challenging during thrombolysis treatment, where the exact time point of recanalization is more difficult to determine (e.g. with continuous or repeated transcranial Doppler Imaging). However, there are also clinical cases with prolonged periods of hypoperfusion even after recanalization. These can be interpreted partwise as prolonged hypoaemia in reaction to PIDs. In addition, prolonged ischemia may impair microcirculatory function and capillary reperfusion after recanalization (the “no-reflow” phenomenon).44 Further experimental studies with models of transient MCA occlusion, i.e. temporary clipping or filament occlusion, are needed to understand the effect of sensory stimulation during reperfusion.
Avenues to clinical translation of sensory stimulation in acute stroke treatment
Further experimental studies need to clarify the mechanisms behind the neuroprotective effect of sensory stimulation and define safety standards. Clinical pilot trials should await further information from pre-clinical studies as described above. However, uncontrolled sensory stimulation is already mingled with acute stroke management—without even considering beneficial or detrimental effects or a critical time window. Patients are being touched and moved during transport and critical care; they are subject to noise, and actively take part in clinical examination and conversations—even if aphasic. A first step towards more attention to sensory stimulation in acute stroke therapy could be an observational trial where all relevant stimuli are recorded in a digital logbook and then correlated to stroke outcome, adjusting for other known outcome predictors. Although such a trial would most likely lead to heterogenous results, cases with particularly high or low sensory stimulation levels could be analyzed separately providing orientation whether the marked dual effects of sensory stimulation are also to be expected in man.
Once pre-clinical studies have defined reliable and reproducible safety standards and observational studies have been completed, it should be feasible to design randomized clinical trials on the therapeutic role of sensory stimulation in acute stroke. This process should start with identifying appropriate stimulation paradigms for distinct locations and time points of ischemic stroke. Neuroprotective effects have been documented in rat experiments when stimulating the contralesional whisker(s).3 The cortical S1 representation of the whisker includes the core region of an ischemic stroke after occlusion of the MCA.3,20 This spatial relationship is relevant to potential neuroprotective effects of sensory stimulation. Vasodilation in the ischemic territory might lead to reperfusion through increased collateral flow, whereas vasodilation in surrounding cortical areas might redirect blood flow away from the stroke territory, i.e. a “steal” effect. Therefore, sensory stimulation in patients should target the stroke territory and not randomly activate the lesional hemisphere. The core region of a typical MCA M1-segment occlusion in man includes the cortical representations of the contralateral hand as well as the primary auditory cortex, among others (e.g. mouth). Especially, auditory stimulation evoked by playing short stories via headphones has been shown to activate large cortical areas in stroke patients.45 The most standardized method of hand stimulation is electric stimulation of the median nerve.46 The activated area can be expanded to the motor cortex when combined with passive hand movement.47 Taken together, a continuous or alternating stimulation with short stories played over headphones, stimulation of the contralesional median nerve as well as passive hand movement (manually or assisted by a medical device) would seem to have the highest efficiency and clinical feasibility. However, before applying any kind of stimulation to stroke patients, the paradigms should be tested in healthy volunteers to determine the exact degree of cortical activation and potential logistical obstacles. In these studies, new stimulation designs can be studied as well to optimize targeted activation.
Determining the neuroprotective effect of sensory stimulation is challenging due to the overlap with revascularization therapy. To monitor the effects of sensory stimulation, more clinical examinations and cerebral imaging around the therapeutic time window are necessary. Additional MR imaging, immediately after the termination of sensory stimulation and 24 h after stimulation, is proposed as the most sensitive endpoints to determine the effect of neuronal activation on infarct progression. Additionally, the NIH Stroke Scale should be obtained after each 30-min interval of stimulation and at least twice during the following days in order to ascertain potential treatment-related deterioration or early improvement. The proposed mechanism that leads to neuroprotection is an increased blood flow in the activated area. A direct relation between sensory stimulation and cerebral blood flow can be revealed with a functional MRI protocol, using either BOLD or Arterial Spin Labeling.
Conclusion
Early sensory stimulation has been shown to have significant effects, both beneficial and detrimental, on stroke size and functional outcome. Experimental studies in rats suggest that cortical activation within 3 h after stroke onset redirects blood flow through anastomoses between cerebral arteries and thereby reperfuses the ischemic territory. Impaired neurovascular response, inadequate cerebral anastomoses as well as a later time point of stimulation antagonize this neuroprotective mechanism and might even worsen stroke outcome. Towards clinical translation, more experimental data are needed to define standards of safe sensory stimulation. However, stroke patients are already subject to extensive sensory stimulation during standard care, which underlines the urgency and relevance of clinical pilot trials.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Fondation Leducq Transatlantic Network “Evoked Neuronal Activity: A New Therapy for Acute Ischemic Stroke?” and NEUROWIND Young Scientist Award 2015 (von Bornstädt).
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Dimyan MA, Cohen LG. Neuroplasticity in the context of motor rehabilitation after stroke. Nat Rev Neurol 2011; 7: 76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Von Bornstädt D, Houben T, Seidel JL, et al. Supply-demand mismatch transients in susceptible peri-infarct hot zones explain the origins of spreading injury depolarizations. Neuron 2015; 85: 1117–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frostig RD, Lay CC, Davis MF. A rat’s whiskers point the way toward a novel stimulus-dependent, protective stroke therapy. Neuroscientist 2013; 19: 313–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer’s disease. Nat Rev Neurosci 2004; 5: 347–360. [DOI] [PubMed] [Google Scholar]
- 5.Chen-Bee CH, Agoncillo T, Xiong Y, et al. The triphasic intrinsic signal: implications for functional imaging. J Neurosci 2007; 27: 4572–4586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baker WB, Sun Z, Hiraki T, et al. Neurovascular coupling varies with level of global cerebral ischemia in a rat model. J Cereb Blood Flow Metab 2013; 33: 97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heiss WD, Hayakawa T, Waltz AG. Cortical neuronal function during ischemia. Effects of occlusion of one middle cerebral artery on single-unit activity in cats. Arch Neurol 1976; 33: 813–820. [DOI] [PubMed] [Google Scholar]
- 8.Latchaw RE, Yonas H, Hunter GJ, et al. Guidelines and recommendations for perfusion imaging in cerebral ischemia: a scientific statement for healthcare professionals by the writing group on perfusion imaging, from the Council on Cardiovascular Radiology of the American Heart Association. Stroke 2003; 34: 1084–1104. [DOI] [PubMed] [Google Scholar]
- 9.Lassen NA. Normal average value of cerebral blood flow in younger adults is 50 ml/100 g/min. J Cereb Blood Flow Metab 1985; 5: 347–349. [DOI] [PubMed] [Google Scholar]
- 10.Shiokawa O, Sadoshima S, Kusuda K, et al. Cerebral and cerebellar blood flow autoregulations in acutely induced cerebral ischemia in spontaneously hypertensive rats – transtentorial remote effect. Stroke 1986; 17: 1309–1313. [DOI] [PubMed] [Google Scholar]
- 11.Shen Q, Ren H, Cheng H, et al. Functional, perfusion and diffusion MRI of acute focal ischemic brain injury. J Cereb Blood Flow Metab 2005; 25: 1265–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lay CC, Davis MF, Chen-Bee CH, et al. Mild sensory stimulation reestablishes cortical function during the acute phase of ischemia. J Neurosci 2011; 31: 11495–11504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lay CC, Davis MF, Chen-Bee CH, et al. Mild sensory stimulation protects the aged rodent from cortical ischemic stroke after permanent middle cerebral artery occlusion. J Am Heart Assoc 2012; 1: e001255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bandla A, Le Teng Sherry C, Lim F, et al. Peripheral sensory stimulation is neuroprotective in a rat photothrombotic ischemic stroke model. Conf Proc IEEE Eng Med Biol Soc 2016; 2016: 6086–6089. [DOI] [PubMed] [Google Scholar]
- 15.Davis MF, Lay CC, Chen-Bee CH, et al. Amount but not pattern of protective sensory stimulation alters recovery after permanent middle cerebral artery occlusion. Stroke 2011; 42: 792–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wardlaw JM, Murray V, Berge E, et al. Thrombolysis for acute ischaemic stroke. Cochrane Database Syst Rev 2014; 7: CD000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hossmann KA. Viability thresholds and the penumbra of focal ischemia. Ann Neurol 1994; 36: 557–565. [DOI] [PubMed] [Google Scholar]
- 18.Heiss WD, Rosner G. Functional recovery of cortical neurons as related to degree and duration of ischemia. Ann Neurol 1983; 14: 294–301. [DOI] [PubMed] [Google Scholar]
- 19.Weimar C, Mieck T, Buchthal J, et al. Neurologic worsening during the acute phase of ischemic stroke. Arch Neurol 2005; 62: 393–397. [DOI] [PubMed] [Google Scholar]
- 20.Petersen CCH. The functional organization of the barrel cortex. Neuron 2007; 56: 339–355. [DOI] [PubMed] [Google Scholar]
- 21.Pyke KE, Tschakovsky ME. The relationship between shear stress and flow-mediated dilatation: implications for the assessment of endothelial function. J Physiol (Lond) 2005; 568(Pt 2): 357–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shin HK, Dunn AK, Jones PB, et al. Vasoconstrictive neurovascular coupling during focal ischemic depolarizations. J Cereb Blood Flow Metab 2006; 26: 1018–1030. [DOI] [PubMed] [Google Scholar]
- 23.Lay CC, Davis MF, Chen-Bee CH, et al. Mild sensory stimulation completely protects the adult rodent cortex from ischemic stroke. PLoS One 2010; 5: e11270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hossmann KA. Periinfarct depolarizations. Cerebrovasc Brain Metab Rev 1996; 8: 195–208. [PubMed] [Google Scholar]
- 25.Dreier JP, Fabricius M, Ayata C, et al. Recording, analysis, and interpretation of spreading depolarizations in neurointensive care: review and recommendations of the COSBID research group. J Cereb Blood Flow Metab 2017; 37: 1595–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hancock AM, Frostig RD. Testing the effects of sensory stimulation as a collateral-based therapeutic for ischemic stroke in C57BL/6J and CD1 mouse strains. PLoS One 2017; 12: e0183909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McColl BW, Carswell HV, McCulloch J, et al. Extension of cerebral hypoperfusion and ischaemic pathology beyond MCA territory after intraluminal filament occlusion in C57Bl/6J mice. Brain Res 2004; 997: 15–23. [DOI] [PubMed] [Google Scholar]
- 28.Luckl J, Baker W, Sun Z-H, et al. The biological effect of contralateral forepaw stimulation in rat focal cerebral ischemia: a multispectral optical imaging study. Front Neuroenergetics 2010; 2 DOI: 10.3389/fnene.2010.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krainik A, Hund-Georgiadis M, Zysset S, et al. Regional impairment of cerebrovascular reactivity and BOLD signal in adults after stroke. Stroke 2005; 36: 1146–1152. [DOI] [PubMed] [Google Scholar]
- 30.Manganotti P, Storti SF, Formaggio E, et al. Effect of median-nerve electrical stimulation on BOLD activity in acute ischemic stroke patients. Clin Neurophysiol 2012; 123: 142–153. [DOI] [PubMed] [Google Scholar]
- 31.Lay CC, Frostig RD. Complete protection from impending stroke following permanent middle cerebral artery occlusion in awake, behaving rats. Eur J Neurosci 2014; 40: 3413–3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lay CC, Jacobs N, Hancock AM, et al. Early stimulation treatment provides complete sensory-induced protection from ischemic stroke under isoflurane anesthesia. Eur J Neurosci 2013; 38: 2445–2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Engel O, Kolodziej S, Dirnagl U, et al. Modeling stroke in mice – middle cerebral artery occlusion with the filament model. J Vis Exp 2011; 47 DOI: 10.3791/2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lutz TA and Woods SC. Overview of animal models of obesity. Curr Protoc Pharmacol 2012; Chapter 5: Unit5.61. [DOI] [PMC free article] [PubMed]
- 35.Coyle P, Heistad DD. Blood flow through cerebral collateral vessels one month after middle cerebral artery occlusion. Stroke 1987; 18: 407–411. [DOI] [PubMed] [Google Scholar]
- 36.Chan S-L, Sweet JG, Bishop N, et al. Pial collateral reactivity during hypertension and aging: understanding the function of collaterals for stroke therapy. Stroke 2016; 47: 1618–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xue S, Gong H, Jiang T, et al. Indian-ink perfusion based method for reconstructing continuous vascular networks in whole mouse brain. PLoS One 2014; 9: e88067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Endres M, Gertz K, Lindauer U, et al. Mechanisms of stroke protection by physical activity. Ann Neurol 2003; 54: 582–590. [DOI] [PubMed] [Google Scholar]
- 39.Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 2015; 372: 1019–1030. [DOI] [PubMed] [Google Scholar]
- 40.Kunz A, Ebinger M, Geisler F, et al. Functional outcomes of pre-hospital thrombolysis in a mobile stroke treatment unit compared with conventional care: an observational registry study. Lancet Neurol 2016; 15: 1035–1043. [DOI] [PubMed] [Google Scholar]
- 41.Kunz A, Nolte CH, Erdur H, et al. Effects of ultraearly intravenous thrombolysis on outcomes in ischemic stroke: the STEMO (stroke emergency mobile) group. Circulation 2017; 135: 1765–1767. [DOI] [PubMed] [Google Scholar]
- 42.Ebinger M, Kunz A, Wendt M, et al. Effects of golden hour thrombolysis: a Prehospital Acute Neurological Treatment and Optimization of Medical Care in Stroke (PHANTOM-S) substudy. JAMA Neurol 2015; 72: 25–30. [DOI] [PubMed] [Google Scholar]
- 43.Pan J, Konstas A-A, Bateman B, et al. Reperfusion injury following cerebral ischemia: pathophysiology, MR imaging, and potential therapies. Neuroradiology 2007; 49: 93–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kloner RA, King KS and Harrington M. No-reflow phenomenon in heart and brain. Am J Physiol Heart Circ Physiol. Epub ahead of print 8 June 2018. DOI: 10.1152/ajpheart.00183.2018. [DOI] [PubMed]
- 45.Crinion J, Ashburner J, Leff A, et al. Spatial normalization of lesioned brains: performance evaluation and impact on fMRI analyses. Neuroimage 2007; 37: 866–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Backes WH, Mess WH, van Kranen-Mastenbroek V, et al. Somatosensory cortex responses to median nerve stimulation: fMRI effects of current amplitude and selective attention. Clin Neurophysiol 2000; 111: 1738–1744. [DOI] [PubMed] [Google Scholar]
- 47.Blatow M, Reinhardt J, Riffel K, et al. Clinical functional MRI of sensorimotor cortex using passive motor and sensory stimulation at 3 Tesla. J Magn Reson Imaging 2011; 34: 429–437. [DOI] [PubMed] [Google Scholar]