Abstract
Stroke is the world's second leading cause of mortality, with a high incidence of morbidity. Numerous neuronal membrane receptors are activated by endogenous ligands and may contribute to infarct development. Notch is a well-characterized membrane receptor involved in cell differentiation and proliferation, and now shown to play a pivotal role in cell death during ischemic stroke. Blockade of Notch signaling by inhibition of γ-secretase, an enzyme that generates the active form of Notch, is neuroprotective following stroke. We have also identified that Pin1, a peptidyl-prolyl isomerase that regulates p53 transactivation under stress, promotes the pathogenesis of ischemic stroke via Notch signaling. Moreover, Notch can also mediate cell death through a p53-dependent pathway, resulting in apoptosis of neural progenitor cells. The current study has investigated the interplay between Notch and p53 under ischemic stroke conditions. Using pharmacological inhibitors, we have demonstrated that a Notch intracellular domain (NICD)/p53 interaction is involved in transcriptional regulation of genes downstream of p53 and NICD to modify stroke severity. Furthermore, the NICD/p53 interaction confers stability to p53 by rescuing it from ubiquitination. Together, these results indicate that Notch contributes to the pathogenesis of ischemic stroke by promoting p53 stability and signaling.
Keywords: Ischemic stroke, p53, Notch, Cell death, Neuroprotection
Introduction
The Notch1 signaling pathway plays a crucial role in the development of the nervous system, including neuronal differentiation and glial determination. Besides its role in all phases of brain development and in physiological events in the adult, there is growing evidence that Notch may also play a contributing role in pathological events. Notch is present in cortical post-mitotic neurons and plays a role in dendritic branching and in neural plasticity.1,2 Notch signaling has been implicated in neuronal cell death and microglial activation in stroke.3–6 We have also reported that the activity of the Notch-activating enzyme, γ-secretase, is elevated in cultured primary cortical neurons subjected to ischemic stroke-like conditions,3 and that mice treated with inhibitors of γ-secretase or transgenic for Notch antisense exhibit less brain injury and functional deficits following ischemic stroke.3,5 Besides enhancing apoptotic cascades in neurons, Notch may play a key role in post-stroke microglial activation and leukocyte infiltration.7,8
Collaborative roles of Notch with other transcription factors such as nuclear factor-κB (NF-κB) and hypoxia-inducible factor-1 (HIF-1) α have been demonstrated in multiple tissues under different conditions.4,5,7,9 Numerous lines of evidence also suggest that these interactions could promote cell death under certain conditions, such as stroke.4 A role for Notch1 in the regulation of NFκ-B-mediated cell death and inflammatory signaling pathways has been suggested in several studies.4,7,9 Similarly, a role for Notch/HIF-1α interaction in neuronal cell death has also been documented in ischemic stroke.5 Cell death was potentiated by combined overexpression of Notch intracellular domain (NICD) and HIF-1α compared to that seen with expression of either protein alone.5 The early inhibition of HIF-1α reduced neurological deficits and brain infarct volume, and additional protection was obtained by combined inhibition of Notch-activating γ-secretase and HIF-1α.5 Additional molecular entities likely to play an interacting role with Notch following stroke are the c-Jun N-terminal kinases (JNK).10–12 Notch signaling can modulate mitogen-activated protein kinase (MAPK) pathways and regulate inflammation, cell death, and cell proliferation.10–12 Our group has demonstrated that the peptidyl-prolyl cis/trans isomerase, Pin1, promotes mechanisms associated with Notch-dependent cell death following stroke.6 In that study, Pin1 was found to interact with NICD and increase its stability by inhibiting FBW7-induced polyubiquitination.6
While evidence exists for interactions between Notch and each of NF-κB, HIF-1α and Pin1 in ischemic stroke, it is unknown whether any interaction occurs between Notch and p53 to promote transcriptional activity and the attendant neuronal cell death following ischemia. However, it was shown that conditional expression of a constitutively active form of Notch in early neural progenitor cells selectively induces extensive apoptosis by elevating levels of nuclear p53 and up-regulating transcription of target pro-apoptotic genes, Bax and Noxa.13 p53 expression is maintained at a low level by mouse double minute 2 homolog (MDM2)-mediated ubiquitination and proteasomal degradation under normal conditions, and it can be stabilized by various cellular stresses.14 The severity and duration of hypoxia appear to exert very different influences on the activity, level, and apoptotic function of p53.14,15 Here we provide the first evidence that Notch and p53 interact to activate apoptotic and neurodegenerative pathways during ischemic brain injury, and that suppression of Notch/p53 signaling ameliorates the disease process in a mouse model of ischemic stroke.
Materials and methods
Animals
All in vivo experimental procedures were approved by the National University of Singapore Animal Care and Use Committee and performed according to the guidelines set forth by the National Advisory Committee for Laboratory Animal Research (NACLAR), Singapore. Mice were housed in individual cages under standard laboratory conditions. All efforts were made to minimize suffering and numbers of animals used. All sections of the manuscript were performed in accordance with ARRIVE (Animal Research: Reporting in Vivo Experiments) guidelines.
Primary mice cortical neuronal cultures and cell lines
Dissociated neuron-enriched cell cultures of cerebral cortex were established from day 16 C57BL/6NTac mouse embryos. Experiments were performed in seven to nine day-old cultures. SH-SY5Y and HEK293T cells were cultured in Dulbecco Modified Eagle Medium (DMEM; Life Technologies) supplemented with 10% fetal bovine serum (HyClone Laboratories, GE Healthcare Life Sciences) and 1% penicillin/streptomycin (Life Technologies, Thermo Fischer Scientific). For transfection experiments, cells were seeded at a density of 2 × 105/well onto 6-well plates on the day before transfection. Cells were transfected using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s protocol and then incubated with complete medium for 24 h. Cells were transfected with mouse human cDNAs of GFP-NICD, NICD-Myc, HA-p53, p53 domain mutants HA-p53/ΔN, HA-p53/DBD and HA-p53/ΔC and a control vector for ubiquitination and immunoprecipitation studies.
Oxygen-glucose deprivation
Cultured neurons were incubated in glucose-free Locke’s buffer containing 154 mM NaCl, 5.6 mM KCl, 2.3 mM CaCl2, 1 mM MgCl2, 3.6 mM NaHCO3, 5 mM HEPES, pH 7.2, supplemented with gentamicin (5 mg/L) in an oxygen-free chamber for 1, 3, 6, 9 or 12 h. N-[N-(3,5-Difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl ester (DAPT), a γ-secretase inhibitor (GSI) (Calbiochem-Merck Millipore), a dipeptide inhibitor of the benzodiazepine type, which inhibits the generation of Notch Intracellular Domain (NICD) mediated by γ-secretase enzyme3–5 and the p53 inhibitor pifithrin-α (Calbiochem-Merck Millipore), a small molecule inhibitor of p53 transcriptional activity which inhibits p53-dependent apoptosis16, were dissolved in dimethylsulfoxide (DMSO, Sigma Aldrich) and used at final concentrations of 1 to 50 µM. To observe the effect of GSI (DAPT) or p53 inhibitor (PFTα), DMSO-dissolved drugs were added to cultures during OGD with the glucose-free Locke’s buffer. Control conditions included exposure to vehicle. OGD-only treatment of primary cortical neurons represents a less complex ischemic situation, and is an ideal approach to study the immediate cell death mechanisms of neurons during ischemia. Nevertheless, it is noteworthy that, despite the chamber containing 95% N2 + 5% CO2 (i.e. no O2), the neuronal cultures are treated with non-deoxygenized Locke’s buffer, such that there is a minimal level of oxygen persisting in the cell environment, which mimics a partial peri-infarct region.
Immunoblot analysis
Protein samples were subjected to sodium dodecyl sulfate–polyacrylamide (10%) gel electrophoresis using a Tris-SDS-glycine running buffer. Gels were then electro-blotted using a transfer apparatus (Bio-Rad) in transfer buffer containing 0.025 mol/L Tris base, 0.15 mol/L glycine, and 20% (v/v) methanol for 1.5 h at 500 mA onto a nitrocellulose membrane (Bio-Rad). The membrane was then incubated for 1 h at room temperature (RT) in blocking agent (1% Fish Skin Gelatin – FSG) dissolved in 1 × TBST (Tris-buffered saline-T) constituted of 20 mM Tris-HCl, pH 7.5, 137 mM NaCl and 0.2% Tween-20. The membrane was then incubated overnight at 4℃ with primary antibodies including those that selectively bind NICD1 (Abcam), NICD-Myc (Roche), P-p53 (Thr81) (Bioss), Total p53 (Cell Signaling Technology), Bax (Abcam), Puma (Abcam), Bcl-2 (Cell Signaling Technology), P-Pin-1 (Abcam), Pin-1 (Abcam), Hes1 (Abcam), cleaved caspase-3 (Cell Signaling Technology), Caspase-3 (Cell Signaling Technology) and β-actin (Sigma-Aldrich) diluted in 1 × TBST. Subsequently, the membrane was incubated with secondary antibodies tagged with horseradish peroxidase (HRP) against the primary antibody for 1 h at RT. The membrane was washed with 1 × TBST and incubated in Clarity Western ECL (Chemiluminescent enhancer reagent) before scanning using Chemidoc Plus (Bio-Rad). Quantification of protein levels was achieved by densitometry analysis using Image J software.
Immunocytochemistry and immunohistochemistry
Coverslips containing cortical neurons subjected to either control or OGD conditions were fixed in 4% buffered paraformaldehyde (PFT) in PBS. Fixed cells were permeabilized and incubated in blocking solution (1% BSA and 0.1% Triton-X in PBS) at RT for 1 h before overnight incubation at 4℃ with microtubule-associated protein 2 antibody (MAP2, Millipore) along with primary antibodies that selectively bind NICD (Abcam), P-p53 Thr81 (Bioss) diluted in blocking solution. Following incubation with primary antibodies, cells were incubated with the appropriate Alexa Fluor-conjugated secondary antibodies (In-vitrogen, Life Technologies, Thermo Fisher Scientific) for 1 h at RT. The nuclei were counterstained with DAPI (AbD Serotec) for 10 min at RT. Following secondary antibody incubation, coverslips were sealed with Vectashield Fluorescent Mounting Medium (Vector Laboratories) on glass slides. For immunohistochemistry, frozen cryostat brain sections were obtained from animals subjected to either sham surgery or cerebral ischemia-reperfusion (I/R) followed by trans-cardiac perfusion with 4% PFA and immunostaining with primary antibodies against MAP2 (Millipore) or primary antibodies that selectively bind NICD (Abcam), P-p53 Thr81 (Bioss). Images were acquired using an Olympus FluoView FV1000 confocal laser-scanning inverted microscope (Olympus, Tokyo, Japan) with a 60 × and 100 × oil immersion objective.
Immunoprecipitation and in vitro ubiquitination
SH-SY5Y cells were transfected with NICD-Myc and HA-p53 (WT, ΔN, DBD, ΔC). Transfected cells were lysed in PBS (0.1% Tween-20) after 24 h and total lysates were centrifuged at 10,000 × g for 10 min at 4℃. For ubiquitination, SH-SY5Y cells were transiently transfected with NICD-Myc or HIS-ubiquitin, and HA-p53 and incubated for 2–3 h. Subsequently, cells were treated with MG132 (10 µg/mL) for 9 h. Cells were lysed in radioimmunoprecipitation assay buffer (200 mM NaCl, 50 mM Tris-cl, 1% tritonX-100, 0.1 % SDS) and centrifuged at 10,000 × g for 10 min at 4℃. The supernatant was incubated with Protein A/G Sepharose (GE Healthcare) beads for 3 h at 4℃. The mixture was centrifuged at 2000 r/min for 30 s and then washed three times with 1 ml of radioimmunoprecipitation assay buffer. The supernatant was discarded, and remaining beads were supplemented with 15 µL of 2 × sample buffer and then boiled at 95℃ for 10 min. Subsequently, immunoprecipitated materials were subjected to immunoblotting with anti-HA, anti-Myc, and anti-p53 antibodies.
p53 stability
SH-SY5Y cells were transfected with Myc-tagged NICD and p53 or HA-tagged p53 (WT, ΔN, DBD, ΔC) for 24 h and then treated with cycloheximide (CHX; 40 µg/mL). The cells were harvested at the indicated times, followed by an immunoblot analysis with anti-Myc, anti-p53 and anti-HA antibodies.
Focal middle cerebral artery I/R stroke model
Experiments were carried out on wild-type (WT) C57BL/6NTac mice obtained from In Vivos (Singapore). Three-month-old C57BL/6NTac male mice were subjected to transient middle cerebral artery I/R. Briefly, after making a midline incision in the neck, the left external carotid and pterygopalatine arteries were isolated and ligated with 6–0 silk thread. The peripheral site of the bifurcation of the internal carotid artery (ICA) was occluded with a small clip and the common carotid artery (CCA) was ligated with 6–0 silk thread. The external carotid artery (ECA) was cut, and a 6–0 nylon monofilament with a tip that was blunted (0.20–0.22 mm) with a coagulator was inserted into the ECA. After the clip at the ICA was removed, the nylon thread was advanced to the origin of the middle cerebral artery (MCA) until light resistance was evident. The nylon thread and the CCA ligature were removed after 1 h to initiate reperfusion. In the sham group, surgery was performed until the arteries were visualized. Mice were administered with either 40 mg/kg of a gamma-secretase (γ-sec) inhibitor (GSI, Calbiochem-Merck Millipore), 2 mg/kg of a p53 inhibitor Pifithrin-α (Santa Cruz) or vehicle (DMSO) by infusion into the femoral vein 1 h after the start of reperfusion. Experimental doses were determined based on previously published work by our group and others.3,4,5,16,17 The mice were euthanized with isoflurane after either 6 h (for protein analysis) or 24 h (for infarct analysis) reperfusion. The animals were included in the study if they underwent successful MCA occlusion, defined by an 80% or greater drop in cerebral blood flow seen with laser Doppler flowmetry. The animals were excluded if insertion of the thread resulted in perforation of the vessel wall determined by the presence of sub-arachnoid blood at the scheduled time of euthanasia.
Neurological assessment and infarct size determination
The functional consequences of I/R injury were evaluated using a five-point neurological deficit score (0, no deficit; 1, failure to extend right paw; 2, circling to the right; 3, falling to the right; and 4, unable to walk spontaneously) and were assessed in a blinded fashion. Brains were immediately removed and placed into phosphate-buffered saline (PBS, Sigma-Aldrich) at 4℃ for 15 min, and four 2-mm coronal sections were made from the olfactory bulb to the cerebellum. The brain sections were stained with 2% 2,3,5-triphenyltetrazolium chloride (TTC) in PBS at 37℃ for 15 min. The stained sections were photographed and the digitized images used for analysis. Borders of the infarct in the image of each brain slice were outlined and the area quantified (Image J software). To correct for brain swelling, the infarct area was determined by subtracting the area of undamaged tissue in the left hemisphere from that of the intact contralateral hemisphere. The infarct volume was determined by calculating the percentage of infarcted area in each brain slice, and then integrating the infarct areas for all slices of each brain.
Statistical analysis
Statistical analysis of all data except the behavioral score data was performed using a one-way ANOVA followed by a Bonferroni post hoc test to determine between-group differences. Statistical difference was accepted if P < 0.05. Neurological behavior scores were analyzed using a non-parametric Kruskal–Wallis test and Dunn’s multiple comparison test. Statistical analyses were performed using GraphPad Prism.
Results
Notch and p53 activation are interdependent following ischemia
To investigate whether Notch-1 and p53 cooperate in promoting neuronal death in stroke, we first examined the expression profiles of NICD and p53 in primary cortical neurons under ischemia-like conditions. Levels of both NICD and phosphorylated p53 (P-p53) were increased after 3–6 h of OGD (Figure 1(a) to (c)). Activation of both Notch and p53 induces cell death under ischemic conditions by promoting expression of cell death pathway proteins, such as Bax, Puma and Caspase-3.4,15 Levels of Bax, Puma, and cleaved Caspase-3, which is an active form of an apoptotic protease, increased following 1–12 h of OGD (Figure 1(a), (e) and (f)). Following ischemia, NICD migrates into the nucleus and associates with transcription factors, such as RBP-Jk, HIF-1α and NF-κB, and up-regulates expression of target genes.18,19 Similarly, it was reported that P-p53 is activated in the ischemic areas of brain, contributing to neuronal apoptosis by up-regulating expression of its target genes.14 Confocal imaging of cortical neurons immunostained with NICD and P-p53 antibodies confirmed that both NICD and P-p53 accumulate in the nucleus following OGD (Figure 1(i) and (j)).
Figure 1.
Simulated ischemia increases the activation of Notch and p53 in primary cortical neurons. Neurons were subjected to OGD and analyzed for NICD, P-p53, and cell death pathway proteins. (a–h), Representative immunoblots and analysis of NICD, phosphorylated p53 (P-p53), p53, apoptotic marker cleaved-caspase3, Bax, Aif, and Puma at the indicated time points during OGD. β-actin was used as the loading control. Data are represented as mean ± S.E.M. n = 3–5 cultures. *P < 0.05, **P < 0.005 and ***P < 0.001 compared with normal cell culture. (i and j), Immunofluorescence images showing confocal images of NICD (i, green), P-p53 (j, green), the neuronal marker MAP2 (red) and the nuclear marker 4′6-diamidino-2-phenylindol (DAPI) (blue). Both NICD and P-p53 staining showed an increase during OGD. Scale bar: 20 µm.
We next examined whether Notch and p53 are functionally interdependent. Thus, using SH-SY5Y cell lines under simulated ischemic conditions (OGD), we determined if inhibition of the Notch activating enzyme, γ-secretase, affects p53 activation. As expected, the OGD-induced increase in NICD was reduced by the GSI (DAPT, 30 μM) (Figure 2(a), (b), (d) and (e)). Intriguingly, the OGD-induced increase in P-p53 was also attenuated by the GSI (Figure 2(a), (c), (d) and (f)). To confirm these findings, we next examined the expression of P-p53 and cell death pathway proteins in primary cortical neurons treated with GSI (DAPT, 10–30 μM) during OGD. Indeed, the OGD-induced increases in NICD, P-p53, cleaved Caspase-3, and Bax were all reduced by GSI treatment (Figure 2(g) to (l)). Next, we tested if blocking p53 might affect expression of NICD in cortical neurons during OGD. Our data indeed indicate that the p53 inhibitor Pifithrin-α (PFT, 20 μM) reduces expression of P-p53, NICD, and the Notch target gene, Hes1 (Figure 2(m) to (q)). We also assessed in cortical neurons the expression of cleaved Caspase-3 as well as lactate dehydrogenase (LDH) release into the media during OGD, and found that both were reduced by the p53 inhibitor Pifithrin-α (PFT, 1–20 μM) (Figure 2(r) and (s)).
Figure 2.
Notch receptor activation and p53-phosphorylation-dependent signaling are inter-dependent. (a–c), Representative immunoblots and analysis of NICD and P-p53 in SH-SY5Y culture treated with a γ-secretase inhibitor (GSI) compared to vehicle treatment. (d–f), Representative immunoblots and analysis of NICD and P-p53 in SH-SY5Y cultured cells treated with GSI compared to the vehicle treated groups. (g–l), Representative immunoblots and quantification of NICD, P-p53, Bax, and cleaved-Caspase3 in primary cortical neuronal cultures treated with GSI or vehicle during 3 h of OGD. (m–r), Representative immunoblots and analysis of P-p53, NICD, Hes-1, and cleaved-Caspase3 in primary cortical neuronal cultures treated with the p53 inhibitor Pifithrin-α (PFT) or vehicle during 3 h of OGD. β-actin was used as the loading control. (s), Cytotoxicity evaluation of PFT treatment or vehicle by lactate dehydrogenase assay during 6 h of OGD. Data are represented as mean ± S.E.M. n = 3–5 cultures. *P < 0.05, **P < 0.005 and ***P < 0.001 compared with vehicle-treated control.
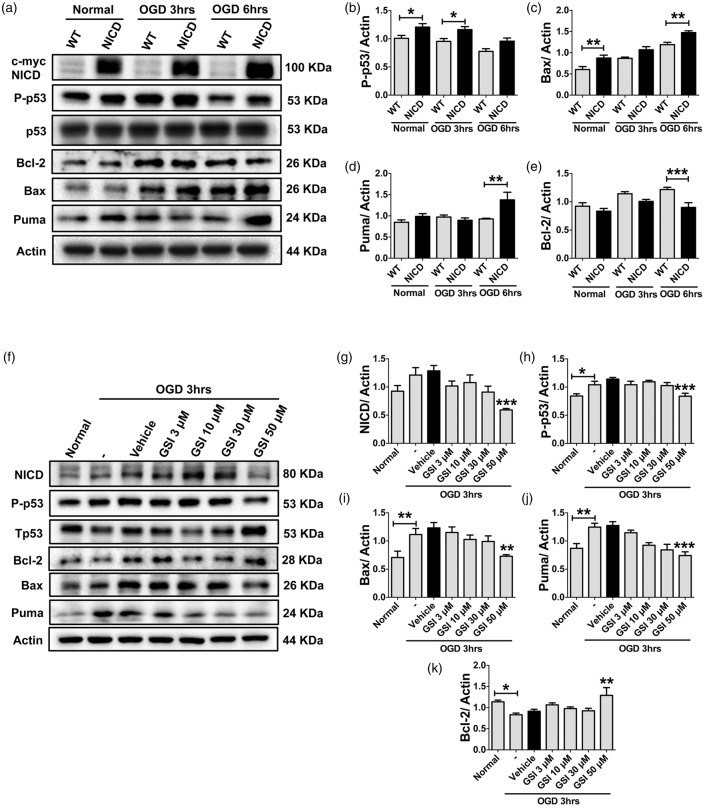
To further confirm Notch and p53 to be functionally interdependent under ischemic conditions, we studied c-Myc-tagged NICD-overexpressing HEK293T cells and assessed the expression of p53 and cell death proteins during OGD. Our data show that transfection with NICD increases expression of P-p53 under normal conditions and after 3 h of OGD (Figure 3(a) and (b)) compared with non-transfected WT control cells. In addition, expression of Bax and Puma was also increased compared to WT controls following 6 h of OGD (Figure 3(a), (c) and (d)). On the other hand, expression of the anti-apoptotic protein, Bcl-2, was lower in NICD-overexpressing cells compared to WT cells (Figure 3(a) and (e)). As we had employed Myc-tagged NICD-overexpressing HEK293T cells above, we next tested whether blocking Notch using the GSI (DAPT, 3–50 μM) can also reduce expression of p53 and cell death proteins during OGD. Our data indeed demonstrate that γ-secretase inhibition reduced expression of NICD, P-p53, Bax, and Puma during 3 h of OGD (Figure 3(f) to (j)). Furthermore, we found that GSI increased expression of the anti-apoptotic protein, Bcl-2, during OGD compared to vehicle treatment (Figure 3(f) and (k)).
Figure 3.
NICD overexpression triggers p53-mediated cell signaling, while Notch inhibition regulates p53 cell signaling. (a–e), Representative immunoblots and analysis of P-p53, Bax, Puma, and Bcl-2 in HEK 293 T NICD-overexpressing cells at the indicated time-points during OGD. (f–k), Representative immunoblots and analysis of NICD, p53,Bax, Puma, and Bcl-2 in HEK 293 T cells treated with GSI or vehicle during 3 h of OGD. β-actin was used as the loading control. Data are represented as mean ± S.E.M. n = 3–5 cultures. *P < 0.05, **P < 0.005 and ***P < 0.001 compared with vehicle treated control.
NICD associates with p53 under basal and ischemic conditions
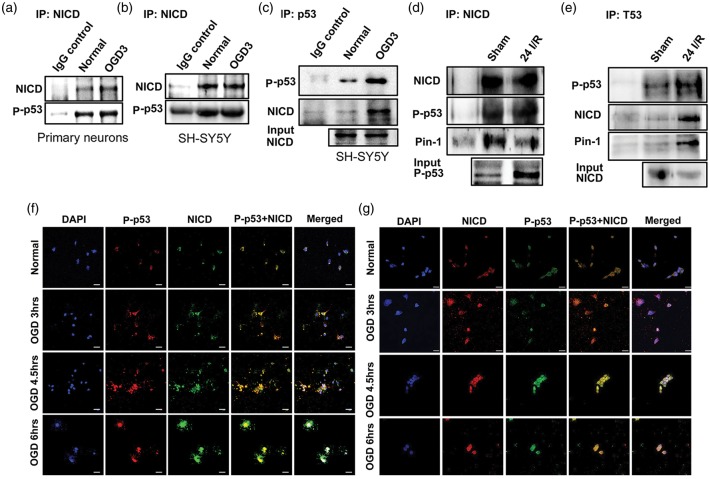
To further establish that Notch and p53 collaboration promotes cell death under ischemic conditions, we next performed co-immunoprecipitation experiments in neuronal cells and in brain tissue following ischemia in vivo. Our data demonstrate that NICD and p53 physically bind together in primary mouse cortical neurons (Figure 4(a)) and in SH-SY5Y cells (Figure 4(b) and (c)) under both normal and OGD conditions. Furthermore, NICD and p53 are co-immunoprecipitated in brain tissue from mice subjected to focal ischemic stroke (Figure 4(d) and (e)). We also observed co-immunoprecipitation of Pin1 with both NICD (Figure 4(d)) and p53 (Figure 4(e)) in post-ischemic brain tissue from mice. Confocal images of primary neurons (Figure 4(f)) and SH-SY5Y cells (Figure 4(g)) immunostained with antibodies against NICD and P-p53 confirmed increased levels and co-localization of NICD and p53 in the nucleus in response to OGD (Figure 4(f) and (g)).
Figure 4.
NICD associates with p53. (a–e), Representative immunoblots of NICD and p53 co-immunoprecipitate in primary cortical neurons (a) and a neuroblastoma SH-SY5Y cell line (b and c) following 3 h of OGD and in ischemic brain 24 h following ischemia and reperfusion (I/R) or sham surgery (d and e). Confocal immunofluorescence images of primary cortical neurons (f) and neuroblastoma SH-SY5Y cells (g), show staining of NICD (f, green; g, Red), P-p53 (f, red; g, green), and nuclear marker 4′6-diamidino-2-phenylindol (DAPI) (blue). Both NICD and P-p53 staining are increased and co-localized at the indicated OGD time-points. Scale bar: 20 µm.
Notch reduces p53 ubiquitination and increases p53 stability
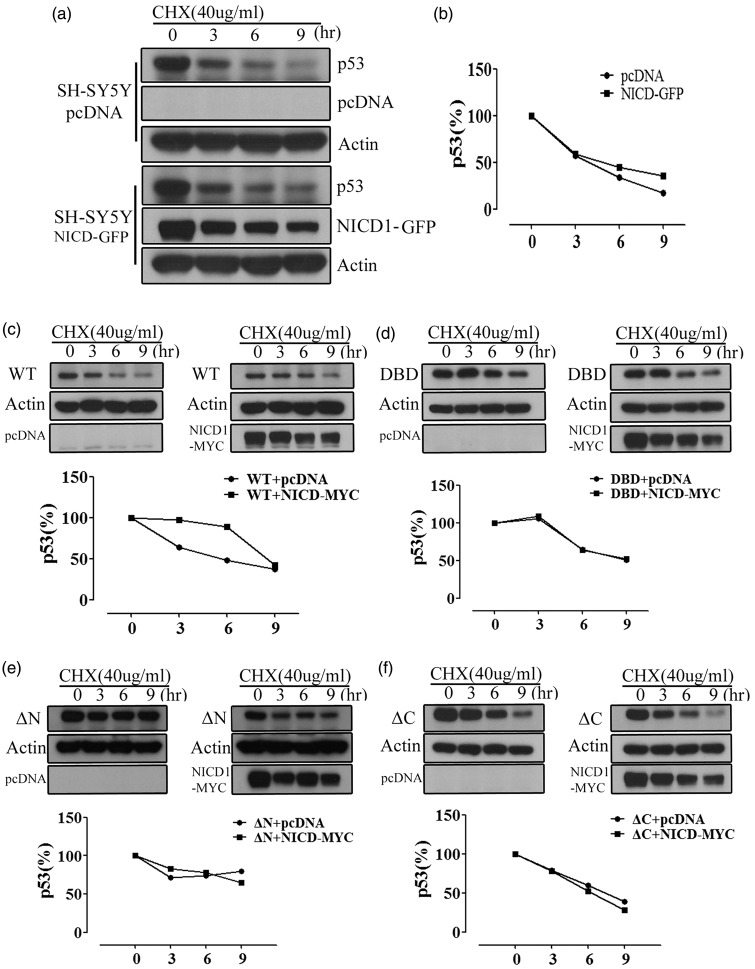
To test whether NICD regulates p53 stability, a GFP-tagged NICD-overexpressed SH-SY5Y cell line was employed, and p53 stability was assessed using cycloheximide (CHX) chases. We found that NICD overexpression increased p53 stability in comparison to pcDNA-transfected cells (Figure 5(a) and (b)). Next, we examined p53 turnover in NICD-Myc overexpressing or pcDNA transfected SH-SY5Y cells transiently transfected with p53-HA, DNA binding domain deleted (DBD)-p53-HA, N-terminal deleted p53 (ΔNp53)-HA or C-terminal deleted (ΔCp53)-HA in the presence of CHX chases. Our data demonstrate that NICD overexpression increased p53 stability in WT over 9 h in the presence of CHX chases (Figure 5(c)), while cells with deletion of DBD (Figure 5(d)), N terminal (Figure 5(e)) or C terminal (Figure 5(f)) domains were unaffected by NICD overexpression-induced p53 stability. To identify the role of NICD in p53 regulation, we performed co-immunoprecipitation in SH-SY5Y cells transiently transfected with p53-HA, ΔNp53-HA, DBD-p53-HA, ΔCp53-HA or NICD-Myc. After 24 h of transfection, cells were subjected to immunoprecipitation with anti-HA. NICD binding with p53 was decreased in ΔNp53-HA, DBD-p53-HA and ΔCp53-HA domain-deleted mutants (Figure 6(a) and (b)). p53 protein stability is regulated by MDM2 mediation of the ubiquitin proteasome pathway (UPP).20,21 Because NICD was found to regulate p53 stability, we next tested for effects of NICD on p53 ubiquitination. SH-SY5Y cells were transfected with HIS-tagged ubiquitin (Ub-HIS), Myc-tagged NICD, or HA-tagged p53, and then treated with MG132 for 12 h. MG132 blocked the proteolytic activity of the 26 S proteasome complex. Co-immunoprecipitation using an anti-HA antibody showed that polyubiquitinated p53 was markedly reduced by NICD overexpression. In addition, interaction with E3 ubiquitin-protein ligase MDM2 was markedly reduced by NICD overexpression (Figure 6(c)).
Figure 5.
NICD increases stability of p53. (a and b), pcDNA and NICD-GFP were transiently transfected in SH-SY5Y cells and after 24 h, the cells were treated with 40 ug cycloheximide (CHX) with the indicated time. Representative immunoblot and analysis showing p53 stabilization in NICD-Myc overexpressing cells. (c–d), SH-SY5Y cells were transiently transfected with p53-HA, ΔNp53-HA, DBD-p53, ΔCp53-HA and NICD-Myc. After 24 h of transfection, the cells were treated with 40 µg CHX with the indicated time. (c) Representative immunoblot and analysis showing increased stability of WT p53 by over-expression of NICD-Myc. (d–f), Representative immunoblots and analysis showing stability of p53 deletion mutants following over-expression of NICD-Myc.
Figure 6.
Interaction of NICD-p53 reduces p53 ubiquitination. (a and b), SH-SY5Y cells were transiently transfected with p53-HA, ΔNp53-HA, DBD-p53, ΔCp53-HA and NICD-Myc. Immunoprecipitation with anti-HA shows that NICD binding with p53 is decreased in ΔNp53-HA, DBD-p53 and ΔCp53-HA domain-deleted mutants. (c), pcDNA, NICD-Myc, Ub-HIS and p53-HA were transiently transfected into SH-SY5Y cells and the cells were then treated with 10 µg MG132. A co-immunoprecipitation representative blot shows reduced p53-polyubiquitination and a reduced interaction between p53 and MDM2 upon overexpression of NICD.
γ-secretase and p53 inhibition protects against neuronal cell death in vitro and ischemic stroke-induced brain injury in vivo
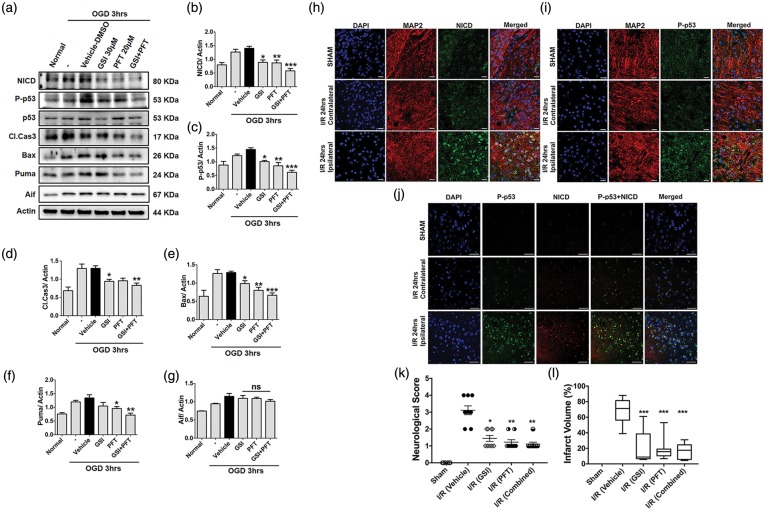
To determine whether blockade of γ-secretase-mediated Notch activation or p53 individually can protect against cell death and stroke-induced brain injury by reducing the NICD-p53 interaction, we used γ-secretase inhibitor (GSI) DAPT and PFT (p53 inhibitor). Indeed, we found that SH-SY5Y cells treated with either DAPT or PFT had lower expression of NICD, P-p53, pro-apoptotic cleaved-Caspase-3, Bax, and Puma compared to vehicle-treated cells following 3 h of OGD (Figure 7(a) to (f)). No changes were observed in expression of apoptosis inducing factor (Aif) among cells treated with vehicle, DAPT, or PFT (Figure 7(a) and (g)).
Figure 7.
NICD and p53 co-localize in the neuronal nucleus and inhibition of NICD and P-p53 reduces ischemic brain injury in vivo. (a–g), Representative immunoblots of activated NICD, p53, Bax, Puma, Aif, and cleaved-Caspase-3 in primary cortical neurons treated with GSI and/or PFT compared with vehicle treatment during 3 h of OGD. β-actin was used as the loading control. Data are represented as mean ± S.E.M. n = 3-5 cultures. *P < 0.05, **P < 0.005 and ***P < 0.001 compared with control. (h and i), Figures showing immunofluorescent staining of NICD (h, green), P-p53 (i, green), the neuronal marker MAP2 (red), and the nuclear marker 4′6-diamidino-2-phenylindol (DAPI) (blue) in the cortical penumbra region of the ischemic hemisphere of mice. (j), Immunofluorescent staining of NICD (red), P-p53 (green), and the nuclear marker 4′6-diamidino-2-phenylindol (DAPI) (blue) in the cortical penumbra region of the ipsilateral brain of mice. Both NICD and P-p53 staining showed an increase and co-localization after 1 h of ischemia and 24 h reperfusion (I/R). Scale bar: 20 µm. (k and l), Mice underwent 1 h of MCAO and 24 h reperfusion and were analysed for neurological deficit and infarct volume. Mice treated with GSI (n = 9) and PFT (n = 9), alone or in combination (n = 9), exhibited improved neurological function and reduced brain injury after stroke, compared to vehicle-treated (n = 9) mice. Neurological score and infarct volume were measured at 24 h following reperfusion. Data are represented as mean ± SEM. *P < 0.05, **P < 0.005 and ***P < 0.001 compared with I/R (Vehicle).
We next clarified the expression patterns of NICD and p53 in vivo following 1 h ischemia and 24-h reperfusion. In the cerebral cortex of sham-operated control mice, very little immunoreactivity with NICD and p53 antibodies was observed, and both NICD and p53 appeared restricted to the neuronal cytoplasm (Figure 7(h) and (i)). After 24 h of post-ischemic reperfusion, neurons in the ischemic cortex (ipsilateral) exhibited robust NICD and p53 immunoreactivity in both nucleus and cytoplasm (Figure 7(h) and (i)). In addition, we observed strong co-localization of NICD and p53 in the nucleus of neurons in the ischemic cortex (Figure 7(j) and Supplementary Figure 1). Finally, we studied the effect of either individual or combined inhibition of γ-secretase (using DAPT) and p53 (using PFT) on the degree of stroke-induced brain injury in vivo. Intravenous infusion of either DAPT or PFT alone immediately after ischemia reduced the extent of neurological deficit and brain damage compared to vehicle treatment as assessed after 24 h (Figure 7(k) and (l)). Interestingly, combined DAPT and PFT treatment did not provide greater protection than did the administration of the individual drugs (Figure 7(k) and (l)).
Discussion
The major finding of this study is that the Notch-p53 interaction leads to compromised neuronal viability after ischemic stroke. We showed that the NICD-p53 interaction is involved in transcriptional regulation of genes downstream of p53 and NICD to modify disease outcome following stroke. Additionally, we demonstrated that the NICD-p53 interaction confers stability to p53 by rescuing it from ubiquitination.
Notch signaling is involved in the maintenance of neural progenitors in an undifferentiated state, in part by inhibition of neurogenesis, and it influences synaptic plasticity, learning, and memory in the adult brain.19,22 NICD translocates to the nucleus where it functions as a transcriptional coactivator in conjunction with the CBF-1/Su(H)/LAG-1 (CSL) family of DNA-binding proteins by recruiting general transcription factors, including CBP/p300.23–25 NICD-mediated transcription induces expression of genes such as Hairy-Enhancer of Split (Hes) and Hes-related protein (Herp).26 Notch-mediated neuronal cell fate determination or other events could be influenced not only by Hes but also by NICD’s interaction with other targets, such as NF-κB,4,27 HIF-1α5,28 and c-Jun.12,29 A pro-apoptotic role for Notch in focal ischemic stroke was supported by studies showing that mice overexpressing Notch1 antisense (NAS) and normal mice treated with inhibitors of the Notch-activating enzyme, γ-secretase, exhibit reduced damage to brain cells and improved functional outcome.3–5 Consequently, the mechanisms underlying Notch-mediated apoptosis in ischemic stroke were further investigated here. Notch was shown to bind to P-p65 and to prolong NF-κB-mediated pro-inflammatory responses in immune cells.30 Notch signaling was also shown to interact with NF-κB and to endanger neurons after ischemic stroke by modulating NF-κB activity.4,7,31 Accordingly, inhibition of γ-secretase protects against ischemic neuronal cell death by targeting NF-κB and the pro-death BH3-only protein, Bcl-2-interacting mediator of cell death (Bim).4,31 Furthermore, inhibitors of γ-secretase reduce activation of NF-κB and expression of proinflammatory mediators, resulting in marked attenuation of neurotoxic actions of microglia.7,31
It seemed plausible that other death-promoting transcription factors may also interact with NICD and contribute to brain injury following stroke, and we previously showed that NICD and HIF-1α bind directly to each other to form part of a multi-protein transcriptional complex that may include NF-κB and other NICD binding partners.5 Levels of NF-κB, p-p65, and cleaved Caspase-3 were all reduced by inhibition of γ-secretase/Notch, HIF-1α alone or incombination under hypoxic conditions.5 Interestingly, Caspase-3 levels were further reduced by combined inhibition of γ-secretase/Notch and HIF-1α. Transfection with NICD, HIF-1α, or both, increased expression of the phosphorylated (p-p65) and non-phosphorylated (p65) forms of the 65 kDa subunit of NF-κB under both normal and hypoxic/ischemic conditions.5 Likewise, HIF-1α inhibition in neuronal cells transfected with either NICD, HIF-1α, or both, resulted in decreased levels of total and P-p65 under both normal and hypoxic conditions.5 Independent of NICD, an interaction between HIF-1α and NF-κB was also demonstrated to exist in normal and other disease conditions.4,30,32 Collectively, the evidence described above suggests that NICD, HIF-1α, and NF-κB signaling pathways communicate with each other, but interplay between NICD and p53 has not been established under conditions of ischemic stroke. Thus, our current findings represent the first evidence that interactions between NICD and p53 promote cell death pathways in the brain following ischemic stroke.
The Notch-p53 association in apoptosis was previously established in early neural progenitor cells by Yang et al.13 who showed that conditional expression of a constitutively active form of Notch1 selectively induces extensive apoptosis by elevating levels of nuclear p53 and up-regulating transcription of target pro-apoptotic genes, such as Bax and Noxa. p53 is normally maintained at a low level by MDM2-mediated ubiquitination and proteasomal degradation, and it can be stabilized by various cellular stresses. The p53 protein is also involved in the cellular response to ischemia. The severity and the duration of hypoxia/ischemia exert very different influences on the activity, level, and apoptotic function of p53.33,34 Early studies reported that the total absence of p53 was marginally protective in ischemic stroke.35 Another study found that a majority of p53-deficient mice displayed no signs of cell damage following subcutaneous injection of kainic acid compared to the extensive hippocampal cell loss in wild type animals.36 Furthermore, it was shown that pifithrin-α protected neurons against apoptosis induced by the DNA-damaging agents, amyloid beta-peptide and glutamate.37 Animals treated with pifithrin-α displayed less cell death, decreased expression of the p53 target gene, BAX37 and dose-dependently increased the number and size of new neurospheres formed following ischemic stroke.38 In the present study, we employed a comparable dose of pifithrin-α (2 mg/kg) and also observed a similar degree of neuroprotection in ischemic stroke. We also observed an additive protective effect by combined Notch/p53 inhibition in a cell culture model of simulated ischemic stroke, but such an additive effect was not replicated in the degree of attenuation of infarct size and neurological deficit in vivo. It is possible that this was due to the severity of our ischemic model such that the maximum possible protective effect could be obtained with just a single treatment targeting either Notch or p53. In addition, the interdependent mechanism of p53 and NICD in vivo could be maximally inhibited (if the inhibitor dose is sufficiently high) due to a maximal inhibitory effect achieved by targeting one of the proteins.
During severe hypoxia, induction of p53 is HIF-1α-dependent39 because p53 stabilization is mediated through its binding with HIF-1α.40 HIF-1α-mediated stabilization and activation of p53 may result from HIF-1α binding of MDM2, leading to accumulation of p53.39 Other reports propose that HIF-1α-mediated p53 stabilization promotes p53-dependent apoptosis during hypoxia.41 Our current data show that overexpression of NICD increases stability of p53 in comparison to pcDNA-transfected cells and deletion of N terminal, C terminal, or DNA binding domain (DBD) abolished the p53 stability resulting from NICD overexpression. The interaction between NICD and p53 was reduced in mutants containing N terminal, C terminal, or DBD deletion. Furthermore, polyubiquitinated p53 was markedly reduced by NICD overexpression in neuronal cells following conditions of simulated ischemia. The interaction between the E3 ubiquitin-protein ligase MDM2 and p53 was also noticeably reduced by NICD overexpression. This body of new evidence strongly suggests that the NICD-p53 interplay may play a pivotal role in determining pro-apoptotic signaling in the brain after ischemic stroke.
We recently reported that Pin1 also plays a crucial role in neuronal death following ischemic stroke.6 Specifically, the Pin1-FBW7-Notch1 axis compromises neuronal vulnerability after ischemic stroke, and neuronal injury can be ameliorated in vitro and in vivo by either a Pin1 inhibitor or Pin1 deficiency.6 Pin1 interacts with NICD in the brain to increase its stability after ischemic stroke by inhibiting FBW7-mediated polyubiquitination, resulting in facilitation of NICD-induced neuronal death. Thus, the coordinated interaction between Pin1/FBW7 and NICD, and the well-established function of Notch1 in ischemia-induced neuronal damage, provides strong evidence that Pin1 regulates neuronal death by stabilizing NICD following ischemic stroke. Furthermore, it was shown that the phosphorylated p53 induced by stress forms a complex with Pin1 and then undergo a conformational change in order to fulfill its biological roles.42 Our current and previous observations imply that the coordinated interactions between Pin1-NICD, and NICD with p53, NFκ-B, and HIF1α, may each impact the stability of the other protein interactions to promote ischemia-induced neuronal cell death. The factors that determine whether the interplay of NICD-p53 is detrimental for neurons following ischemic stroke need further investigation. It is likely that the NICD-p53 interaction plays a key functional role in the multiprotein transcriptional complex, which ultimately drives the expression of inflammatory and pro-apoptotic genes in the brain following ischemic stroke.
Supplementary Material
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Singapore Ministry of Education Tier 1 grant [T1-BSRG-2015-01; R-185-000-300-112], NUHS Bench to Bedside and Bench to Bedside to Product Grant [R-185-000-276-515], Singapore National Medical Research Council Research Grant (NMRC-CBRG-0102/2016), National Research Foundation (NRF) (2015R1A2A1A01003530), Republic of Korea, the Ministry of Education, Science and Technology, and a grant of the Korean Health 21 R&D Project (HI14C2539), Ministry of Health & Welfare, Republic of Korea.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors’ contributions
The experimental set-up was designed by PB, SHB, CGS, DGJ, and TVA. The experiments were conducted by PB, and SHB. Data analysis was made by PB, SHB, KM, DGJ, and TVA. All authors discussed the results. Manuscript was written by PB, SHB, and TVA edited by CGS, and DGJ, and approved by all authors.
Supplementary material
Supplementary material for this paper can be found at the journal website: http://journals.sagepub.com/home/jcb
References
- 1.Redmond L, Oh SR, Hicks C, et al. Nuclear Notch1 signaling and the regulation of dendritic development. Nat Neurosci 2000; 3: 30–40. [DOI] [PubMed] [Google Scholar]
- 2.Wang Y, Chan SL, Miele L, et al. Involvement of Notch signaling in hippocampal synaptic plasticity. Proc Natl Acad Sci U S A 2004; 101: 9458–9462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arumugam TV, Chan SL, Jo DG, et al. Gamma secretase-mediated Notch signaling worsens brain damage and functional outcome in ischemic stroke. Nat Med 2006; 12: 621–623. [DOI] [PubMed] [Google Scholar]
- 4.Arumugam TV, Cheng YL, Choi Y, et al. Evidence that gamma-secretase-mediated Notch signaling induces neuronal cell death via the nuclear factor-kappaB-Bcl-2-interacting mediator of cell death pathway in ischemic stroke. Mol Pharmacol 2011; 80: 23–31. [DOI] [PubMed] [Google Scholar]
- 5.Cheng YL, Park JS, Manzanero S, et al. Evidence that collaboration between HIF-1α and Notch-1 promotes neuronal cell death in ischemic stroke. Neurobiol Dis 2014; 62: 286–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baik SH, Fane M, Park JH, et al. Pin1 promotes neuronal death in stroke by stabilizing Notch intracellular domain. Ann Neurol 2015; 77: 504–516. [DOI] [PubMed] [Google Scholar]
- 7.Wei Z, Chigurupati S, Arumugam TV, et al. Notch activation enhances the microglia-mediated inflammatory response associated with focal cerebral ischemia. Stroke 2011; 42: 2589–2594. [DOI] [PubMed] [Google Scholar]
- 8.Marumo T, Takagi Y, Muraki K, et al. Notch signaling regulates nucleocytoplasmic Olig2 translocation in reactive astrocytes differentiation after ischemic stroke. Neurosci Res 2013; 75: 204–209. [DOI] [PubMed] [Google Scholar]
- 9.Cheng YL, Choi Y, Sobey CG, et al. Emerging roles of the gamma-secretase-notch axis in inflammation. Pharmacol Ther 2015; 147: 80–90. [DOI] [PubMed] [Google Scholar]
- 10.Pallavi SK, Ho DM, Hicks C, et al. Notch and Mef2 synergize to promote proliferation and metastasis through JNK signal activation in Drosophila. EMBO J 2012; 31: 2895–2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamashita AS, Geraldo MV, Fuziwara CS, et al. Notch pathway is activated by MAPK signaling and influences papillary thyroid cancer proliferation. Transl Oncol 2013; 6: 197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng YL, Choi Y, Seow WL, et al. Evidence that neuronal Notch-1 promotes JNK/c-Jun activation and cell death following ischemic stress. Brain Res 2014; 1586: 193–202. [DOI] [PubMed] [Google Scholar]
- 13.Yang X, Klein R, Tian X, et al. Notch activation induces apoptosis in neural progenitor cells through a p53-dependent pathway. Dev Biol 2004; 269: 81–94. [DOI] [PubMed] [Google Scholar]
- 14.Sermeus A, Michiels C. Reciprocal influence of the p53 and the hypoxic pathways. Cell Death Dis 2011; 2: e164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Culmsee C, Mattson MP. p53 in neuronal apoptosis. Biochem Biophys Res Commun 2005; 331: 761–777. [DOI] [PubMed] [Google Scholar]
- 16.Komarov PG, Komarova EA, Kondratov RV, et al. A chemical inhibitor of p53 that protects mice from the side effects of cancer therapy. Science 1999; 285: 1733–1737. [DOI] [PubMed] [Google Scholar]
- 17.Leker RR, Aharonowiz M, Greig NH, et al. The role of p53-induced apoptosis in cerebral ischemia: effects of the p53 inhibitor pifithrin alpha. Exp Neurol 2004; 187: 478–486. [DOI] [PubMed] [Google Scholar]
- 18.Artavanis-Tsakonas S, Muskavitch MA, Yedvobnick B. Molecular cloning of Notch, a locus affecting neurogenesis in Drosophila melanogaster. Proc Natl Acad Sci U S A 1983; 80: 1977–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lathia JD, Mattson MP, Cheng A. Notch: from neural development to neurological disorders. J Neurochem 2008; 107: 1471–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levine AJ. p53, the cellular gatekeeper for growth and division. Cell 1997; 88: 323–331. [DOI] [PubMed] [Google Scholar]
- 21.Wu L, Levine AJ. Differential regulation of the p21/WAF-1 and mdm2 genes after high-dose UV irradiation: p53-dependent and p53-independent regulation of the mdm2 gene. Mol Med 1997; 3: 441–451. [PMC free article] [PubMed] [Google Scholar]
- 22.Tan T, Lu B, Zhang J, et al. Notch1 signaling antagonizes transforming growth factor-beta pathway and induces apoptosis in rabbit trophoblast stem cells. Stem Cells Dev 2014; 23: 813–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schroeter EH, Kisslinger JA, Kopan R. Notch-1 signalling requires ligand-induced proteolytic release of intracellular domain. Nature 1998; 393: 382–386. [DOI] [PubMed] [Google Scholar]
- 24.Brou C, Logeat F, Gupta N, et al. A novel proteolytic cleavage involved in Notch signaling: the role of the disintegrin-metalloprotease TACE. Mol Cell 2000; 5: 207–216. [DOI] [PubMed] [Google Scholar]
- 25.Miele L. Notch signaling. Clin Cancer Res 2006; 12: 1074–1079. [DOI] [PubMed] [Google Scholar]
- 26.Borggrefe T, Oswald F. The Notch signaling pathway: transcriptional regulation at Notch target genes. Cell Mol Life Sci 2009; 66: 1631–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng P, Zlobin A, Volgina V, et al. Notch-1 regulates NF-kappaB activity in hemopoietic progenitor cells. J Immunol 2001; 167: 4458–4467. [DOI] [PubMed] [Google Scholar]
- 28.Mukherjee T, Kim WS, Mandal L, et al. Interaction between Notch and Hif-alpha in development and survival of Drosophila blood cells. Science 2011; 332: 1210–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen M, Sun J, Lu C, et al. The impact of neuronal Notch-1/JNK pathway on intracerebral hemorrhage-induced neuronal injury of rat model. Oncotarget 2016; 7: 73903–73911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shin HM, Minter LM, Cho OH, et al. Notch1 augments NF-kappaB activity by facilitating its nuclear retention. EMBO J 2006; 25: 129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li S, Zyang X, Wang Y, et al. DAPT protects brain against cerebral ischemia by down-regulating the expression of Notch 1 and nuclear factor κB in rats. Neurol Sci 2012; 33: 1257–1264. [DOI] [PubMed] [Google Scholar]
- 32.Wang J, Shelly L, Miele L, et al. Human Notch-1 inhibits NF-kappa B activity in the nucleus through a direct interaction involving a novel domain. J Immunol 2001; 167: 289–295. [DOI] [PubMed] [Google Scholar]
- 33.Hubert A1, Paris S, Piret JP, et al. Casein kinase 2 inhibition decreases hypoxia-inducible factor-1 activity under hypoxia through elevated p53 protein level. J Cell Sci 2006; 119: 3351–3362. [DOI] [PubMed] [Google Scholar]
- 34.Sano M, Minamino T, Toko H, et al. p53-induced inhibition of Hif-1 causes cardiac dysfunction during pressure overload. Nature 2007; 446: 444–448. [DOI] [PubMed] [Google Scholar]
- 35.Crumrine RC, Thomas AL, Morgan PF. Attenuation of p53 expression protects against focal ischemic damage in transgenic mice. J Cereb Blood Flow Metab 1994; 14: 887–891. [DOI] [PubMed] [Google Scholar]
- 36.Morrison RS, Wenzel HJ, Kinoshita Y, et al. Loss of the p53 tumor suppressor gene protects neurons from kainate-induced cell death. J Neurosci 1996; 16: 1337–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Culmsee C, Zhu X, Yu QS, et al. A synthetic inhibitor of p53 protects neurons against death induced by ischemic and excitotoxic insults, and amyloid beta-peptide. J Neurochem 2001; 77: 220–228. [DOI] [PubMed] [Google Scholar]
- 38.Luo Y, Kuo CC, Shen H, et al. Delayed treatment with a p53 inhibitor enhances recovery in stroke brain. Ann Neurol 2009; 65: 520–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.An WG, Kanekal M, Simon MC, et al. Stabilization of wild-type p53 by hypoxia-inducible factor 1alpha. Nature 1998; 392: 405–408. [DOI] [PubMed] [Google Scholar]
- 40.Suzuki H, Tomida A, Tsuruo T. Dephosphorylated hypoxia-inducible factor 1alpha as a mediator of p53-dependent apoptosis during hypoxia. Oncogene 2001; 20: 5779–5788. [DOI] [PubMed] [Google Scholar]
- 41.Halterman MW, Miller CC, Federoff HJ. Hypoxia-inducible factor-1alpha mediates hypoxia-induced delayed neuronal death that involves p53. J Neurosci 1999; 19: 6818–6824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zacchi P, Gostissa M, Uchida T, et al. The prolyl isomerase Pin1 reveals a mechanism to control p53 functions after genotoxic insults. Nature 2002; 419: 853–857. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.