Abstract
Clinicians in pulmonary medicine frequently confront the challenge of screening, diagnosis and management of pulmonary hypertension (PH) in sarcoidosis patients who present with unexplained dyspnea. Sarcoidosis associated pulmonary hypertension (SAPH) is most prevalent in patients with pulmonary fibrosis, though it can be independent of airflow obstruction or restriction. SAPH independently associates with significantly increased mortality and decreased functional capacity, outcomes which can be mitigated by early detection and focused treatment. In this review, we discuss the pathophysiology of SAPH, which may resemble pulmonary arterial hypertension as well as secondary causes of PH. We offer a screening algorithm for SAPH, and advocate for detailed assessment of the cause of PH in each patient prior to choice of an individualized treatment plan. We note that treatment of sarcoidosis via immune suppression is typically insufficient to adequately treat SAPH. We discuss secondary causes of SAPH such as left heart disease, sleep disordered breathing, and thromboembolic disease, and the evidence for use of PH-specific therapy in select cases of SAPH. Management of SAPH by clinicians experienced in PH, with early referral to transplantation in refractory cases is advised.
Keywords: Sarcoidosis, Pulmonary hypertension, Treatment, Diagnosis, Pathophysiology
Introduction
Sarcoidosis is an immune mediated disease thought to be precipitated by unknown environmental triggers in patients with a susceptible genetic background1,2. The multi-systemic manifestations of sarcoidosis are unified by the presence of sterile, typically non-caseating granulomas on biopsy of involved tissue3. Granulomatous inflammation and fibrosis in the pulmonary vasculature, airways, interstitium and other anatomic locations results in pulmonary hypertension (PH) for a significant number of sarcoidosis patients.
The epidemiology of sarcoidosis and PH demonstrates that sarcoidosis associated pulmonary hypertension (SAPH) is prevalent, highly morbid, and deadly in patients that are commonly seen by pulmonologists. Between 5.7 and 28.3% of all sarcoidosis patients develop SAPH, with a wide range of prevalence reported across several single center studies4–6. Sarcoidosis patients with pulmonary fibrosis have the highest prevalence of SAPH7, though SAPH can also occur in the absence of significant lung disease. In sarcoidosis patients referred for lung transplant, SAPH was present in 73.8%8. Patients with SAPH have higher oxygen requirements and more functional disabilities in comparison to sarcoidosis patients with end-stage lung disease but without PH8. SAPH ultimately results in excess mortality9. Patients with SAPH incur a striking 7 fold increase in risk for all-cause mortality when compared to sarcoidosis patients without PH, even when adjusted for age and pulmonary function10.
Patients with SAPH suffer high morbidity and mortality for several reasons. First, SAPH is a complex disease mechanism. As discussed below, sarcoidosis falls within the 2013 World Health Organization (WHO) group V category of pulmonary hypertensive disorders due to multiple etiologies11. Thus, each sarcoidosis patient requires an individualized assessment of mechanisms driving their pulmonary hypertension. For example, SAPH can result from pulmonary vascular infiltration or obliteration, altered flow dynamics due to bulky lymphadenopathy or lung fibrosis, as well as cardiac or extra cardiac sarcoidosis. Astute clinicians recognize which factors are most contributing to SAPH for the individual, and direct the treatment plan accordingly. Secondly, treatment of SAPH is informed by a select few high-quality studies, with most evidence for PH-specific therapy extrapolated from studies in group 1 pulmonary arterial hypertension (PAH) patients. Robust phenotypic clustering of SAPH patients followed by randomized controlled trials of PH-specific therapies in SAPH remains a great unmet clinical need.
In this review we summarize an evidence based approach to diagnosis and management of SAPH. We describe the disease pathogenesis of SAPH in detail because understanding these mechanisms practically informs SAPH phenotype categorization and treatment decisions. Where appropriate, we highlight areas of controversy and future research in the field.
Pathogenesis of pulmonary hypertension in sarcoidosis
The WHO defines PH by a mean pulmonary artery pressure (MPAP) of ≥ 25 mm Hg at rest11,12. Exercise-based definitions are excluded due to lack of standardized exercise measurements and consensus regarding cutoff values for exercise-induced PH11. PH is then further divided into 5 distinct WHO groups, classified by etiology (Table 1). SAPH is classified as group V PH because of its multifactorial mechanisms. Thus management of SAPH may encompass elements of each of the other 4 categories of PH, depending on the particular clinical manifestations present in the patient. Treatment will vary based on the manifestations of sarcoidosis particular to each SAPH patient. In the following sections we will review the potential pathophysiologic manifestations of SAPH as seen in other WHO groups
Table 1. World Health Organization Classification of Pulmonary Hypertension11.
Pulmonary hypertension is defined by the World Health Organization as a mean pulmonary artery pressure ≥ 25 as measured by right heart catheterization. The diagnosis of pulmonary arterial hypertension (Group 1) also requires measured pulmonary vascular resistance of >3 Wood units. Items in bold represent etiologies which have been implicated in SAPH.
|
|
|
|
|
|
|
|
|
|
|
|
|
Pulmonary arterial hypertension
PAH comprises a subset of PH patients that have low mean pulmonary artery (PA) wedge pressure ≤ 15 mm Hg and high pulmonary vascular resistance (PVR) >3 Wood units. PAH patients demonstrate panvasculopathy of the pulmonary arterial circulation, with all layers of the vessels demonstrating pathology. The hallmark pathologic features of PAH include adventitial and medial thickening, smooth muscle hyperplasia, pathologic muscularization of non-muscularized arterioles, and intimal fibrosis and proliferation13. PAH is also characterized by marked endothelial dysfunction, including impaired nitric oxide generation and in situ thrombosis. In late stage disease, disorganized whorls of endothelial cells called plexiform lesions develop. Pathologic angiogenesis is also a hallmark of PAH14. Whether endothelial dysfunction is a primary causative feature of PH or a secondary response to elevated PA pressures remains controversial. However, it is believed that aberrant vascular remodeling over time elevates PA pressures.
On pathologic review, SAPH frequently demonstrates granulomas in the walls of the pulmonary vasculature. Granulomatous vessel involvement may affect the entirety of the pulmonary vascular tree, from elastic arteries to the collecting venules15,16. Granulomas are most commonly found in the lymphatic and venous systems, where they can mimic pulmonary veno-occlusive disease16. Granulomatous inflammation has been described in all layers of the vasculature and can circumferentially encase the vessel lumen and cause vessel fibrosis, leading to increased pulmonary vascular resistance. Some pathologic hallmarks of PAH are noted in SAPH, including the findings of plexiform lesions and intimal fibrosis16,17.
Right heart catheterization (RHC) is required to establish the diagnosis of PH. Current guidelines recommend vasoreactivity testing for patients with idiopathic, heritable or drug toxic PH only, in an experienced center setting and in patients who are not in right heart failure. Vasoreactivity is defined by a decrease in the MPAP to ≤40 mm Hg accompanied by an absolute decrease of 10 mm Hg in response to inhaled nitric oxide or IV epoprostenol during RHC18. While vasoreactivity has been described in SAPH there is currently no significant amount of data regarding the benefit of calcium channel blockers in vasoreactive SAPH patients19,20. The vasodilatory response to inhaled nitric oxide in some patients nonetheless suggests the possibility of successfully treating select SAPH patients with pulmonary vasodilators. As in patients with PAH, patients with SAPH should never be treated empirically with calcium channel blockers or pulmonary vasodilators, as these may precipitate worsening heart failure or pulmonary edema when utilized in the incorrect setting.
Pulmonary hypertension secondary to left heart disease
The prevalence of cardiac sarcoidosis may be under-recognized and it is estimated that 20–25% of sarcoidosis patients have silent cardiac involvement, compared to approximately 5% with manifest disease21. The three most common clinical manifestations of cardiac sarcoid are conduction disease, ventricular arrhythmias, and heart failure21. Sarcoidosis may result in heart failure symptoms with either reduced or preserved left ventricular ejection fraction. The co-occurrence of PH in sarcoidosis patients with left heart failure is unclear, with estimates ranging from 23–79%22. This is true for a variety of reasons, including different diagnostic criteria between studies and heterogeneity of populations studied.
The diagnosis of left heart disease associated PH depends on having a PA wedge pressure > 15 mm Hg11. This retrograde transmission of pressure from the left heart is mostly driven by diastolic dysfunction, and thus the maintenance of euvolemia is a key component of management23. Additionally, increased retrograde pressure can trigger release of vasoconstrictive molecules that lead to a “pre-capillary” component of PH in left sided heart failure24. Furthermore, if pulmonary hypertension from poorly-controlled heart failure persists over time there is adaptive vascular remodeling that is likely permanent13. The treatment for WHO Group II PH is to treat heart failure, and thus medications such as diuretics, afterload reduction agents, and beta blockers, are the mainstays of treatment.
It is important to note that primary impact on the right ventricle from cardiac sarcoidosis is also described in patients without left ventricle involvement or pulmonary function impairment to otherwise explain significant right ventricular strain25,26. The management of primary right ventricular dysfunction is beyond the scope of this review.
Pulmonary hypertension due to chronic lung disease or hypoxemia
Patients who develop parenchymal lung disease from sarcoidosis are at greatest risk of developing pulmonary hypertension, and indeed the majority of patients with SAPH have evidence of pulmonary disease7,8. The primary mechanism for this is believed to be destruction of vasculature from the lung disease, resulting in hypoxemia from ventilation/perfusion mismatch. Thus, supplemental oxygen should be used for patients when necessary. Architectural distortion of the pulmonary vasculature can also increase PVR. Dyspnea out of proportion to lung disease and accompanied by exertional hypoxemia should raise suspicion for the development of SAPH, particularly in sarcoidosis patients whose dyspnea appears refractory to immune suppression. A formal PH workup should be pursued when there is suspicion for SAPH in patients with parenchymal lung disease, as using imaging to diagnose PH in fibrotic lung disease is not sufficiently reliable. This has been demonstrated in idiopathic pulmonary fibrosis, where radiographic markers did not predict pulmonary hypertension27. Additionally, there may be concomitant but separate involvement of the pulmonary vasculature and lung parenchyma in SAPH. While parenchymal lung disease is strongly associated with SAPH, a full evaluation of potential causes of PH should be pursued given the multiple etiologies by which sarcoidosis may cause SAPH.
Sarcoidosis patients are also noted to have increased rates of sleep disordered breathing28–30. As a result, their risk of developing PH secondary to nocturnal hypoxemia from obstructive sleep apnea is increased and is further compounded by the potential worsening of obstructive sleep apnea from corticosteroids used to treat the disease30. Patients who have congestive heart failure also have a higher risk of sleep-disordered breathing, thus providing another mechanism by which sarcoidosis may contribute to the development of PH31.
Pulmonary hypertension due to chronic thromboembolic disease (CTEPH)
Sarcoidosis confers an elevated risk of venous thromboembolism, thought to be commensurate with active inflammation promoting a hypercoagulable state32. Swigris et al. found pulmonary embolism in 2.5% of United States decedents with sarcoidosis33. A recent analysis of the Olmstead county cohort in Minnesota elucidated a 3 fold increase in hazard ratio of venous thromboembolism for patients with sarcoidosis34, while in the United Kingdom, sarcoidosis patients under age 65 had a risk ratio of 2.0 for pulmonary embolism (95% CI: 1.1–3.4)35. Therefore, sarcoidosis patients are at increased risk of developing CTEPH. CTEPH presents on a spectrum ranging from central obstruction of the pulmonary vasculature to small emboli distributed among the microvasculature. Central emboli may be amenable to pulmonary endarterectomy, while disease of the microvasculature requires lifelong anticoagulation and is best detected through ventilation-perfusion scan. An additional medical treatment for CTEPH is the soluble guanylyl cyclase activator riociguat, which in studies of non-sarcoidosis PH was associated with improved WHO functional class, increased 6 minute walk distance, and improved hemodynamics36. Riociguat is currently considered appropriate for use in patients with CTEPH who are non-operable candidates or who have failed pulmonary endarterectomy. Concordant with guidelines for PH evaluation, ventilation perfusion scanning to detect CTEPH is warranted in all patients with PH. If indeterminate or multiple perfusion defects are present, CT angiography, followed by referral to a CTEPH center is advised37,38.
Other Mechanisms of SAPH
Sarcoidosis may present with marked thoracic lymphadenopathy and fibrosing mediastinitis. In these cases, architectural distortion on major branches of the pulmonary circulation imposes a physical impedance to pulmonary blood flow, leading to pulmonary stenosis and segmental pulmonary hypertension39,40. The presence of significant thoracic lymphadenopathy or a chest bruit are clues to anatomic pulmonary arterial obstruction. PA stenting has been demonstrated to be effective by several groups, achieving sustained decrease in the PA pressure41,42.
Although liver involvement has been well-described, cirrhosis is a rare complication of sarcoidosis, comprising fewer than 1% of cases43. No cases of portopulmonary hypertension definitively attributed to sarcoidosis have been reported, although a different cause of hypoxemia, the hepatopulmonary syndrome, has been described43,44. While the possibility of portopulmonary hypertension should be considered in sarcoidosis patients with cirrhosis, the overall incidence of this etiology appears to be quite rare.
Lastly, chronic anemia can lead to high output heart failure. Patients with sarcoidosis may have a mild degree of anemia from their disease which can be exacerbated by agents commonly used to treat sarcoidosis such as methotrexate or azathioprine.
Screening for SAPH
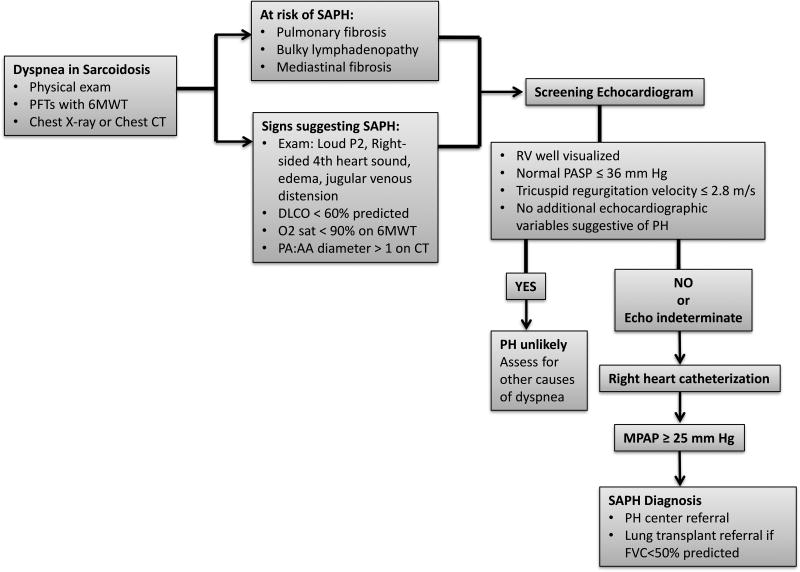
The gold standard for the diagnosis of pulmonary hypertension is RHC. This section focuses on key aspects of the patient’s clinical presentation and screening tests when considering referral for RHC. Figure 1 summarizes a SAPH screening algorithm for sarcoidosis patients presenting with dyspnea.
Figure 1. Screening and diagnostic algorithm for SAPH.
In dyspneic patients with sarcoidosis at risk of PH or who exhibit signs suggestive of PH, screening echocardiography is performed, followed by right heart catheterization if echocardiography is positive or indeterminate. 6MWT 6 minute walk test, CT computed tomography, DLCO diffusing capacity of the lung for carbon monoxide, PA pulmonary artery diameter, AA Aorta diameter, FVC forced vital capacity, PASP pulmonary artery systolic pressure, RV right ventricle, SAPH sarcoidosis associated pulmonary hypertension, PH pulmonary hypertension
Symptomatology and exam
The symptoms of sarcoidosis and pulmonary hypertension may be difficult to distinguish, but the clinician should have a low threshold of suspicion for SAPH and be vigilant for worsening dyspnea or signs of right sided heart failure. Exertional dyspnea is a common complaint in pulmonary sarcoidosis, cardiac sarcoidosis, and in pulmonary hypertension, thus confounding the diagnosis of SAPH1,12. Similarly, chest pain and palpitations are also reported in both diseases independently. Exertional syncope is one criteria for WHO functional class IV PH, but may also occur due to cardiac conduction disease in sarcoidosis.
Signs of pulmonary hypertension on physical exam include a loud P2, evidence of right sided volume overload such as elevated jugular venous pressure or peripheral edema, and a right ventricular heave, but these are often findings that are discovered late in the disease course. A systematic review revealed that the presence of a loud P2 or a right-sided 4th heart sound were the best physical exam correlates of PH, but most reliable in the hands of a specialist45.
Pulmonary Function Testing
Patients with sarcoidosis undergo routine pulmonary function tests (PFTs), which may provide clues to the presence of PH. Diffusing capacity of the lung for carbon monoxide (DLCO), forced vital capacity (FVC), and 6 minute walk test (6MWT) have all been noted to be decreased in patients with SAPH4. Additionally, ambulating hypoxemia is a key feature of PH. In a study of 162 patients with sarcoidosis, Bourbonnais et al demonstrated that oxygen desaturation below 90% on 6MWT correlated with an odds ratio of 12.1 (CI 3.7–19.7) of having SAPH, with a DLCO <60% predicted demonstrating an odds ratio of 7.3 of having PH4. Mirsaedi and others also identified 6MWT desaturation and low DLCO as the strongest correlates with PA systolic pressure as measured by echocardiography46. Among the different parameters for PFT it is logical that DLCO and 6MWT desaturation would be the strongest predictors of SAPH since they can reflect capillary destruction from high pulmonary pressures and circulatory insufficiency, respectively. Additionally, a focus on these parameters may help modulate suspicion when other PFT results are also abnormal.
Imaging
The presence of advanced lung disease on chest x-ray has been associated with SAPH by several groups5,7. However, SAPH can also exist in isolation of significant lung disease47. The presence of significant parenchymal lung disease in patients with sarcoidosis should lead to the obtaining of computed tomography (CT) scan of the chest. Often this imaging has occurred earlier in a patient’s disease course before the diagnosis of sarcoidosis is made.
Numerous studies have investigated the utility of CT scan for the detection of PH, with reported associations between PA diameter and the ratio of PA to aorta27,48,49. In a study specific to sarcoidosis in which over half the patients exhibited Scadding stage IV disease, Huitema and colleagues found CT-measured PA diameter corrected for body surface area was the best predictor of SAPH,50. This recent study counters earlier literature questioning the reliability of CT scanning for detection of PH in patients with pulmonary fibrosis. While RHC remains the gold standard for PH diagnosis and radiographic results can only be suggestive, enlarged PA diameter on CT scan should heighten the clinicians’ pre-test probability of PH being present and prompt further workup.
Echocardiography
Echocardiography remains the most common way to screen for PH of all causes51. It is particularly useful in sarcoidosis because of the simultaneous need for assessment of cardiac sarcoidosis. Bertoli and colleagues were the first to report on the use of echocardiography in SAPH52.
Echocardiography remains a screening test which should not be used for PH diagnosis due to several limitations. One notable pitfall is the use of echocardiography in the presence of fibrotic lung disease, which compromises the accuracy of echocardiography to estimate MPAP. In a cross-sectional study of idiopathic pulmonary fibrosis patients with RHC data and echocardiograms, Nathan and colleagues found that only 40% of patients had echocardiograms that reasonably estimated MPAP53. In addition to this pitfall, not all patients have a regurgitant tricuspid jet which is required for estimation of PA pressure, nor adequate windows for echocardiographic estimation of right heart parameters. Despite these limitations, high estimated pulmonary pressures are likely still helpful. Baughman and colleagues reported an estimated PA systolic pressure > 50 by echocardiogram was associated with worse mortality, while when under 30 mortality was unchanged54. Early detection of PH and cardiac sarcoidosis by noninvasive cardiac imaging is an area of active research. For example, echocardiography derived global longitudinal peak systolic strain (GLS) can be used in the assessment of early RV dysfunction in sarcoidosis patients who do not otherwise demonstrate cardiac involvement or pulmonary hypertension55. GLS on echocardiography is associated with cardiac sarcoidosis on MRI, and high GLS associates with increased risk of adverse cardiovascular events in patients with preserved left ventricular ejection fraction56.
Treatment of SAPH
The pathophysiology of SAPH holds relevance for treatment. With the exception of CTEPH, the only approved medical therapies PH are for group I, or pre-capillary PAH. In PH secondary to left heart disease or chronic lung disease current recommendations are to treat the underlying cause of disease. However, treatment of sarcoidosis alone may be insufficient. For example, steroid use does not affect the burden of disease in the pulmonary vasculature of sarcoidosis patients on autopsy16. Furthermore, in the largest cohort study of SAPH patients, only 4/11 patients treated with immunosuppression alone saw improvements in hemodynamics57. Since granulomatous involvement of the pulmonary vasculature as well as evidence of vasoreactivity suggest that SAPH may in some cases behave similarly to PAH, multiple groups have investigated PAH-directed therapies in the treatment of SAPH.
The selection of patients is paramount when planning the start of PAH therapies for SAPH. Because of the multiple pathophysiologic mechanisms that mediate SAPH, many patients may not respond to PH-specific therapy. Some SAPH patients, notably those with pulmonary veno-occlusive pathophysiology, acutely worsen with pulmonary vasodilator (PV) therapy58. Nevertheless, PVs have demonstrated efficacy in several small studies conducted at expert centers, where patients with a pre-capillary SAPH phenotype most similar to PAH were carefully selected. Additional treatments for secondary PH, including diuretics for volume optimization, surgery or anticoagulation for thromboembolic disease, treatment of sleep disordered breathing, and stenting for mechanical vascular obstruction should be pursued when these concurrent illnesses are present.
The results of the studies investigating treatment of SAPH with PVs demonstrate improvements in hemodynamics and functional status. The PV treatment in SAPH was not associated with harm in patients carefully selected by expert centers, though none were able to clearly demonstrate a mortality benefit. Many studies are limited by small populations of patients and a high dropout rate. Table 2 summarizes major findings.
Table 2. Summary of trials of SAPH treatment.
These nine studies report the use of PH-specific therapy in select SAPH patients with pre-capillary PH.
| Author, year | Study type | Number of patients |
Percentage with lung fibrosis |
Drug | Percentage of patients completing study or average follow up time |
Results |
|---|---|---|---|---|---|---|
| Prostacyclins | ||||||
| Baughman et al, 200968 | Case series, prospective | 22 | 68% | Inhaled iloprost | 68% (16 weeks) | Improvement in RHC, 6MWT distance, quality of life |
| Bonham et al, 201510 | Case series, retrospective | 26 | 81% | Epoprostenol, treprostinil | 12.7 months | Improvement in hemodynamics, reduced BNP |
| Fisher et al, 200667 | Case series, retrospective | 8 | 50% | Epoprostenol | 29 months | Hemodynamic improvement, WHO functional class |
| Endothelin Receptor Antagonists | ||||||
| Baughman et al, 201454 | Multi-center randomized double-blind controlled trial | 35 | 51% | Bosentan | 90% (16 weeks) | Improvement in hemodynamics, no change in quality of life or 6MWT |
| Judson et al, 201162 | Case series, prospective | 21 | 38% | Ambrisentan | 48% (24 weeks) | Improvements in quality of life and WHO functional class, poor tolerance of ambrisentan |
| Phosphodiesterase Inhibitors | ||||||
| Ford et al, 201664 | Case series, prospective | 12 | 33% | Tadalafil | 58% (24 weeks) | No clinical worsening, no improvement in 6MWT, primary endpoint not met, no change in quality of life |
| Milman et al, 200863 | Case series, retrospective | 24 | 75% | Sildenafil | 14 months | Improvement in hemodynamics but not 6MWT distance |
| Studies with multiple pharmacologic categories in combined analysis | ||||||
| Dobarro et al, 201372 | Case series, retrospective | 24 | 63.2% | Sildenafil, bosentan | 22.6 months | No difference in mortality, improved 6MWT, reduced BNP |
| Barnett et al, 200971 | Case series, retrospective | 22 | 68.2% | Sildenafil, bosentan, epoprostenol | 11 months | Improvement in functional class and hemodynamics |
| Boucly et al, 201757 | Cohort, prospective | 126 | 74% | Bosentan/ambri-sentan, Sildenafil/Tadalafil, Epoprostenol/tre-prostinil/iloprost | 28 months | Improvement in hemodynamics, no change in exercise capacity, 4/11 patients treated with only immunosuppression saw improved hemodynamics |
RHC Right Heart Catheterization, BNP brain natriuretic peptide, 6MWT 6 minute walk test.
Endothelin Receptor Antagonists
Bosentan, ambrisentan, and macitentan are endothelin receptor antagonists, which block the activity of endothelin on pulmonary vascular smooth muscle. Endothelin is a potent endogenous vasoactive molecule implicated in the pathophysiology of PAH via its ability to increase pulmonary vascular tone and impact long-term effects of pulmonary vascular remodeling58. Bosentan and ambrisentan are among the first line therapies in the treatment of PAH, while macitentan has not been studied in SAPH.
Bosentan is approved for PAH and also has shown hemodynamic benefit in PH secondary to congenital heart disease and CTEPH59,60. The sole randomized clinical trial for SAPH examined the use of bosentan. This study randomized patients with SAPH diagnosed by RHC to bosentan versus placebo and measured hemodynamic and functional outcomes after 16 weeks of therapy54. It demonstrated an improvement in hemodynamics with the use of bosentan, but no significant improvement in pulmonary function tests or 6MWT. Two patients who were treated with bosentan also required oxygen. Liver toxicity, a common side effect of bosentan, was not observed. The trial had a somewhat high dropout rate, with 8 of the 43 enrolled (19%) patients not being observed for the full 16 weeks. The placebo and treatment groups were similar in WHO functional class, sarcoidosis treatment and degree of lung parenchymal involvement of sarcoidosis.
Ambrisentan has a mechanism of action similar to bosentan but boasts less liver toxicity and has also been studied in SAPH62. Judson et al performed an open-label prospective study to assess the impact of ambrisentan on SAPH patients. At a follow-up period of 24 weeks, there was improvement in WHO functional class and in quality of life. Hemodynamics were not assessed. The study was limited in large part by the high dropout rate: only 10/21 subjects remained in the study through the full follow-up period. Two patients reported liver toxicity.
Phosphodiesterase inhibitors
Phosphodiesterase inhibitors inhibit the degradation of intracellular cyclic guanylyl monophosphate in vascular smooth muscle, which leads to increased nitric oxide generation and subsequent smooth muscle relaxation. Sildenafil and tadalafil are members of this class, along with vardenafil which is less commonly used. The results of phosphodiesterase inhibitor studies are mixed, with one retrospective study demonstrating improvement in hemodynamics but not 6MWT63. A prospective case series highlighting the use of tadalafil in 12 patients noted no change in 6MWT nor quality of life measures, although like many other studies, the dropout rate was significant. Patients on tadalafil did not worsen clinically64.
Riociguat, a soluble guanylyl cyclase activator, also serves to increase intracellular cyclic guanylyl monophosphate and is approved in Group I and Group III PH36. This medication has not been assessed in SAPH.
Prostacyclins
Prostacyclins, including epoprostenol, treprostinil and iloprost, are mainstays of treatment for PAH patients with WHO functional class III or IV symptoms. Epoprostenol was the first medication approved for the treatment of PAH and has demonstrated mortality benefit65. Available formulations for prostacyclin analogs include continuous IV infusion (epoprostenol and treprostinil), subcutaneous (treprostinil), oral (treprostinil), and inhaled (iloprost). More recently, selexipag, a non-prostanoid prostacyclin receptor agonist, demonstrated good tolerance, reduced hospitalization, and slowing of disease progression, but has not been studied in SAPH66.
Fisher first reported on the use of epoprostenol for SAPH in 2006 in a small study of 8 patients, half of whom had Stage IV radiographic disease67. Of the 7 patients who underwent a vasodilator trial, 6 had a >25% reduction in PVR. This was the first report of the long-term use of epoprostenol in SAPH.
Subsequently, inhaled iloprost was studied by Baughman and colleagues in a group of 22 patients and was associated with a decrement in the PVR of >20% and improved quality of life68. This study was limited by a significant dropout rate of 31% due mainly to side effects or difficulty adhering to the regimen. Only 15 subjects completed the 16-week study, of which 6 experienced a significant decrease in PVR, and 5/6 demonstrated a reduction in MPAP. A few patients demonstrated an improved 6MWT, although this did not correlate to hemodynamic parameters. This discrepancy points to the fact that MPAP and PVR are only partially correlated with functional status, with factors such as deconditioning and lung disease also contributing to the dyspnea of SAPH. Despite its limitations, as one of the earliest trials of PAH-directed therapy in SAPH, this study served to illustrate the potential efficacy of prostacyclins.
Bonham and colleagues published a retrospective study regarding the efficacy of prostacyclins in the treatment of SAPH10. In this cohort of 26 patients prostacyclin use was associated with significant decreases in PVR, decreased MPAP, improved cardiac output and functional class. Both intravenous epoprostenol as well as treprostinil were used, often on a background of other PVs. This study also noted the potential for long-term use of prostacyclins in sarcoidosis, as many of the patients remained on prostacyclin therapy for years.
Conclusion
We suggest a stepwise approach to screening, diagnosis and selection of treatment for sarcoidosis patients suspected of having SAPH (Table 3). We emphasize that any patient with sarcoidosis who has dyspnea should receive chest imaging and full PFTs with 6MWT. Key clues that raise suspicion for SAPH include markedly reduced DLCO below 60% of predicted, oxygen desaturation below 90% on 6MWT, as well as dyspnea that seems refractory to immune suppression. Echocardiography is the key noninvasive test to screen for cardiac dysfunction and pulmonary hypertension.
Table 3. Key checkpoints in the evaluation of a dyspneic sarcoidosis patient.
| 1. Establish the suspicion of pulmonary hypertension via physical examination, chest imaging, pulmonary function testing with six-minute walk, and echocardiography |
| 2. Confirm the diagnosis via right heart catheterization |
| 3. Classify the predominant type(s) of pulmonary hypertension present |
| 4. Determine disease severity |
| 5. Select appropriate treatment |
While a positive initial evaluation should prompt referral for RHC, the question remains about what to do regarding negative screening. We recommend that unexplained dyspnea in a sarcoidosis patient, particularly in light of abnormal 6MWT or indeterminate echocardiography should be referred for RHC.
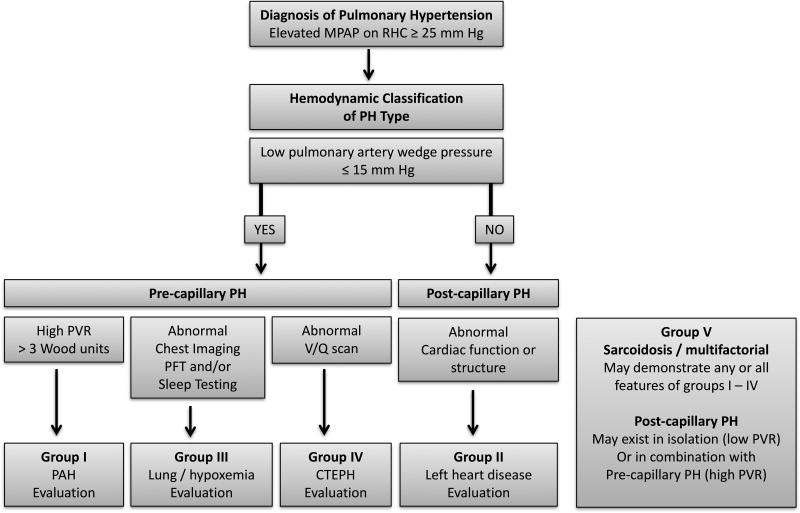
Because of the heterogeneity of SAPH, future studies must clearly phenotype patients’ PH and extent of pulmonary disease if we are to understand which treatments are efficacious for which patients. Figure 2 delineates an algorithm for assessment of the pathophysiology of SAPH based on RHC data. PVs are currently approved for the treatment of PAH, but the majority of patients with SAPH have lung disease, where the role of PVs remains unclear. As discussed above, the literature to date supports a favorable safety profile for the use of PVs in SAPH patients who present with pre-capillary predominant PAH. A clear algorithm for PV initiation is not known. Patient preferences, physician experience and side effect profiles currently drive PV medication selection. In addition, SAPH patients with poor functional class or rapid progression are typically started on combination therapy. This is analogous to the current practice for group 1 PH, which has shown that combination up front therapy gives a longer time to first exacerbation69. Referral to an experienced PH center for RHC and treatment planning is advised.
Figure 2. Diagnostic algorithm for pulmonary hypertension phenotype classification.
Hemodynamic evaluation via RHC aids in grouping patients into pre- and post-capillary etiologies of PH. Individuals with sarcoidosis are classified as group V, and may have predominating features from any group I to IV. CTEPH chronic thromboembolic pulmonary hypertension, MPAP mean pulmonary artery pressure, PAH pulmonary arterial hypertension, PFT pulmonary function test, PH pulmonary hypertension, PVR pulmonary vascular resistance, RHC right heart catheterization.
Finally, when presented with severe or refractory disease, guidelines for lung transplantation in patients with PAH may be applied to SAPH. Early lung transplant referral is made for patients with rapidly progressive PH, functional class III or IV symptoms during escalating therapy, use of parenteral targeted PH therapy, or physiology mimicking pulmonary veno-occlusive disease70. Sarcoidosis patients who have severe lung disease with FVC less than 50% predicted have been shown to respond less favorably to PH specific therapy and should also be referred early for lung transplant consideration71.
Acknowledgments
Disclosure of funding received for this work: Dr. Duong was supported by NIH/NHLBI T32 HL007605 Respiratory Biology Training Grant and Dr. Bonham was supported by NIH/NHLBI Grant K12 HL119995 during preparation of this manuscript.
Abbreviations
- 6MWT
six minute walk test
- CT
computed tomography
- CTEPH
chronic thromboembolic pulmonary hypertension
- FVC
forced vital capacity
- DLCO
diffusing capacity of the lung for carbon monoxide
- MPAP
mean pulmonary artery pressure
- PA
pulmonary artery
- PAH
pulmonary arterial hypertension
- PFT
pulmonary function test
- PH
pulmonary hypertension
- PV
pulmonary vasodilator
- PVR
pulmonary vascular resistance
- RHC
right heart catheterization
- SAPH
sarcoidosis associated pulmonary hypertension
- WHO
World Health Organization
Footnotes
Conflict of interest statement: The authors have no conflicts of interest.
References
- 1.Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med. 1999;160(2):736–755. doi: 10.1164/ajrccm.160.2.ats4-99. [DOI] [PubMed] [Google Scholar]
- 2.Baughman RP, Culver DA, Judson MA. A concise review of pulmonary sarcoidosis. Am J Respir Crit Care Med. 2011;183(5):573–581. doi: 10.1164/rccm.201006-0865CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rosen Y. Pathology of sarcoidosis. Semin Respir Crit Care Med. 2007;28(1):36–52. doi: 10.1055/s-2007-970332. [DOI] [PubMed] [Google Scholar]
- 4.Bourbonnais JM, Samavati L. Clinical predictors of pulmonary hypertension in sarcoidosis. Eur Respir J. 2008;32(2):296–302. doi: 10.1183/09031936.00175907. [DOI] [PubMed] [Google Scholar]
- 5.Handa T, Nagai S, Miki S, et al. Incidence of pulmonary hypertension and its clinical relevance in patients with sarcoidosis. Chest. 2006;129(5):1246–1252. doi: 10.1378/chest.129.5.1246. [DOI] [PubMed] [Google Scholar]
- 6.Maimon N, Salz L, Shershevsky Y, Matveychuk A, Guber A, Shitrit D. Sarcoidosis-associated pulmonary hypertension in patients with near-normal lung function. Int J Tuberc Lung Dis Off J Int Union Tuberc Lung Dis. 2013;17(3):406–411. doi: 10.5588/ijtld.12.0428. [DOI] [PubMed] [Google Scholar]
- 7.Sulica R, Teirstein AS, Kakarla S, Nemani N, Behnegar A, Padilla ML. Distinctive clinical, radiographic, and functional characteristics of patients with sarcoidosis-related pulmonary hypertension. Chest. 2005;128(3):1483–1489. doi: 10.1378/chest.128.3.1483. [DOI] [PubMed] [Google Scholar]
- 8.Shorr AF, Helman DL, Davies DB, Nathan SD. Pulmonary hypertension in advanced sarcoidosis: epidemiology and clinical characteristics. Eur Respir J. 2005;25(5):783–788. doi: 10.1183/09031936.05.00083404. [DOI] [PubMed] [Google Scholar]
- 9.Shorr AF, Davies DB, Nathan SD. Predicting mortality in patients with sarcoidosis awaiting lung transplantation. Chest. 2003;124(3):922–928. [PubMed] [Google Scholar]
- 10.Bonham CA, Oldham JM, Gomberg-Maitland M, Vij R. Prostacyclin and oral vasodilator therapy in sarcoidosis-associated pulmonary hypertension: a retrospective case series. Chest. 2015;148(4):1055–1062. doi: 10.1378/chest.14-2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Simonneau G, Gatzoulis MA, Adatia I, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2013;62(25 Suppl):D34–41. doi: 10.1016/j.jacc.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 12.Hoeper MM, Bogaard HJ, Condliffe R, et al. Definitions and diagnosis of pulmonary hypertension. J Am Coll Cardiol. 2013;62(25 Suppl):D42–50. doi: 10.1016/j.jacc.2013.10.032. [DOI] [PubMed] [Google Scholar]
- 13.Dickinson MG, Bartelds B, Borgdorff MAJ, Berger RMF. The role of disturbed blood flow in the development of pulmonary arterial hypertension: lessons from preclinical animal models. Am J Physiol Lung Cell Mol Physiol. 2013;305(1):L1–14. doi: 10.1152/ajplung.00031.2013. [DOI] [PubMed] [Google Scholar]
- 14.Cool CD, Stewart JS, Werahera P, et al. Three-dimensional reconstruction of pulmonary arteries in plexiform pulmonary hypertension using cell-specific markers. Evidence for a dynamic and heterogeneous process of pulmonary endothelial cell growth. Am J Pathol. 1999;155(2):411–419. doi: 10.1016/S0002-9440(10)65137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoffstein V, Ranganathan N, Mullen JB. Sarcoidosis simulating pulmonary veno-occlusive disease. Am Rev Respir Dis. 1986;134(4):809–811. doi: 10.1164/arrd.1986.134.4.809. [DOI] [PubMed] [Google Scholar]
- 16.Takemura T, Matsui Y, Saiki S, Mikami R. Pulmonary vascular involvement in sarcoidosis: a report of 40 autopsy cases. Hum Pathol. 1992;23(11):1216–1223. doi: 10.1016/0046-8177(92)90288-e. [DOI] [PubMed] [Google Scholar]
- 17.Tayal S, Voelkel NF, Rai PR, Cool CD. Sarcoidois and pulmonary hypertension--a case report. Eur J Med Res. 2006;11(5):194–197. [PubMed] [Google Scholar]
- 18.Galiè N, Corris PA, Frost A, et al. Updated treatment algorithm of pulmonary arterial hypertension. J Am Coll Cardiol. 2013;62(25 Suppl):D60–72. doi: 10.1016/j.jacc.2013.10.031. [DOI] [PubMed] [Google Scholar]
- 19.Milman N, Svendsen CB, Iversen M, Videbaek R, Carlsen J. Sarcoidosis-associated pulmonary hypertension: acute vasoresponsiveness to inhaled nitric oxide and the relation to long-term effect of sildenafil. Clin Respir J. 2009;3(4):207–213. doi: 10.1111/j.1752-699X.2008.00120.x. [DOI] [PubMed] [Google Scholar]
- 20.Preston IR, Klinger JR, Landzberg MJ, Houtchens J, Nelson D, Hill NS. Vasoresponsiveness of sarcoidosis-associated pulmonary hypertension. Chest. 2001;120(3):866–872. doi: 10.1378/chest.120.3.866. [DOI] [PubMed] [Google Scholar]
- 21.Birnie D, Ha ACT, Gula LJ, Chakrabarti S, Beanlands RSB, Nery P. Cardiac Sarcoidosis. Clin Chest Med. 2015;36(4):657–668. doi: 10.1016/j.ccm.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 22.Vachiéry J-L, Adir Y, Barberà JA, et al. Pulmonary hypertension due to left heart diseases. J Am Coll Cardiol. 2013;62(25 Suppl):D100–108. doi: 10.1016/j.jacc.2013.10.033. [DOI] [PubMed] [Google Scholar]
- 23.Harvey RM, Enson Y, Ferrer MI. A Reconsideration of the Origins of Pulmonary Hypertension. Chest. 1971;59(1):82–94. doi: 10.1378/chest.59.1.82. [DOI] [PubMed] [Google Scholar]
- 24.Guazzi M, Borlaug BA. Pulmonary hypertension due to left heart disease. Circulation. 2012;126(8):975–990. doi: 10.1161/CIRCULATIONAHA.111.085761. [DOI] [PubMed] [Google Scholar]
- 25.Otero FJ, Lenihan DJ. Sarcoidosis-induced right ventricular hypertrophy and pulmonary hypertension: echocardiographic imaging. Echocardiogr Mt Kisco N. 2001;18(1):19–20. doi: 10.1046/j.1540-8175.2001.00019.x. [DOI] [PubMed] [Google Scholar]
- 26.Patel MB, Mor-Avi V, Murtagh G, et al. Right Heart Involvement in Patients with Sarcoidosis. Echocardiogr Mt Kisco N. 2016;33(5):734–741. doi: 10.1111/echo.13163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zisman DA, Karlamangla AS, Ross DJ, et al. High-resolution chest CT findings do not predict the presence of pulmonary hypertension in advanced idiopathic pulmonary fibrosis. Chest. 2007;132(3):773–779. doi: 10.1378/chest.07-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Turner GA, Lower EE, Corser BC, Gunther KL, Baughman RP. Sleep apnea in sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis Off J WASOG. 1997;14(1):61–64. [PubMed] [Google Scholar]
- 29.Ahmed M, Sulaiman I, Rutherford R, Gilmartin JJ. First presentation of sarcoidosis with severe obstructive sleep apnoea and epiglottic involvement. Sarcoidosis Vasc Diffuse Lung Dis Off J WASOG. 2013;30(2):146–148. [PubMed] [Google Scholar]
- 30.Lal C, Medarov BI, Judson MA. Interrelationship between sleep-disordered breathing and sarcoidosis. Chest. 2015;148(4):1105–1114. doi: 10.1378/chest.15-0584. [DOI] [PubMed] [Google Scholar]
- 31.Ferrier K, Campbell A, Yee B, et al. Sleep-disordered breathing occurs frequently in stable outpatients with congestive heart failure. Chest. 2005;128(4):2116–2122. doi: 10.1378/chest.128.4.2116. [DOI] [PubMed] [Google Scholar]
- 32.Goljan-Geremek A, Geremek M, Puscinska E, Sliwinski P. Venous thromboembolism and sarcoidosis: co-incidence or coexistence? Cent-Eur J Immunol. 2015;40(4):477–480. doi: 10.5114/ceji.2015.56972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Swigris JJ, Olson AL, Huie TJ, et al. Increased risk of pulmonary embolism among US decedents with sarcoidosis from 1988 to 2007. Chest. 2011;140(5):1261–1266. doi: 10.1378/chest.11-0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ungprasert P, Crowson CS, Matteson EL. Association of Sarcoidosis With Increased Risk of VTE: A Population-Based Study, 1976 to 2013. Chest. 2017;151(2):425–430. doi: 10.1016/j.chest.2016.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Crawshaw AP, Wotton CJ, Yeates DGR, Goldacre MJ, Ho L-P. Evidence for association between sarcoidosis and pulmonary embolism from 35-year record linkage study. Thorax. 2011;66(5):447–448. doi: 10.1136/thx.2010.134429. [DOI] [PubMed] [Google Scholar]
- 36.Ghofrani H-A, D’Armini AM, Grimminger F, et al. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N Engl J Med. 2013;369(4):319–329. doi: 10.1056/NEJMoa1209657. [DOI] [PubMed] [Google Scholar]
- 37.Hoeper MM, Barberà JA, Channick RN, et al. Diagnosis, assessment, and treatment of non-pulmonary arterial hypertension pulmonary hypertension. J Am Coll Cardiol. 2009;54(1 Suppl):S85–96. doi: 10.1016/j.jacc.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 38.Galiè N, Humbert M, Vachiery J-L, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT) Eur Heart J. 2016;37(1):67–119. doi: 10.1093/eurheartj/ehv317. [DOI] [PubMed] [Google Scholar]
- 39.Damuth TE, Bower JS, Cho K, Dantzker DR. Major pulmonary artery stenosis causing pulmonary hypertension in sarcoidosis. Chest. 1980;78(6):888–891. doi: 10.1378/chest.78.6.888. [DOI] [PubMed] [Google Scholar]
- 40.Toonkel RL, Borczuk AC, Pearson GD, Horn EM, Thomashow BM. Sarcoidosis-associated fibrosing mediastinitis with resultant pulmonary hypertension: a case report and review of the literature. Respir Int Rev Thorac Dis. 2010;79(4):341–345. doi: 10.1159/000243786. [DOI] [PubMed] [Google Scholar]
- 41.Hamilton-Craig CR, Slaughter R, McNeil K, Kermeen F, Walters DL. Improvement after angioplasty and stenting of pulmonary arteries due to sarcoid mediastinal fibrosis. Heart Lung Circ. 2009;18(3):222–225. doi: 10.1016/j.hlc.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 42.Condado JF, Babaliaros V, Henry TS, Kaebnick B, Kim D, Staton GW. Pulmonary stenting for the treatment of sarcoid induced pulmonary vascular stenosis. Sarcoidosis Vasc Diffuse Lung Dis Off J WASOG. 2016;33(3):281–287. [PubMed] [Google Scholar]
- 43.Gupta S, Faughnan ME, Prud’homme GJ, Hwang DM, Munoz DG, Kopplin P. Sarcoidosis complicated by cirrhosis and hepatopulmonary syndrome. Can Respir J. 2008;15(3):124–126. doi: 10.1155/2008/412836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ganguli SN, Steinhardt MI, Kundu S. Hepatopulmonary syndrome associated with sarcoidosis of the liver: case report. Can Assoc Radiol J J Assoc Can Radiol. 1998;49(6):411–414. [PubMed] [Google Scholar]
- 45.Colman R, Whittingham H, Tomlinson G, Granton J. Utility of the physical examination in detecting pulmonary hypertension. A mixed methods study. PloS One. 2014;9(10):e108499. doi: 10.1371/journal.pone.0108499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mirsaeidi M, Omar HR, Baughman R, Machado R, Sweiss N. The association between BNP, 6MWD test, DLCO% and pulmonary hypertension in sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis Off J WASOG. 2016;33(4):317–320. [PubMed] [Google Scholar]
- 47.Baughman RP, Engel PJ, Meyer CA, Barrett AB, Lower EE. Pulmonary hypertension in sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis Off J WASOG. 2006;23(2):108–116. [PubMed] [Google Scholar]
- 48.Ng CS, Wells AU, Padley SP. A CT sign of chronic pulmonary arterial hypertension: the ratio of main pulmonary artery to aortic diameter. J Thorac Imaging. 1999;14(4):270–278. doi: 10.1097/00005382-199910000-00007. [DOI] [PubMed] [Google Scholar]
- 49.Devaraj A, Wells AU, Meister MG, Corte TJ, Wort SJ, Hansell DM. Detection of pulmonary hypertension with multidetector CT and echocardiography alone and in combination. Radiology. 2010;254(2):609–616. doi: 10.1148/radiol.09090548. [DOI] [PubMed] [Google Scholar]
- 50.Huitema MP, Spee M, Vorselaars VMM, et al. Pulmonary artery diameter to predict pulmonary hypertension in pulmonary sarcoidosis. Eur Respir J. 2016;47(2):673–676. doi: 10.1183/13993003.01319-2015. [DOI] [PubMed] [Google Scholar]
- 51.Bossone E, D’Andrea A, D’Alto M, et al. Echocardiography in pulmonary arterial hypertension: from diagnosis to prognosis. J Am Soc Echocardiogr Off Publ Am Soc Echocardiogr. 2013;26(1):1–14. doi: 10.1016/j.echo.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 52.Bertoli L, Rizzato G, Merlini R, et al. Can pulmonary hypertension be predicted by non-invasive approach? Echocardiographic and haemodynamic study. Acta Cardiol. 1984;39(2):97–106. [PubMed] [Google Scholar]
- 53.Nathan SD, Shlobin OA, Barnett SD, et al. Right ventricular systolic pressure by echocardiography as a predictor of pulmonary hypertension in idiopathic pulmonary fibrosis. Respir Med. 2008;102(9):1305–1310. doi: 10.1016/j.rmed.2008.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baughman RP, Culver DA, Cordova FC, et al. Bosentan for sarcoidosis-associated pulmonary hypertension: a double-blind placebo controlled randomized trial. Chest. 2014;145(4):810–817. doi: 10.1378/chest.13-1766. [DOI] [PubMed] [Google Scholar]
- 55.Joyce E, Kamperidis V, Ninaber MK, et al. Prevalence and Correlates of Early Right Ventricular Dysfunction in Sarcoidosis and Its Association with Outcome. J Am Soc Echocardiogr Off Publ Am Soc Echocardiogr. 2016;29(9):871–878. doi: 10.1016/j.echo.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 56.Murtagh G, Laffin LJ, Patel KV, et al. Improved detection of myocardial damage in sarcoidosis using longitudinal strain in patients with preserved left ventricular ejection fraction. Echocardiogr Mt Kisco N. 2016;33(9):1344–1352. doi: 10.1111/echo.13281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Boucly A, Cottin V, Nunes H, et al. Management and long-term outcomes of sarcoidosis-associated pulmonary hypertension. Eur Respir J. 2017;50(4) doi: 10.1183/13993003.00465-2017. [DOI] [PubMed] [Google Scholar]
- 58.Palmer SM, Robinson LJ, Wang A, Gossage JR, Bashore T, Tapson VF. Massive pulmonary edema and death after prostacyclin infusion in a patient with pulmonary veno-occlusive disease. Chest. 1998;113(1):237–240. doi: 10.1378/chest.113.1.237. [DOI] [PubMed] [Google Scholar]
- 59.Lee SH, Channick RN. Endothelin antagonism in pulmonary arterial hypertension. Semin Respir Crit Care Med. 2005;26(4):402–408. doi: 10.1055/s-2005-916155. [DOI] [PubMed] [Google Scholar]
- 60.Galiè N, Beghetti M, Gatzoulis MA, et al. Bosentan therapy in patients with Eisenmenger syndrome: a multicenter, double-blind, randomized, placebo-controlled study. Circulation. 2006;114(1):48–54. doi: 10.1161/CIRCULATIONAHA.106.630715. [DOI] [PubMed] [Google Scholar]
- 61.Jaïs X, D’Armini AM, Jansa P, et al. Bosentan for treatment of inoperable chronic thromboembolic pulmonary hypertension: BENEFiT (Bosentan Effects in iNopErable Forms of chronIc Thromboembolic pulmonary hypertension), a randomized, placebo-controlled trial. J Am Coll Cardiol. 2008;52(25):2127–2134. doi: 10.1016/j.jacc.2008.08.059. [DOI] [PubMed] [Google Scholar]
- 62.Judson MA, Highland KB, Kwon S, et al. Ambrisentan for sarcoidosis associated pulmonary hypertension. Sarcoidosis Vasc Diffuse Lung Dis Off J WASOG. 2011;28(2):139–145. [PubMed] [Google Scholar]
- 63.Milman N, Burton CM, Iversen M, Videbaek R, Jensen CV, Carlsen J. Pulmonary hypertension in end-stage pulmonary sarcoidosis: therapeutic effect of sildenafil? J Heart Lung Transplant Off Publ Int Soc Heart Transplant. 2008;27(3):329–334. doi: 10.1016/j.healun.2007.11.576. [DOI] [PubMed] [Google Scholar]
- 64.Ford HJ, Baughman RP, Aris R, Engel P, Donohue JF. Tadalafil therapy for sarcoidosis-associated pulmonary hypertension. Pulm Circ. 2016;6(4):557–562. doi: 10.1086/688775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Barst RJ, Rubin LJ, Long WA, et al. A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. N Engl J Med. 1996;334(5):296–301. doi: 10.1056/NEJM199602013340504. [DOI] [PubMed] [Google Scholar]
- 66.Sitbon O, Channick R, Chin KM, et al. Selexipag for the Treatment of Pulmonary Arterial Hypertension. N Engl J Med. 2015;373(26):2522–2533. doi: 10.1056/NEJMoa1503184. [DOI] [PubMed] [Google Scholar]
- 67.Fisher KA, Serlin DM, Wilson KC, Walter RE, Berman JS, Farber HW. Sarcoidosis-associated pulmonary hypertension: outcome with long-term epoprostenol treatment. Chest. 2006;130(5):1481–1488. doi: 10.1378/chest.130.5.1481. [DOI] [PubMed] [Google Scholar]
- 68.Baughman RP, Judson MA, Lower EE, et al. Inhaled iloprost for sarcoidosis associated pulmonary hypertension. Sarcoidosis Vasc Diffuse Lung Dis Off J WASOG. 2009;26(2):110–120. [PubMed] [Google Scholar]
- 69.Galiè N, Barberà JA, Frost AE, et al. Initial Use of Ambrisentan plus Tadalafil in Pulmonary Arterial Hypertension. N Engl J Med. 2015;373(9):834–844. doi: 10.1056/NEJMoa1413687. [DOI] [PubMed] [Google Scholar]
- 70.Weill D, Benden C, Corris PA, et al. A consensus document for the selection of lung transplant candidates: 2014--an update from the Pulmonary Transplantation Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant Off Publ Int Soc Heart Transplant. 2015;34(1):1–15. doi: 10.1016/j.healun.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 71.Barnett CF, Bonura EJ, Nathan SD, et al. Treatment of sarcoidosis-associated pulmonary hypertension. A two-center experience. Chest. 2009;135(6):1455–1461. doi: 10.1378/chest.08-1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dobarro D, Schreiber BE, Handler C, Beynon H, Denton CP, Coghlan JG. Clinical characteristics, haemodynamics and treatment of pulmonary hypertension in sarcoidosis in a single centre, and meta-analysis of the published data. Am J Cardiol. 2013;111(2):278–285. doi: 10.1016/j.amjcard.2012.09.031. [DOI] [PubMed] [Google Scholar]