Abstract
Most research focuses on overt stroke caused by blockage of major blood vessels. Less attention has been paid to small vessel disease which gives rise to covert stroke that often leads to vascular cognitive impairment (VCI). One reason for this may be the relative lack of relevant animal models. Herein, we describe, a model of VCI induced in middle-aged Sprague-Dawley rats exposed to a diet high in saturated fats, salt and refined sugar (HFSS). In Experiment 1, rats were fed HFSS and subjected to a small mediodorsal (MD) thalamic stroke with or without concomitant permanent bilateral carotid artery occlusion. MD lesions produce significant executive dysfunction in an attention set-shift task (p = 0.012). In Experiment 2, rats were exposed to either HFSS or control diet and functional effects assessed. We found significant hypertension (p = 0.013), blockage of brain microvessels (p = 0.018) and white matter atrophy (p = 0.039) in HFSS diet animals. As in Experiment 1, profound, specific set-shifting executive dysfunction was noted (p = 0.003) following both small MD infarcts (0.332 mm3) and the HFSS diet. In summary, these data describe a middle-aged animal model of VCI that includes clinically relevant metabolic disturbances and small vessel disease and as such may be helpful in developing new cognitive therapies.
Keywords: Cognition, risk factors, small vessel disease, executive function, imaging
Introduction
Vascular cognitive impairment (VCI) refers to a syndrome characterized by executive dysfunction with evidence of psychomotor slowing and to a lesser extent, memory disturbances.1–3 The most common cause of VCI is SVD arising from hypertension and other lifestyle factors that is often associated with white matter lesions seen as hyperintensities on T2-weighted imaging and small “covert” infarcts.4,5 The term covert is used to refer to infarcts, evident on MRI, that do not result in obvious motor stroke like symptoms.4 The development of treatments for VCI has been hampered by the lack of suitable animal models.6 While no model captures all aspects of human SVD, the rat 2-vessel occlusion (2-VO) model creates a state of cerebral hypoperfusion, white matter damage, loss of capillaries, blood–brain barrier breakdown, neuroinflammation and memory impairments as a result of permanent bilateral occlusion of the carotid arteries,7–9 thereby meeting several of the pathological criteria for human VCI.6
However, there is consensus that human risk factors, including disease co-morbidities, should be incorporated into preclinical studies given the widespread failure of stroke neuroprotection trials that were based on positive findings in animal models.10 A number of reviews have emphasized this need and suggested guidelines to mitigate translational failure.11–13 Accordingly, in a previous study,14 we used the 2-VO model in rats chronically fed a diet high in fat and sugar to approximate the dietary habits of much of the developed world and the growing incidence of obesity, hypertension, diabetes and dyslipidemia all of which are risk factors for VCI.2,3 In our previous study, the animals showed evidence of progressive impairment in spatial memory over several months and altered hippocampal CA1 architecture that was partially reversed by a combination of physical and cognitive rehabilitation.14 While encouraging, these results are tempered by the fact that the animals were relatively young (six month age) when subjected to the 2-VO procedure and the diet (high fat, high sugar) was instituted following 2-VO instead of early in life as is the case in humans. In addition, other risk factors (e.g. hypertension) and pathological hallmarks of VCI (e.g. white matter injury, small covert infarcts, microvascular dysfunction) were either not incorporated or not investigated. Most importantly, animal studies of VCI, including our own work, have relied almost exclusively on hippocampal-based tests of spatial memory such as the Morris water maze, whereas in human VCI, the predominant impairment is in executive function.2,3 The present study addresses many of these shortcomings including the use of older animals maintained from weaning on a diet not only high in fat and sugar but also sodium prior to 2-VO induction in middle age. To simulate covert stroke, we used small infusions of endothelin-1 (ET-1) to create ischemic injury of the mediodorsal nucleus of thalamus,15,16 a common site of covert stroke in humans.4,17 In this model, we detected significant abnormalities in physiological, hemodynamic and structural features of the brain and cerebrovasculature that were associated with profound impairments in executive function.
Materials and methods
Subjects
One hundred and fifty (n = 150) male Sprague-Dawley rats (Charles River Laboratories, Montreal, Quebec, Canada) weighing 60–95 g (∼1 month of age) upon arrival were used in this study. Rats were pair-housed in standard rodent cages on a reverse 12:12-h light:dark cycle. Behavioural assessments were conducted during the dark phase. All procedures were approved by Memorial University’s Institutional Animal Care Committee and complied with the Canadian Council on Animal Care (CCAC) Guide to the Care and Use of Experimental Animals. The following experiments and data are reported following the ARRIVE guidelines for animal experimentation.
Experimental diet
Following arrival, animals were randomized to one of two dietary conditions: High saturated fat, salt and refined sugar (HFSS; n = 113) or control (n = 37). The total energy content (kcal) of the HFSS diet comprised protein (17%), fat (43%) and carbohydrates (40%; TestDiet, 5S9E, Richmond, IN USA). The ingredients included 39% sucrose, 7% fiber and 7% salt (% by weight). Animals in this condition also received 50 mL of Pepsi (PepsiCo Beverages Canada, Peterborough, ON, Canada)/day throughout experimentation. Control diet (TestDiet 5P00) consisted of standardized laboratory chow supplied by Memorial University. Rats remained on their respective diet throughout experimentation and fed food and water ad libitum, unless otherwise specified. A total of 17 HFSS and five control diet animals were excluded from the study due to pre-surgical or surgical complications. The subsequent condition numbers were used for the final analyses.
Experimental conditions
Experiment 1
The aim of Experiment 1 was to determine the effects of very small thalamic infarcts and cerebrovascular perturbations on executive function in middle-aged (∼12 month) rats exposed to an unhealthy diet (HFSS). Animals were pseudorandomized to one of four conditions: (1) unilateral, mediodorsal thalamic stroke (MD; n = 21); (2) chronic cerebral hypoperfusion induced by permanent bilateral occlusion of the common carotid arteries (2VO; n = 27); (3) combination of MD + 2VO surgical procedures (n = 22); and (4) surgical sham (n = 26). Pseudorandomization consisted of drawing animal numbers randomly from a hat until each group was of equal size (some rats were subsequently lost during course of experiment).
Experiment 2
Data from Experiment 1 indicated that small, unilateral MD lesions induced significant executive dysfunction. Using data from these animals, we assessed the functional and physiological effects of the HFSS diet in these aged animals using a 2 × 2 (Diet × Lesion) experimental design. Subsets of animals were randomly selected to undergo assessments of blood pressure, glucose tolerance, pro-inflammatory cytokines, cerebral blood flow, cerebral imaging and neuropathologic assessments. Conditions consisted of: (1) MD + HFSS (n = 21); (2) MD + Control diet (n = 17); (3) Sham surgery + HFSS (n = 26); and (4) Sham surgery + Control diet (n = 15).
Surgical procedures
Animals were anesthetized with isoflurane (4.0% induction, 2.0% maintenance in 100% O2: CDMV, St-Hyacinthe, Quebec, Canada). All surgical procedures were conducted using aseptic techniques. Temperature was maintained at 37.0℃ throughout surgery using a self-regulating heating blanket (Harvard Apparatus, Holliston, MA, USA).
2VO surgery
A midline, ventral neck incision was made and both the left and right common carotid arteries were carefully isolated from associated nerve bundles and surrounding musculature. Each artery was doubly ligated, occlusion verified, and then returned to the neck cavity. Sham surgery consisted of similar procedures, with the exception of the ligatures. Animals received topical 2% xylocaine (AstraZeneca, Mississauga, ON, Canada) on the midline incision and were placed in a standard cage on a heating blanket until recovered from surgery.
MD infarcts
Animals were placed in a stereotaxic frame and a midline scalp incision was made. To induce a small MD infarct, 0.25 µL (400 pmol/µL) of the vasoconstrictive peptide, endothelin-1 (ET-1; CalBiochem, Hornby, ON, Canada) was infused at the following coordinates relative to bregma: anterioposterior: −2.8 mm, mediolateral: −0.7 mm, dorsoventral: −6.2 mm. Endothelin-1 was injected over a period of 1 min and the needle remained in place for 4 min prior to removal to minimize backflow. Sham surgery consisted of all manipulations up to, and including, drilling the burr hole.
Sham surgery
In Experiment 1, sham surgery consisted of exposure of the carotid arteries and drilling a burr hole in the skull. In Experiment 2, which did not include bilateral carotid artery occlusion (Figure 1), the Sham procedure consisted of drilling the burr hole.
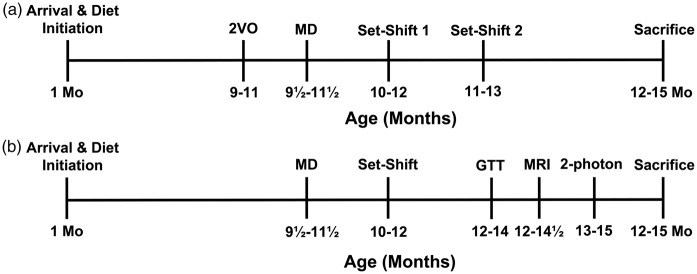
Figure 1.
Timeline for Experiments 1 (a) and 2 (b). 2-VO: 2-vessel occlusion; MD: mediodorsal thalamus; GTT: glucose tolerance test; MRI: magnetic resonance imaging; 2-photon: 2-Photon imaging.
Behavioural assessment
Rats’ executive function was assessed using a paradigm of attention set shifting.18 Animals were assessed at two time points in Experiment 1 (two to five and six to nine week post-MD surgery; Figure 1) and once in Experiment 2 (two to five week post-surgery).
Rats were socialized and habituated to the testing environment before behavioural testing. One week before testing, rats were food restricted and trained to make a series of stimulus discriminations by finding a hidden 1/3 Honey Nut Cheerio© (General Mills, Mississauga, ON, Canada) food reward buried in one of two 10 cm diameter ceramic pots whose location was paired with one of two different odours, external textures and digging media. A successful discrimination consisted of the rat digging with its paws or nose in the pot containing the buried reward on six consecutive trials. The attention set shift test was administered the following day and consisted of seven discriminations (Table 1): (1) a simple discrimination (SD) where rats were rewarded for responding to a stimulus feature of one of the two pots (e.g. patchouli odour); (2) a compound discrimination (CD) where two new sensory dimensions were introduced (digging medium and texture), but the previous exemplars remained constant; (3) an intradimensional (ID) shift, where new relevant and irrelevant stimuli were introduced and a rat was required to discriminate a stimulus in the same sensory dimension as the previous tests (e.g. a nutmeg odour); (4) a reversal (REV1), where the reward was paired with the previously unrewarded feature in the same sensory dimension (e.g. jasmine odour), as a test of perseveration; (5) an extradimensional (ED) shift, where new stimuli were introduced again and the reward was paired with a feature in a sensory dimension that was previously irrelevant (e.g. a soft, external texture), which required a shift of attention to a new sensory dimension; (6) followed by a second reversal (REV2); (7) and a learned irrelevance (LI) test where a change was made in the dimension that had never been rewarded during the test (e.g. the digging medium), assessing the ability of rats to ignore irrelevant stimuli, an important aspect of attention processing.19 The sensory dimensions of ED attention shifts were balanced across rats; 16 HFSS subjects and 8 control diet subjects were excluded for failure to learn the task or complete behavioural testing.
Table 1.
Order of attention set shift discriminations.
| Dimensions | ||
|---|---|---|
| Discriminations | Relevant | Irrelevant |
| Simple discrimination (SD) | Odor 1 | |
| Compound discrimination (CD) | Odor 1 | Medium and texture |
| Intradimensional (ID)shift | Odor 2 | Medium and texture |
| Reversal 1 (REV1) | Odor 3 | Medium and texture |
| Extradimensional (ED) shift | Medium 1 | Odor and texture |
| Reversal 2 (REV2) | Medium 2 | Odor and texture |
| Learned irrelevance (LI) | Texture | Medium and odor |
Physiological assessments
Blood pressure
Two weeks prior to surgery, animals (HFSS = 9; control diet = 11) underwent measurements of systolic blood pressure via tail cuff compression (IITC Life Science Instruments model 29, pulse/pressure amplifier, Woodland Hills, CA, USA).
Glucose tolerance
Overnight (12 h) fasting glucose, insulin and glucose tolerance were assessed prior to MRI assessment (∼14–16 month of age). Blood was obtained via tail nick procedure under brief, light anesthesia (1–2 min 2.0% isoflurane in 100% O2) and glucose was measured using a glucometer (One Touch, UltraMini, Lifescan Inc., Milpitas, CA, USA) and insulin via ELISA (Crystal Chem, Downers Grove, IL, USA). To assess glucose tolerance, following baseline fasting measurements, animals’ compensatory response to an acute glucose stress was assessed by infusing 50% D-Glucose (1 g/kg, i.p.; Sigma-Aldrich, Oakville, ON, Canada). Glucose measurements were taken at 10, 30, 60 and 120 min following injection (MD + HFSS = 17; MD + control diet = 15; sham + HFSS = 25; sham + control = 15).
Cytokines
Immediately prior to sacrifice, serum cytokine concentrations were assessed from blood obtained via cardiac puncture (HFSS = 18; control diet = 16). Three milliliters of blood were collected into a microtainer and allowed to clot for 30 min. Blood was then centrifuged at 3000 r/min for 10 min and serum was decanted and stored at −80℃ until further processing. Serum cytokines: IL-1α, IL-1β, IL-2, IL-6, IL-7, IL-18, IFN-g, GM-CSF, VEGF and TNF-α were analyzed using a Bio-Rad Bio-Plex dual laser flow-based sorting and detection analyzer (Bio-Rad, Mississauga, ON, Canada).
Cerebrovascular assessments
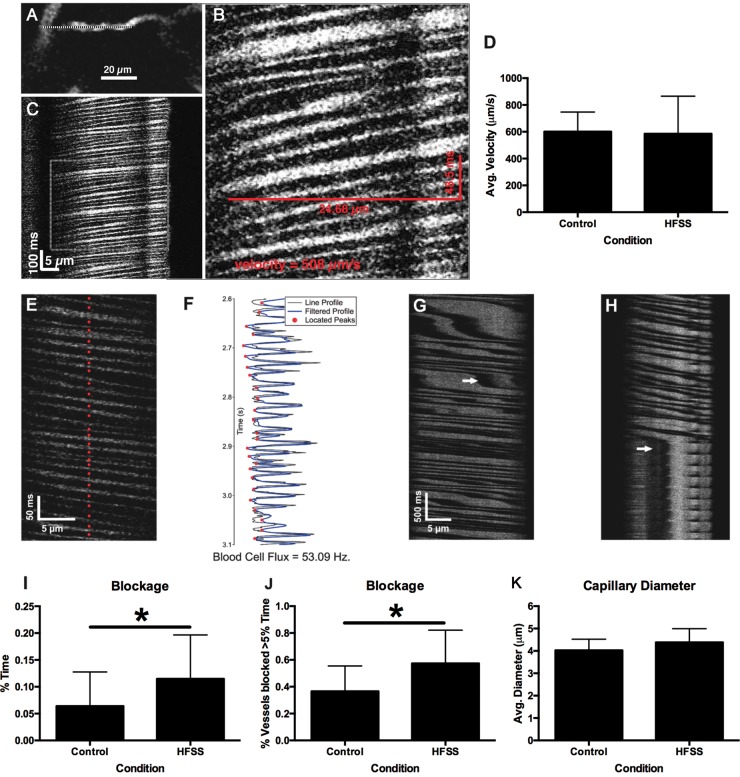
Following MRI analysis (Figure 1(b)), rats (HFSS = 21; control diet = 22) were assessed with 2-photon imaging to quantify cerebral blood flow and capillary structure in frontal cortex (Supplementary Figure 1(d)). Surgical procedures for preparing a cranial window and the in vivo two-photon imaging methods have been described previously.20 Briefly, animals were anesthetized with urethane (0.15 g/100 g) and fitted into a custom-made head holder. Two-photon excitation was performed with a Spectraphysics Mai Tai laser and tuned to 800 nm to excite Fluorescein isothiocyanate (FITC) dextran (1000 kDa; Sigma, St. Louis, MO). The cortex was covered with 1.3% low-melt agarose (at 37–38℃; Type 3-A; A9793; Sigma) dissolved in HEPES-buffered artificial CSF and sealed with a glass coverslip; 0.1-mL bolus of 5 mg/mL FITC-dextran (70 kDa) in brain buffer was injected into the tail vein to permit determination of blood flow velocity.21 A 4× air objective was used to obtain images of the surface vasculature across the entire cranial window. A 40×-magnification water-immersion objective was used for high-resolution imaging and line-scan measurements. Velocity of red blood cell flow (mm/s) and supply rate (cells/s) was measured from radon transform analysis of line scan images22 in which capillaries were identified that were oriented parallel to the scanning axis as described previously.23 Red blood cell blockage was quantified as both the percentage duration of cell flow stoppage and as the proportion of vessels exhibiting blockage for greater than 5% of the time of a 40-s time window. The diameter of each capillary was measured from the scan images and heart rate was quantified from the pulse of cell velocities. A complete dataset was not obtained from two animals in each condition.
Magnetic resonance imaging and neuropathological assessments
Magnetic resonance imaging
Rats (HFSS = 26; control diet = 16) were imaged ∼10 week post-MD surgery with MRI to measure, in vivo, parenchyma and ventricle size. MRI was performed using a Bruker MSL-X Biospec 7/21 cm spectrometer. For each image, rats were anesthetized with pentobarbital (50 mg/kg, i.p.) and placed in a holder with the head positioned in a 3-cm diameter saddle coil using an incisor bar. Spin-echo scout images were acquired first in 16 contiguous coronal slices, centered 1.5 mm posterior to Bregma. Multislice multi-echo T2-weighted spin-echo images were acquired with echo times of 20, 40 and 60 ms and TR of 1500 ms.
Histological procedures
Animals were sacrificed following completion of all behavioral, physiological and imaging assessments. Animals were anesthetized with isoflurane (4.0% isoflurane, 100% O2) and transcardially perfused with ice-cold heparinized 0.9% saline and 4.0% paraformaldehyde. Brains were removed (HFSS = 18; control diet = 20) and stored in PBS with 20% sucrose, frozen and sectioned at 20 µm and stained with cresyl violet to determine injury to MD. The monoclonal antibody, ED-1, was used to assess activated microglia/macrophages and gold chloride to delineate white matter tracts. For ED-1 immunohistochemistry, sections were washed in PBS, treated with 1.0% H2O2, blocked with normal goat serum (5.0%; Jackson Immunoresearch Laboratories, West Grove, PA, USA), and subsequently incubated overnight at 4℃ with monoclonal mouse anti-rat CD68 (ED-1, 1:1000, MCA341R; Serotec, Raleigh, NC, USA). The sections were then exposed to anti-mouse biotinylated secondary antibody (1:1000; Jackson Immunoresearch Laboratories), incubated in 10 µg/mL extravadin (Sigma-Aldrich), and reacted for 3–5 min in a 3,3′-diaminobenzidine tablet set (Sigma-Aldrich).
The number of activated microglia/macrophages (ED-1+ cells) was calculated using unbiased stereological assessments in a similar manner as previously described.24 Briefly, sampling sites were randomly superimposed on the cortex in each hemisphere across seven sections extending from +2.2 mm to −3.8 mm relative to Bregma. White matter volume estimates were calculated using gold chloride stained sections by measuring corpus callosum volume in each hemisphere from the same rostral-caudal coronal sections as the ED-1 counts: Volume = average area of corpus callosum × section interval × number of sections. Volume was calculated using ImageJ software (National Institutes of Health, USA). Data were incomplete for two control diet animals.
Statistical analyses
In Experiment 1, a mixed-factor ANOVA, with two within-subjects factors (Shift & Test repetition) and one between-subjects factor (Lesion: MD, 2VO, MD + 2VO & sham), was used to analyze the effect of surgery on executive function (IBM® SPSS® Statistics; v. 20.0.0, Armonk, NY, USA) with a series of planned comparisons between sham animals and the surgical conditions with Bonferroni correction used as post hoc tests to assess significant differences. Separate analyses were performed on attention shifts (ID and ED) and other aspects of performance including acquisition (SD and CD), reversals (REV1 and REV2) and LI. In Experiment 2, a 2-factor (Lesion X Diet) ANOVA with repeated measures was used to assess the effects of dietary manipulation on lesion outcome in the behavioural analyses. One-tailed, independent t-tests with Bonferroni correction were used to assess the physiological, imaging and neuroanatomical changes associated with dietary manipulations. In cases where the homogeneity of variance was violated, Brown-Forsythe correction was used. Statistical significance was considered at p ≤ 0.05. Sample size was calculated using a ‘large effect size’ (ω2 = 0.1525) for the behavioural assessments using data from Cordova et al.15and Birrell and Brown18 and the β level set at 0.80. Given that these studies implemented similar behavioural assays, but used different CNS injury models, we decided to use a conservative approach and slightly overestimate the number of animals required to detect statistically significant differences among conditions. All figures contain mean ± SD.
Results
All raw data used to generate each of the figures (mean ± SD, Ns) are provided in Supplementary Table 1.
Experiment 1
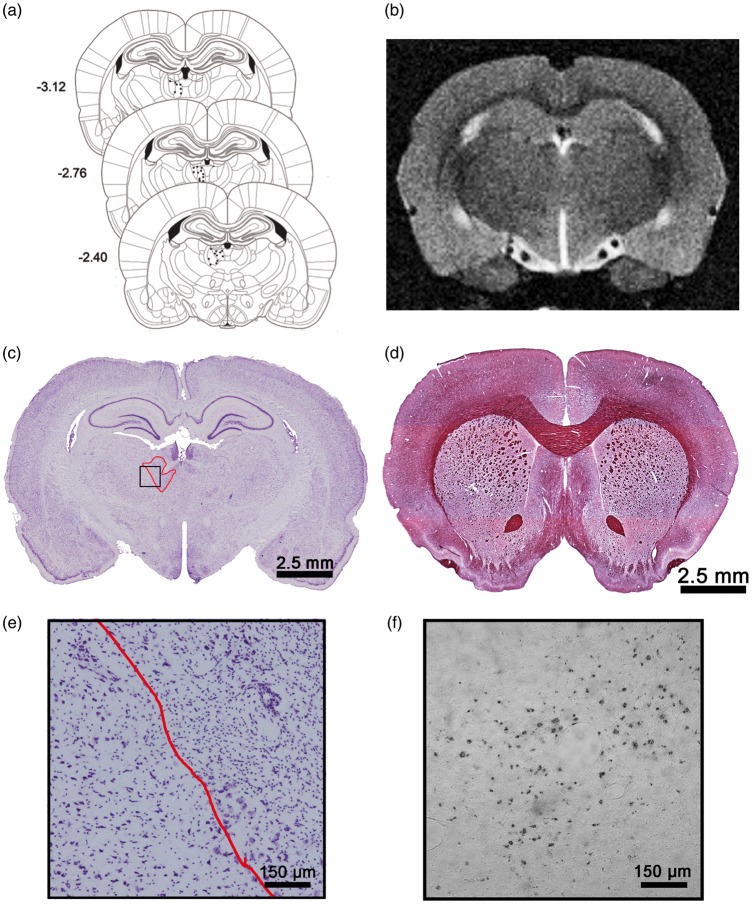
The representative photomicrographs and illustration in Figure 2(a), (c) and (e) demonstrate the small area (0.363 mm3) of ET-1 induced damage that was restricted to the MD nucleus of the thalamus. There was no obvious ischemic damage induced by the 2VO surgery.
Figure 2.
Schematic representing injection sites of ET-1 within MD (a). Example of T2-weighted MRI image used to assess volumetric changes in brain (b). Representative cresyl-violet-stained section at site of ischemic damage in MD nucleus of thalamus (c). Example of gold chloride staining used to estimate corpus callosum white matter volume in rats exposed to HFSS (d). Enlarged view of MD ischemic cell damage and adjacent uninjured thalamus (e). Representative ED-1 stained microglia/macrophages (f).
Unilateral MD lesions disrupt executive function
There was no difference in performance in the set-shifting tests performed at early and late time points (p > 0.05). As a result, we have presented the data from the first time point only.
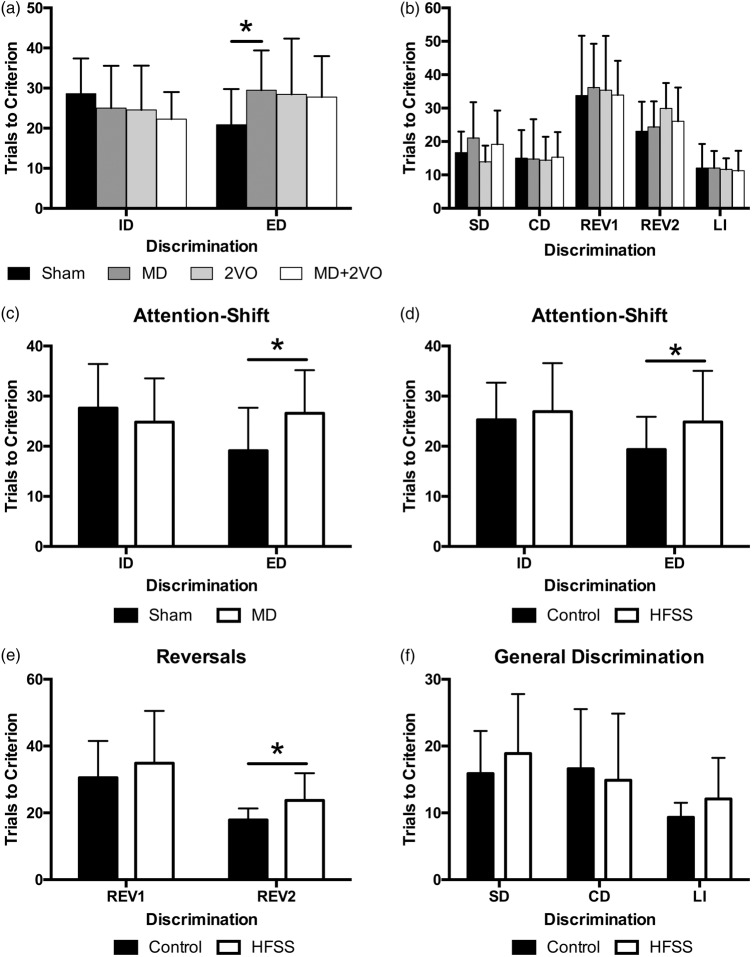
Mixed-factor ANOVA of attention shifts (i.e. ID and ED, within-subjects variable) revealed a significant lesion × shift interaction (F3,50 = 3.10, p = 0.035; Figure 3(a)). A series of planned comparisons with Bonferroni correction demonstrated that the MD stroke-alone resulted in a selective impairment in the ED shift, with rats requiring ∼50% more trials to learn the new discrimination relative to sham-lesion controls (p = 0.012), but no effect of 2VO or the combined MD+2VO surgery on set shifting (p > 0.05). There were no effects of lesion on ID attention shifts (ID, all p > 0.05; Figure 3(a)).
Figure 3.
Performance in the set-shift behavioural assessment for Experiment 1 and 2 (mean ± SD). Average number of trials to criterion in the intradimensional (ID) & extradimensional (ED) attention-shifts. Ischemic damage to the MD thalamic nucleus significantly impaired animals’ ability to shift attention to a new dimension compared to sham surgery (*p = 0.012). There were no other differences among conditions (a). There were no differences among animals in generalized simple (SD) or compound discriminations (CD), perseveration (Reversal test 1, (REV1) and Reversal test 2 (REV2)) or learned irrelevance (b). As in Experiment 1, MD ischemic lesions significantly impaired attention shifting to a new dimension (ED) compared to sham surgery (*p = 0.003) (c). Chronic exposure to HFSS significantly impaired animals’ generalized ability to shift attention (ED; *p = 0.024) (d) and resulted in significant perseveration (e; REV 2; p = 0.005). There was no effect of diet or lesion on discrimination ability or learned irrelevance (f).
A mixed factor ANOVA of the other attention tests (SD, CD, REV1, REV2 and LI) revealed that all animals performed similarly with no main effect of lesion or lesion × attention test interaction, indicating that lesion did not affect discrimination ability (SD and CD; F3,47 = 0.752, p > 0.05), perseveration (REV1 and REV2; F3,48 = 0.378, p > 0.05) or LI (F3,47 = 0.056, p > 0.05; Figure 3(b)).
Experiment 2
Results from Experiment 1 indicated that a small unilateral MD infarct had a pronounced, but specific effect on executive function. Using this lesion model in a 2 × 2 lesion (MD vs. Sham surgery) × diet (HFSS vs. Control diet) design, we assessed the contributory effects of the HFSS diet on executive function. Further, we assessed the physiological and histopathological changes induced by the dietary manipulation. With the exception of the behavioural and glucose homeostasis analyses, there was no effect of lesion and no lesion × diet interaction in these latter analyses. Therefore, lesion data were collapsed and dietary effects were assessed using independent t-tests with Bonferroni correction.
HFSS contributes to executive dysfunction
Attention shift
A 2 × 2 ANOVA with repeated measures revealed a significant shift × lesion interaction (F1,42 = 6.395, p < 0.02), where MD animals required significantly more trials to meet criterion than sham animals in the ED shift (Figure 3(c)), thus confirming the attention shifting deficit results of Experiment 1. Further, there was a main effect of diet (F1,42 = 4.693, p < 0.04) where, overall, HFSS animals experienced significantly greater difficulty in shifting attention to a different dimension (both intra- and extradimensional) than animals fed a control diet (Figure 3(d)).
Reversals
Mixed factor ANOVA revealed a significant main effect of diet (F1,39 = 4.793, p < 0.04), but no other main effects or interactions. Further analysis of this main effect demonstrated that HFSS animals were more likely to persist with the previously rewarded stimulus than control diet animals, demonstrating significant perseveration (Figure 3(e)).
Discrimination and LI
There was no main effect of lesion or diet and no interaction between these independent variables with respect to the SD, CD or LI trials indicating that generalized discrimination abilities remained intact among all animals (Figure 3(f)).
Physiological effects of HFSS diet
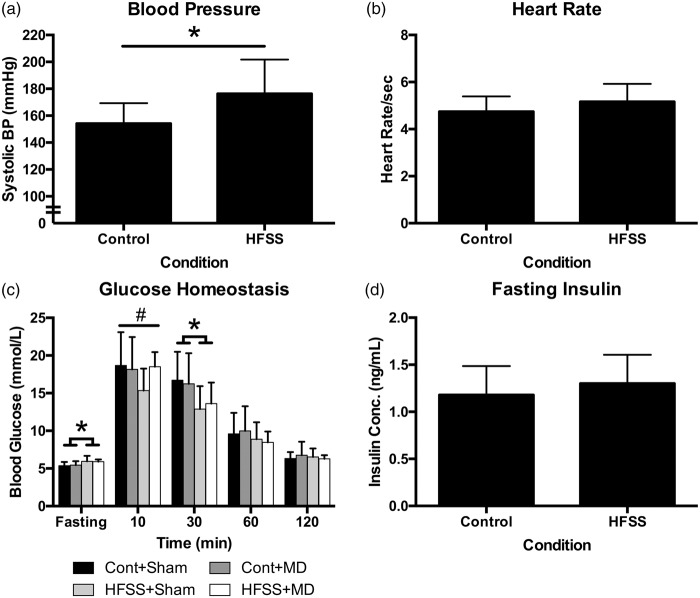
Animals were exposed to the experimental diet for at least nine month prior to physiological assessments. HFSS diet significantly elevated systolic blood pressure (t18 = 2.42, p = 0.013; Figure 4(a), independent of heart rate (t37 = 1.88, p > 0.05; Figure 4(b). With respect to glucose homeostasis, there was a complex three-way interaction among time, diet and surgery at the last time point assessed (F2.311,157.155 = 3.811, p < 0.02). Follow-up analysis indicated that this difference extended from a peculiar interaction at different time points. HFSS animals had significantly elevated baseline glucose levels, HFSS + MD animals expressed lower glucose at 10-min post-glucose administration and control diet animals had elevated readings at 30-min post-administration. There were no differences for the remaining time points (Figure 4(c)). Fasting insulin concentrations were not different among conditions (p > 0.05; Figure 4(d)).
Figure 4.
Chronic exposure to HFSS results in significant elevation in systolic blood pressure (p = 0.013) (a) but no effect on heart rate (b). There was a complex interaction among independent variables with respect to glucose homeostasis measured ∼2 weeks prior to MRI assessment (c). HFSS animals were significantly different than control diet animals at both fasting and 30-min time points (*p < 0.01), HFSS + Sham animals expressed significantly lower glucose at 10-min post-glucose administration (#p < 0.03). There were no differences for the remaining time points. X-axis time data correspond to fasting glucose (overnight, 12 h), 10 -, 30- 60- and 120-min post-50% D-glucose infusion (1 g/kg, i.p.). There was no effect of diet on fasting insulin concentration (d). Cont+Sham – Control diet + Sham surgery; Cont+MD – Control diet + Mediodorsal thalamic stroke; HFSS+Sham – High Fat, Salt, Sugar diet + Sham surgery; HFSS+MD – High Fat, Salt, Sugar diet + Mediodorsal thalamic stroke.
HFSS diet affects cerebral blood flow
Although there were no dietary effects on cerebral blood flow velocity (p > 0.05; Figure 5(a) to (d)) or red blood cell flux rate (p > 0.05, data not shown), the HFSS diet significantly increased the average duration of cell blockage (t37 = 2.17, p = 0.018; Figure 5(e) to (i)) and the percentage of vessels exhibiting blockage for more than 5% of imaged time (t33 = 2.80, p = 0.004; Figure 5(j)), indicating intermittently reduced cerebrovascular flow. There was no effect on capillary diameter (Figure 5(k)).
Figure 5.
Dietary influences on cerebrovasculature measured using two-photon imaging of red blood cell (RBC) velocity. Example of a capillary vessel (with overlaid line scan) selected for two-photon line-scan measurements (a). Line-scan data of RBC motion along the baseline direction of flow during the acquisition of the image (b). Expanded view of RBC motion with velocity calculated from the distance and duration of a red blood cell moving across the frame (c). There was no effect of diet on cerebral blood flow velocity (d). Individual blood cells located from peaks of line-scan cell images (red dots) (e). Image intensity over time and filtered profile peaks used to locate blood cells (f). Stalled blood flow is indicated by white arrows (g). Sustained blood cell blockage is shown in panel (h). Chronic HFSS exposure resulted in increased duration of cell blockage in % time blocked (i*p = 0.018) and increased the percentage of vessels blocked for more than 5% of the time (j*p = 0.004). Effect of HFSS on cerebral capillary diameter (k).
MRI and neuropathological changes induced by HFSS
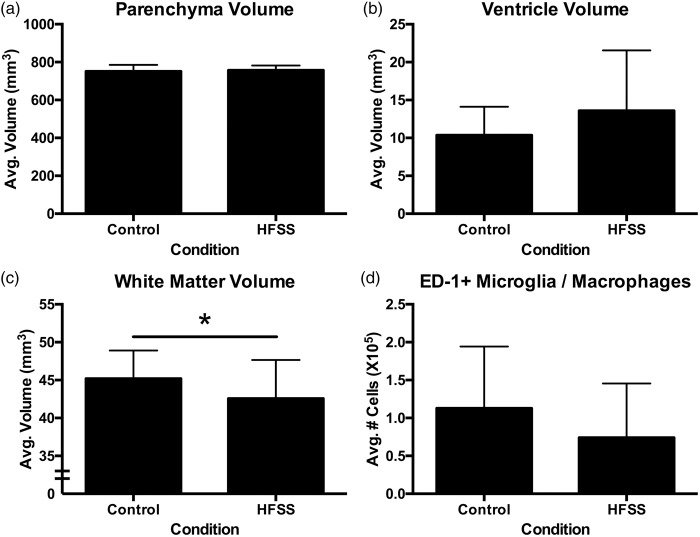
In vivo MRI analysis (Figure 2(b); Supplementary Figure 1(a) to (c)) demonstrated that there were no dietary-induced gross neuroanatomical changes with respect to tissue volume (Figure 6(a)) or ventricle size (p > 0.05; Figure 6(b)). However, gold chloride staining (Figure 2(d)) of the corpus callosum revealed significant white matter atrophy in HFSS animals (t35 = 1.82, p = 0.039; Figure 6(c)). Neuroinflammation as assessed by ED-1 microglia/macrophage staining (Figure 2(f)) did not reveal an effect of diet (p > 0.05; Figure 6(d)). Similarly, serum cytokine analysis (Supplementary Table 2) failed to demonstrate elevated systemic inflammation with exposure to the HFSS diet. There were no differences between HFSS and control diet-MD animals with respect to infarct size (p > 0.05; mean infarct volume ± SD: 0.332 mm3 ± 0.054).
Figure 6.
Neuropathological and MRI analyses of HFSS-induced changes. There was no difference between conditions with respect to MRI assessment of tissue volume (a) or ventricle size (b). HFSS diet did, however, induce significant white matter atrophy (c*p = 0.039). There was no difference in neuroinflammation measured by mean number of microglia/macrophages per brain (d).
Discussion
The primary purpose of this study was to examine how dietary-induced systemic changes interact with small brain infarcts akin to the covert infarcts associated with human VCI. We demonstrated that chronic exposure to a diet high in saturated fats, salt and refined sugar in middle-aged rats (∼12–15 month) causes systemic cardiovascular changes such as hypertension, independent of tachycardia and decreased cerebrovascular compliance. Long-term consumption of this diet also resulted in neuropathological changes that included white matter atrophy as detected with gold chloride staining. The failure to see similar changes with MRI is likely due to the reduced sensitivity of imaging relative to histology especially in the relatively white matter poor rodent brain. The culmination of the above effects resulted in the manifestation of executive dysfunction in the form of generalized attention-shifting impairments as well as perseveration of previously learned stimuli. Additionally, these middle-aged rats displayed pronounced, selective impairments of a separate aspect of executive function, extradimensional shifting, following a unilateral mediodorsal thalamic infarct. This is one of a few studies to induce disease co-morbidities into middle-age animals to produce the hallmark cognitive symptoms of VCI – executive dysfunction.6,26
Particularly striking was the dichotomous executive dysfunction, observed with both independent variables in this study. The unilateral MD infarcts, though quite small, selectively impaired attention shifting to a new dimension, and consumption of the HFSS diet resulted in a generalized attention shifting difficulty combined with a propensity for perseveration. With respect to the HFSS diet, traditional studies of cognition have focused mainly on dietary-induced changes in hippocampal-dependent spatial tasks,27 although other aspects of cognition are also detrimentally influenced by unhealthy diets.28 Importantly, we have shown here that more complex executive functioning, an area of cognition not commonly assessed in preclinical stroke models, is also altered by a diet rich in saturated fats, refined sugar and high salt content.
Notably, we did not observe a generalized increase in ischemic damage as a result of the HFSS diet as noted elsewhere.29,30 In the current study, we sought to model VCI by inducing small (0.363 mm3) infarcts in the MD thalamus. The volume of infarction produced in the current study matches the clinical description of covert stroke with respect to both size and absence of obvious deficit.4,17 This is important for the development of an animal model of VCI.6
Other potential factors contributing to the cognitive dysfunction noted in the current study include atrophic white matter changes in HFSS animals. Clinical evidence suggests that white matter damage31 and subcortical infarcts32 impair executive functioning. Traditional animal models of stroke often spare major white matter tracts16,33 or cause nonspecific white matter damage.34 Thus, the present data corroborate clinical brain changes and support the use of dietary manipulations in animals to model high-risk stroke populations. Studying the effects of covert stroke and the subsequent progression to dementia will further our understanding of the pathophysiology of these multifaceted diseases.4
Interestingly, chronic cerebral hypoperfusion did not exacerbate cognitive dysfunction in Experiment 1, as hypothesized. Although hypoperfusion causes significant white matter changes similar to SVD pathology,35 it primarily affects spatial memory processing8 and targets susceptible hippocampal pyramidal neurons,14 thus potentially sparing executive function.
It is also important to highlight the profound effect that the unilateral MD infarcts had on functional outcome in these middle-aged rats. Due to the general absence of lateralization in rats, many studies induce bilateral brain injury in order to produce cognitive deficits.36,37 In a recent report,15 bilateral MD ischemic lesions in young, healthy rats did not affect executive performance in the set-shift test. These age-related differences in susceptibility to MD induced executive dysfunction emphasize the importance of using more clinically relevant animal models in stroke research.13
Also of note were the physiological changes documented in the current study. The HFSS diet significantly elevated systolic blood pressure, independently of tachycardia. This is important because blood pressure is a function of cardiac output and systemic vascular resistance. With animals exhibiting similar heart rates, one can speculate that the increase in systolic blood pressure is due primarily to increases in systemic vascular resistance in HFSS animals. With respect to rats, dietary-induced hypertension is generally only observed in stroke prone, spontaneously hypertensive rats (SHR-SP)38 and other strains are considered less susceptible.39,40 Although the SHR-SP models particular aspects of SVD, the location and size of resultant infarcts are unpredictable,6,41 making comparisons of functional outcomes impractical. However, we have learned from SHR-SP models that chronic hypertension leads to significant cerebrovasculopathy.42 This is consistent with findings in the current study. Two-photon imaging revealed a small increase in brain capillary diameter with accompanying sporadic increases in capillary blockage in animals exposed to the HFSS diet. It is unknown to what extent endothelial cell function was affected by diet in the current study, but previous reports note that HFSS diets result in chronic Akt activation and subsequent premature vascular senescence, thus altering the dynamics of cerebral perfusion.43 Further exploration of this hypothesis along with an investigation into the effects of caffeine (contained in Pepsi) which also alters cerebral perfusion,44 is warranted.
Other mechanistic changes that occur with similar dietary manipulations include decreases in brain-derived neurotropic factor, synapsin-1, and cyclic adenosine monophosphate response element-binding protein.45 Although not assessed in the current study, these molecules have been linked to repair mechanisms, neuroplasticity and recovery of function following stroke and neurotrauma.46,47 In this study, we did not find any evidence of elevated systemic or central inflammation. Interestingly, rats exposed to the more palatable, and thus more clinically relevant cafeteria-diet exhibits larger epididymal white adipose and brown adipose tissue changes and subsequent systemic inflammation than their high-fat diet counterparts.48 This may explain the relative lack of inflammation noted in the current study.
As noted in the introduction, age is the most important non-modifiable risk factor for cardiovascular disease and stroke.49 As the brain ages, it responds differentially to insults than a younger brain;50,51 fortunately, many of these effects can be mitigated by lifestyle changes.52 Older individuals recruit additional neuronal circuitry when learning new tasks compared to younger counterparts.53 Aged rats exhibit profound deficits in complex executive functioning tasks such as the one used in the current study.54 Considering the data presented here (i.e. age, diet, SVD, MD infarcts), one hypothesis is that these animals experienced a generalized loss of compensatory ability. It may be that aged rats with complex co-morbidities lack the cognitive reserve that is present in younger animals that allows them to compensate for decreased frontal cortex-thalamic function.15 Thus, it is possible that compensatory circuitry is recruited to maintain cognitive performance following injury to the MD thalamus, but this ability is lost with the added burden imposed by age- and diet-induced vascular and brain compromise.
In conclusion, we report here a rat model of VCI that includes aspects of aging, metabolic disturbances, cardiovascular and SVD. Importantly, these animals exhibit profound executive dysfunction following a unilateral, small (0.363 mm3) infarct in the MD, an area commonly affected in humans with SVD. In accordance with the STAIR recommendations,12 this animal model, including the co-morbidities, may be of heuristic value in the development and understanding of treatments and rehabilitation approaches to slow or offset the development of VCI executive dysfunction.
Supplementary Material
Acknowledgements
The authors thank Garry Chernenko, Matthew Jeffers and Krista Hewlett for technical assistance.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by operating grants from Canadian Institutes of Health Research (DC) and the Canadian Stroke Network, Vascular Cognitive Initiative to DC, JP and THM.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
KDL authored the original version of the manuscript. KDL, CAC, SGB, and JDB conducted all experiments and data analyses. JP, THM and DC conceived the original experiment, contributed to the interpretation of results and provided supervision for the project. All authors read and provided feedback on the written manuscript.
Supplementary material
Supplementary material for this paper can be found at the journal website: http://journals.sagepub.com/home/jcb
References
- 1.Hachinski V, Iadecola C, Petersen RC, et al. National institute of neurological disorders and stroke-canadian stroke network vascular cognitive impairment harmonization standards. Stroke 2006; 37: 2220–2241. [DOI] [PubMed] [Google Scholar]
- 2.Iadecola C. The pathobiology of vascular dementia. Neuron 2013; 80: 844–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rincon F, Wright CB. Vascular cognitive impairment. Curr Opin Neurol 2013; 26: 29–36. [DOI] [PubMed] [Google Scholar]
- 4.Vermeer SE, Longstreth WT, Jr, Koudstaal PJ. Silent brain infarcts: a systematic review. Lancet Neurol 2007; 6: 611–619. [DOI] [PubMed] [Google Scholar]
- 5.Jellinger KA. Pathology and pathogenesis of vascular cognitive impairment – a critical update. Front Aging Neurosci 2013; 5: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hainsworth AH, Markus HS. Do in vivo experimental models reflect human cerebral small vessel disease? A systematic review. J Cereb Blood Flow Metab 2008; 28: 1877–1891. [DOI] [PubMed] [Google Scholar]
- 7.de la Torre JC, Fortin T, Park GAS, et al. Chronic cerebrovascular insufficiency induces dementia-like deficits in aged rats. Brain Res 1992; 582: 186–195. [DOI] [PubMed] [Google Scholar]
- 8.Pappas BA, de la Torre JC, Davidson CM, et al. Chronic reduction of cerebral blood flow in the adult rat: late-emerging CA1 cell loss and memory dysfunction. Brain Res 1996; 708: 50–58. [DOI] [PubMed] [Google Scholar]
- 9.Farkas E, Luiten PG, Bari F. Permanent, bilateral common carotid artery occlusion in the rat: a model for chronic cerebral hypoperfusion-related neurodegenerative diseases. Brain Res Rev 2007; 54: 162–180. [DOI] [PubMed] [Google Scholar]
- 10.O'Collins VE, Macleod MR, Donnan GA, et al. 1,026 experimental treatments in acute stroke. Ann Neurol 2006; 59: 467–477. [DOI] [PubMed] [Google Scholar]
- 11.Endres M, Engelhardt B, Koistinaho J, et al. Improving outcome after stroke: overcoming the translational roadblock. Cerebrovasc Dis 2008; 25: 268–278. [DOI] [PubMed] [Google Scholar]
- 12.Fisher M, Feuerstein G, Howells DW, et al. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke 2009; 40: 2244–2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dirnagl U, Hakim A, Macleod M, et al. A concerted appeal for international cooperation in preclinical stroke research. Stroke 2013; 44: 1754–1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Langdon KD, Granter-Button S, Harley CW, et al. Cognitive rehabilitation reduces cognitive impairment and normalizes hippocampal CA1 architecture in a rat model of vascular dementia. J Cereb Blood Flow Metab 2013; 33: 872–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cordova CA, Jackson D, Langdon KD, et al. Impaired executive function following ischemic stroke in the rat medial prefrontal cortex. Behav Brain Res 2014; 258: 106–111. [DOI] [PubMed] [Google Scholar]
- 16.Windle V, Szymanska A, Granter-Button S, et al. An analysis of four different methods of producing focal cerebral ischemia with endothelin-1 in the rat. Exp Neurol 2006; 201: 324–334. [DOI] [PubMed] [Google Scholar]
- 17.Longstreth WT, Jr, Bernick C, Manolio TA, et al. Lacunar infarcts defined by magnetic resonance imaging of 3660 elderly people: the Cardiovascular Health Study. Arch Neurol 1998; 55: 1217–1225. [DOI] [PubMed] [Google Scholar]
- 18.Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci 2000; 20: 4320–4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holland PC, Bouton ME. Hippocampus and context in classical conditioning. Curr Opin Neurobiol 1999; 9: 195–202. [DOI] [PubMed] [Google Scholar]
- 20.Zhang S, Murphy TH. Imaging the impact of cortical microcirculation on synaptic structure and sensory-evoked hemodynamic responses in vivo. PLoS Biol 2007; 5: e119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murphy TH, Li P, Betts K, et al. Two-photon imaging of stroke onset in vivo reveals that NMDA-receptor independent ischemic depolarization is the major cause of rapid reversible damage to dendrites and spines. J Neurosci 2008; 28: 1756–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drew PJ, Blinder P, Cauwenberghs G, et al. Rapid determination of particle velocity from space-time images using the Radon transform. J Comput Neurosci 2010; 29: 5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schaffer CB, Friedman B, Nishimura N, et al. Two-photon imaging of cortical surface microvessels reveals a robust redistribution in blood flow after vascular occlusion. PLoS Biol 2006; 4: e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Langdon KD, Maclellan CL, Corbett D. Prolonged, 24-h delayed peripheral inflammation increases short- and long-term functional impairment and histopathological damage after focal ischemia in the rat. J Cereb Blood Flow Metab 2010; 30: 1450–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohen J. Statistical power analysis for the behavioral sciences, 2nd ed Hillsdale, NJ: Lawerence Earlbaum Associates, 1988. [Google Scholar]
- 26.Madigan JB, Wilcock DM, Hainsworth AH. Vascular contributions to cognitive impairment and dementia: topical review of animal models. Stroke 2016; 47: 1953–1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arvanitidis AP, Corbett D, Colbourne F. A high fat diet does not exacerbate CA1 injury and cognitive deficits following global ischemia in rats. Brain Res 2009; 1252: 192–200. [DOI] [PubMed] [Google Scholar]
- 28.Winocur G, Greenwood CE. The effects of high fat diets and environmental influences on cognitive performance in rats. Behav Brain Res 1999; 101: 153–161. [DOI] [PubMed] [Google Scholar]
- 29.Wang RY, Wang PS, Yang YR. Effect of age in rats following middle cerebral artery occlusion. Gerontology 2003; 49: 27–32. [DOI] [PubMed] [Google Scholar]
- 30.Langdon KD, Clarke J, Corbett D. Long-term exposure to high fat diet is bad for your brain: exacerbation of focal ischemic brain injury. Neuroscience 2011; 182: 82–87. [DOI] [PubMed] [Google Scholar]
- 31.Tullberg M, Fletcher E, DeCarli C, et al. White matter lesions impair frontal lobe function regardless of their location. Neurology 2004; 63: 246–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carey CL, Kramer JH, Josephson SA, et al. Subcortical lacunes are associated with executive dysfunction in cognitively normal elderly. Stroke 2008; 39: 397–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brown CE, Wong C, Murphy TH. Rapid morphologic plasticity of peri-infarct dendritic spines after focal ischemic stroke. Stroke 2008; 39: 1286–1291. [DOI] [PubMed] [Google Scholar]
- 34.Belayev L, Alonso OF, Busto R, et al. Middle cerebral artery occlusion in the rat by intraluminal suture. Neurological and pathological evaluation of an improved model. Stroke 1996; 27: 1616–1622; discussion 1623. [DOI] [PubMed] [Google Scholar]
- 35.Farkas E, Donka G, de Vos RAI, et al. Experimental cerebral hypoperfusion induces white matter injury and microglial activation in the rat brain. Acta Neuropathol 2004; 108: 57–64. [DOI] [PubMed] [Google Scholar]
- 36.Kolb B, Buhrmann K, McDonald R, et al. Dissociation of the medial prefrontal, posterior parietal, and posterior temporal cortex for spatial navigation and recognition memory in the rat. Cereb Cortex 1994; 4: 664–680. [DOI] [PubMed] [Google Scholar]
- 37.Colbourne F, Corbett D. Delayed postischemic hypothermia: a six month survival study using behavioral and histological assessments of neuroprotection. J Neurosci 1995; 15: 7250–7260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yamori Y, Horie R, Sato M, et al. Hypertension as an important factor for cerebrovascular atherogenesis in rats. Stroke 1976; 7: 120–125. [DOI] [PubMed] [Google Scholar]
- 39.Gu JW, Manning RD, Jr., Young E, et al. Vascular endothelial growth factor receptor inhibitor enhances dietary salt-induced hypertension in Sprague-Dawley rats. Am J Physiol Regulatory, integrative and comparative physiology 2009; 297: R142–R148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ufnal M, Drapala A, Sikora M, et al. Early high-sodium solid diet does not affect sodium intake, sodium preference, blood volume and blood pressure in adult Wistar-Kyoto rats. Br J Nutr 2011; 106: 292–296. [DOI] [PubMed] [Google Scholar]
- 41.Fredriksson K, Kalimo H, Nordborg C, et al. Cyst formation and glial response in the brain lesions of stroke-prone spontaneously hypertensive rats. Acta Neuropathol 1988; 76: 441–450. [DOI] [PubMed] [Google Scholar]
- 42.Ogata J, Fujishima M, Tamaki K, et al. Vascular changes underlying cerebral lesions in stroke-prone spontaneously hypertensive rats. A serial section study. Acta Neuropathol 1981; 54: 183–188. [DOI] [PubMed] [Google Scholar]
- 43.Wang CY, Kim HH, Hiroi Y, et al. Obesity increases vascular senescence and susceptibility to ischemic injury through chronic activation of Akt and mTOR. Sci Signal 2009; 2: ra11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clement P, Mutsaerts HJ, Vaclavu L, et al. Variability of physiological brain perfusion in healthy subjects – a systematic review of modifiers. Considerations for multi-center ASL studies. J Cereb Blood Flow Metab. Epub ahead of print 10 April 2017. DOI: 10.1177/0271678X17702156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Molteni R, Barnard RJ, Ying Z, et al. A high-fat, refined sugar diet reduces hippocampal brain-derived neurotrophic factor, neuronal plasticity, and learning. Neuroscience 2002; 112: 803–814. [DOI] [PubMed] [Google Scholar]
- 46.Wu A, Ying Z, Gomez-Pinilla F. The interplay between oxidative stress and brain-derived neurotrophic factor modulates the outcome of a saturated fat diet on synaptic plasticity and cognition. Eur J Neurosci 2004; 19: 1699–1707. [DOI] [PubMed] [Google Scholar]
- 47.Ploughman M, Windle V, MacLellan CL, et al. Brain-derived neurotrophic factor contributes to recovery of skilled reaching after focal ischemia in rats. Stroke 2009; 40: 1490–1495. [DOI] [PubMed] [Google Scholar]
- 48.Sampey BP, Vanhoose AM, Winfield HM, et al. Cafeteria diet is a robust model of human metabolic syndrome with liver and adipose inflammation: comparison to high-fat diet. Obesity 2011; 19: 1109–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Iadecola C, Anrather J. Stroke research at a crossroad: asking the brain for directions. Nat Neurosci 2011; 14: 1363–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li S, Carmichael ST. Growth-associated gene and protein expression in the region of axonal sprouting in the aged brain after stroke. Neurobiol Dis 2006; 23: 362–373. [DOI] [PubMed] [Google Scholar]
- 51.Hachinski VC, Bowler JV. Vascular dementia. Neurology 1993; 43: 2159–2160. [DOI] [PubMed] [Google Scholar]
- 52.Pialoux V, Brown AD, Leigh R, et al. Effect of cardiorespiratory fitness on vascular regulation and oxidative stress in postmenopausal women. Hypertension 2009; 54: 1014–1020. [DOI] [PubMed] [Google Scholar]
- 53.Heuninckx S, Wenderoth N, Swinnen SP. Systems neuroplasticity in the aging brain: recruiting additional neural resources for successful motor performance in elderly persons. J Neurosci 2008; 28: 91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barense MD, Fox MT, Baxter MG. Aged rats are impaired on an attentional set-shifting task sensitive to medial frontal cortex damage in young rats. Learn Mem 2002; 9: 191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.