Abstract
The exact physiological function of amyloid-β precursor protein (APP) in endothelial cells is unknown. Endothelium-specific APP-deficient (eAPP−/−) mice were created to gain new insights into the role of APP in the control of vascular endothelial function. Endothelium-dependent relaxations to acetylcholine were significantly impaired in basilar arteries of global APP knockout (APP−/−) and eAPP−/− mice (P < 0.05). In contrast, endothelium-independent relaxations to nitric oxide (NO)-donor diethylamine-NONOate were unchanged. Western blot analysis revealed that protein expression of endothelial nitric oxide synthase (eNOS) was significantly downregulated in large cerebral arteries of APP−/− mice and eAPP−/− mice as compared to respective wild-type littermates (P < 0.05). Furthermore, basal levels of cyclic guanosine monophosphate (cGMP) were also significantly reduced in large cerebral arteries of APP-deficient mice (P < 0.05). In contrast, protein expression of prostacyclin synthase as well as levels of cyclic adenosine monophosphate (cAMP) was not affected by genetic inactivation of APP in endothelial cells. By using siRNA to knockdown APP in cultured human brain microvascular endothelial cells we also found a significant downregulation of eNOS mRNA and protein expressions in APP-deficient endothelium (P < 0.05). These findings indicate that under physiological conditions, expression of APP in cerebral vascular endothelium plays an important protective function by maintaining constitutive expression of eNOS.
Keywords: Amyloid precursor protein, amyloid-β, cerebral arteries, endothelium, endothelial nitric oxide synthase
Background
Large cerebral arteries contribute significantly to the cerebral vascular resistance thereby participating in the regulation of cerebral blood flow.1 Under physiological conditions, nitric oxide (NO) is continuously produced in endothelial cells by endothelial nitric oxide synthase (eNOS).2 In the brain, basal production of endothelial NO is essential vascular protective mechanism responsible for maintenance of normal cerebrovascular function.3–6 Endothelial dysfunction is characterized by a loss of biologically active NO produced in the endothelium and is one of the most important events in initiation and progression of cerebrovascular disease.7,8
The amyloid-β precursor protein (APP) is an integral membrane protein expressed abundantly in the endothelium of large cerebral blood vessels as well as brain microvessels.9–13 There are three isoforms of APPs, namely, APP695, APP751, and APP770, generated by alternative splicing of exons 7 and 8. APP695, predominantly expressed in neurons, is lacking two exons, Kunitz-type serine protease inhibitor (KPI) domain encoded by exon 7, and immunoregulatory OX-2 antigen domain (OX2) encoded by exon 8. APP751 and APP770 possess KPI domain while APP770 contains an additional OX2 domain.11,14 Both APP751 and APP770 are expressed in endothelial cells. According to amyloid-β hypothesis, APP plays a central role in the pathogenesis of Alzheimer’s disease.15 Dysregulation of amyloidogenic APP processing results in the generation of amyloid-β (Aβ) peptides responsible for development of amyloid plaques. Physiological role of APP in neuronal as well as in endothelial cells is incompletely understood.16–18 In the present study, we tested the hypothesis that APP plays an important role in control of endothelial function of cerebral arteries. To advance this hypothesis, we performed studies on cerebral blood vessels derived from global APP knockout (APP−/−) mice as well as in mice lacking APP only in the endothelium (eAPP−/−).
Materials and methods
Generation of APP−/− and eAPP−/− mice
All experimental protocols were reviewed and approved by the Institutional Animal Care and Use Committee of the Mayo Clinic and complied with the National Institute of Health Guide for Care and Use of Laboratory Animals and with the ARRIVE guidelines. Homozygous APP (APP−/−) mice on C57BL/6 background (stock #004133) and C57BL/6 (stock #000664) were obtained from the Jackson Laboratory (Bar Harbor, ME) and were bred together to generate heterozygous APP+/− mice, which then were used to generate wild-type littermates and APP−/− offspring. PCR of tail snips was performed to identify the genotype by using wild-type and APP primers according to the protocol from the Jackson Laboratory.
The heterozygous APPflox/+ mice on C57BL/6 background were kindly provided by Dr H. Zheng (Baylor College of Medicine, Houston, TX, USA). Originally, mice harboring loxP sites on either side of exon 3 of the APP gene were generated and backcrossed onto C57BL/6 background.19 Floxed APP allele (APPflox/flox) mice were generated in our laboratory and were identified by PCR using previously described primers.19 The female floxed offspring were crossed with male mice expressing Cre recombinase driven by the Tie2 promoter (Tie2-Cre; stock #004128, The Jackson Laboratory) to generate APPflox/+;Tie2-Cre− and APPflox/+;Tie2-Cre+ mice. The latter were used to breed with female APPflox/flox mice to generate APPflox/flox;Tie2-Cre− (control littermates) mice and APPflox/flox;Tie2-Cre+ (eAPP−/−) mice, and the genotyping was performed by using Cre and floxed APP primers (Figure 1(a)). In order to confirm endothelium-specific deletion of APP, mRNA was isolated from endothelial cells of mouse aorta for cDNA synthesis as described.20 A PCR analysis using primers spanning the deletion site (exon 3) in the APP gene (Genbank accession NM_001198823.1): forward: 5′-GCAGATCACAAACGTGGTGG-3′ and reverse: 5′-AGGAACTTGCACTTGTCGGG-3′ was performed (Figure 1(b)). The following PCR conditions were used: 94℃ for 30 s, 55℃ for 30 s, and 72℃ for 1 min (30 cycles). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) primer forward: 5′-TGCCAAGGCTGTGGGCAAGG-3′ and reverse: 5′-TGGGCCCTCAGATGCCTGCT-3′ was used as reference controls (Figure 1(b)).20
Figure 1.
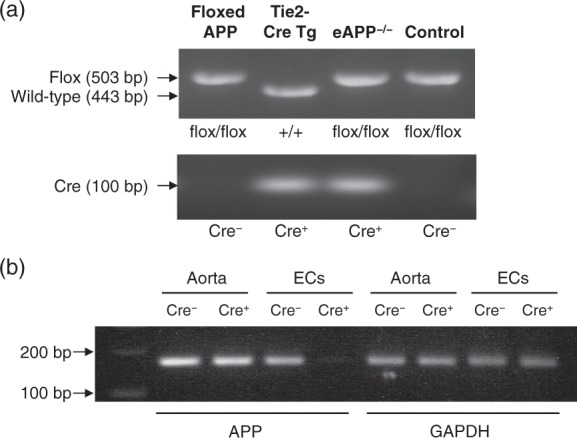
(a) Representative genotyping analysis of endothelium-specific amyloid precursor protein-deficient mice (eAPP−/− mice). Mice carrying loxP sites of APP gene (floxed APP) were bred with mice carrying the Tie2-Cre transgene (Tie2-Cre Tg) to generate eAPP−/− mice and wild-type littermates (control). The PCR products were 503 bp for floxed allele and 443 bp for wild-type allele (upper blot) as well as 100 bp for presence of Cre recombinase (Cre+, lower blot) Cre− indicates absence of Cre recombinase. (b) PCR analysis of APP mRNA in the aorta of wild-type littermates (APPflox/flox;Tie2-Cre−) and eAPP−/− (APPflox/flox;Tie2-Cre+) mice. Endothelial cells (ECs) were isolated from mouse aortas and subjected to PCR analysis. The remaining aortas without endothelium were also analyzed. Please note that the PCR product was 168 bp for the exon 3-deleted APP allele and it was absent in eAPP−/− mice ECs. GAPDH was used as a loading control (168 bp).
In addition, quantitative real-time (qRT)-PCR (CFX Connect, BioRad, Hercules, CA, USA) was performed to evaluate APP expression in large cerebral arteries with and without endothelium. Briefly, endothelial cells were mechanically removed by gently rubbing of internal lumen (of isolated large cerebral arteries from eAPP−/− mice and wild-type littermates) over a stainless steel wire with diameter of 0.005″ as previously described.21,22 Thereafter, one side of arteries was cannulated with glass micropipette and was flushed with phosphate buffered saline (Gibco, Thermo Fisher, Waltham, MA, USA) to remove remaining endothelial cells. The methods for isolation of total RNA and reverse transcription-PCR as well as primers for qRT-PCR were same as described above. The obtained mRNAs were analyzed with qRT-PCR using universal SYBR green supermix (BioRad) under the following conditions: denaturalization at 95℃ for 15 s and annealing at 58℃ for 30 s. Copy numbers of the target gene were expressed as 2−ΔCT (where ΔCT = CT of targeted gene (APP) − CT of internal control gene (GAPDH), and CT represents the threshold cycle).23
All mice were maintained on standard chow with free access to drinking water. Sixteen- to 24-week-old male mice were anaesthetized with overdose of pentobarbital (200–250 mg/kg BW, i.p.) followed by exsanguination via cardiac puncture for blood collection. Investigations were not blinded regarding groups of animals studied (wild-type versus knockout mice). The brains were removed and placed in cold (4℃) Krebs solution [composition (in mmol/L): NaCl 118.6; KCl 4.7; CaCl2 2.5; MgSO4 1.2; KH2PO4 1.2; NaHCO3 25.1; glucose 10.1; EDTA 0.026]. The basilar arteries were carefully isolated for vascular reactivity studies, and the remaining large cerebral arteries (middle cerebral, anterior cerebral, and posterior cerebral arteries) were isolated for biochemical and molecular biological analyses.
Systolic blood pressure
Systolic blood pressure was recorded in non-anesthetized mice by a tail-cuff method (Harvard Apparatus Ltd., Kent, UK) as described previously.24
Blood cell count
Blood cell count was performed in whole blood using VetScan® HM5 Hematology System (Abaxis, Union City, CA, USA).25
Plasma levels of Aβ1-40, sAPPα, and sAPPβ
Blood samples were transferred to a tube containing EDTA, centrifuged at 2000 r/min (10 min, 4℃), and stored at −80℃ until assayed. Mouse-specific Aβ1-40 ELISA kit (Invitrogen, Camarillo, CA, USA) as well as highly sensitive mouse/rat-specific sAPPα ELISA and mouse-specific sAPPβ ELISA kits (IBL America, Minneapolis, MN, USA) were used to perform the measurements of circulating levels of Aβ1-40, sAPPα, and sAPPβ, respectively.26
Vascular reactivity studies in isolated basilar arteries
Basilar artery was dissected free from surrounding tissues in cold Krebs solution under a microscope (Leica Wild M3C) and was transferred to small vessel chamber filled with Krebs solution. Vascular reactivity was studied in-vitro under pressurized conditions using a video dimension analyzer system (Living Systems Instrumentation, Burlington, VT, USA) as described elsewhere.25,27 Endothelium-dependent relaxations to acetylcholine (10−9 to 10−5 mol/L; Sigma, St. Louis, MO, USA) and endothelium-independent relaxations to the NO donor diethylammonium (Z)-1-(N,N-diethylamino) diazen-1-ium-1,2-diolate (DEA-NONOate; 10−9 to 10−5 mol/L; Cayman Chemical, Ann Arbor, MI, USA) were obtained during submaximal contractions to 9,11-dideoxy-9α,11α-methanoepoxy prostaglandin F2α (U46619; 3 × 10−8–3 × 10−7 mol/L; Cayman Chemical). Between each protocol, the system was washed out with Krebs solution and then equilibrated for 30 min. In separate experiments, contractions to endothelin-1 (10−11–10−7 mol/L; EMD Millipore, Billerica, MA, USA) were performed. At the end of experiments, the basilar arteries were washed out with Ca2+ free Krebs solution of the following composition (in mmol/L): NaCl 130.8; KCl 4.7; MgSO4 1.2; KH2PO4 1.2; NaHCO3 25.1; glucose 10.1; EGTA 2.0] in the presence of 10−4 mol/L papaverine in order to achieve maximal dilatation used for measurements of lumen diameter.
Knockdown studies of APP by small interfering RNA
Human brain microvascular endothelial cells (hBMECs) obtained from Applied Cell Biology Research Institute (Kirkland, WA, USA) were grown in endothelial growth medium 2 (EGM-2; Lonza, Basel, Switzerland). At 30–50% confluence, hBMECs were transfected with 30 nM of human ON-TARGETplus APP siRNA or control siRNA (GE Dharmacon, Lafayette, CO) in the presence of Lipofectamine 2000 (Invitrogen, Camarillo, CA, USA) for three days as described.10
Western blot studies
Large cerebral arteries and hBMECs were homogenized in lysis buffer [composition (in mmol/L) 50 NaCl, 50 NaF, 50 sodium pyrophosphate, 5 EDTA, 5 EGTA, 0.1 Na3VO4, 10 HEPES, 0.5 PMSF, and 10 µg/mL leupeptin, and 1% Triton X-100, pH 7.4] and stored at −80℃ until assayed.26 Equal amount of protein (15 µg) were separated using SDS-polyacrylamide gel electrophoresis and transferred onto nitrocellulose membrane. Primary antibodies against APP (Invitrogen), eNOS (BD Biosciences, San Jose, CA, USA), and prostaglandin I2 (PGI2) synthase (Cayman) were used. As a loading control, blots were rehybridized with β-actin (Sigma). Densitometry was carried out using Odyssey imaging system (Li-Cor Biosciences, Lincoln, NE, USA).
Quantitation of eNOS mRNA
Total RNA was extracted from hHBMECs using RNeasy Plus Mini kit (Qiagen, Redwood City, CA, USA), and a reverse transcription of RNA to cDNA was performed using SuperScript III First-Strand Synthesis System kit (Invitrogen) as described.10 The amount of target cDNA was quantified (in triplicate for each sample) using PCR with human specific eNOS (forward: 5′-GAGATGCTGTTGAAGCGGAT-3′ and reverse: 5′- GGTAACCAGCACATTTGGGA-3′ and β-actin primers.28
Measurement of intracellular superoxide anions
Intracellular superoxide anion levels in large cerebral arteries were quantified using a HPLC-based fluorescence detection of the oxidative product 2-hydroxyethidium.12,24 In brief, large cerebral arteries were equilibrated for 15 min in Krebs-HEPES buffer at 37℃ in CO2 incubator after which they were incubated with 50 µM dihydroethidium (Molecular Probes, Eugene, OR, USA) for additional 15 min. The samples were then washed of dihydroethidium and incubated for additional 1 h in fresh Krebs-HEPES (20 mM) buffer at 37℃. The arteries were homogenized in 4℃ cold methanol and centrifuged at 10,000 r/min. The supernatants were analyzed by HPLC/fluorescence (Beckman Coulter, Brea, CA, USA) in 37% acetonnitrile in 0.1% trifluoroacetic acid aqueous solution. Data were quantified using 2-hydroxyethidium standard from the reaction between dihydroethidium and Fremy’s salt29 and normalized against tissue protein levels.
Determination of cyclic nucleotides levels
Colorimetric ELISA kits (Cell Biolabs Inc., San Diego, CA, USA) were used for measurements of cyclic guanosine 3′,5′-monophosphate (cGMP) and cyclic adenosine 3′,5′-monophosphate (cAMP) concentrations. Large cerebral arteries were incubated for 120 min in GIBCO® minimal essential medium (containing 0.1% BSA, 100 U/mL penicillin and 100 µg/mL streptomycin; Thermo Fischer Scientific Inc., Waltham, MA, USA) in the presence of 100 µmol/L 3-isobutyl-1-methylxanthine in order to inhibit the degradation of cyclic nucleotides by phosphodiesterases. After incubation, the arteries were removed, quickly frozen in liquid N2, and stored at −80℃ until assayed. The arteries were homogenized in lysis buffer provided by the kit and after centrifugation the supernatants were subjected to cGMP and cAMP measurements according to the manufacturer’s instructions.
Calculations and statistical analyses
All results are expressed as means ± SD and “n” denotes the number of animals from which tissues were harvested. Efficacy (maximal relaxation expressed as % of increase in intraluminal diameter measured during contraction with U46619 or maximal contraction expressed as percentage of decrease in the basal diameter) as well as the potency of the drugs (expressed as negative logarithm of the concentration that caused half-maximal response [pEC50 value]30) was determined for each individual concentration–response curve by non-linear regression analysis.31 An unpaired Student’s t-test was used to compare APP−/− and eAPP−/− mice with their wild-type littermate control mice. In addition, two-way ANOVA was used for multiple comparisons where appropriate. The concentration–response curves of the different groups were compared by ANOVA for repeated measurements followed by Bonferroni’s correction (StatView). For these analyses, the group-by-concentration interaction effect was used to assess whether the response to a given agent differed between groups. A value of P < 0.05 was considered statistically significant.
Results
Characteristics of APP−/− and eAPP−/− mice
Body weight was significantly reduced in APP−/− as reported previously18,32 (P < 0.05 vs. wild-type littermates) but not in eAPP−/− mice (Table 1). Systolic blood pressure was unaltered in eAPP−/− mice (118 ± 5 mmHg; P = n.s. vs. wild-type littermates: 112 ± 4 mmHg; n = 5 per group). Moreover, lumen diameter of completely relaxed isolated basilar arteries was not different between wild-type littermates and APP−/− mice (181 ± 13 µm [n = 7] and 185 ± 13 µm [n = 6], respectively) as well as between wild-type littermates and eAPP−/− (178 ± 11 µm [n = 13] and 182 ± 10 µm [n = 14], respectively).
Table 1.
Characteristics of APP−/− and eAPP−/− mice.
| Parameters | Wild-type littermates | APP−/− | Wild-type littermates (APPflox/flox;Tie2-Cre−) | eAPP−/− (APPflox/flox;Tie2-Cre+) |
|---|---|---|---|---|
| Body weight (g) | 31 ± 2 (6) | 26 ± 1 (6)* | 29 ± 1 (10) | 28 ± 1 (10) |
| White blood cells (109/L) | 12.9 ± 2.8 (8) | 12.0 ± 5.0 (7) | 11.0 ± 2.9 (9) | 12.7 ± 2.7 (10) |
| Lymphocytes (109/L) | 11.9 ± 2.4 (8) | 10.9 ± 4.7 (7) | 9.3 ± 2.8 (9) | 11.1 ± 2.3 (10) |
| Monocytes (109/L) | 0.3 ± 0.1 (8) | 0.4 ± 0.3 (7) | 0.4 ± 0.3 (9) | 0.3 ± 0.2 (10) |
| Granulocytes (109/L) | 0.7 ± 0.4 (8) | 0.9 ± 0.9 (7) | 1.3 ± 0.9 (9) | 1.2 ± 0.8 (10) |
| Red blood cells (1012/L) | 9.3 ± 0.3 (8) | 9.0 ± 0.3 (7) | 9.4 ± 0.9 (9) | 9.7 ± 0.7 (10) |
| Hematocrit (%) | 40 ± 1 (8) | 39 ± 2 (7) | 41 ± 3 (9) | 41 ± 2 (10) |
| Hemoglobin (g/dL) | 14.6 ± 0.5 (8) | 14.2 ± 0.9 (7) | 14.5 ± 0.8 (9) | 14.6 ± 1.1 (10) |
| Platelets (109/L) | 745 ± 66 (8) | 515 ± 168 (7)* | 483 ± 180 (9) | 577 ± 81 (10) |
| Aβ1-40 (pg/mL) | 124 ± 40 (3) | n.d. (3) | 194 ± 53 (12) | 165 ± 50 (12) |
| sAPPα (pg/mL) | 271 ± 63 (3) | n.d. (3) | 338 ± 32 (15) | 232 ± 66 (15)* |
| sAPPβ (pg/mL) | ND | ND | 64 ± 5 (16) | 59 ± 5 (16)* |
Note: Data are means ± SD, and the numbers of mice are indicated in the parentheses. ND: not determined; n.d.: not detectable.
P < 0.05 vs. wild-type littermates (unpaired Student’s t-test).
Blood cell counts were performed as there are reported studies showing that loxP/Cre technology may also affect hematopoiesis.33,34 However, analysis of peripheral blood cell count indicated that the profile of blood cells was unaltered in eAPP−/− mice as well as in APP−/− mice as compared to their respective wild-type littermates (Table 1). An exception was cell number of platelets that was significantly reduced in APP−/− mice (P < 0.05 vs. wild-type littermates; Table 1).
We also examined the plasma levels of Aβ1-40 as well as alpha- and beta-secretase cleaved soluble APP (sAPPα and sAPPβ, respectively). Levels of Aβ1-40 and sAPPα were undetectable in plasma of APP−/− mice (Table 1). However, circulating levels of sAPPα and sAPPβ were significantly reduced in eAPP−/− mice (P < 0.05 as compared to wild-type littermates; Table 1). Circulating levels of Aβ1-40 tended to decrease in eAPP−/− mice but they did not reach statistical significance (P = 0.1779; Table 1).
Endothelial function
Endothelium-dependent relaxations to acetylcholine were significantly impaired in isolated basilar arteries of APP−/− mice compared to wild-type littermates (group-by-concentration interaction P < 0.05; Figure 2(a)). Moreover, endothelium-specific deletion of APP reduced relaxations to acetylcholine in eAPP−/− mice compared to wild-type littermates (group-by-concentration interaction P < 0.05; Figure 2b). Indeed, the efficacy of acetylcholine was significantly decreased in APP−/− (28 ± 15%; n = 6) as well as in eAPP−/− (30 ± 13%; n = 7) mice (P < 0.05 vs. respective wild-type littermates: 54 ± 16% [n = 8] and 47 ± 16% [n = 8], respectively). However, the responses to acetylcholine were equipotent between wild-type littermates (pEC50: 7.2 ± 0.7; n = 8) and APP−/− mice (pEC50: 7.3 ± 0.7; n = 6) as well as between wild-type littermates (pEC50: 7.2 ± 0.2; n = 8) and eAPP−/− mice (pEC50: 6.9 ± 0.4; n = 7). Contractions to U46619 were not different between wild-type littermates and APP−/− mice (18 ± 6% [n = 8] and 16 ± 5% [n = 6], respectively) as well as between littermates and eAPP−/− mice (23 ± 5% [n = 8] and 21 ± 3% [n = 7], respectively). Endothelium-independent relaxations to NO-donor DEA-NONOate were unaffected in APP−/− mice or in eAPP−/− mice as compared to their respective wild-type littermates (Figure 2(c) and (d), respectively).
Figure 2.
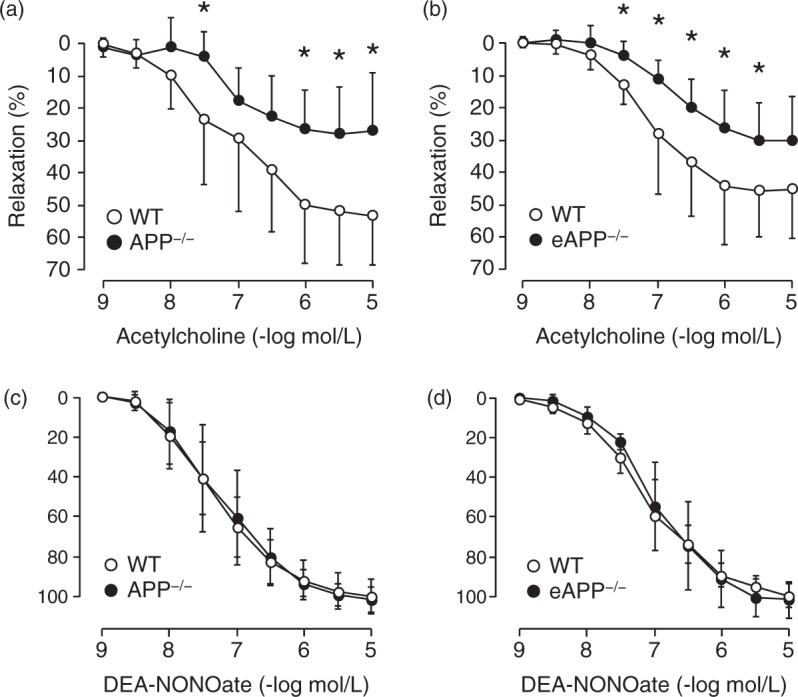
Endothelial function in isolated basilar arteries of APP−/− and eAPP−/− mice. Endothelium-dependent relaxations to acetylcholine were significantly reduced in APP−/− mice compared to wild-type littermates (a; n = 6 APP−/−; n = 8 wild-type; group-by-concentration interaction P < 0.05) and eAPP−/− mice compared to wild-type littermates (b; n = 7 eAPP−/−; n = 8 wild-type; group-by-concentration interaction P < 0.05). In contrast, endothelium-independent relaxations to NO-donor DEA-NONOate were unaffected in APP−/− mice (c; n = 6 APP−/−; n = 8 wild-type; group-by-concentration interaction P > 0.05) and eAPP−/− mice (d; n = 5 eAPP−/−; n = 6 wild-type; group-by-concentration interaction P > 0.05). All results are shown as mean ± SD and relaxations are expressed as percentage of the increase in intraluminal diameter from contraction with U46619 (10−8 to 3 × 10−8 mol/L). *Differences with respect to single concentration between wild-type littermates and APP−/− mice or between wild-type littermates and eAPP−/− mice are statistically significant (P < 0.05; unpaired Student’s t-test).
Expressions of APP, eNOS, and PGI2 synthase
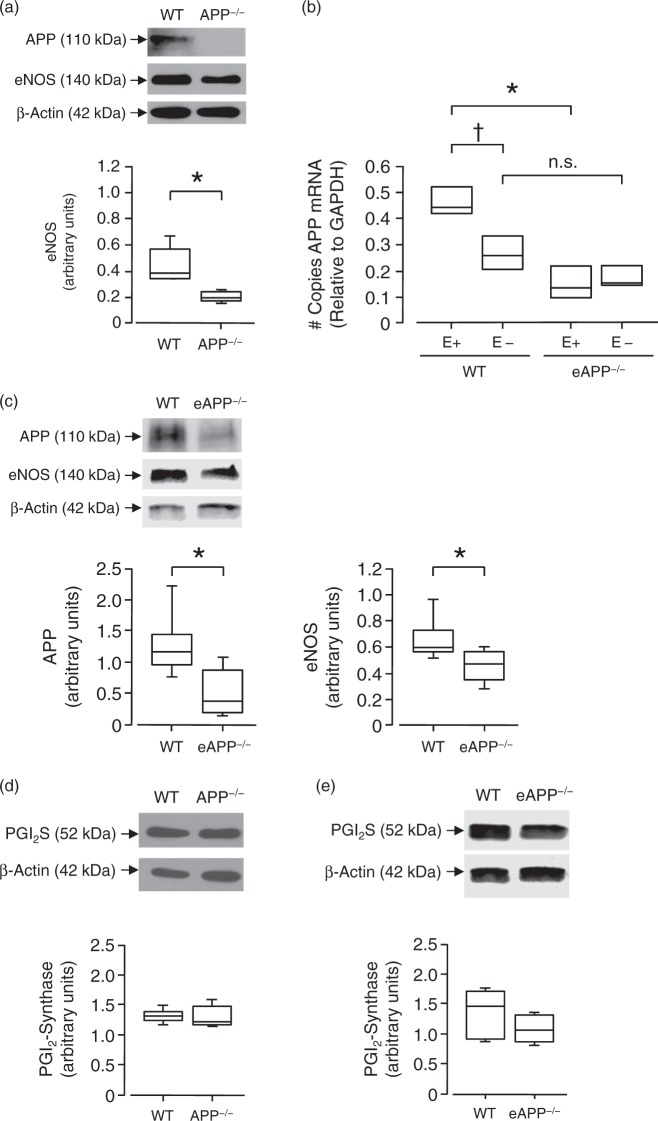
Western Blot analyses confirmed that APP protein expression is absent in large cerebral arteries of APP−/− mice (Figure 3(a)). qRT-PCR analysis revealed that expression of APP mRNA was significantly reduced in large cerebral arteries of eAPP−/− mice (P < 0.05 vs. wild-type littermates; Figure 3(b)). Furthermore, removal of endothelium significantly reduced expression of APP in large cerebral arteries of wild-type littermates but did not affect APP expression in eAPP−/− mice (Figure 3(b)). Moreover, there is no significant difference between wild-type littermates and eAPP−/− mice arteries without endothelium (Figure 3(b)) suggesting that expression of APP is abolished in endothelium of eAPP−/− mice. Protein expression of APP is also reduced by 60% in large cerebral arteries of eAPP−/− mice (P < 0.05 vs. wild-type littermates; Figure 3(c)).
Figure 3.
Representative Western blot and qRT-PCR analyses of large cerebral arteries. (a) Protein expressions of APP and eNOS in wild-type littermates and APP−/− mice (n = 5 for each group). *P < 0.05 vs. wild-type (WT) littermates (unpaired Student’s t-test). (b) qRT-PCR analysis of APP mRNA in large cerebral arteries of wild-type littermates (APPflox/flox;Tie2-Cre−) and eAPP−/− (APPflox/flox;Tie2-Cre+) mice in the presence and absence of endothelial cells (n = 3 independent experiments for each group). Please note that APP mRNA was significantly reduced in wild-type mice without endothelium (E-) while it was unchanged in eAPP−/− mice without endothelium. *P < 0.05 vs. wild-type (WT) littermates; †P < 0.05 vs. wild-type littermates with endothelium (E+); n.s. indicates not significant (two-way ANOVA). (c) Protein expressions of APP and eNOS in wild-type littermates and eAPP−/− mice (n = 6 for each group). *P < 0.05 vs. wild-type (WT) littermates (unpaired Student’s t-test). (d) Protein expression of prostaglandin I2 (PGI2) synthase in wild-type littermates and APP−/− mice (n = 5 for each group). (e) Protein expression of PGI2 synthase in wild-type littermates and eAPP−/− mice (n = 5 for each group). All western blot results are the relative densitometry compared with β-actin protein. All results are represented as box plots with whiskers showing the median, the 25th to 75th percentiles, and min–max range.
Most importantly, protein expression of eNOS was significantly reduced in large cerebral arteries of APP−/− mice (P < 0.05 vs. wild-type littermates; Figure 3(a)). Moreover, eNOS expression was significantly reduced in eAPP−/− mice (P < 0.05 vs. wild-type littermates; Figure 3(c)). In contrast, protein expression of PGI2 synthase was not affected in large cerebral arteries derived from both APP−/− and eAPP−/− mice (Figure 3(d) and (e), respectively).
Levels of cyclic nucleotides
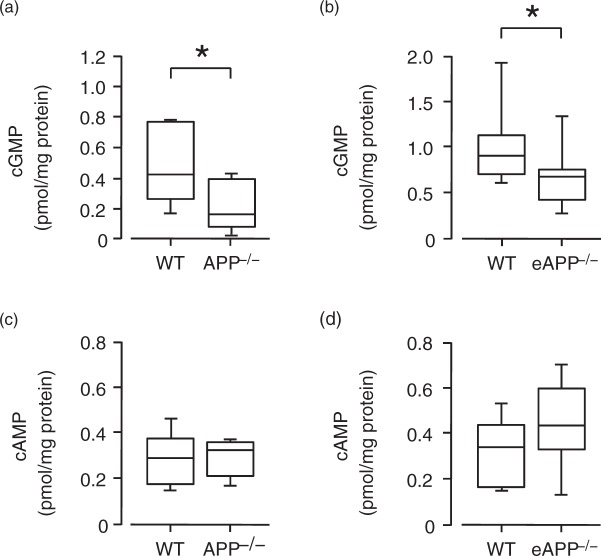
Basal cGMP levels were significantly reduced in large cerebral arteries of APP−/− and eAPP−/− mice as compared to their respective wild-type littermates (P < 0.05; Figure 4(a) and (b), respectively). Contrary to cGMP, basal cAMP levels were unchanged in APP−/− and eAPP−/− mice (P > 0.05; Figure 4(c) and (d), respectively).
Figure 4.
Basal levels of cGMP and cAMP in large cerebral arteries. (a) cGMP levels were significantly reduced in APP−/− mice as compared to wild-type (WT) littermates (n = 7 per group). (b) cGMP levels were significantly reduced in eAPP−/− mice as compared to wild-type (WT) littermates (n = 11 per group). (c) cAMP levels in wild-type (WT) littermates and APP−/− mice (n = 6 per group). (d) cAMP levels in wild-type (WT) littermates and eAPP−/− mice (n = 7 per group). Results were normalized against tissue protein levels, and box plots with whiskers are showing the median, the 25th to 75th percentiles, and min–max range. *P < 0.05 vs. wild-type (WT) littermates (unpaired Student’s t-test).
Superoxide anion production
Levels of intracellular superoxide anion were not different between large cerebral arteries isolated from eAPP−/− mice and their wild-type littermates (1.44 ± 0.55 nmol/mg and 1.23 ± 0.25 nmol/mg, respectively; P > 0.05; n = 7 per group).
Cell culture studies of hBMECs
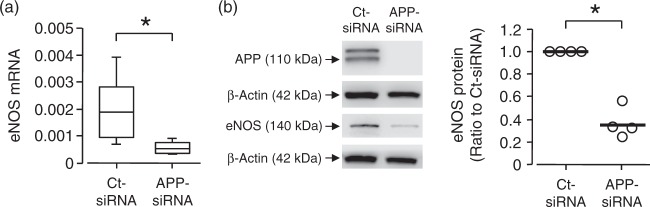
To confirm that downregulation of eNOS is caused by genetic inactivation of APP in endothelium rather than some systemic alterations in APP-deficient mice, we treated hBMECs with siRNA against APP for three days. Treatment of hBMECs with APP siRNA resulted in significant attenuation of eNOS mRNA expression in hBMECs (P < 0.05 vs. control siRNA, Figure 5(a)). Moreover, Western blot analysis revealed that knockdown of APP decreased protein expression of eNOS in hBMECs (P < 0.05 vs. control siRNA, Figure 5(b)).
Figure 5.
Expression of eNOS mRNA and protein in human BMECs. Cells were transduced with siRNA against APP (APP-siRNA) or control siRNA (Ct-siRNA) for three days. Exposure of HBMECs to APP-siRNA resulted in significantly attenuated expression of eNOS mRNA (A; n = 5 per group) and protein (B; n = 4 per group). Please note that APP expression is absent in APP-siRNA treated BMECs. eNOS expression was normalized to β-actin. Box plots with whiskers are showing the median, the 25th to 75th percentiles, and min–max range. Bars in dot plot are representing the mean. *P < 0.05 vs. Ct-siRNA (unpaired Student’s t-test).
Contractions to endothelin-1
Concentration-dependent contractions to endothelin-1 were significantly enhanced in basilar arteries of eAPP−/− mice compared to wild-type littermates (group-by-concentration interaction P < 0.05; Figure 6). Moreover, the efficacy of ET-1 responses was increased in eAPP−/− mice (51 ± 6%; n = 7) as compared to their wild-type littermates (36 ± 8%; n = 6; P < 0.05). In contrast, the responses to ET-1 were equipotent among groups of mice (pEC50 for wild-type: 8.7 ± 0.4 [n = 6] and eAPP−/−: 8.8 ± 0.4 [n = 7]).
Figure 6.
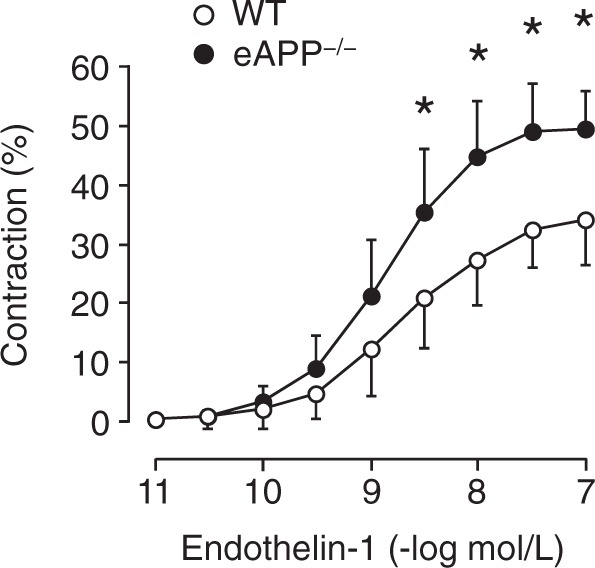
Concentration-dependent contractions to endothelin-1 in isolated basilar arteries of wild-type littermates and eAPP−/− mice. The contractions to endothelin-1 were expressed as percentage of the decrease in the basal intraluminal diameter. Results are mean ± SD (n = 6 for wild-type (WT) littermates and n = 7 for eAPP−/− mice). *Difference with respect to single concentration between wild-type littermates and eAPP−/− mice is statistically significant (P < 0.05; unpaired Student’s t-test).
Discussion
This is the first study to examine the effects of APP deficiency on vascular function in mice and in cultured cerebrovascular endothelial cells. We report several major new findings. First, endothelium-dependent relaxations to acetylcholine were impaired in basilar arteries of APP−/− and eAPP−/− mice while endothelium-independent relaxations to NO donor were unaltered. Second, eNOS protein expression and cGMP levels were significantly reduced in large cerebral arteries of APP−/− and eAPP−/− mice. Third, knockdown of the APP gene by siRNA led to downregulation of eNOS mRNA and protein in cultured hBMECs. Our results demonstrate that in cerebrovascular endothelium, APP regulates expression and function of eNOS thereby exerting vascular protective properties under physiological conditions.
There are numerous lines of evidence demonstrating expression and processing of APP in aortic and brain endothelial cells.9,11,17,26,35 Notably, endothelial cells of cerebral blood vessels express higher levels of APP protein as compared to peripheral endothelial cells.11 However, functional relevance of this observation is unknown and remains to be determined. The non-amyloidogenic processing of APP is constitutive in endothelium, and cleavage fragments of APP produced and released by the endothelium are part of normal cellular metabolism.36 Hence, the presence of sAPPα and sAPPβ in circulation suggests that APP and its cleavage products might play a role in normal human physiology.16,37
In the present study, we found that endothelium-dependent relaxations to acetylcholine were impaired in basilar arteries derived from APP−/− mice while relaxations to DEANONOate were not affected. These findings suggest that loss of APP in the endothelium causes selective impairment of endothelial function. Indeed, reactivity of smooth muscle cells to NO is not impaired in arteries derived from APP−/− mice. Moreover, our studies showed that eNOS protein expression was significantly downregulated in large arteries of APP−/− mice thereby providing the most likely mechanism responsible for observed alterations in vasomotor function in APP−/− mice. In order to test the selectivity of APP effects on endothelial function, we generated eAPP−/− mice lacking APP exclusively in endothelial cells. Consistent with findings in APP−/− mice, eNOS protein expression is downregulated and endothelium-dependent relaxations to acetylcholine were significantly impaired in basilar arteries of eAPP−/− mice. In contrast, endothelium-independent relaxations to DEANONOate were unchanged. It is also important to note that APP as well as all enzymes required for APP processing are present in vascular smooth muscle cells.38 However, our findings clearly indicate that the endothelium (rather than smooth muscle) is responsible for the impaired vasomotor function in APP−/− mice. Moreover, systolic blood pressure was unchanged in eAPP−/− mice demonstrating that endothelial dysfunction observed in eAPP−/− mice was not caused by chronic increase in arterial blood pressure.
Our in vivo observations were confirmed by in vitro experiments demonstrating that genetic silencing of APP expression by siRNA significantly reduced expression of eNOS mRNA and protein in hBMECs. The observations made in cultured endothelium, support the interpretation that endothelial APP-deficiency per se (rather than alteration of some systemic factors) is responsible for loss of eNOS and endothelial dysfunction. We further investigated whether increased vascular superoxide anion production may be responsible for reduced bioavailability of NO and impaired endothelium-dependent vasodilatation.7 However, we did not detect any changes in superoxide anion levels in large cerebral arteries of eAPP−/− mice. In aggregate, these findings suggest that reduced expression of eNOS is the most likely explanation for the endothelial dysfunction observed in APP-deficient cerebral arteries. On the other hand, endothelial dysfunction was also observed in Tg2576 transgenic mice overexpressing of mutated human APP.25,39 However, upregulated eNOS expression and increased production of superoxide anion were detected in these mice thereby causing reduced bioavailability of tetrahydrobiopterin and eNOS uncoupling.25,39 Thus, in agreement with hypothesis of the present study, overexpression of APP increases expression of eNOS. Our studies showed that basal levels of cGMP, the second messenger of NO, were significantly reduced in APP−/− mice and in eAPP−/− mice. This is in line with the observations made in eNOS-deficient mice demonstrating that loss of endogenous NO production via eNOS in vascular endothelium is responsible for reduction in basal cGMP levels.40 Furthermore, in agreement with our findings, exogenous NO administration by NO-donor induced comparable increase in cyclic GMP and vasodilatation in wild type mice and eNOS-deficient mice. Likewise, it is important to note that loss of endogenous NO and reduced basal concentration of cyclic GMP does not inhibit, but in some studies even enhances, vasodilator responses to NO-donors.41,42
We also demonstrated that protein expression of PGI2 synthase as well as levels of cAMP were not altered in large cerebral arteries of APP−/− or eAPP−/− mice indicating that the metabolism of PGI2, which dilates vascular smooth muscle cells directly, is unaffected. These findings suggest that selective loss of eNOS/cGMP signaling is present in APP-deficient cerebral arteries. However, one limitation of our study is that we did not test other vasodilators such as adenosine, which may relax vascular smooth muscle cells of large cerebral arteries independently of cAMP signaling.43
Endothelin-1 is a potent vasoconstrictor and mitogen both in vitro and in vivo.44 Interestingly, our study shows that contractions to endothelin-1 are significantly enhanced in isolated basilar arteries of eAPP−/− mice. Reduced expression of eNOS observed in cerebral arteries is the most likely explanation for enhancement of endothelin-1 induced contractions. Our findings are consistent with the results of previous studies showing that under physiological conditions, NO inhibits endothelin-1 production via a cGMP-dependent mechanism and reduces endothelin-1-induced vasoconstriction of vascular smooth muscle cells.45–47
As blood–brain barrier prevents the influx of soluble forms of APP770 and APP751 from the circulation into the brain, their concentrations are markedly lower in cerebrospinal fluid as compared to their circulating levels indicating that circulating cleavage products of APP are mostly derived from peripheral sources of APP770 and APP751 including platelets and endothelial cells.14,48 Notably, circulating levels of sAPPα were reduced (∼30%) in eAPP−/− mice demonstrating that endothelial cells synthetize and release sAPPα. We also detected a slight decrease in circulating sAPPβ in eAPP−/− mice; however, the difference was much smaller as compared to sAPPα. Similarly, levels of Aβ1-40 tended to decrease but observed difference did not reach statistical significance thereby reinforcing the concept that non-amyloidogenic processing of APP is major constitutively active pathway in APP metabolism in endothelium.
Platelets count was significantly decreased in APP−/− mice but not in eAPP−/− mice. The reason is unclear but this could be related to the adaptive mechanisms designed to prevent pro-thrombotic function of platelets during APP deficiency. It is well known that human platelets contain high levels of APP which are mainly cleaved by non-amyloidogenic APP processing upon stimulation.49–51 Since release of soluble APP has been reported to inhibit platelet activation via negative feedback regulation, global loss of APP might increase the risk of platelet aggregation and thrombosis.52,53 Furthermore, several studies indicate that platelets can produce up to 90% of circulating Aβ (mainly Aβ1-40 peptide).54,55 However, it is well established that generation of sAPPα in platelets is higher than Aβ peptides.55,56 Since the circulating levels of Aβ1-40 were unaltered in eAPP−/− mice, but undetectable in APP−/− mice, an involvement of circulating Aβ1-40 in downregulation of eNOS expression could be excluded. Whether APP and/or other cleavage products of APP have direct or indirect effects on expression and function of eNOS remains to be determined.17
Our findings may help to explain beneficial effects of APP in experimental models of cerebral ischemia. Indeed, APP−/− mice are vulnerable to hypoxic injury and mortality rate is significantly higher in APP−/− mice exposed to cerebral ischemia as compared to wild-type animals.57 Conversely, overexpression of wild-type APP in neuronal tissue exerts neuroprotective effect during ischemic injury induced by middle cerebral artery occlusion.58 Notably, prior studies demonstrated that APP maturation and endogenous APP processing were impaired during cellular aging59 thereby suggesting that aging-induced loss of APP function might increase the susceptibility to ischemia. The exact relative contribution of APP expression in vascular endothelium to protective effect of APP against brain ischemia will have to be determined in the future studies.
In conclusion, the present in vivo and in vitro studies demonstrate that the loss of APP in vascular endothelium decreases expression and function of eNOS. Our observations provide entirely novel insights into the previously unrecognized vascular protective function of APP.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by National Institutes of Health R01 grants HL-111062 and HL-131515 and by the Mayo Foundation.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
Ld’U contributed to the experimental design and performance, generated mice, performed vascular reactivity experiments, performed studies in eAPP−/− mice, data collection and analysis, and was took part in manuscript writing. TH performed cell culture experiments, data collection and analysis, and manuscript writing; AS performed studies in APP−/− mice, data collection and analysis; ZK took part in the experimental design, data interpretation, and manuscript writing.
References
- 1.Faraci FM, Heistad DD. Regulation of large cerebral arteries and cerebral microvascular pressure. Circ Res 1990; 66: 8–17. [DOI] [PubMed] [Google Scholar]
- 2.Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev 1991; 43: 109–142. [PubMed] [Google Scholar]
- 3.Atochin DN, Demchenko IT, Astern J, et al. Contributions of endothelial and neuronal nitric oxide synthases to cerebrovascular responses to hyperoxia. J Cereb Blood Flow Metab 2003; 23: 1219–1226. [DOI] [PubMed] [Google Scholar]
- 4.Ma J, Meng W, Ayata C, et al. L-NNA-sensitive regional cerebral blood flow augmentation during hypercapnia in type III NOS mutant mice. Am J Physiol 1996; 271: H1717–1719. [DOI] [PubMed] [Google Scholar]
- 5.Sobey CG, Faraci FM. Effects of a novel inhibitor of guanylyl cyclase on dilator responses of mouse cerebral arterioles. Stroke 1997; 28: 837–842; discussion 842–833. [DOI] [PubMed] [Google Scholar]
- 6.Santhanam AV, d’Uscio LV, Smith LA, et al. Uncoupling of eNOS causes superoxide anion production and impairs NO signaling in the cerebral microvessels of hph-1 mice. J Neurochem 2012; 122: 1211–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Faraci FM. Protecting against vascular disease in brain. Am J Physiol Heart Circ Physiol 2011; 300: H1566–H1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Atochin DN, Huang PL. Role of endothelial nitric oxide in cerebrovascular regulation. Curr Pharm Biotechnol 2011; 12: 1334–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Austin SA, Santhanam AV, Katusic ZS. Endothelial nitric oxide modulates expression and processing of amyloid precursor protein. Circ Res 2010; 107: 1498–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He T, Santhanam AV, Lu T, et al. Role of prostacyclin signaling in endothelial production of soluble amyloid precursor protein-α in cerebral microvessels. J Cereb Blood Flow Metab 2017; 37: 106–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kitazume S, Tachida Y, Kato M, et al. Brain endothelial cells produce amyloid β from amyloid precursor protein 770 and preferentially secrete the O-glycosylated form. J Biol Chem 2010; 285: 40097–40103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Santhanam AV, d’Uscio LV, He T, et al. Uncoupling of endothelial nitric oxide synthase in cerebral vasculature of Tg2576 mice. J Neurochem 2015; 134: 1129–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haass C, Koo EH, Mellon A, et al. Targeting of cell-surface β-amyloid precursor protein to lysosomes: alternative processing into amyloid-bearing fragments. Nature 1992; 357: 500–503. [DOI] [PubMed] [Google Scholar]
- 14.Kitazume S, Yoshihisa A, Yamaki T, et al. Soluble amyloid precursor protein 770 is released from inflamed endothelial cells and activated platelets: a novel biomarker for acute coronary syndrome. J Biol Chem 2012; 287: 40817–40825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Selkoe DJ, Podlisny MB, Joachim CL, et al. β-amyloid precursor protein of Alzheimer disease occurs as 110- to 135-kilodalton membrane-associated proteins in neural and nonneural tissues. Proc Natl Acad Sci U S A 1988; 85: 7341–7345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haass C, Schlossmacher MG, Hung AY, et al. Amyloid β-petide is produced by cultured cells during normal metabolism. Nature 1992; 259: 322–325. [DOI] [PubMed] [Google Scholar]
- 17.d’Uscio LV, He T, Katusic ZS. Expression and processing of amyloid precursor protein in vascular endothelium. Physiology (Bethesda) 2017; 32: 20–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zheng H, Jiang M, Trumbauer ME, et al. β-Amyloid precursor protein-deficient mice show reactive gliosis and decreased locomotor activity. Cell 1995; 81: 525–531. [DOI] [PubMed] [Google Scholar]
- 19.Wang Z, Wang B, Yang L, et al. Presynaptic and postsynaptic interaction of the amyloid precursor protein promotes peripheral and central synaptogenesis. J Neurosci 2009; 29: 10788–10801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.d’Uscio LV, He T, Santhanam AV, et al. Mechanisms of vascular dysfunction in mice with endothelium-specific deletion of the PPAR-δ gene. Am J Physiol Heart Circ Physiol 2014; 306: H1001–H1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gericke A, Steege A, Manicam C, et al. Role of the M3 muscarinic acetylcholine receptor subtype in murine ophthalmic arteries after endothelial removal. Invest Ophthalmol Vis Sci 2014; 55: 625–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Onoue H, Tsutsui M, Smith L, et al. Adventitial expression of recombinant endothelial nitric oxide synthase gene reverses vasoconstrictor effect of endothelin-1. J Cereb Blood Flow Metab 1999; 19: 1029–1037. [DOI] [PubMed] [Google Scholar]
- 23.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 2008; 3: 1101–1108. [DOI] [PubMed] [Google Scholar]
- 24.d’Uscio LV, Smith LA, Katusic ZS. Differential effects of eNOS uncoupling on conduit and small arteries in GTP-cyclohydrolase I-deficient hph-1 mice. Am J Physiol Heart Circ Physiol 2011; 301: H2227–2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Santhanam AV, d’Uscio LV, Katusic ZS. Characterization of cerebral microvasculature in transgenic mice with endothelium targeted over-expression of GTP-cyclohydrolase I. Brain Res 2015; 1625: 198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.d’Uscio LV, Das P, Santhanam AV, et al. Activation of PPARδ prevents endothelial dysfunction induced by overexpression of amyloid-β precursor protein. Cardiovasc Res 2012; 96: 504–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.d’Uscio LV, Barton M, Shaw S, et al. Structure and function of small arteries in salt-induced hypertension: effects of chronic endothelin-subtype-A-receptor blockade. Hypertension 1997; 30: 905–911. [DOI] [PubMed] [Google Scholar]
- 28.Goodwin JE, Feng Y, Velazquez H, et al. Endothelial glucocorticoid receptor is required for protection against sepsis. Proc Natl Acad Sci U S A 2013; 110: 306–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zielonka J, Zhao H, Xu Y, et al. Mechanistic similarities between oxidation of hydroethidine by Fremy’s salt and superoxide: stopped-flow optical and EPR studies. Free Radic Biol Med 2005; 39: 853–863. [DOI] [PubMed] [Google Scholar]
- 30.Neubig RR, Spedding M, Kenakin T, et al. International Union of Pharmacology Committee on Receptor Nomenclature and Drug Classification. XXXVIII. Update on terms and symbols in quantitative pharmacology. Pharmacol Rev 2003; 55: 597–606. [DOI] [PubMed] [Google Scholar]
- 31.DeLean A, Munson PJ, Rodbard D. Simultaneous analysis of families of sigmoidal curves: application to bioassay, radioligand assay, and physiological dose-response curves. Am J Physiol 1978; 235: E97–102. [DOI] [PubMed] [Google Scholar]
- 32.Duce JA, Ayton S, Miller AA, et al. Amine oxidase activity of β-amyloid precursor protein modulates systemic and local catecholamine levels. Mol Psychiatr 2013; 18: 245–254. [DOI] [PubMed] [Google Scholar]
- 33.Korhonen J, Polvi A, Partanen J, et al. The mouse tie receptor tyrosine kinase gene: expression during embryonic angiogenesis. Oncogene 1994; 9: 395–403. [PubMed] [Google Scholar]
- 34.Risau W. Differentiation of endothelium. FASEB J 1995; 9: 926–933. [PubMed] [Google Scholar]
- 35.Golde TE, Estus S, Usiak M, et al. Expression of β amyloid protein precursor mRNAs: recognition of a novel alternatively spliced form and quantitation in Alzheimer’s disease using PCR. Neuron 1990; 4: 253–267. [DOI] [PubMed] [Google Scholar]
- 36.Haass C, Kaether C, Thinakaran G, et al. Trafficking and proteolytic processing of APP. Cold Spring Harb Perspect Med 2012; 2: a006270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seubert P, Vigo-Pelfrey C, Esch F, et al. Isolation and quantification of soluble Alzheimer’s β-peptide from biological fluids. Nature 1992; 359: 325–327. [DOI] [PubMed] [Google Scholar]
- 38.Coma M, Guix FX, Ill-Raga G, et al. Oxidative stress triggers the amyloidogenic pathway in human vascular smooth muscle cells. Neurobiol Aging 2008; 29: 969–980. [DOI] [PubMed] [Google Scholar]
- 39.Park L, Zhou P, Pitstick R, et al. Nox2-derived radicals contribute to neurovascular and behavioral dysfunction in mice overexpressing the amyloid precursor protein. Proc Natl Acad Sci U S A 2008; 105: 1347–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brandes RP, Kim D, Schmitz-Winnenthal FH, et al. Increased nitrovasodilator sensitivity in endothelial nitric oxide synthase knockout mice: role of soluble guanylyl cyclase. Hypertension 2000; 35: 231–236. [DOI] [PubMed] [Google Scholar]
- 41.Faraci FM, Sigmund CD, Shesely EG, et al. Responses of carotid artery in mice deficient in expression of the gene for endothelial NO synthase. Am J Physiol 1998; 274: H564–570. [DOI] [PubMed] [Google Scholar]
- 42.Hussain MB, Hobbs AJ, MacAllister RJ. Autoregulation of nitric oxide-soluble guanylate cyclase-cyclic GMP signalling in mouse thoracic aorta. Br J Pharmacol 1999; 128: 1082–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murphy K, Gerzanich V, Zhou H, et al. Adenosine-A2a receptor down-regulates cerebral smooth muscle L-type Ca2+ channel activity via protein tyrosine phosphatase, not cAMP-dependent protein kinase. Mol Pharmacol 2003; 64: 640–649. [DOI] [PubMed] [Google Scholar]
- 44.Yanagisawa M, Kurihara H, Kimura S, et al. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 1988; 332: 411–415. [DOI] [PubMed] [Google Scholar]
- 45.Boulanger C, Lüscher TF. Release of endothelin from the porcine aorta: Inhibition by endothelium-derived nitric oxide. J Clin Invest 1990; 85: 587–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lerman A, Sandok EK, Hildebrand FL, Jr., et al. Inhibition of endothelium-derived relaxing factor enhances endothelin-mediated vasoconstriction. Circulation 1992; 85: 1894–1898. [DOI] [PubMed] [Google Scholar]
- 47.Moreau P, Takase H, Küng CF, et al. Blood pressure and vascular effects of endothelin blockade in chronic nitric oxide-deficient hypertension. Hypertension 1997; 29: 763–769. [DOI] [PubMed] [Google Scholar]
- 48.Lang IM, Moser KM, Schleef RR. Expression of Kunitz protease inhibitor-containing forms of amyloid β-protein precursor within vascular thrombi. Circulation 1996; 94: 2728–2734. [DOI] [PubMed] [Google Scholar]
- 49.Cole GM, Galasko D, Shapiro IP, et al. Stimulated platelets release amyloid β-protein precursor. Biochem Biophys Res Commun 1990; 170: 288–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Van Nostrand WE, Schmaier AH, Farrow JS, et al. Protease nexin-II (amyloid β-protein precursor): a platelet α-granule protein. Science 1990; 248: 745–748. [DOI] [PubMed] [Google Scholar]
- 51.Smirnov A, Trupp A, Henkel AW, et al. Differential processing and secretion of Aβ peptides and sAPPα in human platelets is regulated by thrombin and prostaglandine 2. Neurobiol Aging 2009; 30: 1552–1562. [DOI] [PubMed] [Google Scholar]
- 52.Henry A, Li QX, Galatis D, et al. Inhibition of platelet activation by the Alzheimer’s disease amyloid precursor protein. Br J Haematol 1998; 103: 402–415. [DOI] [PubMed] [Google Scholar]
- 53.Xu F, Davis J, Miao J, et al. Protease nexin-2/amyloid β-protein precursor limits cerebral thrombosis. Proc Natl Acad Sci U S A 2005; 102: 18135–18140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen M, Inestrosa NC, Ross GS, et al. Platelets are the primary source of amyloid β-peptide in human blood. Biochem Biophys Res Commun 1995; 213: 96–103. [DOI] [PubMed] [Google Scholar]
- 55.Li QX, Whyte S, Tanner JE, et al. Secretion of Alzheimer’s disease Abeta amyloid peptide by activated human platelets. Lab Invest 1998; 78: 461–469. [PubMed] [Google Scholar]
- 56.Catricala S, Torti M, Ricevuti G. Alzheimer disease and platelets: how’s that relevant. Immun Ageing 2012; 9: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Koike MA, Lin AJ, Pham J, et al. APP knockout mice experience acute mortality as the result of ischemia. PloS One 2012; 7: e42665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Clarke J, Thornell A, Corbett D, et al. Overexpression of APP provides neuroprotection in the absence of functional benefit following middle cerebral artery occlusion in rats. Eur J Neurosci 2007; 26: 1845–1852. [DOI] [PubMed] [Google Scholar]
- 59.Kern A, Roempp B, Prager K, et al. Down-regulation of endogenous amyloid precursor protein processing due to cellular aging. J Biol Chem 2006; 281: 2405–2413. [DOI] [PubMed] [Google Scholar]