Abstract
Objectives:
This study examined the association between reward processing, as measured by performance on the probabilistic reversal learning (PRL) task and avoidance/rumination in depressed older adults treated with Engage, a psychotherapy that uses “reward exposure” to increase behavioral activation.
Methods:
Thirty older adults with major depression received 9 weeks of Engage treatment. At baseline and treatment end, the 24-item Hamilton Depression Rating Scale (HAM-D) was used to assess depression severity and the Behavioral Activation for Depression Scale (BADS) to assess behavioral activation and avoidance/rumination. Participants completed the PRL task at baseline and at treatment end. The PRL requires participants to learn stimulus-reward contingencies through trial and error, and switch strategies when the contingencies unexpectedly change.
Results:
At the end of Engage treatment, the severity of depression was lower (HAM-D: t(19) = −7.67, P < .001) and behavioral activation was higher (BADS: t(19) = 2.23, P = .02) compared to baseline. Response time following all switches (r(19) = −0.63, P = .003) and error switches (r(19) = −0.57, P = .01) at baseline was negatively associated with the BADS avoidance/rumination subscale score at the end of Engage treatment.
Conclusions:
Impaired reward learning, evidenced by slower response following all switches and error switches, contributes to avoidant, ruminative behavior at the end of Engage therapy even when depression improves. Understanding reward processing abnormalities of avoidance and rumination may improve the timing and targeting of interventions for these symptoms, whose persistence compromises quality of life and increases the risk of depression relapse.
Keywords: behavioral activation, late-life depression, reward learning, rumination
1 |. INTRODUCTION
Symptoms of avoidance and rumination are central to the expression of late-life depression. Rumination may be described as a self-directed focus on one’s depressed mood, which can lead to longer periods of depression and difficulty disengaging from negative thoughts.1–3 Rumination is commonly accompanied by behavioral avoidance, in which the ruminative focus on one’s depressed mood leads to refraining from certain behaviors and activities to reduce exposure to aversive stimuli.4 During depressive episodes, the interaction of avoidance and rumination contributes to avoidance of events perceived as negative, withdrawal from social experiences, and an inability to recognize and repair these dysfunctional patterns of behavior.5,6
During depressive episodes, avoidant behavior and rumination are associated with higher severity of depression, decline in quality of life, incomplete remission, and high likelihood of relapse. Previous research demonstrated that depressed patients with ruminations have more severe depression than nonruminative depressed patients.1,7,8 Avoid ance and rumination may compromise quality of life as a result of social withdrawal, inactivity, and avoidance of events and experiences previously perceived as rewarding.9–11 Further, avoidance and rumination often persist into remission of depression3,12 and forecast the onset of new depressive episodes.13 Thus, treatments targeting avoidance and ruminative thoughts have been developed to prevent relapse or recurrence of depressive episodes.14–16
Neuroimaging studies of rumination support the involvement of brain regions involved in negative affect and self-referential processing.11,17 Rumination is associated with greater activation in cognitive control regions, particularly the dorsal anterior cingulate cortex, which is associated with impaired ability to inhibit responses to negative information.18,19 Additionally, ruminative participants present increased medial prefrontal cortex activation in response to negative stimuli, which is indicative of self-referential processing of stimuli,20 as well as greater activation of the amygdala in response to negative stimuli.21 The amygdala has also been implicated in the expression of behavioral avoidance, with increased amygdala activation in studies investigating avoidance in response to aversive, threatening stimuli.22–24 These self-referential processing and cognitive control regions are also preferentially susceptible to the effects of aging, with older adults showing age-related disruptions in white matter structure and abnormal patterns of functional connectivity.25–30 Dysfunction in the neural circuits underlying avoidance and rumination may be critical to the maintenance of negative affect in the depressed state, contributing to expression of depressive syndrome in older adults.
The neurobiological dysfunction related to avoidance and rumination may disrupt reward processing and impair performance on a probabilistic reversal learning (PRL) task. The PRL task is a reward processing task that requires participants to learn stimulus-reward contingencies through trial and error and switch strategies when the contingencies unexpectedly change.31 A challenging component of the PRL task is the “probabilistic error” trials, during which participants receive negative feedback regardless of their response. The error trials may prompt erroneous switching and premature abandonment of previously successful response strategies in reaction to negative feedback. Thus, this task is particularly useful in studying altered reward processing in the depressed state, because depressed patients place emphasis on negative feedback and deemphasize positive feedback during reward processing tasks.32–36 Avoidance and rumination accompanying depressive episodes may adversely influence PRL task performance. Avoidance is associated with poor learning and problem-solving strategies,37 while rumination can lead to impaired learning of probabilities and stimulus-reward contingencies38 and contribute to fixed, perseverative focus on negative feedback.39–41
Here, we examined the relationship of reward processing and reports of avoidance and rumination following treatment with Engage. Engage is a psychotherapy for late-life depression that grew out of the need for streamlined therapies that can be accurately used by community therapists. It was built on a theory that implicates a dysfunction of reward networks as the principal mechanism mediating depressive symptoms.42–45 Accordingly, Engage uses reward exposure as its principal therapeutic intervention. Engage also recognizes that negativity bias, apathy, and inadequate emotional regulation are common in late-life depression and originate from dysfunction of the negative valence network, arousal network, and cognitive control networks respectively. In response, Engage uses a stepped approach and offers interventions targeting these behavioral abnormalities, when in the course of treatment they prevent patients from using reward exposure.
The primary goal of the study was to examine the association between reward processing during the PRL task and reports of avoidance and rumination in late-life depression following treatment with Engage. We expected, however, that a ruminative focus on negative information and diminished motivation would persist despite reward exposure during Engage therapy and even after improvement of depression. Accordingly, we hypothesized that abnormalities in PRL task performance prior to treatment would be associated with remaining avoidance and rumination at the end of Engage therapy.
2 |. METHODS
2.1 |. Participants
Older adults were recruited by the Weill Cornell Institute of Geriatric Psychiatry by advertisement for a psychosocial treatment study for late-life depression. The study was approved by the Weill Cornell Medicine Institutional Review Board. Inclusion criteria were (1) age greater than or equal to 60 years; (2) diagnosis of unipolar nonpsychotic major depression by the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) and Structured Clinical Interview for DSM Disorders46; (3) Mini-Mental State Examination47 score greater than or equal to 24; (4) not currently taking an antidepressant or on a stable dose of an antidepressant for 12 weeks, with no plan to change dose in the next 10 weeks; and (5) capacity to consent. Exclusion criteria were (1) intent or plan to commit suicide; (2) history or presence of psychiatric diagnoses other than major depression or generalized anxiety disorder; (3) use of psychotropic drugs or cholinesterase inhibitors other than mild doses of benzodiazepines; and (4) current engagement in psychotherapy. For participants who met inclusion criteria, no other treatment options were offered other than those of the Engage study.
2.2 |. Systematic assessment
Rating scales were administered by trained interviewers. Diagnosis was assigned in research conferences by agreement of 2 clinician investigators after review of the participants’ psychiatric history and Structured Clinical Interview for DSM Disorders. Overall cognitive impairment was assessed with the Mini-Mental State Examination. Depression severity was quantified with the 24-item Hamilton Depression Rating Scale (HAM-D).48 Behavioral activation was rated with the Behavioral Activation for Depression Scale (BADS).49 The BADS consists of 25 items rated on a scale of 0 to 6 and consists of 4 subscales that target sets of behavior related to Engage. The activation subscale measures the degree of engagement in activities; the avoidance/rumination subscale measures negative thoughts and avoidance of unpleasant situations; the work/school impairment sub-scale measures failure to fulfill work, school, or chore responsibilities, and the social impairment subscale measures failure to engage in social functions. One item (item 22: My work/schoolwork suffered because I was not as active as I needed to be.) was not relevant to most of our depressed older adults and was not administered. Thus, we used 24 of the 25 items of the BADS. The HAM-D and BADS were administered at baseline and following 9 weeks of treatment with Engage.
2.3 |. Engage
Engage is a stepped “reward exposure” therapy consisting of 9 weekly sessions. Therapists included community social workers (MSWs and LCSWs) and research therapists who were trained in Engage. During the training period, therapists were instructed to read the Engage Manual, and the trainer offered 2 45-minute didactics. Then, therapists had one-to-one role-play sessions in which a trainer first demonstrated a role play, and after role reversal, evaluated each trainee’s fidelity to Engage with the Engage Adherence Scale. Therapists were assigned “practice cases” and required to achieve Engage Adherence Scale scores of 4 or higher on 2 consecutive sessions to be certified. Post-training fidelity to treatment manuals was examined by reviewing audiotapes of Engage sessions.43
During each session, therapists work with patients to select 2 or 3 activities meaningful to them and develop a list of goals related to pursing these rewarding activities. Therapists guide patients in selecting the most feasible goals and make plans with concrete steps to overcome barriers to implementation. In patients who do not adequately pursue planned rewarding activities during the initial 3 sessions of Engage, therapists identify and target “barriers” to reward exposure in 3 domains: negativity bias, apathy, and inadequate emotional regulation. Therapists add interventions for negativity bias, apathy, and/or inadequate emotional regulation only when they impede the pursuit of activities planned during reward exposure.
2.4 |. Probabilistic reversal learning task
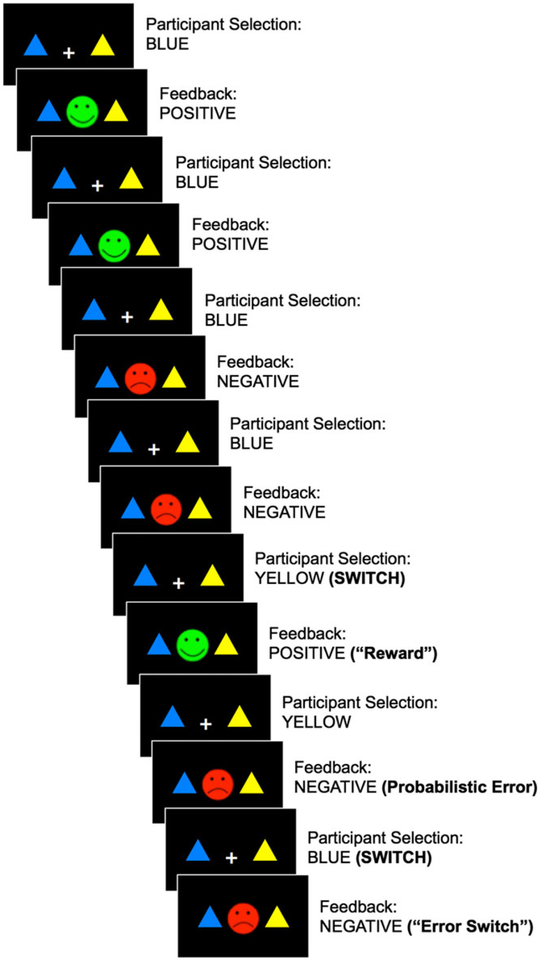
To investigate reward processing abnormalities in late-life depression, participants performed the PRL task at baseline and treatment end (Figure 1).31,50 In the PRL task, participants make an arbitrary choice between a blue and yellow triangle and identify the “rewarding” triangle color through trial and error. The rewarding or correct color selections are followed by positive feedback (a green happy face), while incorrect color selections are followed by negative feedback (a red sad face). Participants are instructed to keep selecting the color most consistently associated with positive feedback, and only switch their color selection when the previously rewarding color begins to be associated with consistent negative feedback. Probabilistic errors comprise 20% of the trials, on which participants receive negative feedback regardless of their color selection. Thus, successful task performance requires a switch from a previously rewarding color in response to consistent negative feedback, while avoiding premature or random switches unrelated to reward history.
FIGURE 1.
A schematic representation of the probabilistic reversal learning (PRL) task. Participants choose the blue or yellow triangle and receive positive (green happy face) or negative (red sad face) feedback. Switches indicate a change in triangle color selection, which may be followed by positive (reward switch) or negative (error switch) feedback. Probabilistic error trials result in negative feedback regardless of response [Colour figure can be viewed at wileyonlinelibrary.com]
The PRL task was programmed in e-Prime51 and administered on a Dell laptop with a 14-in monitor during assessment sessions. Blue and yellow triangle stimuli were presented against a black background in the left or right side of the visual field, along with a central white fixation cross. Left versus right triangle location was randomized across trials. Participants made their color selections by pressing the “x” key for the triangle displayed on the left side and the “m” key for the triangle on the right side. Stimuli were presented for 2000 milliseconds. (If a color selection was not made during this time, a late message was displayed.) Following a color selection, positive or negative feedback was displayed on the center of the screen for 500 milliseconds while the triangle stimuli also remained on the screen. The PRL task consisted of 80 trials and lasted approximately 7 minutes.
2.5 |. Data analysis
We conducted paired sample t-tests to examine change in clinical scores (HAM-D and BADS) and PRL task performance from baseline to the end of Engage treatment. We used Pearson’s correlations with a 2-tailed significance value of 0.05 to examine the association between baseline PRL variables of interest and clinical scores following 9 weeks of Engage treatment.
3 |. RESULTS
The participants were 30 older adults (8 males, 22 females) with major depression (Table 1). At the end of 9 weeks of treatment with Engage, 20 participants completed their clinical ratings and 18 participants completed the PRL task. Reasons for missing data at the end of Engage treatment included early termination of treatment (10 participants) and refusal to complete the PRL task (2 participants).
TABLE 1.
Participant demographic and clinical characteristics
| Baseline (N = 30) | Treatment end (N = 20) | ||
|---|---|---|---|
| Variable | Mean (SD) | Mean (SD) | t(df), P value |
| Age | 70.5 (8.1) | N/A | N/A |
| Education | 15.3 (3.0) | N/A | N/A |
| MMSE | 28.7 (1.0) | N/A | N/A |
| HAM-D | 23.6 (4.4) | 14.9 (7.4) | −5.68 (19), .001* |
| BADS total | 111.7 (21.5) | 124.7 (23.5) | 2.63 (19), .02* |
| BADS subscale | |||
| Activation | 18.0 (9.5) | 18.6 (9.8) | 0.06 (19), .95 |
| Avoidance/rumination | 42.4 (8.9) | 48.6 (8.6) | 3.14 (19), .005* |
| Work/school impairment | 21.6 (5.0) | 23.6 (5.7) | 2.51 (19), .02* |
| Social impairment | 29.7 (5.8) | 33.9 (5.3) | 1.83 (19), .08 |
MMSE, Mini-Mental State Examination; HAM-D, 24-item Hamilton Depression Rating Scale; BADS, Behavioral Activation for Depression Scale.
P < .05.
At the end of treatment with Engage (week 9), overall behavioral activation and depression severity improved (Table 1). At treatment end, both BADS total scores (r(18) = −0.74, P < .001) and avoidance/rumination subscale scores (r(18) = −0.49, P = .03) were correlated with depression severity (HAM-D scores).
At treatment end, 4 participants achieved remission (HAM-D score <10), 11 participants had mild depression (HAM-D score of 10–19), and 5 participants had depression of moderate severity (HAM-D score of 20–29). The BADS total score (F(2, 19) = 5.69, P = .01) and BADS avoidance/rumination subscale score (F(2, 19) = 4.87, P = .02) differed among these participants at treatment end, an expected finding because BADS and HAM-D scores at treatment end were significantly correlated. However, there was no significant difference in BADS scores between participants with mild and moderate depression at treatment end (BADS total: t(14) = 0.92, P = .37; BADS avoidance/rumination: t(14) = 0.67, P = .51).
Performance on the PRL task was divided into all switches (any change in color selection, regardless of feedback), rewarded switches (color changes followed by positive feedback), and error switches (color changes after probabilistic error trials, followed by negative feedback regardless of response). There was a difference in all switches from baseline to treatment end (t(17) = −2.51, P = .02); participants made more switches at the end of Engage treatment. There was a greater number of both rewarded switches and error switches at treatment end compared to baseline, but the difference did not reach significance (rewarded switches: t(17) = −1.62, P = .12; error switches: t(17) = −1.37, P = .19).
Probabilistic reversal learning task accuracy (mean percent correct) was 64.7% at baseline and 68.6% at treatment end; the number of correct trials did not significantly differ from baseline to treatment end (t(17) = −1.36, P = .19). PRL task accuracy was not correlated with total BADS at baseline (r(28) = 0.22, P = .24), with total BADS at treatment end (r(16) = 0.44, P = .08) or with BADS avoidance/rumination sub-scale score at baseline (r(28) = 0.31, P = .10) or at treatment end (r(16) = 0.22, P = .39).
While overall response times on the PRL task decreased from baseline to treatment end (t(17) = 2.24, P = .04), change in overall response time was not significantly correlated with change in HAM-D score (r(16) = −0.09, P = .72) or change in BADS score (r(16) = −0.13, P = .62) from baseline to treatment end.
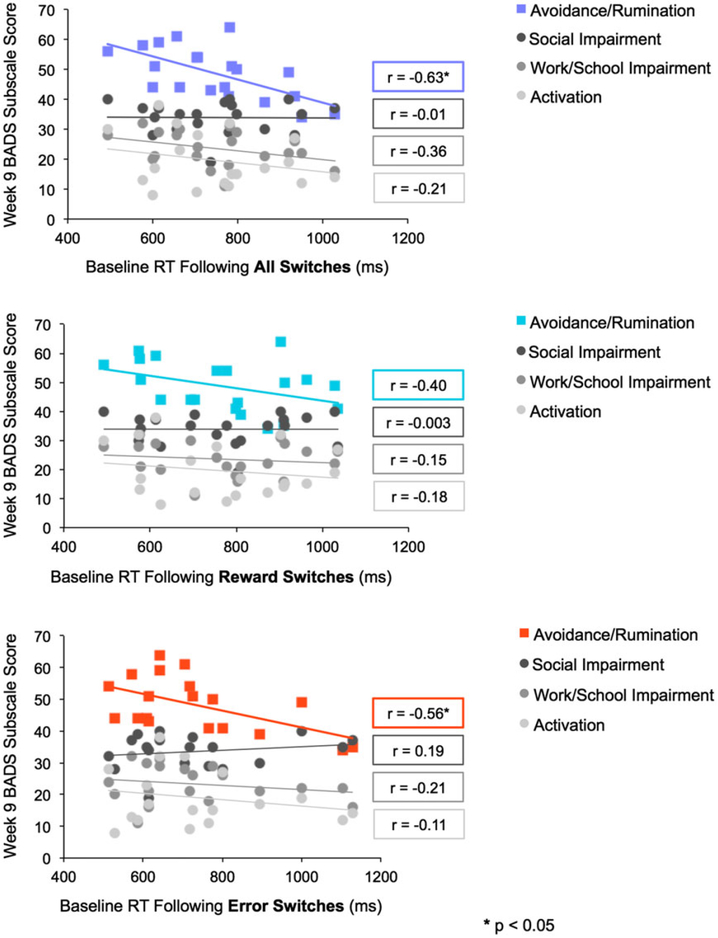
Slow feedback response (response time following switch trials) at baseline was associated with remaining BADS Avoidance/Rumination scores at the end of Engage treatment. Response time following all (rewarded and nonrewarded) switches at baseline was negatively associated with avoidance/rumination subscale score at the end of Engage treatment (r(18) = −0.63, P = .003; 95% CI: −0.84, −0.26). Response time following error switches at baseline was also negatively correlated with avoidance/rumination subscale score at treatment end (r(18) = −0.57, P = .01; 95% CI: −0.81, −0.17). The correlation between response time following rewarded switches at baseline and avoidance/rumination subscale score at the end of Engage treatment, although rather high, did not reach significance (r(18) = −0.40, P = .08, 95% CI: −0.72, −0.05). Thus, slower response times following all switches and error switches at baseline were associated with avoidance and rumination at the end of Engage treatment; the avoidance/rumination sub-scale of the BADS is reverse scored.
There was no significant correlation between response time following all switches, rewarded switches, or error switches at baseline and scores on the BADS subscales of activation, work/school impairment, and social impairment at the end of Engage treatment (Figure 2). Further, response times did not significantly differ across participants who remitted or remained mildly or moderately depressed at treatment end (RT following all switches: F(2, 19) = 1.32, P = .39; RT following rewarded switches: F(2, 19) = 0.62, P = .25; RT following error switches: F(2, 19) = 3.16, P = .07).
FIGURE 2.
Plots indicating the correlation between baseline response time following switches, reward switches, and error switches and score at the end of treatment with Engage on each subscale of the BADS [Colour figure can be viewed at wileyonlinelibrary.com]
4 |. DISCUSSION
The main finding of this study is that slow response times following all switches and error switches on the PRL task at baseline predicted remaining avoidance and rumination responses at the end of Engage therapy in patients with late-life major depression. Response times following switches at baseline had a specific relationship to remaining avoidance and rumination, as this performance measure was not associated with scores on the BADS subscales of activation, work/school impairment, and social impairment at treatment end. Slow response time to all switches and to error switches during late-life depressive episodes may be a reward processing abnormality contributing to remaining avoidant and ruminative, even after increase in behavioral activation and improvement of depression.
Slower response times in participants with avoidance and rumination at treatment end could have been a result of psychomotor slowing that often accompanies late-life depression.52 However, PRL task response time was not associated with improvement in depression or increase in behavioral activation during Engage treatment. This observation suggests that our findings are specific to response times to switches and not to overall time on task and to psychomotor speed.
This is the first study to report an association between PRL task performance and avoidant/rumination responses following a treatment designed to increase behavioral activation in late-life depression. Perseverative errors during reward processing tasks, and especially errors following negative feedback, were shown to be associated with rumination in patients with late-life depression.34,53,54 Such errors arise from a processing bias for negative stimuli and inability to disengage from negative information and shift attention toward positive information.3 Our findings suggest that a broader dysfunction in the reward learning process contributes to avoidant and ruminating responses remaining at the end of Engage treatment because the response time was slow in all switches regardless of the valence of earlier feedback.
The origins of disrupted reward learning in ruminative late-life depression are unclear. Aging-related dysfunction of the reward system may be a contributor. The brain’s reward system, comprised by the amygdala, ventral tegmental area, nucleus accumbens, ventral striatum, medial prefrontal cortex, and orbitofrontal cortex, is particularly susceptible to the effects of aging evidenced by microstructural changes in white matter and in functional connectivity.26,55,56 These neural abnormalities are strongly associated with disrupted reward-related behaviors during aging.57,58 Further, the link between impaired reward processing, negativity bias, and ruminative symptoms of late-life depression has been further supported by neuroimaging research indicating reward system dysfunction, particularly attenuated ventral striatum activation for rewarded switches, which may represent a neural substrate of poor PRL performance in the depressed state and indicate diminished reward responsiveness in depressed individuals.59
This study’s findings cannot be used in clinical care. However, our observations, along with findings of others,38,60 suggest that further understanding of reward processing abnormalities may lead to the development of clinically usable tests of reward processing to identify depressed older patients in need of interventions targeting avoidant and ruminative behavior. Perseveration on a negative representation of the environment and increased focus on negative stimuli34,36,61 may be addressed with an intervention that targets negativity bias early and consistently during treatment. These strategies include training patients to recognize when they are disproportionately focused on negative expectations and redirect their attention to positive or rewarding outcomes.42,45
A strength of this study is the use of PRL, a challenging reward processing task that requires learning of stimulus-reward probabilities for successful task performance. Its error trials result in negative feedback regardless of response and encourage perseveration on negative feedback and erroneous reversal of previously effective response strategies.50 These qualities make the PRL well suited for the investigation of behavioral correlates of avoidance and rumination. Previous work indicated a link between PRL performance and the expression of depressive symptoms.35–36,61 Our study expanded upon these findings and identified specific aspects of PRL task performance (speed of response following all switches and error switches) in contributing to remaining symptoms of avoidance and rumination in late-life depression at the end of Engage therapy.
The findings of this study should be viewed in the context of its limitations. These include the study’s small sample and the absence of a comparison group receiving a different therapy. It is unclear whether the association of slow response following switches during the PRL task and remaining avoidant and rumination responses at treatment end is specifically related to Engage therapy, or is a broader property. However, Engage is a stepped therapy that includes an intervention for negativity bias offered to patients in whom therapists identify negativity bias as a barrier to reward exposure. The relationship of speed of responses to PRL switches to remaining avoidant rumination was identified even though some Engage-treated patients received interventions for negativity bias. Therefore, it is less likely that another therapy would have obscured this relationship would have obscured this relationship (most therapies do not include a specific negativity bias intervention). Another limitation is the study’s exclusive focus on PRL task performance.
In conclusion, our findings suggest that dysfunctional reward learning contributes to avoidant, ruminative behavior remaining at the end of a behavioral activation therapy even when depression improves. Studies of activation and processing by reward-related brain structures during probabilistic reward learning may clarify the neural basis of avoidant ruminative behavior in late-life depression. Treatment studies may examine whether successful treatment targeting avoidant, ruminative behavior can repair disrupted reward processes that interfere with PRL task performance, improve maintenance of stimulus-reward probabilities, and increase responsiveness to positive task feedback.
Key points.
Reward learning is impaired in late-life depression by a ruminative focus on negative feedback.
Slow response to feedback on a reward processing task at baseline was associated with remaining avoidance and rumination following 9 weeks of treatment with Engage psychotherapy.
Further understanding of reward learning deficits in late-life depression may help inform clinical tests to identify depressed older adults who may benefit from early interventions for avoidant and ruminative behavior.
ACKNOWLEDGEMENTS
This paper was supported by National Institute of Mental Health grants P50 MH113838 (Alexopoulos), R01 MH102252 (Alexopoulos), T32 MH019132 (Alexopoulos), and the Sanchez Foundation.
Funding information
National Institute of Mental Health, Grant/Award Numbers: T32 MH019132, R01 MH102252 and P50 MH113838; Sanchez Foundation
Footnotes
CONFLICT OF INTEREST
G.S. Alexopoulos has served on the speakers’ bureaus of Allergan, Otsuka, and Takeda. No other authors report conflicts of interest.
REFERENCES
- 1.Nolen-Hoeksema S, Morrow J. A prospective study of depression and posttraumatic stress symptoms after a natural disaster: The 1989 Loma Prieta Earthquake. J Pers Soc Psychol. 1991;61(1):115–121. [DOI] [PubMed] [Google Scholar]
- 2.Nolen-Hoeksema S, Morrow J, Fredrickson BL. Response styles and the duration of episodes of depressed mood. J Abnorm Psychol. 1993; 102(1):20–28. [DOI] [PubMed] [Google Scholar]
- 3.Koster EH, De Lissnyder E, Derakshan N, De Raedt R. Understanding depressive rumination from a cognitive science perspective: The impaired disengagement hypothesis. Clin Psychol Rev. 2011;31(1): 138–145. [DOI] [PubMed] [Google Scholar]
- 4.Cribb G, Moulds ML, Carter S. Rumination and experiential avoidance in depression. Behaviour Change. 2006;23(3):165–176. [Google Scholar]
- 5.Jacobson NS, Dobson KS, Traux PA, et al. A component analysis of cognitive-behavioral treatment for depression. J Consult Clin Psychol. 1996;64(2):295–304. [DOI] [PubMed] [Google Scholar]
- 6.Jacobson NS, Martell CR, Dimidjian S. Behavioral activation treatment for depression: Returning to contextual roots. Clin Psychol Sci Prac. 2001;8(3):255–270. [Google Scholar]
- 7.Just N, Alloy LB. The response styles theory of depression: Tests and an extension of the theory. J Abnorm Psychol. 1997;106(2):221–229. [DOI] [PubMed] [Google Scholar]
- 8.Nolan SA, Roberts JE, Gotlib IH. Neuroticism and ruminative response style as predictors of change in depressive symptomatology. Cognit Ther Res. 1998;22(5):445–455. [Google Scholar]
- 9.Lewinsohn PM, Graf M. Pleasant activities and depression. J Consult Clin Psychol. 1973;41(2):261–268. [DOI] [PubMed] [Google Scholar]
- 10.Watson PJ, Andrews PW. Toward a revised evolutionary adaptationist analysis of depression: The social navigation hypothesis. J Affect Disord. 2002;72(1):1–4. [DOI] [PubMed] [Google Scholar]
- 11.Nolen-Hoeksema S, Wisco BE, Lyubomirsky S. Rethinking rumination. Perspect Psychol Sci. 2008;3(5):400–424. [DOI] [PubMed] [Google Scholar]
- 12.Dichter GS, Kozink RV, McClernon FJ, Smoski MJ. Remitted major depression is characterized by reward network hyperactivation during reward anticipation and hypoactivation during reward outcomes. J Affect Disord. 2012;136(3):1126–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spasojević J, Alloy LB. Rumination as a common mechanism relating depressive risk factors to depression. Emotion. 2001;1(1):25–37. [DOI] [PubMed] [Google Scholar]
- 14.Teasdale JD, Segal Z, Williams JM. How does cognitive therapy prevent depressive relapse and why should attentional control (mindfulness) training help? Behav Res Ther. 1995;33(1):25–39. [DOI] [PubMed] [Google Scholar]
- 15.Teasdale JD. Metacognition, mindfulness and the modification of mood disorders. Clin Psychol Psychother. 1999;6(2):146–155. [Google Scholar]
- 16.Watkins E, Scott J, Wingrove J, Rimes K, Bathurst N, Steiner H, Kennell–Webb S, Moulds M, Malliaris Y. Rumination-focused cognitive behaviour therapy for residual depression: a case series. Behav Res Ther 2007;45(9):2144–2154. [DOI] [PubMed] [Google Scholar]
- 17.Burkhouse KL, Jacobs RH, Peters AT, Ajilore O, Watkins ER, Langenecker SA. Neural correlates of rumination in adolescents with remitted major depressive disorder and healthy controls. Cogn Affect Behav Neurosci. 2017;17(2):394–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cooney RE, Joormann J, Eugène F, Dennis EL, Gotlib IH. Neural correlates of rumination in depression. Cogn Affect Behav Neurosci. 2010;10(4):470–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacobs RH, Jenkins LM, Gabriel LB, et al. Increased coupling of intrinsic networks in remitted depressed youth predicts rumination and cognitive control. PloS one. 2014;9(8):e104366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ray RD, Ochsner KN, Cooper JC, Robertson ER, Gabrieli JD, Gross JJ. Individual differences in trait rumination and the neural systems supporting cognitive reappraisal. Cogn Affect Behav Neurosci. 2005; 5(2):156–168. [DOI] [PubMed] [Google Scholar]
- 21.Siegle GJ, Steinhauer SR, Thase ME, Stenger VA, Carter CS. Can’t shake that feeling: event-related fMRI assessment of sustained amygdala activity in response to emotional information in depressed individuals. Biol Psychiatry. 2002;51(9):693–707. [DOI] [PubMed] [Google Scholar]
- 22.LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA. Human amygdala activation during conditioned fear acquisition and extinction: A mixed-trial fMRI study. Neuron. 1998;20(5):937–945. [DOI] [PubMed] [Google Scholar]
- 23.Evans KC, Wright CI, Wedig MM, Gold AL, Pollack MH, Rauch SL. A functional MRI study of amygdala responses to angry schematic faces in social anxiety disorder. Depress Anxiety. 2008;25(6):496–505. [DOI] [PubMed] [Google Scholar]
- 24.Schlund MW, Cataldo MF. Amygdala involvement in human avoidance, escape and approach behavior. Neuroimage. 2010;53(2):769–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aizenstein HJ, Butters MA, Wu M, et al. Altered functioning of the executive control circuit in late-life depression: episodic and persistent phenomena. Am J Geriatr Psychiatry. 2009;17(1):30–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gunning-Dixon FM, Brickman AM, Cheng JC, Alexopoulos GS. Aging of cerebral white matter: A review of MRI findings. Int J Geriatr Psychiatry. 2009;24(2):109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Aizenstein HJ, Andreescu C, Edelman KL, et al. fMRI correlates of white matter hyperintensities in late-life depression. Am J Psychiatry. 2011; 168(10):1075–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alexopoulos GS, Hoptman MJ, Kanellopoulos D, Murphy CF, Lim KO, Gunning FM. Functional connectivity in the cognitive control network and the default mode network in late-life depression. J Affect Disord. 2012;139(1):56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alexopoulos GS, Hoptman MJ, Yuen G, et al. Functional connectivity in apathy of late-life depression: A preliminary study. J Affect Disord. 2013;149(1):398–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yuen GS, Gunning-Dixon FM, Hoptman MJ, et al. The salience network in the apathy of late-life depression. Int J Geriatr Psychiatry. 2014; 29(11):1116–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Swainson R, Rogers RD, Sahakian BJ, Summers BA, Polkey CE, Robbins TW. Probabilistic learning and reversal deficits in patients with Parkinson’s disease or frontal or temporal lobe lesions: Possible adverse effects of dopaminergic medication. Neuropsychologia. 2000;38(5):596–612. [DOI] [PubMed] [Google Scholar]
- 32.Nelson RE, Craighead WE. Selective recall of positive and negative feedback, self-control behaviors, and depression. J Abnorm Psychol. 1977;86(4):379–388. [DOI] [PubMed] [Google Scholar]
- 33.Shah PJ, O’Carroll RE, Rogers A, Moffoot AP, Ebmeier KP. Abnormal response to negative feedback in depression. Psychol Med. 1999; 29(1):63–72. [DOI] [PubMed] [Google Scholar]
- 34.Steffens DC, Wagner HR, Levy RM, Horn KA, Krishnan KR. Performance feedback deficit in geriatric depression. Biol Psychiatry. 2001;50(5):358–363. [DOI] [PubMed] [Google Scholar]
- 35.Murphy FC, Michael A, Robbins TW, Sahakian BJ. Neuropsychological impairment in patients with major depressive disorder: The effects of feedback on task performance. Psychol Med. 2003;33(3):455–467. [DOI] [PubMed] [Google Scholar]
- 36.Dombrovski AY, Szanto K, Clark L, Reynolds CF, Siegle GJ. Reward signals, attempted suicide, and impulsivity in late-life depression. JAMA Psychiat. 2013;70(10):1020–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.D’Zurilla TJ, Nezu AM. Problem-solving therapy: A social competence approach to clinical intervention. Springer Publishing Company; 1999. [Google Scholar]
- 38.Whitmer AJ, Frank MJ, Gotlib IH. Sensitivity to reward and punishment in major depressive disorder: Effects of rumination and of single versus multiple experiences. Cognit Emot. 2012;26(8):1475–1485. [DOI] [PubMed] [Google Scholar]
- 39.Segerstrom SC, Tsao JC, Alden LE, Craske MG. Worry and rumination: Repetitive thought as a concomitant and predictor of negative mood. Cognit Ther Res. 2000;24(6):671–688. [Google Scholar]
- 40.Robinson MS, Alloy LB. Negative cognitive styles and stress-reactive rumination interact to predict depression: A prospective study. Cognit Ther Res. 2003;27(3):275–291. [Google Scholar]
- 41.Ciesla JA, Roberts JE. Rumination, negative cognition, and their interactive effects on depressed mood. Emotion. 2007;7(3):555–565. [DOI] [PubMed] [Google Scholar]
- 42.Alexopoulos GS, Arean P. A model for streamlining psychotherapy in the RDoC era: The example of ‘engage’. Mol Psychiatry. 2014;19(1):14–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alexopoulos GS, Raue PJ, Kiosses DN, Seirup JK, Banerjee S, Arean PA. Comparing engage with PST in late-life major depression: A preliminary report. Am J Geriatr Psychiatry. 2015;23(5):506–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alexopoulos GS, Raue PJ, Gunning F, et al. “Engage” therapy: Behavioral activation and improvement of late-life major depression. Am J Geriatr Psychiatry. 2016;24(4):320–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alexopoulos GS, O’Neil R, Banerjee S, et al. “Engage” therapy: Prediction of change of late-life major depression. J Affect Disord. 2017; 221:192–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.First MB, Gibbon M, Spitzer RL, Benjamin LS. User’s guide for the structured clinical interview for DSM-IV axis II personality disorders: SCID-II. American Psychiatric Pub; 1997. [Google Scholar]
- 47.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. [DOI] [PubMed] [Google Scholar]
- 48.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23(1):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kanter JW, Mulick PS, Busch AM, Berlin KS, Martell CR. The behavioral activation for depression scale (BADS): Psychometric properties and factor structure. Journal of Psychopathology and Behavioral Assessment. 2007;29(3):191–202. [Google Scholar]
- 50.Cools R, Clark L, Owen AM, Robbins TW. Defining the neural mechanisms of probabilistic reversal learning using event-related functional magnetic resonance imaging. J Neurosci. 2002;22(11):4563–4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schneider W, Eschman A, Zuccolotto A. E-Prime: User’s guide. Psychology Software Incorporated; 2002. [Google Scholar]
- 52.Butters MA, Whyte EM, Nebes RD, et al. The nature and determinants of neuropsychological functioning in late-life depression. Arch Gen Psychiatry. 2004;61(6):587–595. [DOI] [PubMed] [Google Scholar]
- 53.Beats BC, Sahakian BJ, Levy R. Cognitive performance in tests sensitive to frontal lobe dysfunction in the elderly depressed. Psychol Med. 1996;26(3):591–603. [DOI] [PubMed] [Google Scholar]
- 54.Davis RN, Nolen-Hoeksema S. Cognitive inflexibility among ruminators and nonruminators. Cognit Ther Res. 2000;24(6):699–711. [Google Scholar]
- 55.Lamar M, Charlton RA, Ajilore O, et al. Prefrontal vulnerabilities and whole brain connectivity in aging and depression. Neuropsychologia. 2013;51(8):1463–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Viswanathan V, Lee S, Gilman JM, et al. Age-related striatal BOLD changes without changes in behavioral loss aversion. Front Hum Neurosci. 2015;9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.von Hippel W, Henry JD, Matovic D. Aging and social satisfaction: Offsetting positive and negative effects. Psychol Aging. 2008; 23(2):435–439. [DOI] [PubMed] [Google Scholar]
- 58.Eppinger B, Hämmerer D, Li SC. Neuromodulation of reward-based learning and decision making in human aging. Ann N Y Acad Sci. 2011;1235(1):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Robinson OJ, Cools R, Carlisi CO, Sahakian BJ, Drevets WC. Ventral striatum response during reward and punishment reversal learning in unmedicated major depressive disorder. Am J Psychiatry. 2012; 169(2):152–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chase HW, Frank MJ, Michael A, Bullmore ET, Sahakian BJ, Robbins TW. Approach and avoidance learning in patients with major depression and healthy controls: Relation to anhedonia. Psychol Med. 2010;40(3):433–440. [DOI] [PubMed] [Google Scholar]
- 61.Pizzagalli DA, Iosifescu D, Hallett LA, Ratner KG, Fava M. Reduced hedonic capacity in major depressive disorder: Evidence from a probabilistic reward task. J Psychiatr Res. 2008;43(1):76–87. [DOI] [PMC free article] [PubMed] [Google Scholar]