Abstract
Background & Aims
Infections are life-threatening to patients with acute decompensation and acute-on-chronic liver failure (AD/ACLF). Patients with AD/ACLF have prostaglandin E2–mediated immune suppression, which can be reversed by administration of albumin; infusion of 20% human albumin solution (HAS) might improve outcomes of infections. We performed a feasibility study to determine optimal trial design, assess safety, and validate laboratory assessments of immune function to inform design of a phase 3 trial.
Methods
We performed a prospective multicenter, single-arm, open-label trial of 79 patients with AD/ACLF and levels of albumin lower than 30 g/L, seen at 10 hospitals in the United Kingdom from May through December 2015. Patients were given daily infusions of 20% HAS, based on serum levels, for 14 days or until discharge from the hospital. Rates of infection, organ dysfunction, and in-hospital mortality were recorded. The primary end point was daily serum albumin level during the treatment period. Success would be demonstrated if 60% achieved and maintained serum albumin levels at or above 30 g/L on at least one third of days with recorded levels.
Results
The patients’ mean model for end-stage disease score was 20.9 ± 6.6. The primary end point (albumin ≥30 g/L on at least one third of days recorded) was achieved by 68 of the 79 patients; 75% of administrations were in accordance with suggested dosing regimen. Mean treatment duration was 10.3 days (104 ± 678 mL administered). There were 8 deaths and 13 serious adverse events, considered by the independent data-monitoring committee to be consistent with those expected. Twelve of 13 patients that developed either respiratory or cardiovascular dysfunction (based on ward-based clinical definitions) as their only organ dysfunction were alive at 30 days compared with 1 of 3 that developed renal dysfunction. Only 1 case of brain dysfunction was recorded.
Conclusions
In a feasibility trial, we found that administration of HAS increased serum levels of albumin in patients with AD/ACLF. The dosing regimen was acceptable at multiple sites and deemed safe by an independent data-monitoring committee. We also developed a robust system to record infections. The poor prognosis for patients with renal dysfunction was confirmed. However, patients with cardiovascular or respiratory dysfunction had good outcomes, which is counterintuitive. Severe encephalopathy appeared substantially under-reported, indicating that ward-based assessment of these parameters cannot be recorded with sufficient accuracy for use as a primary outcome in phase 3 trials. Trial registration no: EudraCT 2014-002300-24 and ISRCTN14174793.
Keywords: Cirrhosis, Treatment, Mortality, Immune Response
Abbreviations used in this paper: ACLF, acute-on-chronic liver failure; AD, acute decompensation; ATTIRE, Albumin To PrevenT Infection In Chronic LiveR FailurE; HAS, human albumin solution; IDMC, Independent Data Monitoring Committee; PGE2, prostaglandin E2; RCT, randomized controlled trial; SAE, serious adverse event; TNF, tumor necrosis factor
See editorial on page 633, and related article on page 738.
Liver disease represents 2% to 3% of deaths globally1, 2 and was estimated to be responsible for more than 1 million deaths in 2010.1 Incidence rates are predicted to double over the next 20 years.3
Cirrhosis patients with liver failure are termed acute decompensation (AD) or acute-on-chronic-liver failure4 (ACLF). They are highly prone to bacterial infection5, 6 secondary to immune dysfunction,7 with nosocomial infection rates of 35% compared with 5% in noncirrhotic patients.8 Bacterial infection with organ dysfunction carries a mortality risk ranging from 60% to 95%.9, 10 Currently, there is no immune-restorative treatment for patients with AD/ACLF.
Intravenous albumin is used commonly in patients with AD/ACLF as a volume expander11, 12, 13 and has been proven to prevent and improve renal failure.14, 15 Many hepatologists believe it has additional properties,16 however, no multicenter evidence exists for these putative properties and critical-care studies have failed to establish a role.17
In our previous work, we showed that increased circulating prostaglandin E2 (PGE2) was a potential key immune-suppressive mediator in AD.18 We also developed an immune assay to assess the effects of patient plasma on healthy monocyte-derived macrophage tumor necrosis factor (TNF) production because monocyte deactivation is a key feature of AD/ACLF and reduced monocyte TNF production predicts poor outcomes in sepsis.11 We recently showed, using a whole-blood stimulation assay, a reduction in TNF production by fresh circulating monocytes taken from ACLF patients. These data match our observations made when monocyte-derived macrophages are treated with AD/ACLF plasma, validating our approach.12 The only clinical characteristic that predicted immune suppression using this approach was a serum albumin value less than 30 g/L. Albumin has been reported to bind and catalyze inactivation of PGE2,13 and both circulating levels and binding capacity19 decrease in AD/ACLF, making PGE2 potentially more bioavailable. In our pilot study, ex vivo immune function improved after 20% human albumin solution (HAS) transfusions.18 Therefore, we hypothesized that infusing albumin in hospitalized AD/ACLF patients with a serum albumin value less than 30 g/L with a target to increase this value to 35 g/L or higher would improve immune function, prevent new infections, and thus improve mortality. In view of the current discrepancies in ACLF definition20 we believed that large-scale recruitment required simple inclusion criteria and wished to test whether basing these on a serum albumin level less than 30 g/L would select appropriate patients.
There were several other uncertainties regarding our protocol that required clarification before embarking on a large, randomized controlled trial (RCT) comparing albumin infusions with standard care. These included the use of a 72-hour window from admission to treatment, our end point definitions, and obtaining daily data and sample collection. Furthermore, only 1 previous UK multicenter interventional AD/ACLF trial has been performed, the Steroids or Pentoxifylline for Alcoholic Hepatitis trial.21 Furthermore, a recent HAS infusion in sepsis study did not achieve the 30 g/L target22 and a HAS in cirrhosis trial was stopped early because of potential adverse effects. Therefore, a feasibility study was essential to determine whether our suggested protocol was achievable, effective, and did not suggest safety concerns. We did not include a standard-care arm in this feasibility trial and the study was not powered to detect clinical outcomes.
Materials and Methods
Study Design
We conducted this prospective, multicenter, open-label, feasibility trial of targeted 20% HAS infusions in UK AD/ACLF patients to inform the design of a phase 3 RCT. A protocol report was published23 and the full protocol is available online. All authors had access to the study data, and reviewed and approved the manuscript.
Inclusion Criteria
All patients admitted to the hospital with AD or severe worsening of the complications of cirrhosis, who were older than age 18 years, with a serum albumin level less than 30 g/L, a predicted hospital admission longer than 5 days, and that were for full active management at admission were eligible. Patients were recruited within 72 hours of admission; the full inclusion criteria are listed in Supplementary Table 1. We sought written informed consent from patients or their representatives if patients were lacking decision-making capacity. Research ethical approval was granted by London-Brent research ethics committee (ref: 15/LO/0104).
Intervention
In-patients received daily 20% HAS infusions intravenously for the treatment period, with a maximum of 14 days or until the patient was considered medically fit for discharge if fewer than 14 days. The volume of HAS prescribed was determined by the patients’ serum albumin level that day (Supplementary Table 2), based on the Albumin Replacement in Patients with Severe Sepsis study22 and clinical experience. All clinicians could deviate from the suggested regimen, but were requested to document their reasons for doing so.
Clinical Outcomes
The primary end point was daily serum albumin value for the treatment period. Success was defined as 60% of patients achieving and maintaining serum albumin levels at or greater than 30 g/L on at least one third of the days in the study with recorded levels.
The rates of infection, organ dysfunction (for definitions see Supplementary Table 3, Supplementary Table 4), and in-hospital mortality, the component elements of the planned composite end point for the RCT, also were recorded. Definitions of organ failure were chosen from the European Foundation for the study of chronic liver failure Sequential Organ Failure Assessment score.24 We used a recent definition of renal dysfunction in cirrhosis25 reflecting deterioration in patient renal function rather than static values. Because studies have shown that up to 50% of liver patients considered to be infected may have negative microbiological culture,26 we used antibiotic initiation as a surrogate for infection diagnosis. A subset of patients had clinical, biochemical, and microbiological data recorded for validation of infection diagnosis.
Data were summarized further within groups defined by baseline serum albumin levels (<20, 20–25, and 26–29 g/L) to investigate any apparent differences in outcome by group. This subgroup analysis was considered crucial to potentially identify whether any protocol amendments were necessary. The information was summarized for the total volume of albumin infused and of fluid administered, days spent in the intensive care unit, and the duration of hospital stay. Safety was assessed by our Independent Data Monitoring Committee (IDMC) who considered the number of serious adverse events (SAEs) reported during the trial.
Independent Data Monitoring Committee
The IDMC received monthly updates of the SAEs to ensure robust monitoring and met every 6 months to report to the Trial Steering Committee. The SAEs were recorded from entry into study until discharge or the end of treatment.
Statistical Methods
A sample size of 80 ensured 72 evaluable patients (this assumed a 10% loss to follow-up evaluation/withdrawal), with a probability of achieving 44 or more successes of 80%. Calculations assumed each patient had a 65% chance of albumin level being 30 g/L or greater on at least one third of the days when the level was recorded. If 44 successes or more were observed, then a single-sided 90% CI would suggest that the true rate is higher than 50%. Because this was a feasibility study, the emphasis was on producing relevant data summaries rather than on formal modeling or hypothesis testing. All analyses were undertaken by an intention-to-treat, including all consented and enrolled patients for whom outcomes were available, and according to a prespecified (approved before database lock) statistical analysis plan that is included in the Supplementary Materials and Methods section.
Trial Registration
The trial is registered with the European Medicines Agency (European Clinical Trials Database 2014-002300-24) and adopted by National Institute for Health Research (International Standard Randomised Controlled Trial Number 14174793).
Results
Recruitment and Baseline Clinical Characteristics
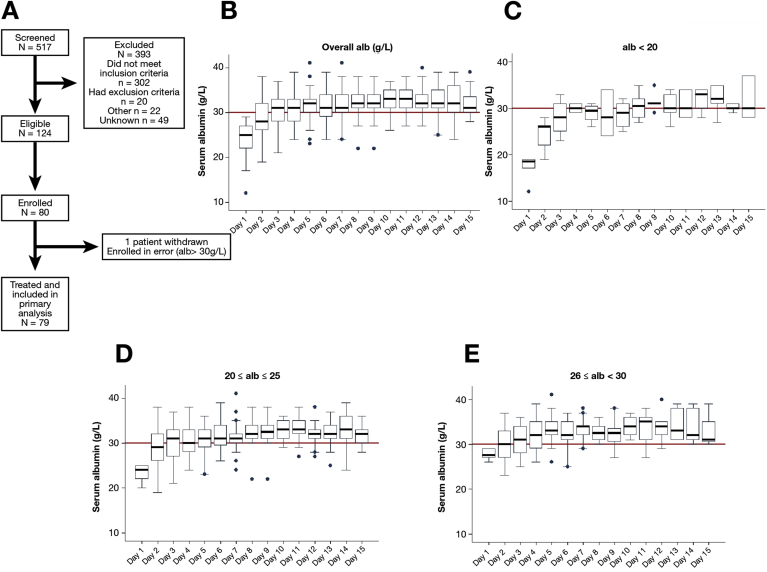
Between May and December 2015, there were 517 AD/ACLF patients who were screened at 10 UK hospitals, with 124 eligible for inclusion and 80 enrolled. One patient was excluded from analysis (Figure 1A) because their baseline albumin level was greater than 30 g/L. Their mean age was 53.4 years, with a male predominance (66%), and alcohol was the primary underlying cause in 96% (Table 1). The mean Model for End-stage Liver Disease score was 20.9 (SD, 6.62), 17 of 79 patients had 1 or more extrahepatic organ dysfunction(s) at baseline according to our definitions, and 21 patients had ACLF grades 1 to 3 according to the European Association for the Study of the Liver-CLIF definition.27 Sixty-seven percent of patients had baseline albumin levels less than 25 g/L. The mean time from admission to enrollment was 1.8 days. The most common reasons for ineligibility during screening were as follows: (1) albumin level of 30 g/L or greater; (2) admission more than 72 hours before screening; and (3) predicted hospital stay of fewer than 5 days. The most common reasons for nonparticipation of eligible patients were as follows: (1) patient declined; (2) informed consent was not possible; and (3) the site was unable to randomize within 72 hours.
Figure 1.
Targeted 20% HAS infusions according to the Albumin To PrevenT Infection In Chronic LiveR FailurE protocol effectively increase serum albumin to 30 g/L or greater. (A) Albumin To PrevenT Infection In Chronic LiveR FailurE feasibility study Consolidated Standards of Reporting Trials flowchart. (B) Median serum albumin levels throughout the study period. (C–E) Data are expressed according to baseline serum albumin (alb) level. Day 1 was defined as the time of recruitment (pretreatment). The horizontal line in the boxes indicates the median, the top and bottom of the box indicate the interquartile range; dots represent individual outliers, defined as data points greater than 1.5 times the interquartile range from the median.
Table 1.
Baseline Demographics and Clinical Characteristics of the Analysis Population (n = 79)
| Characteristic | Mean (SD) |
|---|---|
| Age, y | 53.41 (11.63) |
| Serum albumin level, g/L | 23.95 (3.51) |
| Days since admission | 1.81 (0.88) |
| MELD | 20.90 (6.62) |
| Creatinine level | 91.2 (78.2) |
| n (%) | |
| Male | 52 (66) |
| Admitted to ICU | 2 (3) |
| Prescribed antibiotics | 41 (52) |
| Diagnosis of infection | 27 (34) |
| Etiology of cirrhosisa | |
| Alcohol | 76 (96) |
| Hepatitis B | 1 (1) |
| Hepatitis C | 11 (14) |
| NAFLD | 4 (5) |
| Other etiologies | 2 (3) |
| Organ dysfunctionb | |
| Renal | 8 (10) |
| Respiratory | 9 (11) |
| Circulatory | 13 (16) |
| Cerebral | 3 (4) |
| ACLF gradec | |
| Grade 0 | 58 (73) |
| Grade 1 | 11 (14) |
| Grade 2 | 6 (8) |
| Grade 3 | 4 (5) |
ACLF, acute-on-chronic liver failure; ICU, intensive care unit; MELD, Model for End-stage Liver Disease; NAFLD, nonalcoholic fatty liver disease.
Some patients have more than 1 cirrhosis etiology.
Organ dysfunction at baseline was determined according to the following criteria: renal, creatinine level > 133 μmol/L; respiratory, oxygen saturations divided by inspired oxygen < 357; circulatory, mean arterial pressure < 60 mm Hg or patient is receiving inotropic/vasopressor support; cerebral, grade III or IV encephalopathy.
According to European Association for the Study of the Liver-CLIF criteria.
Targeted 20% Human Albumin Solution Infusions Increase Serum Albumin Level to 30 g/L or More
A total of 68 of 79 patients achieved a serum albumin level of 30 g/L or greater on at least one third of days recorded, providing a success rate of 86% (95% CI, 76%–92%) (Table 2, and Supplementary Table 5). All patients enrolled contributed to the analysis. The regimen was effective across all serum albumin subgroups, with the highest success in the 26 to 29 g/L group (96% success; 95% CI, 80%–100%) compared with less than 20 g/L (50% success; 95% CI, 19%–81%). The mean treatment length was 10.3 days and a mean of 1042 mL 20% HAS (SD, 678 mL) was administered; more than 50% of patients had their albumin level restored to 30 g/L or greater after 3 days, and more than 75% after 7 days. Deaths (n = 8) or SAEs (n = 12) during the trial period were not considered directly related to the HAS infusions by the IDMC (Table 3). Sixty-four percent of administrations were in accordance with the suggested protocol, with 88% within ±100 mL of the suggested dose (Supplementary Table 6). No patient underwent a liver transplantation. On 161 of 657 occasions, 20% HAS was not administered despite a serum albumin level less than 35 g/L, suggesting an adherence rate of 75%.
Table 2.
Primary Outcome of Daily Albumin Level for Subgroups Defined by Baseline Albumin Level (<20, 20–25, and 26–29 g/L)
| Baseline albumin level, g/L | Successes | Estimated success probability (%), mean (95% CI) |
|---|---|---|
| <20 | 5/10 | 50 (19–81) |
| 20–25 | 38/43 | 88 (75–96) |
| 26–29 | 25/26 | 96 (80–100) |
NOTE. The following successes were observed in the patient population.
Table 3.
Details of Reported Serious Adverse Events Throughout Trial Treatment Period (Days 1–15)
| SAE description | Events, n |
|---|---|
| New ascites | 1 |
| Renal impairment | 1 |
| Variceal bleeding (death) | 3 |
| Variceal bleeding (death) | 1 |
| Pneumonia (death) | 1 |
| Death (decompensated cirrhosis) | 4 |
| Bronchogenic carcinoma and pleural effusion (death) | 1 |
| Total deaths in trial treatment period | 8 (10%) |
SAE, serious adverse event.
Infection, Organ Dysfunction, Death, and Duration of Hospital Stay
Clinical end points were reported from days 3 to 15 after study entry (termed the treatment period) to ensure at least 24 hours since the patients first received 20% HAS treatment before measuring possible outcomes. The average time from admission to death or discharge was 16.9 days (SD, 15.7 d; range, 2–102 d). Forty-two of 817 patient days recorded were in the intensive care unit (approximately 5%). Supplementary Table 7 shows the data for days 2 to 15.
Infection defined by new/change in antibiotic prescription
Forty-one of 79 patients were prescribed antibiotics at recruitment to the study, with 27 of 41 given an infection diagnosis by their clinician. During the treatment period, 21 of 79 patients (27%) were diagnosed with a new infection (Table 4). The patients who had been prescribed antibiotics on admission had increased subsequent nosocomial infection rates compared with those who were not prescribed antibiotics on admission (24% vs 8%, respectively).
Table 4.
Number of Patients Reported as Having Infection, Organ Dysfunction, and Death During the Trial Treatment Period
| End point | Patients, n, days 3–15 of trial treatment |
|---|---|
| Infection | 21/79 (27%) |
| Extrahepatic organ dysfunction | |
| Renal | 7/79 (9%) |
| Respiratory | 19/79 (24%) |
| Circulatory | 15/79 (19%) |
| Cerebral | 1/79 (1%) |
| Death | 5/79 (6%) |
NOTE. Data were recorded throughout the study period, but data were reported from day 3 of treatment onward so that patients had at least 24 hours of intravenous 20% HAS treatment. Patients may have achieved more than 1 end point (eg, infection and death). Organ dysfunction at baseline was determined according to the following criteria: renal, creatinine level > 133 μmol/L; respiratory, oxygen saturations divided by inspired oxygen < 357; circulatory, mean arterial pressure < 60 mm Hg or patient is receiving inotropic/vasopressor support; cerebral, grade III or IV encephalopathy.
Infection case report forms were completed for 35 of the antibiotic prescriptions (either at baseline or after recruitment). A blinded microbiology review showed that 4 of 35 did not fulfill infection predefined criteria (Supplementary Table 4). Supplementary Table 8 details these case report form data.
Extrahepatic organ dysfunction
Overall, 7 of 79 (9%) patients met renal dysfunction criteria, 19 of 79 (24%) met respiratory dysfunction criteria, 15 of 79 (19%) met circulatory dysfunction criteria, and 1 of 79 (1%) met cerebral dysfunction criteria during the treatment period.
Death
Five patients (6%) died during the treatment period, and 14 patients died within 30 days of study entry (18%).
The planned composite end point for the randomized controlled trial
Thirty-eight of 79 patients (48%) reached the planned composite end point during the treatment period (Table 5). Of these, the breakdown of components that triggered the composite end point first were as follows.
-
1.
Thirteen patients had an infection recorded as the first end point component, with 8 patients developing subsequent organ dysfunction and 4 of these patients had died by 30 days. Of the 5 patients who did not develop subsequent organ dysfunction, 1 patient died within 30 days. The median hospital stay was 19 days after infection diagnosis (Supplementary Table 9).
-
2.
Three patients developed renal dysfunction as the first end point, of these, 2 patients died during admission and the other patient was alive at 30 days. A further 4 patients developed renal dysfunction after another organ dysfunction, with 2 patients dying as inpatients (1 patient during the treatment period), another patient shortly after discharge, and the other patient was alive at 30 days (Supplementary Table 9).
-
3.
Twelve patients developed respiratory dysfunction as the first end point, with 5 of these patients triggering subsequent other end points (4 infections, 1 died). Of the 7 patients who developed solely respiratory dysfunction, the vast majority had a good outcome, with 6 alive at 30 days, 1 death during admission, and a median stay from respiratory dysfunction diagnosis of only 5 days, with 3 patients discharged within 3 days of diagnosis with respiratory dysfunction (Supplementary Table 9).
-
4.
Nine patients developed circulatory dysfunction as the first end point, with 3 patients triggering other end points, and 1 patient died during admission. All 6 patients who solely developed circulatory dysfunction were alive at 30 days, and the median stay after circulatory dysfunction diagnosis was 7 days, with 2 patients discharged within 2 days of diagnosis (Supplementary Table 9).
-
5.
One patient died without previously triggering another end point.
Table 5.
Incidence of Planned and Revised RCT Composite End Point and Contributing Components (Days 3–15 of Trial Treatment)
| Patients (N = 79) | Planned RCT composite end point | Revised RCT composite end point, excluding respiratory, circulatory, and cerebral dysfunction |
|---|---|---|
| Composite end point | 38 (48%) | 25 (32%) |
| Infectiona | 13 | 19 |
| Extrahepatic organ dysfunctionb | ||
| Renal | 3 | 4 |
| Respiratory | 12 | |
| Circulatory | 9 | |
| Cerebral | 0 | |
| Death | 1 | 2 |
RCT, randomized controlled trial.
Infection indicated by new or change in prescription for antibiotics.
Organ dysfunction at baseline was determined according to the following criteria: renal, creatinine level > 133 μmol/L; respiratory, oxygen saturations divided by inspired oxygen < 357; circulatory, mean arterial pressure < 60 mm Hg or patient is receiving inotropic/vasopressor support; cerebral, grade III or IV encephalopathy.
Only 1 patient developed grade 3 hepatic encephalopathy after an infection, therefore no brain dysfunction diagnoses contributed to the end point.
Serious Adverse Events
The SAEs (n = 13) during treatment were deemed to be unrelated to HAS by our IDMC (Supplementary Table 10).
Discussion
Targeted albumin infusions effectively increased serum albumin levels to 30 g/L or greater in hospitalized AD/ACLF patients; a level below which we previously identified as predicting immune dysfunction.18 Our infusion protocol was effective, acceptable at multiple sites, and deemed safe by the IDMC.
Our suggested protocol allowed dose reduction (eg, if safety concerns), as clinical practice remains variable.28 We achieved the primary end point with 75% adherence, increasing and maintaining albumin even in patients with very low baseline values. Although a single-arm study, the rates of adverse events of particular concern were low, in particular there were no reports of pulmonary edema. Four variceal bleeds were reported, which potentially can be precipitated by increased portal pressure after albumin. Although not reported in previous albumin trials, this remains a concern. However, a 5% incidence during treatment is similar to expected rates.21, 24, 29 The IDMC had no reason to alter our regimen; the absolute safety of our albumin regimen will be determined in our RCT vs standard care.
Our inclusion criteria were broad and straightforward and selected patients with almost exclusively alcohol-induced liver disease, a substantial spread of albumin values, and an ACLF score of 1 to 3 in 25% of cases. We reasoned that our intervention would be more successful in ward-based patients rather than in patients with multi-organ failure, and these criteria appeared to capture exactly this population. The majority were recruited within 2 days of admission, which is crucial because early intervention is widely believed to be imperative to improve outcomes in critically ill patients. As a trial aimed at the prevention of nosocomial infection, the primary composite end point will be reported from day 3 onward in line with established diagnostic criteria, which also ensures at least 24 hours of 20% HAS therapy. The observed in-hospital and 30-day mortality were similar to previous studies.21, 30, 31 Survival at 3 and 6 months were not recorded but will be reported in the RCT. We included a medically fit for discharge category at the end of the trial period because many patients have social needs that prolong hospital stay beyond their medical needs.
Our RCT primary composite end point was planned as infection, organ dysfunction, and death because infection commonly triggers organ dysfunction and the combination substantially increases mortality. However, the feasibility of recording such data in a ward-based trial of AD/ACLF at multiple sites is not commonplace. Other than renal failure,32 there is also no universally accepted definition for early (reversible) organ dysfunction/failures in patients with cirrhosis.
A robust diagnosis of infection in AD/ACLF is challenging because of the high rates of culture-negative sepsis.26 The on-site clinician-reported infection rate on admission was 34%, which is in line with other studies; however, antibiotics were prescribed in substantially more patients (52%). This perhaps reflects a tendency to overprescribe, as reported elsewhere,33, 34 and therefore using a new/changed antibiotic prescription as a surrogate for infection diagnosis, as originally intended, appeared subject to potential bias and this cannot be standardized across multiple sites. In the RCT we therefore will define infection according to clinician diagnosis, triggering completion of an infection case report form, with a substantial proportion blindly scrutinized by a microbiology panel. This approach appeared feasible in a subset of 35 patients tested. Our data suggest that patients prescribed antibiotics at admission have a 3 times increased risk of subsequent nosocomial infection. This has been reported previously6 and justifies inclusion of these patients in an RCT to prevent infection; furthermore, an antibiotic prescription will be included in stratification at randomization. Whether our reported nosocomial infection rate of 27% represents a beneficial effect of 20% HAS infusions will be determined in the RCT.
Renal dysfunction uses an objective measurement, creatinine, and patients developing this had a poor prognosis, as expected. Renal dysfunction rates were lower than anticipated (9% vs up to ≈60%35), perhaps related to additional fluid.30 Cerebral, respiratory, and cardiac dysfunctions were recorded daily. Only 1 patient developed cerebral dysfunction reflecting severe hepatic encephalopathy (≥grade 3), suggesting under-reporting, and objective assessment of encephalopathy is recognized as challenging.34, 36 The majority of patients who solely triggered respiratory and cardiovascular end points had a good outcome, with several discharged within a few days. This is counterintuitive because organ dysfunction is a key predictor of poor prognosis. Assessment may be subject to technical difficulties such as a standard-size blood pressure cuff for patients with sarcopenia and a oxygen saturation/fraction of inspired oxygen recording of respiratory dysfunction is influenced greatly by the amount of oxygen delivered by mask or nasal cannulae. We believe our data cast significant doubt over whether these dysfunctions can be recorded accurately in largely ward-based patients across multiple sites and therefore precludes use as part of our RCT primary composite end point; although these will be reported. Table 5 shows the incidence of our revised primary composite end point of infection, renal dysfunction, and mortality.
In summary, our multicenter feasibility study represents a highly pragmatic feasibility study in a challenging group of patients. The data presented confirm that our suggested protocol was deliverable across multiple sites and the dosing regimen increased serum albumin without significant safety concern. Albumin To PrevenT Infection In Chronic LiveR FailurE stage 2 is a multicenter, open-label, interventional RCT and began recruitment in April 2016. This is a phase 3 RCT to verify whether targeting a serum albumin level of 35 g/L or greater in patients admitted with acutely decompensated cirrhosis using repeated intravenous infusions of 20% HAS will reduce the incidence of infection, renal dysfunction, and mortality for the treatment period (maximum 14 days, or discharge if <14 days) compared with standard medical care. The trial will recruit 866 patients at more than 30 sites across the United Kingdom.
Large multicenter studies in patients with advanced liver disease rarely are performed and require significant resources. This feasibility study has enabled substantial improvement in design and end point definition/selection as well as providing important safety information and mechanistic insight. This approach could serve as a model for future trials in such patients.
Acknowledgments
The authors appreciate the support of the following individuals via trial oversight committees: Professor Graeme Alexander, Professor Stephen Brett, Professor Mauro Bernardi, Professor Dominique Valla, Dr Vipul Jaraith, Mr Tim Clayton, Mr Brennan Kahan, Mr John Crookenden, and Ms Susan Tebbs. A list of trial sites and Principal Investigators can be obtained by contacting attire@ucl.ac.uk.
Footnotes
Conflicts of interest The authors disclose no conflicts.
Funding This work was supported by the Health Innovation Challenge fund (Wellcome Trust and UK Department of Health) award number 164699. This publication presents independent research commissioned by the Health Innovation Challenge Fund, a parallel funding partnership between the Department of Health and Wellcome Trust. The views expressed in this publication are those of the author(s) and not necessarily those of the Department of Health or Wellcome Trust. The trial sponsor is University College London, with trial management activities conducted by the University College London Comprehensive Clinical Trials Unit.
Note: To access the supplementary material accompanying this article, visit the online version of Clinical Gastroenterology and Hepatology at www.cghjournal.org, and at https://doi.org/10.1016/j.cgh.2017.09.012.
Supplementary Materials and Methods
Trial Management and Monitoring
Research steering group
The Research Steering Group operates on behalf of the funders to ensure that appropriate milestones have been met in the delivery of the trial. It consists of the chief investigator, an independent expert, and representatives of the Wellcome Trust and Department of Health.
Trial Management Group
The Trial Management Group comprises the chief investigator, Clinical Research Fellow, Clinical Project Manager, Trial Statistician, Trial Manager, Data Manager, Health Economist, and 5 trial site Principal Investigators. The Trial Management Group is responsible for developing the design, coordination, and strategic management of the trial.
Trial Steering Committee
The Trial Steering Committee is the independent group responsible for oversight of the trial to safeguard the interests of trial patients. The Trial Steering Committee provides advice to the chief investigator, Clinical Trials Unit, funder, and sponsor on all aspects of the trial through its independent chair.
Independent Data Monitoring Committee
The IDMC is responsible for safeguarding the interests of trial patients, monitoring the accumulating data, and making recommendations to the Trial Steering Committee on whether the trial should continue as planned. It comprises a clinical chair (independent hepatologist), independent gastroenterologist, and an independent statistician, all with expertise in Clinical Trials.
The participating hospitals and personnel were as follows: Dr G. Wright at Basildon University Hospital, 17 patients recruited; Dr J. Portal at Bristol Royal Infirmary, 14 patients recruited; Dr L. Corless at Hull Royal Infirmary, 11 patients recruited; Dr C. Lye Ch'ng at Singleton Hospital Swansea, 8 patients recruited; Dr P. Richardson at Royal Liverpool University Hospital, 7 patients recruited; Dr S. McPherson at Newcastle Freeman Hospital, 6 patients recruited; Dr Y. Kallis at Royal London Hospital, 6 patients recruited; Professor J. Metcalf at North Tees and Hartlepool, 6 patients recruited; Professor R. Jalan at Royal Free Hospital, 3 patients recruited; and Dr A. Elsharkawy at University Hospitals Birmingham, 2 patients recruited.
Supplementary Table 1.
ATTIRE Feasibility Study Patient Inclusion and Exclusion Criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
| All patients admitted to the hospital with acute onset or worsening of complications of cirrhosis | Advanced hepatocellular carcinoma with life expectancy of <8 weeks |
| Older than 18 years of age | Patients who will receive palliative treatment only during their hospital admission |
| Predicted hospital admission >5 days at trial enrollment, which must be within 72 hours of admission | Patients who are pregnant |
| Serum albumin level <30 g/L at screening | Known or suspected severe cardiac dysfunction |
| Documented informed consent to participate (or consent given by a legal representative) | Any clinical condition that the investigator considers would make the patient unsuitable for the trial |
| The patient has been involved in a clinical trial of Investigational Medicinal Products within the previous 30 days that would impact their participation in this study | |
| Trial investigators unable to identify the patient (by NHS number) |
ATTIRE, Albumin To PrevenT Infection In Chronic LiveR FailurE; NHS, National Health Service.
Supplementary Table 2.
Suggested Daily Dosing Protocol for 20% HAS Administration According to Measured Serum Albumin Level
| Patient serum albumin level, g/L | Suggested volume of 20% HAS to be infused at rate of 100 mL/h |
|---|---|
| ≥35 | None |
| 30–34 | 100 mL |
| 26–29 | 200 mL |
| 20–25 | 300 mL |
| <20 | 400 mL |
Supplementary Table 3.
Definitions of Extrahepatic Organ Dysfunction
| Organ | Definition of new dysfunction |
|---|---|
| Renal | Serum creatinine level increases by ≥50% as compared with serum creatinine level at baseline |
| Respiratory | Any single point increase in oxygen saturations divided by inspired oxygen as classified on the following scoring system compared with oxygen saturations divided by inspired oxygen at randomization: 0, >357; 1, >214 to ≤357; 2, ≤214 or mechanical ventilation |
| Circulatory | MAP decreases to <60 mm Hg, OR patient is started on inotropic/vasopressor support If the patient has MAP < 60 mm Hg at baseline, they will need to be started on inotropic/vasopressor support to reach this end point |
| Cerebral | Grade III (drowsy) or grade IV encephalopathy (coma) using the Westhaven criteria to grade hepatic encephalopathy If the patient has grade III encephalopathy (somnolent but rousable at baseline), they will need to progress to grade IV to reach this end point |
NOTE. Organ dysfunction at baseline was determined according to the following criteria: renal, creatinine level > 133 μmol/L; respiratory, oxygen saturations divided by inspired oxygen < 357; circulatory, MAP < 60 mm Hg or patient is receiving inotropic/vasopressor support; cerebral, grade III or IV encephalopathy.
MAP, mean arterial pressure.
Supplementary Table 4.
Definition of Infections–Infection Can Be Defined According to the Following Peer-reviewed Criteria6, 14
| Type of infection | Definition |
|---|---|
| Spontaneous bacteremia | Positive blood cultures without a source of infection |
| SBP | Ascitic fluid polymorphonuclear cells >250 cells/mm3 |
| Lower respiratory tract infections | New pulmonary infiltrates in the presence of: at least 1 respiratory symptom (cough, sputum production, dyspnea, pleuritic pain), with at least 1 finding on auscultation (rales or crepitation) or 1 sign of infection (core body temperature >38°C or <36°C, shivering, or leukocyte count >10,000/mm3 or <4000/mm3) in the absence of antibiotics |
| Clostridium difficile infection | Diarrhea with a positive C difficile assay |
| Bacterial enterocolitis | Diarrhea or dysentery with a positive stool culture for Salmonella, Shigella, Yersinia, Campylobacter, or pathogenic Escherichia coli |
| Soft-tissue/skin infection | Fever with cellulitis |
| Urinary tract infection | Urine white blood cell count >15/high-power field with either positive urine Gram stain or culture |
| Intra-abdominal infections | Diverticulitis, appendicitis, cholangitis, and so forth |
| Other infections | Not covered in 1–8 |
| Fungal infections | |
| Nosocomial infections | Are diagnosed after 48 hours of admission, whereas secondary infections are diagnosed after a separate first infection has been documented |
SBP, spontaneous bacterial peritonitis.
Supplementary Table 5.
Daily Albumin Level (g/L) From Day 1 (Baseline) to Day 15: All Patients
| Albumin level, g/L | Day |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | |
| Median | 25 | 28 | 31 | 31 | 32 | 31 | 31 | 32 | 32 | 33 | 33 | 32 | 32 | 32 | 31 |
| IQR | 22–27 | 26–32 | 28–33 | 28–33 | 30–33 | 29–34 | 30–34 | 31–34 | 31–34 | 31–35 | 31–35 | 31–34 | 31–35 | 30–36 | 30–34 |
| Range | 12–29 | 19–38 | 21–37 | 24–39 | 23–41 | 24–39 | 24–41 | 22–38 | 22–38 | 26–37 | 27–38 | 27–40 | 25–39 | 24–39 | 28–39 |
| Mean (SD) | 24 (4) | 28 (4) | 30 (4) | 31 (3) | 32 (3) | 31 (3) | 32 (3) | 32 (3) | 32 (3) | 33 (3) | 33 (3) | 32 (3) | 33 (3) | 33 (3) | 32 (3) |
| N | 79 | 74 | 63 | 57 | 53 | 51 | 51 | 51 | 41 | 34 | 35 | 31 | 32 | 27 | 20 |
| ≥30, N | 0 | 30 | 35 | 36 | 40 | 38 | 40 | 47 | 38 | 30 | 31 | 25 | 29 | 25 | 18 |
| Study, N | 79 | 77 | 74 | 72 | 65 | 60 | 57 | 55 | 48 | 44 | 43 | 42 | 39 | 34 | 28 |
IQR, interquartile range.
Supplementary Table 6.
Number of Occasions Albumin Was Neither Prescribed nor Administered When Albumin Level <35 g/L
| Days | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neither prescribed nor administered | 1 | 5 | 10 | 10 | 11 | 7 | 9 | 4 | 9 | 10 | 13 | 11 | 7 | 7 | 114 |
| Prescribed but not administered | 10 | 6 | 8 | 2 | 1 | 2 | 2 | 3 | 2 | 4 | 2 | 1 | 3 | 1 | 47 |
| Administered but not prescribed | 2 | 2 | 1 | 1 | 3 | 0 | 0 | 0 | 0 | 2 | 0 | 2 | 1 | 0 | 14 |
| Total | 13 | 13 | 19 | 13 | 15 | 9 | 11 | 7 | 11 | 16 | 15 | 14 | 11 | 8 | 175 |
| Albumin level <35, N | 79 | 73 | 64 | 58 | 56 | 48 | 48 | 44 | 40 | 32 | 29 | 34 | 28 | 24 | 657 |
Supplementary Table 7.
Incidence of Proposed Composite End Point and Contributing Components, With and Without Respiratory/Circulatory Dysfunctions From Days 2–15
| Patients (N = 79) | Existing composite end point, days 2–15, n (%) | Excluding respiratory, circulatory, and brain dysfunction from the composite end point, days 2–15, n (%) |
|---|---|---|
| Composite end point | 44 (56) | 30 (38) |
| Infection | 10 | 21 |
| Renal | 4 | 5 |
| Respiratory | 17 | |
| Circulatory | 10 | |
| Brain | 0 | |
| Death | 3 | 4 |
Supplementary Table 8.
Details From Infection Data Matched to 35 of 62 Antibiotic Prescriptions
| Classified infection | Times confirmed, na | Antibiotic sensitivity |
||
|---|---|---|---|---|
| Resistant | Sensitiveb | Unknown | ||
| Spontaneous bacterial peritonitis | 4 | 0 | 1 | 3 |
| Pneumonia | 6 | 0 | 1 | 5 |
| Cellulitis | 4 | 0 | 1 | 1 |
| Bacterial enterocolitis | 1 | 1c | 0 | 0 |
| Fungal infection | 1 | 0 | 0 | 1 |
| Spontaneous bacteremia | 7 | 2d | 2 | 3 |
| Other infection | 8 | 3e | 0 | 5 |
| Urinary tract infection | 0 | N/A | ||
| Other intra-abdominal infection | 0 | |||
| C difficile | 0 | |||
| Total | 31 | 6 | 5 | 18 |
Some patients had multiple infections (4 of 35 cases did not meet criteria for an infection diagnosis).
The following were included: Enterobacter cloacae, Staphylococcus aureus, and E coli.
Vancomycin-resistant enterococci.
Klebsiella oxytoca.
Methicillin-resistant Staphylococcus aureus.
Supplementary Table 9.
Outcomes for Individual Patients Who Triggered the Planned Composite End Point for the RCT
| First component recorded | Day | Subsequent or concurrent component | Day | Subsequent or concurrent component | Day | Days in hospital, n | Days from composite endpoint to discharge, n | Alive at 30 days |
|---|---|---|---|---|---|---|---|---|
| Respiratory | 13 | 13 | 0 | Yes | ||||
| Respiratory | 7 | 23 | 16 | Yes | ||||
| Respiratory | 15 | 16 | 1 | Yes | ||||
| Respiratory | 8 | 23 | 15 | No | ||||
| Respiratory | 3 | 12 | 9 | Yes | ||||
| Respiratory | 9 | 17 | 8 | Yes | ||||
| Respiratory | 4 | 6 | 2 | Yes | ||||
| Respiratory | 3 | Infection | 9 | 15 | 12 | Yes | ||
| Respiratory | 3 | Infection | 5 | 28 | 25 | Yes | ||
| Respiratory | 3 | Infection | 6 | 17 | 14 | Yes | ||
| Respiratory | 3 | Death | 3 | 3 | 0 | No | ||
| Respiratory | 3 | Infection | 5 | Circulation | 9 | 23 | 20 | Yes |
| Circulatory | 6 | 15 | 9 | Yes | ||||
| Circulatory | 9 | 21 | 12 | Yes | ||||
| Circulatory | 3 | 3 | 0 | Yes | ||||
| Circulatory | 3 | 6 | 3 | Yes | ||||
| Circulatory | 3 | 15 | 12 | Yes | ||||
| Circulatory | 9 | 14 | 5 | Yes | ||||
| Circulatory | 4 | Infection | 8 | 32 | 28 | Yes | ||
| Circulatory | 11 | Infection | 15 | 27 | 16 | Yes | ||
| Circulatory | 10 | Renal | 12 | 14 | 8 | Yes | ||
| Renal | 3 | 11 | 8 | Yes | ||||
| Renal | 3 | Infection | 4 | Death | 5 | 5 | 2 | No |
| Renal | 5 | Infection + respiratory + circulatory | 8 | Cerebral | 9 | 24 | 19 | No |
| Infection | 3 | 31 | 28 | No | ||||
| Infection | 11 | 18 | 7 | Yes | ||||
| Infection | 8 | 13 | 5 | Yes | ||||
| Infection | 3 | 22 | 19 | Yes | ||||
| Infection | 13 | 43 | 30 | Yes | ||||
| Infection | 3 | 88 | 85 | Yes | ||||
| Infection | 13 | Respiratory | 13 | 101 | 88 | Yes | ||
| Infection | 3 | Respiratory | 3 | 23 | 20 | No | ||
| Infection | 3 | Circulatory | 10 | 14 | 11 | Yes | ||
| Infection | 7 | Renal | 14 | Death | 15 | 15 | 8 | No |
| Infection | 3 | Circulatory | 5 | Respiratory | 6 | 34 | 31 | Yes |
| Infection | 3 | Respiratory + circulatory | 3 | Renal | 4 | 13 | 10 | No |
| Infection | 3 | Respiratory + circulatory | 3 | Renal Death |
5 10 |
10 | 7 | No |
| Death | 6 | 6 | 1 | No |
Supplementary Table 10.
SAEs as Individually Reported
| SAE description | Events, n | Causality determined by IDMC |
|---|---|---|
| New ascites | 1 | Unrelated |
| Renal impairment | 1 | Unrelated |
| Variceal bleeding (death) | 3 | Unrelated |
| Variceal bleeding (death) | 1 | Unlikely to be related |
| Pneumonia (death) | 1 | Unrelated |
| Death (decompensated cirrhosis) | 4 | Unrelated |
| Bronchogenic carcinoma and pleural effusion (death) | 1 | Unrelated |
References
- 1.Mokdad A.A., Lopez A.D., Shahraz S. Liver cirrhosis mortality in 187 countries between 1980 and 2010: a systematic analysis. BMC Med. 2014;12:145. doi: 10.1186/s12916-014-0145-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ge P.S., Runyon B.A. Treatment of patients with cirrhosis. N Engl J Med. 2016;375:767–777. doi: 10.1056/NEJMra1504367. [DOI] [PubMed] [Google Scholar]
- 3.Williams R., Aspinall R., Bellis M. Addressing liver disease in the UK: a blueprint for attaining excellence in health care and reducing premature mortality from lifestyle issues of excess consumption of alcohol, obesity, and viral hepatitis. Lancet. 2014;384:1953–1997. doi: 10.1016/S0140-6736(14)61838-9. [DOI] [PubMed] [Google Scholar]
- 4.Moreau R., Jalan R., Gines P. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology. 2013;144:1426–1437. doi: 10.1053/j.gastro.2013.02.042. 1437 e1421–e1429. [DOI] [PubMed] [Google Scholar]
- 5.Fernandez J., Navasa M., Gomez J. Bacterial infections in cirrhosis: epidemiological changes with invasive procedures and norfloxacin prophylaxis. Hepatology. 2002;35:140–148. doi: 10.1053/jhep.2002.30082. [DOI] [PubMed] [Google Scholar]
- 6.Bajaj J.S., O'Leary J.G., Reddy K.R. Second infections independently increase mortality in hospitalized patients with cirrhosis: the North American consortium for the study of end-stage liver disease (NACSELD) experience. Hepatology. 2012;56:2328–2335. doi: 10.1002/hep.25947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rajkovic I., Williams R. Abnormalities of neutrophil phagocytosis, intracellular killing and metabolic activity in alcoholic cirrhosis and hepatitis. Hepatology. 1986;6:252–262. doi: 10.1002/hep.1840060217. [DOI] [PubMed] [Google Scholar]
- 8.Fernandez J., Acevedo J., Castro M. Prevalence and risk factors of infections by multiresistant bacteria in cirrhosis: a prospective study. Hepatology. 2012;55:1551–1561. doi: 10.1002/hep.25532. [DOI] [PubMed] [Google Scholar]
- 9.O'Brien A.J., Welch C.A., Singer M. Prevalence and outcome of cirrhosis patients admitted to UK intensive care: a comparison against dialysis-dependent chronic renal failure patients. Intensive Care Med. 2012;38:991–1000. doi: 10.1007/s00134-012-2523-2. [DOI] [PubMed] [Google Scholar]
- 10.Bajaj J.S., O'Leary J.G., Reddy K.R. Survival in infection-related acute-on-chronic liver failure is defined by extrahepatic organ failures. Hepatology. 2014;60:250–256. doi: 10.1002/hep.27077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moore K.P., Aithal G.P. Guidelines on the management of ascites in cirrhosis. Gut. 2006;55(Suppl 6):vi1–vi12. doi: 10.1136/gut.2006.099580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Runyon B.A. American Association for the Study of Liver Diseases. Introduction to the revised American Association for the Study of Liver Diseases Practice Guideline management of adult patients with ascites due to cirrhosis 2012. Hepatology. 2013;57:1651–1653. doi: 10.1002/hep.26359. [DOI] [PubMed] [Google Scholar]
- 13.Arroyo V., Garcia-Martinez R., Salvatella X. Human serum albumin, systemic inflammation, and cirrhosis. J Hepatol. 2014;61:396–407. doi: 10.1016/j.jhep.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 14.Patel A., Laffan M.A., Waheed U. Randomised trials of human albumin for adults with sepsis: systematic review and meta-analysis with trial sequential analysis of all-cause mortality. BMJ. 2014;349:g4561. doi: 10.1136/bmj.g4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Brien A.J., Fullerton J.N., Massey K.A. Immunosuppression in acutely decompensated cirrhosis is mediated by prostaglandin E2. Nat Med. 2014;20:518–523. doi: 10.1038/nm.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ploder M., Pelinka L., Roth E. Lipopolysaccharide induced TNF production and monocyte human leucocyte antigen-DR expression is correlated with survival in septic trauma patients. Shock. 2006;25:129–134. doi: 10.1097/01.shk.0000191379.62897.1d. [DOI] [PubMed] [Google Scholar]
- 17.Maini A., China L., Gilroy D. Progression of cirrhotic liver disease towards acute-on-chronic liver failure triggers changes in innate immune cell phenotype and their response to pro-inflammatory stimuli. J Hepatol. 2017;66:S1–S876. [Google Scholar]
- 18.Yang J., Petersen C.E., Ha C.E. Structural insights into human serum albumin-mediated prostaglandin catalysis. Protein Sci. 2002;11:538–545. doi: 10.1110/ps.28702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jalan R., Schnurr K., Mookerjee R.P. Alterations in the functional capacity of albumin in patients with decompensated cirrhosis is associated with increased mortality. Hepatology. 2009;50:555–564. doi: 10.1002/hep.22913. [DOI] [PubMed] [Google Scholar]
- 20.Hernaez R., Sola E., Moreau R. Acute-on-chronic liver failure: an update. Gut. 2017;66:541–553. doi: 10.1136/gutjnl-2016-312670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thursz M.R., Richardson P., Allison M. Prednisolone or pentoxifylline for alcoholic hepatitis. N Engl J Med. 2015;372:1619–1628. doi: 10.1056/NEJMoa1412278. [DOI] [PubMed] [Google Scholar]
- 22.Caironi P., Tognoni G., Masson S. Albumin replacement in patients with severe sepsis or septic shock. N Engl J Med. 2014;370:1412–1421. doi: 10.1056/NEJMoa1305727. [DOI] [PubMed] [Google Scholar]
- 23.China L., Muirhead N., Skene S.S. ATTIRE: Albumin To prevenT Infection in chronic liveR failurE: study protocol for a single-arm feasibility trial. BMJ Open. 2016;6:e010132. doi: 10.1136/bmjopen-2015-010132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jalan R., Saliba F., Pavesi M. Development and validation of a prognostic score to predict mortality in patients with acute-on-chronic liver failure. J Hepatol. 2014;61:1038–1047. doi: 10.1016/j.jhep.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 25.Angeli P., Ginès P., Wong F. Diagnosis and management of acute kidney injury in patients with cirrhosis: revised consensus recommendations of the International Club of Ascites. J Hepatol. 2015;62:968–974. doi: 10.1016/j.jhep.2014.12.029. [DOI] [PubMed] [Google Scholar]
- 26.Piano S., Fasolato S., Salinas F. The empirical antibiotic treatment of nosocomial spontaneous bacterial peritonitis: results of a randomized, controlled clinical trial. Hepatology. 2016;63:1299–1309. doi: 10.1002/hep.27941. [DOI] [PubMed] [Google Scholar]
- 27.Jalan R., Pavesi M., Saliba F. The CLIF Consortium Acute Decompensation score (CLIF-C ADs) for prognosis of hospitalised cirrhotic patients without acute-on-chronic liver failure. J Hepatol. 2015;62:831–840. doi: 10.1016/j.jhep.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 28.Bajaj J.S., O'Leary J.G., Wong F. Variations in albumin use in patients with cirrhosis: an AASLD members survey. Hepatology. 2015;62:1923–1924. doi: 10.1002/hep.27789. [DOI] [PubMed] [Google Scholar]
- 29.Gustot T., Fernandez J., Garcia E. Clinical course of acute-on-chronic liver failure syndrome and effects on prognosis. Hepatology. 2015;62:243–252. doi: 10.1002/hep.27849. [DOI] [PubMed] [Google Scholar]
- 30.Sort P., Navasa M., Arroyo V. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. N Engl J Med. 1999;341:403–409. doi: 10.1056/NEJM199908053410603. [DOI] [PubMed] [Google Scholar]
- 31.Thevenot T., Bureau C., Oberti F. Effect of albumin in cirrhotic patients with infection other than spontaneous bacterial peritonitis. A randomized trial. J Hepatol. 2015;62:822–830. doi: 10.1016/j.jhep.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 32.Fagundes C., Barreto R., Guevara M. A modified acute kidney injury classification for diagnosis and risk stratification of impairment of kidney function in cirrhosis. J Hepatol. 2013;59:474–481. doi: 10.1016/j.jhep.2013.04.036. [DOI] [PubMed] [Google Scholar]
- 33.Bajaj J.S., O'Leary J.G., Wong F. Bacterial infections in end-stage liver disease: current challenges and future directions. Gut. 2012;61:1219–1225. doi: 10.1136/gutjnl-2012-302339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pan H.C., Chien Y.S., Jeng C.C. Acute kidney injury classification for critically ill cirrhotic patients: a comparison of the KDIGO, AKIN, and RIFLE classifications. Sci Rep. 2016;6:23022. doi: 10.1038/srep23022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bass M., Mullen K.D., Sanyal A. Rifaximin treatment in hepatic encephalopathy. N Engl J Med. 2010;362:1071–1081. doi: 10.1056/NEJMoa0907893. [DOI] [PubMed] [Google Scholar]
- 36.Hassanein T., Blei A.T., Perry W. Performance of the hepatic encephalopathy scoring algorithm in a clinical trial of patients with cirrhosis and severe hepatic encephalopathy. Am J Gastroenterol. 2009;104:1392–1400. doi: 10.1038/ajg.2009.160. [DOI] [PubMed] [Google Scholar]