Acute myeloid leukemia (AML) is a blood cancer resulting from the enhanced proliferation and impaired differentiation of hematopoietic stem and progenitor cells. Chemotherapy consisting of cytarabine and anthracyclines has been the standard of AML care for decades, with a 5-year overall survival rate of 25% [1]. Outcomes in older patients, who represent the majority of patients with this disease, are poor with a median survival of 5 to 10 months. Owing to their inability to tolerate intensive chemotherapy, many older patients do not receive any anti-leukemic therapy [2]. Although the development of drugs targeted to specific pathways offers the promise of improved treatment options, resistance to individual inhibitors has limited their effectiveness. This is in part the result of the substantial disease heterogeneity and clonality underlying AML and underscores the need for combinations of targeted therapies to achieve durable responses.
Our group and others have previously established the utility of ex vivo screening platforms to identify effective targeted treatments for leukemia patients [3]. We adapted this approach to test combinations of targeted drugs in a fixed molar concentration series of seven dose points. This work identified several candidate combinations with enhanced efficacy in myeloid leukemia samples, many of which included the BCL2 inhibitor venetoclax [4]. Given the many complex subtypes of AML, we performed expanded studies on the combination of ruxolitinib plus venetoclax (Rux + Ven) because of its broad effectiveness on a majority of AML samples tested.
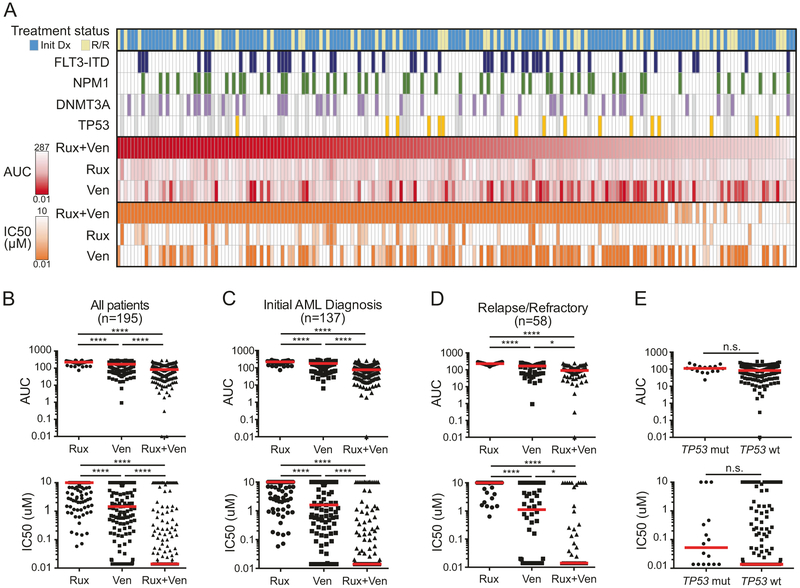
A total of 195 unique AML patients were screened ex vivo for sensitivity to the Rux + Ven combination. Briefly, freshly isolated mononuclear cells were cultured in presence of inhibitors alone or in combination for 3 days before assessing viability as a measure of sensitivity. Under these assay conditions, the cells are viable but not proliferating. The cohort included patients with newly diagnosed AML (n = 137) or relapsed/refractory AML (n = 58). While relapsed/refractory patients surveyed were slightly older than those with newly diagnosed disease (median [range] age of 55.5 (2–87) yrs vs. 62.5 (12–86) yrs, respectively; Mann–Whitney test, p = 0.0371), no significant differences were observed between newly diagnosed and relapsed/refractory patients with respect to sex, WBC count, bone marrow blasts, or ELN prognostic risk (Supplemental Table S1). Among all AML patient specimens tested, the Rux + Ven combination is significantly more effective than either respective single agent by two different effect measures (IC50 and AUC; Fig. 1a, b). For example, median IC50 for Rux + Ven across all patient specimens was 0.014 μM, compared with 10 and 1.45 μM for ruxolitinib and venetoclax, respectively (Friedman test with Dunn’s multiple comparisons test; adjusted p < 0.0001). Furthermore, 80% (156/195) of samples tested demonstrate IC50 values for Rux + Ven <0.5 μM. Previously reported levels of each drug in plasma (ruxolitinib Cmax for 10 mg BID: 0.562 μM [5]; venetoclax Cmax for 400 mg QD: 2.51 μM [6]) suggest effective combination concentrations would be achievable in patients.
Fig. 1.
Sensitivity of Rux + Ven combination on 195 unique AML patient samples. a Heatmap of sensitivities measured by area under the dose–response curve (AUC, red) and by IC50 (orange) for 195 patient samples with the Rux + Ven combination and respective single agents. Treatment status (initial diagnosis (initial Dx) or relapsed/refractory (R/R)) and mutation status for FLT3-ITD, NPM1, DNMT3A, and TP53 are indicated by colored boxes (gray indicates status not available). b Plots by AUC and IC50 for sensitivities on all patients (n =195). Red horizontal bars indicate the median value. c Plots by AUC and IC50 for sensitivities on Initial Dx patients (n = 137). d Plots by AUC and IC50 for sensitivities on R/R patients (n = 58). e Plots by AUC and IC50 for sensitivities on TP53 mutant (n = 16) and TP53 wild type (n = 141) patient specimens. Statistical significance: comparisons for b–d were done using Friedman test adjusted for multiple comparisons; comparison in e utilized two-tailed Mann–Whitney test. **** denotes p < 0.0001; ***p < 0.001; *p < 0.05; n.s. not significant
We used clinical and genetic data to determine potential associations with combination sensitivity. Importantly, specimens from patients with newly diagnosed disease and those with relapsed/refractory AML both demonstrate superior sensitivity to the Rux + Ven combination compared to either single agent (Fig. 1c, d). Additionally, there is no significant difference in IC50 or AUC between newly diagnosed and relapsed/refractory patient specimens for this combination (p = 0.6323 and p = 0.3049, respectively; Mann–Whitney test). Together, these findings suggest that effectiveness of this combination is not confined to previous treatment status and may offer benefit to a broad spectrum of AML patients.
Within the well-established genetic complexity of AML, recurrent cytogenetic abnormalities largely define both disease subtypes and prognostic risk. While several genes including FLT3, NPM1, and DNMT3A are commonly mutated in AML, mutations in the tumor suppressor gene TP53 are known to independently confer a poor prognostic risk with respect to overall and disease-free survival [7]. We compared TP53 mutational status, which was mutant in 16 of 157 patients (10%) with sequencing available, with respect to sensitivity to the Rux + Ven combination and found no significant differences in either IC50 or AUC (Mann–Whitney test; Fig. 1e). Additionally, FLT3-ITD, NPM1, and DNMT3A mutations occurred in 25%, 28%, and 26% of patients, respectively (Fig. 1a). Patients harboring mutations in DNMT3A were slightly more sensitive to Rux + Ven (AUC p = 0 .0242, IC50 p = 0.0569; Mann–Whitney test). Neither FLT3-ITD nor NPM1 mutation status was associated with differences in Rux + Ven sensitivity. We also compared sensitivity to Rux + Ven based on transformation to AML from a prior myeloproliferative neoplasm, a very poor risk group of patients with AML [1], and found no significant difference by IC50 or AUC.
For eight patients in our cohort, specimens were available from both diagnosis and disease progression following standard-of-care 7 + 3 treatment. Ex vivo sensitivity to Rux + Ven was assessed at both timepoints. In 7 of 8 patients, the IC50 of Rux + Ven remained below 0.5 μM, indicating a trend that sensitivity to Rux + Ven was observed over the course of clinical treatment (Supplemental Fig. S1A). In 3 of these 7 patients, increased ex vivo sensitivity by IC50 to single-agent venetoclax was observed in conjunction with preserved combination sensitivity at the time of relapse. While such analysis was only possible for the subset of patients with serial samples, these results suggest that mechanisms that generate resistance to standard-of-care therapies would not generally hinder the effectiveness of this combination.
To validate these findings and to establish whether the efficacy of the Rux + Ven combination represents a synergistic relationship, the combination was tested for sensitivity on the human AML cell line HL-60 using a dose matrix, which included all possible concentration pairings for each drug’s 7-point dose series, under identical assay conditions. Synergy scores were calculated using the zero interaction potency (ZIP) model for each dose pair of the 7 × 7 matrix, whereby a positive score is indicative of synergy relative to the expected cell inhibition when assuming no interaction [8]. By this method, the Rux + Ven combination shows promising synergy across the surveyed dose matrix (average ZIP score: +6.65; Supplemental Fig. S1B). Independent studies have also observed in vivo synergy with the Rux + Ven combination using mouse xenograft models of AML [9] and T-cell acute lymphoblastic leukemia [10].
The combination of venetoclax plus a hypomethylating agent has been reported as an emerging therapeutic strategy for elderly, treatment-naive AML patients for whom standard-of-care chemotherapy is not an option [11]. Furthermore, the combined inhibition of JAK2 and BCL2/BCL-xL has shown superior efficacy in adult T-cell leukemia [12] and enhanced suppression of resistance compared to single-agent JAK2 inhibition in models of JAK2 mutant-driven myeloproliferative neoplasm [13]. Our ex vivo assay screens the bulk mononuclear cell population within a given specimen, which includes both tumor and stromal components. Stromal-derived growth conditions have been shown to enhance sensitivity to JAK1/2 inhibitors in combination with venetoclax [9], which may also be reflected in our mixed cell assay. Additionally, it has been established that MCL-1 levels, which are JAK-dependent [14], exhibit a rapid turnover rate [15], and are required for AML cell viability [16]. It may be that ruxolitinib is causing AML tumor cells to adapt to different transcriptional programs that reduce MCL-1 levels and sensitize cells to BCL- 2 inhibitors. Further mechanistic profiling will be required to determine whether expression-based signatures predict for drug combination efficacy. These findings suggest the simultaneous inhibition of JAK1/2 and BCL2, as embodied by the Rux + Ven combination, may provide an opportunity to offer a targeted therapy combination with activity that extends broadly to patients with both treatment-naive and relapsed/refractory disease.
Supplementary Material
Acknowledgements
Funding for this project was provided in part by a Leukemia Lymphoma Therapy Acceleration Grant to BJD and JWT, and by support provided by the Knight Cancer Research Institute (Oregon Health & Science University, OHSU). Also, this study was supported by grants from the National Cancer Institute (1U01CA217862, 1U54CA224019, 3P30CA069533-18S5). JWT received grants from the V Foundation for Cancer Research, the Gabrielle’s Angel Foundation for Cancer Research, and the National Cancer Institute (1R01CA183947).
Footnotes
Compliance with ethical standards
Conflict of interest JWT receives research support from Agios, Aptose, Array, AstraZeneca, Constellation, Genentech, Gilead, Incyte, Janssen, Seattle Genetics, Syros, Takeda; JWT is a co-founder of Leap Oncology. BJD serves on the advisory boards for Gilead, Aptose, and Blueprint Medicines. BJD is principal investigator or coinvestigator on Novartis and BMS clinical trials. His institution, OHSU, has contracts with these companies to pay for patient costs, nurse and data manager salaries, and institutional overhead. He does not derive salary, nor does his laboratory receive funds from these contracts. The authors certify that the drugs tested in this study were chosen without input from any of our industry partners. The remaininig authors declare that they have no conflict of interest.
Electronic supplementary material The online version of this article (https://doi.org/10.1038/s41375-018-0225-7) contains supplementary material, which is available to authorized users.
References
- 1. Dohner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. N Engl J Med. 2015;373:1136–52. [DOI] [PubMed] [Google Scholar]
- 2.Medeiros BC, Satram-Hoang S, Hurst D, Hoang KQ, Momin F, Reyes C Big data analysis of treatment patterns and outcomes among elderly acute myeloid leukemia patients in the United States. Ann Hematol. 2015;94:1127–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Friedman AA, Letai A, Fisher DE, Flaherty KT Precision medicine for cancer with next-generation functional diagnostics. Nat Rev Cancer. 2015;15:747–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kurtz SE, Eide CA, Kaempf A, Khganna V, Savage SL, Rofelty A, et al. Molecularly targeted drug combinations demonstrate selective effectiveness for myeloid- and lymphoid-derived hematologic malignancies. Proc Natl Acad Sci USA. 2017;114:e7554–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ogama Y, Mineyama T, Yamamoto A, Woo M, Shimada N, Amagasaki T, et al. A randomized dose-escalation study to assess the safety, tolerability, and pharmacokinetics of ruxolitinib (INC424) in healthy Japanese volunteers. Int J Hematol. 2013;97:351–9. [DOI] [PubMed] [Google Scholar]
- 6.Roberts AW, Davids MS, Pagel JM, Kahl BS, Puvvada SD, Gerecitano JF, et al. Targeting BCL2 with venetoclax in relapsed chronic lymphocytic leukemia. N Engl J Med. 2016;374:311–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.TCGA. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med. 2013;368:2059–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yadav B, Wennerberg K, Aittokallio T, Tang J. Searching for drug synergy in complex dose-response landscapes using an interaction potency model. Comput Struct Biotechnol J. 2015;13:504–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karjalainen R, Pemovska T, Popa M, Liu M, Javarappa KK, Majumder MM, et al. JAK1/2 and BCL2 inhibitors synergize to counteract bone marrow stromal cell-induced protection of AML. Blood. 2017;130:789–802. [DOI] [PubMed] [Google Scholar]
- 10.Senkevitch E, Li W, Hixon JA, Andrews C, Cramer SD, Pauly GT, et al. Inhibiting Janus Kinase 1 and BCL-2 to treat T cell acute lymphoblastic leukemia with IL7-Ralpha mutations. Onco-target. 2018;9:22605–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DiNardo CD, Pratz KW, Letai A, Jonas BA, Wei AH, Thirman M, et al. Safety and preliminary efficacy of venetoclax with decitabine or azacitidine in elderly patients with previously untreated acute myeloid leukaemia: a non-randomised, open-label, phase 1b study. Lancet Oncol. 2018;19:216–28. [DOI] [PubMed] [Google Scholar]
- 12. Zhang M, Mathew Griner LA, Ju W, Dubeau DY, Guha R, Petrus MN, et al. Selective targeting of JAK/STAT signaling is potentiated by Bcl-xL blockade in IL-2-dependent adult T-cell leukemia. Proc Natl Acad Sci USA. 2015;112:12480–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Waibel M, Solomon VS, Knight DA, Rallia RA, Kim SK, Banks KM, et al. Combined targeting of JAK2 and Bcl-2/Bcl-xL to cure mutant JAK2-driven malignancies and overcome acquired resistance to JAK2 inhibitors. Cell Rep. 2013;5:1047–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buettner R, Mora LB, Jove R. Activated STAT signaling in human tumors provides novel molecular targets for therapeutic intervention. Clin Cancer Res. 2002;8:945–54. [PubMed] [Google Scholar]
- 15.Ertel F, Nguyen M, Roulston A, Shore GC. Programming cancer cells for high expression levels of Mcl1. EMBO Rep. 2013;14:328–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glaser SP, Lee EF, Trounson E, Bouillet P, Wei A, Fairlie WD, et al. Anti-apoptotic Mcl-1 is essential for the development and sustained growth of acute myeloid leukemia. Genes Dev. 2012;26:120–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.