Abstract
Background:
Neuroblastoma survivors may be at elevated risk of psychological impairment because of their young age at diagnosis and neurotoxic treatment, but these sequelae are not well described.
Methods:
A total of 859 5+year survivors of neuroblastoma <18 years old (diagnosed 1970–1999), median age at diagnosis 0.8 years (range 0.0–7.3), median follow-up 13.3 years (8.0–17.9) were compared to 872 siblings of childhood cancer survivors <18 years old with the parent-reported Behavior Problem Index (BPI) for psychological functioning. Age/sex-adjusted multivariable log-binomial models were used to identify factors associated with impairment on the BPI domains (scores worse than sibling 10th percentile). Impact of psychological impairment on educational outcomes was examined among survivors.
Results:
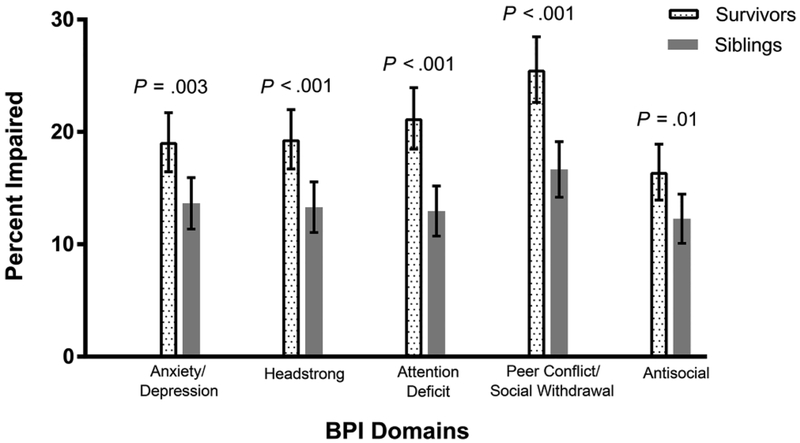
Compared to siblings, neuroblastoma survivors had a higher prevalence of impairment on domains of anxiety/depression (19% vs. 14%, p=0.003), headstrong behavior (19% vs. 13%, p<0.001), attention deficits (21% vs. 13%, p<0.001), peer conflict/social withdrawal (26% vs. 17%, p<0.001), and antisocial behavior (16% vs. 12%, p=0.01). Common treatment exposures (vincristine, cisplatin, retinoic acid) were not associated with impairment. Having ≥2 chronic health conditions predicted impairment on four domains (p<0.001). Specifically, pulmonary disease predicted impairment on all five domains (p≤0.004), and endocrine disease (p≤0.004) and peripheral neuropathy (p≤0.02) each predicted impairment on three domains. Psychological impairment was significantly associated with special education service usage and educational attainment less than college.
Conclusions:
Neuroblastoma survivors are at elevated risk for psychological impairment, which is in turn associated with childhood use of special education services and lower adult educational attainment. Those with chronic health conditions may represent a high-risk group for targeted screening and intervention.
Keywords: neuroblastoma, cancer survivorship, chronic health, psychological outcomes, educational outcomes
Condensed abstract:
A retrospective cohort of neuroblastoma survivors were found to have increased prevalence of psychological impairment compared to siblings. Those with chronic health conditions may represent a particularly high-risk group for future targeted screening and intervention.
Introduction:
Since 1975, treatment advances for neuroblastoma have resulted in an increase in five-year overall survival from 46% to 71% 1. During this time, standard of care for these patients has evolved with lower-risk patients receiving progressively de-escalated therapy and higher-risk patients receiving increasingly more intensive treatments 2, 3. Subsets of neuroblastoma survivors may be vulnerable to long-term health impairments because of their young age at diagnosis and the specific therapies they receive. However, psychological late effects from treatment are understudied in this population 4–7.
In the United States, children with neuroblastoma are diagnosed at a median age of 17.3 months 8, 9. This young age may place them at increased risk for psychological impairment due to contributions from both the early biologic insult to a developing central nervous system 10, as well as disruption of normal psychosocial development by intensive cancer treatment 11, 12. There are known associations between cognitive functioning and neurologic vulnerability at younger ages after receiving cranial radiation and/or chemotherapy alone in survivors of other pediatric cancers 10, 13, 14. Neuroblastoma chemotherapy regimens typically contain agents that are directly neurotoxic and/or can result in cardiovascular insufficiency, which is hypothesized to cause psychological impairment 2, 3, 15.
Long-term psychological impairment in neuroblastoma survivors is not well characterized because, until recently, many succumbed to their disease. The Childhood Cancer Survivor Study (CCSS) can address this knowledge gap because detailed treatment exposures over the last three decades of evolving therapeutic protocols were abstracted and uniform ascertainment of psychological outcomes with standardized instruments were collected. The specific aims of this study were to (1) characterize overall patterns and severity of psychological difficulties in long-term survivors of neuroblastoma, (2) identify survivor and treatment-related predictors of these impairments, and (3) describe education and employment outcomes and their relationship to psychological impairment in these survivors.
Methods:
Study population
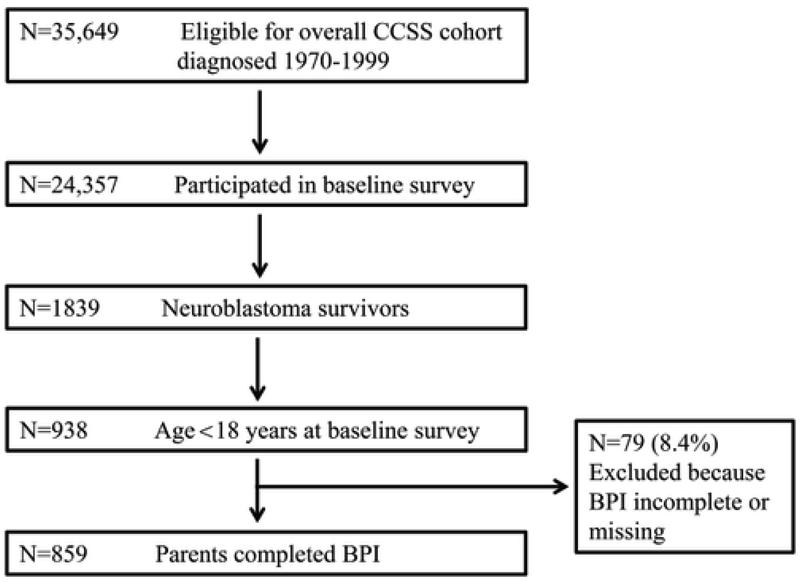
The CCSS is a retrospective, multi-institutional cohort study with detailed methodology and design previously described 16, 17. Survivors were diagnosed with neuroblastoma before age 21 years old, treated at one of 27 participating institutions in the United States and Canada between January 1, 1970 and December 31, 1999, and greater than five years from diagnosis at recruitment. The CCSS was approved by the institutional review board at each institution. For this analysis, participants consisted of 859 5+ year survivors of neuroblastoma <18 years old at the completion of the baseline survey and a comparison population of 872 siblings of cancer survivors from the CCSS. Consent was provided by a parent/guardian who completed a baseline questionnaire that included self-reported demographic information, medical outcomes, and psychosocial outcomes. The study population is detailed in a consort diagram (Figure 1). A subset of the survivors in this study (n=293) diagnosed 1970–1986 completed a follow-up CCSS Survey approximately 15 years after the baseline survey that included information regarding adult educational attainment and employment status among other life and health updates 16, 18. All surveys are available on the CCSS website (www.stjude.org/ccss) 19.
Figure 1.
Consort diagram
Outcome Measures
The primary outcome for this study was the parent-reported Behavior Problem Index (BPI), a 32-item standardized questionnaire included in the CCSS baseline questionnaire that was originally developed for the National Health Survey to describe cognitive, behavioral and emotional functioning 20. For each item, parents were asked about their child’s behavior on a Likert scale ranging from 1 (“Not True”) to 3 (“Often True”). This instrument examines five domains: depression/anxiety, headstrong behavior, attention deficit, peer conflict/social withdrawal, and antisocial behavior, and has been previously validated in adolescent childhood cancer survivors 21. Scores for each domain are calculated as the mean of the weighted response to questions in that domain. Higher scores indicate worse behavioral symptoms. These raw scores were then converted to z-scores using age- and sex-matched means and standard deviations from the sibling control group. Consistent with a past study, impairment was defined as scores elevated beyond the highest tenth percentile of age-matched sibling controls 21.
Secondary outcomes included use of special education services, collected for all survivors from parent report on the baseline questionnaire. Longitudinal assessment of highest level of educational attainment (some college or higher vs. less than college) and unemployment were collected for the subset of survivors (n=293) who completed a follow-up survey as described earlier 18. We defined unemployment as “unable to work due to illness or disability” or “unemployed but seeking work” in the last twelve months. We did not include voluntary unemployment (i.e., student, retirement, maternity leave, military service) as unemployment for this analysis. We further restricted educational attainment and unemployment analysis to survivors who were ≥25 years old at the time of follow-up survey completion. The follow-up survey assessed self-reported outcomes.
Independent Variables
Demographic information was collected for both survivors and siblings from the baseline questionnaire 22. For survivors, cancer related information (including date of diagnosis, chemotherapy exposures, radiation exposures, and surgeries) was collected via medical record abstraction 17. For chemotherapy exposures, we analyzed vincristine, platinum agents, alkylating agents, and/or anthracyclines (yes/no, as well as by cumulative dose over the 5 years since diagnosis). For radiation exposures, we examined whether survivors received primary or stray radiation to the brain, chest/neck, abdominal, or total body (yes/no, as well as by cumulative dose over the 5 years from diagnosis). We also considered whether survivors developed certain chronic health conditions: any cardiac disease, any endocrine disease, any pulmonary disease, any renal disease, peripheral neuropathy, hearing loss, and overweight/obese body mass index (≥85th percentile for age- and sex- specific distribution for US Children) 23, 24. These conditions were ascertained by parent report on the baseline questionnaire and graded using the National Cancer Institute’s Common Terminology Criteria for Adverse Events version 4.03: none (grade 0); mild or asymptomatic (grade 1); moderate (for example, minimal local or noninvasive intervention indicated) (grade 2); severe, medically significant, or disabling (grade 3); and life-threatening (grade 4) 25–27. Peripheral neuropathy was defined as any of the following, in the absence of a history of stroke or paralysis: weakness in the arm; weakness in the leg; and sensory neuropathy. In addition, we evaluated activity limitations, one of the six domains of health status as defined in previous CCSS analyses 28, 29. Activity limitations were considered present among participants who reported problems executing moderate activities (e.g. walking 1 block, carrying groceries, climbing a few flights of stairs) for 3 months or more in the past 2 years.
Statistical Analysis
Neuroblastoma survivors with BPI outcomes were compared to those with incomplete/missing BPI using chi-square tests in terms of age at diagnosis, sex, race, radiation, alkylating agent, platinum agent. Descriptive statistics for demographic and treatment characteristics of neuroblastoma survivors and CCSS siblings were compared using chi-square test statistic with bootstrapping families accounting for potential within-family correlation between survivors and siblings from the same families. The proportion of individuals with scores in the impaired range were described and compared between survivors and siblings for each domain of the BPI. Linear and logistic regression models adjusted for sex and age at evaluation were used for the survivor-sibling comparisons of continuous scores and binary impairment outcomes, respectively, with modifications by generalized estimating equations (GEE) accounting for potential within-family correlation 30. We also compared these outcomes within our sample of neuroblastoma survivors, using the same models without GEE, by treatment modality (surgery only vs. surgery+chemotherapy vs. surgery+chemotherapy+radiation vs. other) and by decade of diagnosis (1970–1970 vs. 1980–1989 vs. 1990–1999).
Within the survivor cohort, we performed multivariable analyses examining impairment on each of the BPI domains using three separate log-binomial models because of the co-linearity between treatment exposures and certain chronic health conditions. In the first model, we restricted analyses to sociodemographic factors: age at diagnosis (<1 year vs. ≥1 year), sex, race (white vs. other), annual household income (<$20,000 vs. $20,000–$39,999 vs. $40,000–$59,999 vs. ≥$60,000). In the second model, we restricted analyses to treatment factors (yes/no): retinoic acid, anthracycline, vincristine, any platinum-agent, any alkylating agent, cranial radiation, chest/neck radiation, abdominal radiation, and total body irradiation. We additionally examined the interaction of exposures to vincristine and any platinum agent. In the third model, we restricted analyses to chronic health conditions and health status: any cardiac disease (grade 2 or higher), any pulmonary disease (grade 2 or higher), any renal disease (grade 2 or higher), any endocrine disease (grade 2 or higher), hearing loss (grade 3 or 4); peripheral neuropathy (any grade, sensory or motor); body mass index (overweight/obese); and moderate-severe activity limitation (yes/no). These chronic health conditions and health status outcome were selected a priori given literature suggesting potential effects on psychological functioning in neuroblastoma survivors, survivors of other childhood cancers, as well as other populations 15, 31–38. In each model, backward selection was used to retain variables with p-value <0.05 in the final model. Prevalence ratios were calculated for each of these sociodemographic, treatment exposures and chronic health conditions with p-value <0.05. All models were adjusted for age at evaluation, sex, and annual household income.
We also examined the overall effect of chronic health conditions (any grade, at least one grade 3 or 4, and having 2 or more chronic health conditions [any grade]), radiation by quartile, and chemotherapy by dosing stratification on each BPI domain using log-binomial model.
We then assessed the relationship of impairment on each of the BPI domains with use of special education services among the survivors, using log-binomial models. In a subset of survivors for whom we collected educational attainment and unemployment in the last 12 months (detailed above), their associations with impairment on each of the BPI domains were examined.
All analyses were conducted using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results:
Study population characteristics
A total of 859 five-year survivors of neuroblastoma were included in this analysis (Figure 1). Participants and non-participants were similar in terms of sex and race. Participants were younger at diagnosis, and less likely to have received radiation, alkylating agents, and platinum agents (all p’s<0.01). Table 1 displays baseline demographic and treatment characteristics of survivors and the sibling comparison group. Siblings did not differ from survivors in terms of sex or race, but were slightly older at baseline evaluation (p=0.04).
Table 1.
Demographic and treatment characteristics for pediatric/adolescent survivors of neuroblastoma and siblings
| Characteristic | Survivors N (%) |
Siblings N (%) |
P-value |
|---|---|---|---|
| Total number | 859 | 872 | N/A |
| Sex | 0.35 | ||
| Female | 429 (49.9%) | 416 (47.7%) | |
| Male | 430 (50.1%) | 456 (52.3%) | |
| Race/ethnicity | 0.07 | ||
| White, non-Hispanic | 706 (82.2%) | 724 (83.0%) | |
| Black, non-Hispanic | 54 (6.3%) | 34 (3.9%) | |
| Hispanic/Latino | 44 (5.1%) | 45 (5.2%) | |
| Other | 55 (6.4%) | 69 (7.9%) | |
| Age at diagnosis (years) | N/A | N/A | |
| <1 | 534 (62.2%) | ||
| 1–1.99 | 184 (21.4%) | ||
| 2–4.99 | 123 (14.3%) | ||
| 5 and older | 18 (2.1%) | ||
| Age at baseline (years) | 0.04 | ||
| 8–11 | 157 (18.3%) | 145 (16.6%) | |
| 12–13 | 206 (24.0%) | 172 (19.7%) | |
| 14–15 | 250 (29.1%) | 261 (30.0%) | |
| 16–17 | 246 (28.6%) | 294 (33.7%) | |
| Overall treatmenta | |||
| Surgery only | 259 (32.8%) | N/A | N/A |
| Surgery+chemotherapy | 292 (37.0%) | ||
| Surgery+radiation | 59 (7.5%) | ||
| Surgery+chemotherapy+radiation | 163 (20.6%) | ||
| None/Other combinations | 17 (2.1%) | ||
| Specific treatment exposures, median cumulative dose for those who received dose > 0 |
|||
| Cranial radiation (cGy, n=220) | 20 (IQR: 20 – 525) | N/A | N/A |
| Chest radiation (cGy, n=221) | 700 (IQR: 200 – 1600) | ||
| Abdominal radiation (cGy, n=220) | 1200 (IQR: 200 – 2100) | ||
| Alkylating agent (gramsb, n=410) | 6180 (IQR: 4018 – 11434) | ||
| Platinum agent (mg/m2, n=238) | 755 (IQR: 360 – 1882.3) | ||
| Anthracycline (mg/m2, n=321) | 142 (IQR: 93.3 – 193.3) |
IQR = interquartile range
69 subjects did not consent to release of medical records for treatment exposures and were not included
Alkylating agent (i.e. cyclophosphamide, ifosfamide, procarbazine, chlorambucil, BCNU, CCNU, melphalan, Thio-TEPA, nitrogen mustard, busulfan) exposure was determined using cyclophosphamide equivalent dose, methodology previously published 49
Impairment on Behavior Problem Index and associated factors among survivors
Compared with siblings, survivors had a greater proportion impaired on every domain of the BPI (Figure 2), including: anxiety/depression (p=0.003), attention deficit (p<0.001), peer conflict/social withdrawal (p<0.001), headstrong (p<0.001), antisocial (p=0.01).
Figure 2.
Percent impairment and 95% confidence intervals for Behavior Problem Index (BPI) domains in neuroblastoma survivors compared to siblings. Error bars signify 95% confidence intervals. Because BPI scores fall on an ordinal scale and multiple siblings achieve exactly the same score at the tenth percentile, the proportion of siblings found to be impaired on any given domain exceeds 10%.
Demographic factors:
Lower household income was associated with impairment in headstrong behavior (adjusted prevalence ratio [PR]=1.72, 95% confidence interval [95%CI] 1.12–2.72), attention deficit (PR=2.12; 95%CI 1.37–3.44), and peer conflict/social withdrawal (PR=1.44, 95%CI 1.02–2.07) when comparing annual incomes between $20,000-$39,999 to those greater than $60,000 (Table 2).
Table 2.
Multivariable analyses of demographic and treatment factors with domains of cognitive and behavioral impairment among neuroblastoma survivors
| Anxiety/Depression | Headstrong | Attention Deficit | Peer Conflict/Social Withdrawal | Antisocial | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PR | P-value | PR | P-value | PR | P-value | PR | P-value | PR | P-value | ||
| Model 1: Patient characteristics | |||||||||||
| Sex | N/S | N/S | N/S | <0.001 | N/S | ||||||
| Male | 0.66 (0.52–0.83) | ||||||||||
| Female | Referent | ||||||||||
| Age at diagnosis | N/S | N/S | N/S | N/S | N/S | ||||||
| <1 year old | |||||||||||
| ≥1 year old | |||||||||||
| Annual household income | 0.05 | 0.02 | 0.002 | 0.04 | N/S | ||||||
| Unknown | 0.89 (0.40–1.73) | 1.46 (0.74–2.69) | 1.66 (0.83–3.13) | 1.55 (0.92–2.45) | |||||||
| <$20,000 | 1.45 (0.90–2.33) | 1.43 (0.84–2.41) | 2.07 (1.26–3.49) | 1.10 (0.69–1.70) | |||||||
| $20,000 - $39,999 | 1.32 (0.87–2.04) | 1.72 (1.12–2.72) | 2.12 (1.37–3.44) | 1.44 (1.02–2.07) | |||||||
| $40,000 – $59,999 | 0.85 (0.57–1.29) | 1.02 (0.67–1.61) | 1.34 (0.87–2.15) | 0.97 (0.70–1.38) | |||||||
| $60,000+ | Referent | Referent | Referent | Referent | |||||||
| Model 2: Treatment exposuresa,b | |||||||||||
| Cranial radiation | N/S | N/S | N/S | 0.003 | N/S | ||||||
| Yes | 2.03 (1.32–2.37) | ||||||||||
| No | Referent | ||||||||||
| Abdominal radiation | N/S | N/S | N/S | N/S | 1.58 (1.01–2.36) | 0.04 | |||||
| Yes | |||||||||||
| No | Referent | ||||||||||
| Total body irradiation | N/S | N/S | N/S | 0.51 (0.27–0.99) | 0.05 | N/S | |||||
| Yes | |||||||||||
| No | Referent | ||||||||||
| Anthracycline | N/S | N/S | N/S | N/S | 1.58 (1.07–2.30) | 0.02 | |||||
| Yes | |||||||||||
| No | Referent | ||||||||||
| Interaction between age at diagnosis and platinum agent | N/S | N/S | N/S | N/S | |||||||
| <1 year old and yes | 0.97 (0.62–1.53) | 0.90 | |||||||||
| <1 year old and no | Referent | ||||||||||
| ≥1 year old and yes | 0.41 (0.20–0.81) | 0.01 | |||||||||
| ≥1 year old and no | Referent | ||||||||||
| Interaction between age at diagnosis and anthracycline | N/S | N/S | N/S | N/S | |||||||
| <1 year old and yes | 1.28 (0.90–1.82) | 0.17 | |||||||||
| <1 year old and no | Referent | ||||||||||
| ≥1 year old and yes | 0.55 (0.32–0.95) | 0.03 | |||||||||
| ≥1 year old and no | Referent | ||||||||||
| Model 3: Chronic conditionsa,c | |||||||||||
| Peripheral neuropathy | 0.002 | 0.005 | 0.02 | N/S | N/S | ||||||
| Yes | 1.86 (1.28–2.56) | 1.78 (1.21–2.48) | 1.58 (1.08–2.19) | ||||||||
| No | Referent | Referent | Referent | ||||||||
| Grade ≥2 pulmonary disease | |||||||||||
| Yes | 1.92 (1.31–2.65) | <0.001 | 1.75 (1.16–1.91) | 0.003 | 1.71 (1.17–2.36) | 0.002 | 1.62 (1.20–2.09) | <0.001 | 1.83 (1.17–2.27) | 0.004 | |
| No | Referent | Referent | Referent | Referent | Reference | ||||||
| Grade ≥2 endocrine disease | N/S | N/S | |||||||||
| Yes | 1.74 (1.16–1.91) | 0.004 | 1.55 (1.17–1.98) | 0.001 | 1.80 (1.13–2.09) | 0.006 | |||||
| No | Referent | Referent | Referent | ||||||||
| Interaction between age at diagnosis and peripheral neuropathy | N/S | N/S | N/S | N/S | |||||||
| <1 year old and yes | 1.81 (1.35–2.44) | <0.001 | |||||||||
| <1 year old and no | Referent | ||||||||||
| ≥1 year old and yes | 0.75 (0.41–1.37) | 0.35 | |||||||||
| ≥1 year old and no | Referent | ||||||||||
PR = prevalence ratio; N/S = not significant;
Adjusted for age at diagnosis and sex;
Also examined but not significant: retinoic acid, vincristine, chest/neck radiation, alkylating agents, platinum agents, interaction of age at diagnosis with sex, interaction of age at diagnosis with each of the treatment factors;
Also examined but not significant: Grade ≥2 cardiac disease, Grade ≥2 renal disease, hearing loss, body mass index, physical fitness limiting daily activities exceeding three months, interaction of age at diagnosis with sex, interaction of age at diagnosis with each of the chronic conditions
Treatment factors:
Survivors treated with anthracyclines had more impairment in the antisocial domain (PR=1.58; 95%CI 1.07–2.30, Table 2) compared to survivors without such treatment. Cranial radiation (PR=2.03; 95%CI 1.32–2.37) predicted impairment in the headstrong domain, while abdominal radiation (PR=1.58; 95%CI 1.01–2.36) was associated with increased risk of impairment in the antisocial domain. No other differences associated with treatment exposures were identified (Supplemental Table 1). There were also no statistical differences in BPI outcomes in survivors when compared by treatment group (surgery only vs. surgery+chemotherapy vs. surgery+chemotherapy+radiation vs. other; Supplemental Table 2) or across decades of diagnosis.
Chronic health conditions:
Survivors with pulmonary disease had an increased prevalence of impairment in all five domains: anxiety/depression (PR=1.92, 95%CI 1.31–2.65, Table 2), headstrong (PR=1.75, 95%CI 1.16–1.91), attention deficit (PR=1.71, 95%CI 1.17–2.36), peer conflict/social withdrawal (PR=1.62, 95%CI 1.20–2.09), antisocial (PR=1.83, 95%CI 1.17–2.27) compared to survivors without pulmonary disease. Survivors with peripheral neuropathy had an 86% increased prevalence of impairment in anxiety/depression (PR=1.86; 95%CI 1.28–2.56), as well as increased prevalence of headstrong behavior (PR=1.78; 95%CI 1.21–2.48) and attention deficit (PR=1.58; 95%CI 1.08–2.19), compared to survivors without neuropathy. Additionally, among survivors diagnosed <1 year old, those who developed peripheral neuropathy had over a two-fold increased prevalence of impairment in peer conflict/social withdrawal (PR=2.29; 95%CI 1.63–3.23), compared to those who did not develop peripheral neuropathy. Similarly, survivors with endocrine disease had increased prevalence of impairment on three domains compared to survivors without endocrine disease: headstrong (PR=1.74, 95%CI 1.16–1.91), peer conflict/social withdrawal (PR=1.55, 95%CI 1.17–1.98), antisocial (PR=1.80, 95%CI 1.13–2.09). There were no significant associations with renal or cardiac disease. Having at least one chronic health condition (any grade, Table 3) was associated with impairment in all five domains: anxiety/depression (PR=2.09; 95%CI 1.51–2.98), headstrong (1.64, 95%CI 1.21–2.27), attention deficit (PR=1.80; 95%CI 1.34–2.47), peer conflict/social withdrawal (PR=1.70; 95%CI 1.31–2.23), and antisocial (PR=1.65; 95%CI 1.18–2.37). Having two or more chronic health conditions (any grade) was associated with impairment in four domains.
Table 3.
Prevalence ratios of BPI impairment in neuroblastoma survivors based on burden of chronic health conditions
| Anxiety/Depression | Headstrong | Attention Deficit | Peer Conflict/ Social Withdrawal |
Antisocial | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PR | P-value | PR | P-value | PR | P-value | PR | P-value | PR | P-value | |
| Any grade 1–4 event | ||||||||||
| Yes | 2.09 (1.51–2.98) | <0.001 | 1.64 (1.21–2.27) | <0.001 | 1.80 (1.34–2.47) | <0.001 | 1.70 (1.31–2.23) | <0.001 | 1.65 (1.18–2.37) | 0.003 |
| No | Referent | Referent | Referent | Referent | Referent | |||||
| Any grade 3–4 event | ||||||||||
| Yes | 1.05 (0.74–1.44) | 0.79 | 1.22 (0.88–1.65) | 0.23 | 1.01 (0.73–1.37) | 0.94 | 1.27 (0.98–1.63) | 0.07 | 1.18 (0.82–1.65) | 0.36 |
| No | Referent | Referent | Referent | Referent | Referent | |||||
| Having 2 or more grade 1–4 events | ||||||||||
| Yes | 1.97 (1.49–2.62) | <0.001 | 1.65 (1.25–2.17) | <0.001 | 1.81 (1.40–2.36) | <0.001 | 1.98 (1.57–2.51) | <0.001 | 1.34 (0.99–1.81) | 0.06 |
| No | Referent | Referent | Referent | Referent | Referent | |||||
Special education services, educational attainment, and unemployment
Compared to siblings, survivors were more likely to use special education services during childhood/adolescence (PR=2.25; 95%CI 1.84–2.74), and more likely to not attend college (PR=1.71; 95%CI 1.17–2.50). There was no significant difference in terms of unemployment (Table 4). Impairment in each of the BPI domains was associated with a 66–126% increase in use of special education services (p<0.001), as well as an 89–173% increased risk of attainment of less than college education (p≤0.04, Table 4). Specifically, impairment in attention deficit was associated with more than a two-fold increase in the use of special education services (PR=2.26; 95%CI 1.86–2.74).
Table 4.
aPrevalence ratios of concurrent use of special education services, and future educational attainment and unemployment in neuroblastoma survivors compared to siblings and based on impairment in psychological functioning
| Use of special education services | Educational attainment lower than collegeb | Unemployment in the last 12 monthsb | ||||
|---|---|---|---|---|---|---|
| PR | P-value | PR | P-value | PR | P-value | |
| Survivors vs. siblings | ||||||
| Survivors | 2.25 (1.84 – 2.74) | <0.001 | 1.71 (1.17–2.50) | 0.007 | 1.42 (0.79–2.53) | 0.24 |
| Siblings | Referent | Referent | Referent | |||
| Psychological functioning in survivors by BPI domain | ||||||
| Anxiety/Depression | ||||||
| Impaired | 1.77 (1.43–2.16) | <0.001 | 2.37 (1.40–3.81) | 0.002 | 2.27 (0.92–5.08) | 0.07 |
| No Impairment | Referent | Referent | Referent | |||
| Headstrong | ||||||
| Impaired | 1.72 (1.38–2.10) | <0.001 | 2.05 (1.22–3.35) | 0.008 | 3.21 (1.42–7.18) | 0.006 |
| No Impairment | Referent | Referent | Referent | |||
| Attention Deficit | ||||||
| Impaired | 2.26 (1.86–2.74) | <0.001 | 1.89 (1.05–3.14) | 0.04 | 2.12 (0.82–4.91) | 0.11 |
| No Impairment | Referent | Referent | Referent | |||
| Peer Conflict/Social Withdrawal | ||||||
| Impaired | 2.03 (1.66–2.47) | <0.001 | 2.47 (1.50–4.07) | <0.001 | 1.61 (0.67–3.60) | 0.27 |
| No Impairment | Referent | Referent | Referent | |||
| Antisocial | ||||||
| Impaired | 1.66 (1.32–2.06) | <0.001 | 2.73 (1.60–4.29) | <0.001 | 2.74 (1.10–6.16) | 0.03 |
| No Impairment | Referent | Referent | Referent | |||
All models adjusted for sex and age at baseline
Survivors had to have completed both the CCSS baseline questionnaire as well as a Follow-up Survey in 2007. They additionally had to be at least 25 years or older at Follow-up in 2007
Discussion:
This retrospective cohort of 859 pediatric/adolescent survivors of neuroblastoma had a higher prevalence of poor psychological outcomes compared to siblings on standardized parent-report. Survivors had higher rates of impairment in anxiety/depression, headstrong, attention deficit, peer conflict/withdrawal, and antisocial domains. We found lower household income to be associated with greater risk of impairment in three out of the five domains measured. While there was no clear pattern of association between psychological impairment with treatment intensity or specific treatment risk factors, our results suggest that these impairments may be driven by the burden of chronic health conditions. Having two or more chronic health conditions was associated with impairment in multiple psychological domains. Psychological impairment was associated with the use of special education services during childhood/adolescence, and longitudinally with lower educational attainment, indicating that there are potentially downstream concerns that affect adult day-to-day functioning.
Our analysis builds on previous studies, which suggested that neurocognitive functioning and psychosocial well-being were affected in survivors of neuroblastoma 4, 21, 34, 35. Consistent with previous work in survivors of different cancers, we found lower household income to be associated with worse outcomes 39–41. The potential effect of lower socioeconomic status on worse psychological functioning adds to similar publications in children with leukemia 42, 43. With more data demonstrating the effects of health disparity on both survival and overall functioning post-treatment, future studies must focus on addressing these gaps given the economic burden of cancer care and prevalence of financial hardship in this patient population 44, 45.
Interestingly, there was no clear pattern for specific treatment-related risk factors or dose effects for psychological impairment. It is possible that our instrument was not sensitive to some aspects of psychological functioning that may require more comprehensive testing to detect. Also surprisingly, total body irradiation was associated with better, not worse, functioning in peer conflict/social withdrawal. We could find no explanation in our analyses, but speculate that this association may be confounded by other factors that we could not measure, e.g., psychosocial or other supportive care interventions.
The lack of association between treatment intensity and worse psychological outcomes in our sample offers the possibility that these outcomes may be a general effect of the cancer/survivorship experience. Stein and colleagues propose a model in which the long-term psychological response to the cancer experience is broken down into the stress and burden of the experience balanced with the resources available to each individual 46. They detail the multifaceted components of the burden of the cancer experience including physical (e.g., late effects like fatigue, weight gain, infertility) and psychological aspects (e.g., fear of recurrence, baseline cognitive function), both of which likely play a role in our neuroblastoma survivor cohort. Specifically, the BPI impairments we observed in this population may be driven by the overall burden of chronic health conditions. We found that having two or more chronic health conditions significantly predicted impairment in four out of the five domains. There is also some evidence suggesting survivors specifically with pulmonary disease, peripheral neuropathy, and endocrine disease may be at particularly increased risk for psychological impairment given >50% increased risk of impairment in these subgroups on at least three domains. However, this finding needs to be confirmed in studies with the capacity for more comprehensive clinical assessment for these conditions, and future work may need to incorporate more nuanced analysis of these associations.
Compared to siblings, neuroblastoma survivors in our study were more likely to use special education services and have worse outcomes in terms of eventual educational attainment. These long-term outcomes contribute to a body of literature demonstrating that these patients are at increased risk of academic learning problems, which may contribute to lower quality of life and social development concerns 34, 47, 48. Impairment on multiple BPI domains was associated with childhood use of special education services, educational attainment less than college, and unemployment as adults. These data suggest that future studies should explore early psychological screening, educational interventions, and supportive services to help maximize success later in life for subsets of this patient population.
To our knowledge, this is the largest analysis focusing on psychological outcomes in neuroblastoma survivors. The CCSS benefits from having a sizeable, multi-institutional patient cohort with detailed medical record abstracted treatment information, as well as longitudinal follow-up spanning several decades 16. This study was additionally strengthened by standardized ascertainment of these outcomes with a validated instrument 20, 21. These results, however, must be interpreted in light of its limitations. The primary outcomes were based on a parent-report instrument. There are no specific data on agreement between parent-proxy and child self-report for the BPI. A systematic review by Upton et al. of parent-child agreement across child health-related quality of life instruments found that parents of children with health conditions tended to underestimate their quality of life 49. However, they cautioned that the data remain limited and that parent and children characteristics are not consistently considered in these studies. Moreover, the authors acknowledge that parental perception may add different, but valuable, information that enriches the understanding of a child’s functioning. Other authors have similarly concluded that parent proxy-reports remain fundamentally important in pediatric clinical research 50, 51. It is also important to note that our study cohort ends with survivors diagnosed in 1999. Since that time there have been changes in standard of care protocol, including the adoption of immunotherapy and cytokines plus isotretinoin for high-risk neuroblastoma52. While our analysis includes retinoic acid, it does not capture the effects of immunotherapy and thus may be more limited in its generalizability.
Results from this study demonstrate that survivors of neuroblastoma are at risk for worse psychological functioning and educational outcomes. Lower household income and having multiple chronic health conditions are notable risk factors. Future studies will benefit from even larger patient samples as overall survival continues to improve. This work should integrate more comprehensive assessment of neurocognitive and mental health function to better characterize specific deficits in this patient population, as well as evaluate approaches for early screening and intervention to identify individuals at higher risk of worse educational and employment outcomes in adulthood.
Supplementary Material
Acknowledgments
Funding Sources: This work was supported by the National Cancer Institute (CA55727, G.T. Armstrong, Principal Investigator), as well as the St. Baldrick’s Summer Fellowship (D.J. Zheng). Support to St. Jude Children’s Research Hospital also provided by the Cancer Center Support (CORE) grant (CA21765, C. Roberts, Principal Investigator) and ALSAC.
Footnotes
Conflicts of Interest: No conflicts to disclose
References:
- 1.Horner M, Ries L, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2006, National Cancer Institute. Bethesda, MD, 2009. [Google Scholar]
- 2.Weinstein JL, Katzenstein HM, Cohn SL. Advances in the diagnosis and treatment of neuroblastoma. The Oncologist. 2003;8: 278–292. [DOI] [PubMed] [Google Scholar]
- 3.Maris JM. Recent advances in neuroblastoma. New England Journal of Medicine. 2010;362: 2202–2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laverdière C, Cheung NKV, Kushner BH, et al. Long‐term complications in survivors of advanced stage neuroblastoma. Pediatric blood & cancer. 2005;45: 324–332. [DOI] [PubMed] [Google Scholar]
- 5.Carpentieri S, Diller L. Neuropsychological resiliency after treatment for advanced stage neuroblastoma. Bone marrow transplantation. 2005;35: 1117–1122. [DOI] [PubMed] [Google Scholar]
- 6.Cohen L, Gordon J, Popovsky E, et al. Late effects in children treated with intensive multimodal therapy for high-risk neuroblastoma: High incidence of endocrine and growth problems. Bone marrow transplantation. 2014;49: 502–508. [DOI] [PubMed] [Google Scholar]
- 7.Laverdière C, Liu Q, Yasui Y, et al. Long-term outcomes in survivors of neuroblastoma: a report from the Childhood Cancer Survivor Study. Journal of the National Cancer Institute. 2009;101: 1131–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goodman MT, Gurney J, Smith M, Olshan A. Sympathetic nervous system tumors. Cancer incidence and survival among children and adolescents: United States SEER Program. 1975;1995: 65–72. [Google Scholar]
- 9.Pizzo PA, Poplack DG, Adamson PC, Blaney SM, Helman LJ. Principles and practice of pediatric oncology. Lippincott Williams & Wilkins; Philadelphia, PA:, 2006. [Google Scholar]
- 10.Taylor HG, Alden J. Age-related differences in outcomes following childhood brain insults: an introduction and overview. Journal of the International Neuropsychological Society. 1997;3: 555–567. [PubMed] [Google Scholar]
- 11.Wallander JL, Varni JW. Effects of pediatric chronic physical disorders on child and family adjustment. Journal of Child Psychology and Psychiatry. 1998;39: 29–46. [PubMed] [Google Scholar]
- 12.Patenaude AF, Kupst MJ. Psychosocial functioning in pediatric cancer. Journal of pediatric psychology. 2005;30: 9–27. [DOI] [PubMed] [Google Scholar]
- 13.Buizer AI, De Sonneville LM, Van Den Heuvel-eibrink MM, Njiokiktjien C, Veerman AJ. Visuomotor control in survivors of childhood acute lymphoblastic leukemia treated with chemotherapy only. Journal of the International Neuropsychological Society. 2005;11: 554–565. [DOI] [PubMed] [Google Scholar]
- 14.Buizer AI, de Sonneville LM, van den Heuvel–Eibrink MM, Veerman AJ. Chemotherapy and attentional dysfunction in survivors of childhood acute lymphoblastic leukemia: effect of treatment intensity. Pediatric blood & cancer. 2005;45: 281–290. [DOI] [PubMed] [Google Scholar]
- 15.Krull KR, Sabin ND, Reddick WE, et al. Neurocognitive function and CNS integrity in adult survivors of childhood hodgkin lymphoma. Journal of Clinical Oncology. 2012;30: 3618–3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robison LL, Armstrong GT, Boice JD, et al. The Childhood Cancer Survivor Study: a National Cancer Institute–supported resource for outcome and intervention research. Journal of clinical oncology. 2009;27: 2308–2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leisenring WM, Mertens AC, Armstrong GT, et al. Pediatric cancer survivorship research: experience of the Childhood Cancer Survivor Study. Journal of clinical oncology. 2009;27: 2319–2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gerhardt CA, Yopp JM, Leininger L, et al. Brief report: post-traumatic stress during emerging adulthood in survivors of pediatric cancer. J Pediatr Psychol. 2007;32: 1018–1023. [DOI] [PubMed] [Google Scholar]
- 19.Robison LL, Mertens AC, Boice JD, et al. Study design and cohort characteristics of the childhood cancer survivor study: A multi‐institutional collaborative project. Medical and pediatric oncology. 2002;38: 229–239. [DOI] [PubMed] [Google Scholar]
- 20.Zill N Behavior problems index based on parent report. Child Trends, 1990. [Google Scholar]
- 21.Schultz KAP, Ness KK, Whitton J, et al. Behavioral and social outcomes in adolescent survivors of childhood cancer: a report from the childhood cancer survivor study. Journal of clinical oncology. 2007;25: 3649–3656. [DOI] [PubMed] [Google Scholar]
- 22.Lozoff B, Jimenez E, Hagen J, Mollen E, Wolf AW. Poorer behavioral and developmental outcome more than 10 years after treatment for iron deficiency in infancy. Pediatrics. 2000;105: e51–e51. [DOI] [PubMed] [Google Scholar]
- 23.Kuczmarski RJ, Ogden CL, Guo SS, et al. 2000. CDC Growth Charts for the United States: methods and development. Vital and health statistics. Series 11, Data from the national health survey. 2002: 1–190. [PubMed] [Google Scholar]
- 24.Ogden CL, Flegal KM. Changes in terminology for childhood overweight and obesity. Age. 2010;12: 12. [PubMed] [Google Scholar]
- 25.Diller L, Chow EJ, Gurney JG, et al. Chronic disease in the Childhood Cancer Survivor Study cohort: a review of published findings. Journal of Clinical Oncology. 2009;27: 2339–2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. New England Journal of Medicine. 2006;355: 1572–1582. [DOI] [PubMed] [Google Scholar]
- 27.Health UDo, Services H. National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) Version 4.03, 2010, 2016. [Google Scholar]
- 28.Ness KK, Hudson MM, Jones KE, et al. Effect of Temporal Changes in Therapeutic Exposure on Self-reported Health Status in Childhood Cancer SurvivorsTemporal Changes in Exposure and Health Status. Annals of internal medicine. 2017;166: 89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hudson MM, Mertens AC, Yasui Y, et al. Health status of adult long-term survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Jama. 2003;290: 1583–1592. [DOI] [PubMed] [Google Scholar]
- 30.Zeger SL, Liang K-Y. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986: 121–130. [PubMed] [Google Scholar]
- 31.Copeland DR, Dowell RE, Fletcher JM, et al. Neuropsychological effects of childhood cancer treatment. Journal of Child Neurology. 1988;3: 53–62. [DOI] [PubMed] [Google Scholar]
- 32.Hockenberry M, Krull K, Moore K, Gregurich MA, Casey ME, Kaemingk K. Longitudinal evaluation of fine motor skills in children with leukemia. Journal of pediatric hematology/oncology. 2007;29: 535–539. [DOI] [PubMed] [Google Scholar]
- 33.Kaemingk KL, Carey ME, Moore IM, Herzer M, Hutter JJ. Math weaknesses in survivors of acute lymphoblastic leukemia compared to healthy children. Child Neuropsychology. 2004;10: 14–23. [DOI] [PubMed] [Google Scholar]
- 34.Gurney JG, Tersak JM, Ness KK, Landier W, Matthay KK, Schmidt ML. Hearing loss, quality of life, and academic problems in long-term neuroblastoma survivors: a report from the Children’s Oncology Group. Pediatrics. 2007;120: e1229–e1236. [DOI] [PubMed] [Google Scholar]
- 35.Kadan-Lottick NS, Zeltzer LK, Liu Q, et al. Neurocognitive functioning in adult survivors of childhood non-central nervous system cancers. Journal of the National Cancer Institute. 2010;102: 881–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vuotto SC, Krull KR, Li C, et al. Impact of chronic disease on emotional distress in adult survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. Cancer. 2017;123: 521–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bugnicourt J-M, Godefroy O, Chillon J-M, Choukroun G, Massy ZA. Cognitive disorders and dementia in CKD: the neglected kidney-brain axis. Journal of the American Society of Nephrology. 2013;24: 353–363. [DOI] [PubMed] [Google Scholar]
- 38.Zammit AR, Katz MJ, Lai JY, Zimmerman ME, Bitzer M, Lipton RB. Association between renal function and cognitive ability domains in the Einstein aging study: a cross-sectional analysis. Journals of Gerontology Series A: Biomedical Sciences and Medical Sciences. 2014;70: 764–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zebrack BJ, Zevon MA, Turk N, et al. Psychological distress in long‐term survivors of solid tumors diagnosed in childhood: A report from the childhood cancer survivor study. Pediatric blood & cancer. 2007;49: 47–51. [DOI] [PubMed] [Google Scholar]
- 40.Nathan PC, Ness KK, Greenberg ML, et al. Health‐related quality of life in adult survivors of childhood Wilms tumor or neuroblastoma: A report from the childhood cancer survivor study. Pediatric blood & cancer. 2007;49: 704–715. [DOI] [PubMed] [Google Scholar]
- 41.Zeltzer LK, Lu Q, Leisenring W, et al. Psychosocial outcomes and health-related quality of life in adult childhood cancer survivors: a report from the childhood cancer survivor study. Cancer Epidemiology Biomarkers & Prevention. 2008;17: 435–446. [DOI] [PubMed] [Google Scholar]
- 42.Bona K, Blonquist TM, Neuberg DS, Silverman LB, Wolfe J. Impact of Socioeconomic Status on Timing of Relapse and Overall Survival for Children Treated on Dana‐Farber Cancer Institute ALL Consortium Protocols (2000–2010). Pediatric blood & cancer. 2016. [DOI] [PubMed] [Google Scholar]
- 43.Petridou ET, Sergentanis T, Perlepe C, et al. Socioeconomic disparities in survival from childhood leukemia in the United States and globally: a meta-analysis. Annals of Oncology. 2014: mdu572. [DOI] [PubMed] [Google Scholar]
- 44.Pelletier W, Bona K. Assessment of financial burden as a standard of care in pediatric oncology. Pediatric blood & cancer. 2015;62: S619–S631. [DOI] [PubMed] [Google Scholar]
- 45.Bona K, London WB, Guo D, Frank DA, Wolfe J. Trajectory of material hardship and income poverty in families of children undergoing chemotherapy: a prospective cohort study. Pediatric blood & cancer. 2016;63: 105–111. [DOI] [PubMed] [Google Scholar]
- 46.Stein KD, Syrjala KL, Andrykowski MA. Physical and psychological long‐term and late effects of cancer. Cancer. 2008;112: 2577–2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barrera M, Shaw AK, Speechley KN, Maunsell E, Pogany L. Educational and social late effects of childhood cancer and related clinical, personal, and familial characteristics. Cancer. 2005;104: 1751–1760. [DOI] [PubMed] [Google Scholar]
- 48.Mitby PA, Robison LL, Whitton JA, et al. Utilization of special education services and educational attainment among long‐term survivors of childhood cancer. Cancer. 2003;97: 1115–1126. [DOI] [PubMed] [Google Scholar]
- 49.Upton P, Lawford J, Eiser C. Parent–child agreement across child health-related quality of life instruments: a review of the literature. Quality of Life Research. 2008;17: 895–913. [DOI] [PubMed] [Google Scholar]
- 50.Pickard AS, Topfer L-A, Feeny DH. A structured review of studies on health-related quality of life and economic evaluation in pediatric acute lymphoblastic leukemia. Journal of the National Cancer Institute Monographs. 2004;2004: 102–125. [DOI] [PubMed] [Google Scholar]
- 51.Varni JW, Limbers CA, Burwinkle TM. Parent proxy-report of their children’s health-related quality of life: an analysis of 13,878 parents’ reliability and validity across age subgroups using the PedsQL™ 4.0 Generic Core Scales. Health and quality of life outcomes. 2007;5: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pinto NR, Applebaum MA, Volchenboum SL, et al. Advances in risk classification and treatment strategies for neuroblastoma. Journal of Clinical Oncology. 2015;33: 3008–3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.