Abstract
Activation of the secretin (Sct)/secretin receptor (SR) axis stimulates ductular reaction and liver fibrosis, which are hallmarks of cholangiopathies. Our aim was to define the role of Sct-regulated cellular senescence, and we demonstrated that both ductular reaction and liver fibrosis are significantly reduced in Sct−/−, SR−/−, and Sct−/−/SR−/− bile duct ligated (BDL) mice compared with BDL wild-type mice. The reduction in hepatic fibrosis in Sct−/−, SR−/−, and Sct−/−/SR−/− BDL mice was accompanied by reduced transforming growth factor-β1 levels in serum and cholangiocyte supernatant, as well as decreased expression of markers of cellular senescence in cholangiocytes in contrast to enhanced cellular senescence in hepatic stellate cells compared with BDL wild-type mice. Secretin directly stimulated the senescence of cholangiocytes and regulated, by a paracrine mechanism, the senescence of hepatic stellate cells and liver fibrosis via modulation of transforming growth factor-β1 biliary secretion. Targeting senescent cholangiocytes may represent a novel therapeutic approach for ameliorating hepatic fibrosis during cholestatic liver injury.
In addition to modifying canalicular bile by modification of water content and electrolyte composition,1 cholangiocytes are also the target cells of cholangiopathies [eg, primary sclerosing cholangitis (PSC) and primary biliary cholangitis], which are characterized by biliary damage, cellular senescence, and liver fibrosis.2, 3, 4 The secretin/secretin receptor (Sct/SR) axis, expressed only by cholangiocytes in rodent and human liver,5, 6, 7, 8, 9 plays a key role in biliary bicarbonate secretion1, 10 as well in the maintenance of biliary homeostasis by regulating the balance between enhanced biliary growth/loss.11, 12, 13, 14 For example, enhanced biliary bicarbonate secretion during ductular reaction is mediated by interaction of Sct with its basolateral receptors, leading to enhanced phosphorylation of protein kinase A and activation of cystic fibrosis transmembrane conductance regulator (CFTR) that induces the activation of the apical chloride bicarbonate anion exchanger 2 (AE2).1, 6, 15, 16, 17, 18 The Sct/SR axis plays a key role in the regulation of bile duct mass and liver fibrosis in animal models of cholestatic liver injury as well as human liver samples from PSC patients.13 Biliary proliferation (ductular reaction) and hepatic fibrosis are reduced when the Sct/SR axis is absent, a finding that is consistent with our previous studies demonstrating that secretin stimulates ductular reaction and liver fibrosis.11, 13, 19 Remarkably, the Sct/SR axis regulates hepatic fibrosis during cholestasis via paracrine activation of hepatic stellate cells (HSCs) through enhanced secretion of transforming growth factor (TGF)-β1 by cholangiocytes.13 In addition, the administration of an SR antagonist to multidrug-resistant 2 (Mdr2)−/− mice induces a decrease in ductular reaction and activation of HSCs.11, 13 In our previous study, significantly higher expression of the Sct/SR axis and TGF-β1 is also observed in liver samples from PSC patients compared with healthy controls.19 These previous studies support the concept that the Sct/SR axis plays a key role in the regulation of ductular reaction and the progression of liver fibrosis during chronic cholestasis.
Cellular senescence—a hallmark of cholangiopathies—has been shown to play a key role in the pathogenesis of PSC.20, 21, 22 We have demonstrated that cellular senescence is increased in subpopulations of large cholangiocytes (G.A., unpublished data) in the bile duct ligated (BDL) model of biliary injury; and enhanced biliary senescence increases the expression of markers of fibrosis but decreases cellular senescence of HSCs.2 The objective of the current study was to investigate the mechanisms by which the Sct/SR axis regulates ductular reaction and liver fibrosis via coordinated modulation of cholangiocyte senescence and both activation and senescence of HSCs through changes in the secretion of TGF-β1 by cholangiocytes.
Materials and Methods
Materials
All reagents were obtained from Sigma-Aldrich (St. Louis, MO) unless otherwise stated. The RNeasy Mini Kit for RNA isolation and mRNA PCR primers were purchased from Qiagen (Valencia, CA). microRNA-125b and snRNA-U6 primers were obtained from Thermo Fisher Scientific (Mountain View, CA). The antibodies for cytokeratin-19 (CK-19), collagen I (Col1a1), vascular endothelial growth factor-A (VEGF-A), desmin (Y66; Alexa Fluor 488), and cyclin-dependent kinase inhibitor 2A (p16INK4a; p16) were obtained from Abcam (Cambridge, MA). The monoclonal antibody for EGF-like, module-containing, mucin-like hormone receptor-like 1 (F4/80) was produced by Cell Signaling Technology (Danvers, MA). The polyclonal antibody against CFTR was purchased from Cell Signaling Technology (Danvers, MA). The polyclonal antibody against AE2 was purchased from LifeSpan Biosciences, Inc. (Seattle, WA). Enzyme-linked immunosorbant assay (ELISA) kits to measure TGF-β1 levels in serum and biliary supernatants were obtained from Affymetrix Inc. (Santa Clara, CA). Mouse primers were generated for GenBank nucleotide sequences (https://www.ncbi.nlm.nih.gov/nuccore) for the listed accession numbers: proliferating cell nuclear antigen (PCNA; accession number NM_011045); TGF-β1 (accession number NM_011577); TGF-β1 receptor (TGF-β1R; accession number NM_009370); α-smooth muscle actin (α-SMA; accession number NM_007392); fibronectin-1 (Fn1; accession number NM_010233); Col1a1 (accession number NM_007742); p16 (accession number NM_001040654); p21 (accession number NM_001111099); glyceraldehyde-3-phosphate dehydrogenase (GAPDH; accession number NM_008084); miR-125b (accession number NR_029822.1); and U6 (used to normalize the expression of miR-125b; accession number NR_004394). The list of human primers is as follows: TGF-β1 (accession number NM_000660); TGF-β1R (accession number NM_001130916); α-SMA (accession number NM_001141945); Fn1 (accession number NM_002026); Col1a1 (accession number NM_000088); p16 (accession number NM_000077); p21 (accession number NM_000389); and GAPDH (accession number NM_001256799). Detailed primer information is included in Table 1.
Table 1.
List of Used Commercially Available Real-Time PCR Primers
| Primer | Species | Catalog number | RefSeq∗ | Source |
|---|---|---|---|---|
| PCNA | Mouse | PPM03456F | NM_011045 | QIAGEN |
| TGF-β1 | Mouse | PPM02991B | NM_011577 | QIAGEN |
| TGF-β1R | Mouse | PPM03072C | NM_009370 | QIAGEN |
| α-SMA | Mouse | PPM04483A | NM_007392 | QIAGEN |
| Fn1 | Mouse | PPM03786A | NM_010233 | QIAGEN |
| Col1α1 | Mouse | PPM03845F | NM_007742 | QIAGEN |
| p16 | Mouse | PPM02906F | NM_001040654 | QIAGEN |
| p21 | Mouse | PPM02901B | NM_001111099 | QIAGEN |
| GAPDH | Mouse | PPM02946E | NM_008084 | QIAGEN |
| miR-125b | Mouse | 000449 | MI0000725 | ThermoFisher |
| U6 | Mouse | 001973 | NR_004394 | ThermoFisher |
| TGF-β1 | Human | PPH00508A | NM_000660 | QIAGEN |
| TGF-β1R | Human | PPH00237C | NM_001130916 | QIAGEN |
| α-SMA | Human | PPH01300B | NM_001141945 | QIAGEN |
| Fn1 | Human | PP H00143B | NM_002026 | QIAGEN |
| Col1α1 | Human | PPH01299F | NM_000088 | QIAGEN |
| p16 | Human | PPH00207C | NM_000077 | QIAGEN |
| p21 | Human | PPH00211E | NM_000389 | QIAGEN |
| GAPDH | Human | PPH00150F | NM_001256799 | QIAGEN |
Animal Models
The animal experiments were performed according to protocols approved by the Baylor Scott & White Institutional Animal Care and Use Committee. Male mice were used in our studies. C57BL/6 wild-type (WT) mice (25 to 30 g) were obtained from Charles River Laboratories (Wilmington, MA). The Sct−/− and SR−/− mouse colonies are established in our facility.11, 12 The established mouse strains, Sct−/− and SR−/−, were crossed until the homozygous double-knockout (Sct−/−/SR−/−) mice were obtained after several generations. The genotype of each Sct−/−/SR−/− mouse was confirmed by PCR amplification of genomic DNA obtained from the tail. Genotyping was performed by Charles River Laboratories. The Sct−/− genotype was identified using three primers: Sct-WT-forward, 5′-GAGTGCCACCTTGCCCTG-3′; Sct-knockout-forward, 5′-GATTTGAGTTTCGGTGCTGG-3′; and Sct-COM-reverse, 5′-GGTTTGGGGAGCCAGTATCT-3′. Migration positions for WT were at 508 bp; and for Sct−/−, at 743 bp. The sequence of primers for identification of SR−/− is as follows: SR-WT-forward, 5′-CAAGCCTGCATTCATCAAGA-3′; SR-knockout-forward, 5′-GCCAGAGGCCACTTGTGTAG-3′; and SR-COM-reverse, 5′-TCATACTCAGGCCCAGTTCC-3′. Migration positions for WT were at 536 bp; and for SR−/−, at 240 bp.
Mice were maintained in a temperature-controlled environment (20°C to 22°C) with 12:12-hour light/dark cycles and with free access to standard chow as well as drinking water. The experiments were performed in normal (sham) and BDL (1 week) WT and Sct−/−, SR−/−, and Sct−/−/SR−/− mice (Table 2). Before each procedure, animals were treated with euthasol (200 to 250 mg/kg body weight). We also measured liver and body weight and liver/body weight ratio, an index of liver cell growth.1
Table 2.
Evaluation of Liver and Body Weight, Liver/Body Weight Ratio, Serum and Bile Sct Levels, and Levels of TGF-β1 in Serum and Cholangiocyte Supernatant
| Treatment | Normal WT | Normal Sct−/− | Normal SR−/− | Normal Sct−/−/SR−/− | BDL WT | BDL Sct−/− | BDL SR−/− | BDL Sct−/−/SR−/− |
|---|---|---|---|---|---|---|---|---|
| Liver weight, g (n) | 1.4 ± 0.04 (52) | 1.6 ± 0.05 (52) | 1.8 ± 0.06 (51) | 1.8 ± 0.07 (52) | 1.7 ± 0.07 (50) | 1.7 ± 0.06 (50) | 1.8 ± 0.09 (29) | 1.8 ± 0.06 (37) |
| Body weight, g (n) | 24.5 ± 0.4 (52) | 27.4 ± 0.7 (52) | 32.0 ± 1.0 (51) | 32.3 ± 0.9 (52) | 21.1 ± 0.3 (50) | 23.0 ± 0.5 (50) | 25.3 ± 0.9 (29) | 25.7 ± 0.6 (37) |
| Liver/body weight, % (n) | 5.9 ± 0.1 (52) | 5.9 ± 0.1 (52) | 5.7 ± 0.1 (51) | 5.6 ± 0.1 (52) | 8.0 ± 0.3 (50)∗ | 7.3 ± 0.2 (50)† | 7.0 ± 0.3 (29)† | 7.0 ± 0.2 (37)† |
| Sct, ng/mL (n) | ||||||||
| Serum | 0.13 ± 0.02 (3) | 0.14 ± 0.03 (3) | 0.13 ± 0.01 (3) | 0.24 ± 0.02 (3) | 67.54 ± 3.79 (3)∗ | 0.73 ± 1.56 (3)† | 0.42 ± 0.04 (3)† | 0.40 ± 0.02 (3)† |
| Bile | 0.79 ± 0.08 (3) | 1.18 ± 0.18 (3) | <0.01 (3) | 0.51 ± 0.02 (3) | 21.16 ± 2.97 (3)∗ | 3.90 ± 1.14 (3)† | 5.01 ± 1.74 (3)† | 4.95 ± 0.59 (3)† |
| TGF-β1, pg/mL (n) | ||||||||
| Serum | 148.3 ± 4.6 (3) | 181.5 ±0 .34.6 (3) | 128.4 ± 3.9 (3) | 132.6 ± 3.9 (3) | 3252 ± 564 (3)∗ | 231.2 ± 11.0 (3)† | 86.8 ± 1.8 (3)† | 80.7 ± 1.0 (3)† |
| Cholangiocyte supernatant | 37.8 ± 0.6 (3) | 37.7 ± 0.5 (3) | 37.2 ± 0.2 (3) | 37.7 ± 0.5 (3) | 145.4 ± 9.7 (6)∗ | 37.8 ± 0.2 (3)† | 37.9 ± 0.5 (3)† | 37.5 ± 1.2 (3)† |
BDL, bile duct ligated; TGF-β1, transforming growth factor-β1; WT, wild type.
P < 0.05 versus normal WT mice.
P < 0.05 versus BDL WT mice.
Purified Cholangiocytes and LCM-Isolated HSCs
Cholangiocytes were obtained by immunoaffinity separation11, 12, 23 using a monoclonal antibody (a gift from Dr. Robert Faris, Brown University, Providence, RI). HSCs were isolated by laser capture microdissection (LCM).13 Frozen liver sections (n = 3, 10 μm thick) were incubated overnight with an antibody reacting with desmin (a marker of stellate cells).24 Then, desmin-positive cells were dissected from the slides by the LCM system Leica LMD7000 (Leica Microsystems Inc., Buffalo Grove, IL) and collected for RNA extraction with the Arcturus PicoPure RNA isolation kit (Thermo Fisher Scientific CO, Mountain View, CA). The in vitro studies were performed in immortalized murine cholangiocyte lines (IMCLs) derived from large cholangiocytes lining large ducts11, 25; and in human hepatic stellate cell lines (HHSteCs; ScienCell Research Laboratories, Carlsbad, CA).13
Immunoreactivity for Sct and SR in Liver Sections and Measurement of Sct Levels in Serum and Bile and Bicarbonate Levels in Bile
The immunoreactivity of Sct and SR was measured by immunohistochemistry in paraffin-embedded liver sections (4 to 5 μm thick) incubated overnight at 4°C with the selected primary antibody after deparaffinization, hydration, antigen unmasking, quenching of endogenous peroxidase activity, and avidin/biotin as well as normal serum blocking; appropriate negative controls were included. After washes in 1× phosphate-buffered saline, sections were incubated for 20 minutes with a secondary biotinylated antibody (Dako Cytomation LSAB Plus System-HRP; Dako, Glostrup, Denmark), subsequently with Dako ABC for 20 minutes, and developed with 3-3′-diaminobenzidine (Dako Cytomation Liquid DAB Plus Substrate Chromogen System). Sections were examined with Leica Microsystems DM 4500 B Microscopy (Wetzlar, Germany), equipped with a JenoptikProg Res C10 Plus Videocam (Jenoptik, Jena, Germany). Observations were processed with an Image Analysis System (Delta Sistemi, Rome, Italy), in a blinded manner, by a board-certified pathologist (E.G.). Secretin levels in serum and bile were measured by ELISA kits (Phoenix Pharmaceuticals, Inc., Burlingame, CA).12 Bile was collected from the selected groups of mice, as previously described.26 Bile bicarbonate levels were measured by the National Mouse Metabolic Phenotyping Centers (Yale University School of Medicine, New Haven, CT).
Evaluation of Intrahepatic Bile Duct Mass, Immunoreactivity for CFTR and AE2, Liver Fibrosis, and Cellular Senescence
Measurement of TGF-β1 Levels in Serum and Biliary Supernatant
The histology of liver, stomach, small and large intestine, pancreas, lung, spleen, and kidney was evaluated in paraffin-embedded sections (4 to 5 μm) by hematoxylin and eosin staining. Slides were evaluated, in a blinded manner, by a board-certified pathologist (E.G.). Intrahepatic bile duct mass was measured in paraffin-embedded liver sections (4 to 5 μm thick, 10 fields evaluated from three samples from three animals) as the area occupied by CK-19–positive bile ducts/total area × 100.
Sections were examined with Leica Microsystems DM 4500 B Microscopy. The immunoreactivity of CFTR and AE2 (functional markers of biliary growth),1, 15, 18, 27 whose expression is enhanced after cholangiocyte hyperplasia but decreased during biliary damage/loss, was evaluated in paraffin-embedded liver sections (4 to 5 μm thick, 10 fields were evaluated from three samples from three animals). A negative score was assigned when 0% to 5% of bile ducts were positive for Sct, SR, CFTR, or AE2; a +/− score was assigned when 6% to 10% of bile ducts were positive; a + score was assigned when 11% to 30% of bile ducts were positive; a ++ score was assigned when 31% to 60% of intrahepatic bile ducts were positive; and a +++ score was assigned when 61% or >61% of bile ducts were positive (Table 3). The evaluations were performed independently by a board-certified pathologist in a blinded manner (E.G.).
Table 3.
Semiquantitative Immunoreactivity for Sct, SR, CFTR, or AE2 Immunoreactivity in Bile Ducts Was Evaluated in Paraffin-Embedded Liver Sections
| Staining | Normal WT | Normal Sct−/− | Normal SR−/− | Normal Sct−/−/SR−/− | BDL WT | BDL Sct−/− | BDL SR−/− | BDL Sct−/−/SR−/− |
|---|---|---|---|---|---|---|---|---|
| Sct | +/− | − | +/− | − | ++ | − | +/− | − |
| SR | +/− | +/− | − | − | ++ | +/− | − | − |
| CFTR | + | ND | ND | ND | +++ | +/− | +/− | − |
| AE2 | +/− | ND | ND | ND | +++ | +/− | +/− | − |
A negative score was assigned when 0% to 5% of bile ducts were positive for Sct, SR, CFTR, or AE2; a +/− score was assigned when 6% to 10% of bile ducts were positive; a + score was assigned when 11% to 30% of bile ducts were positive; a ++ score was assigned when 31% to 60% of intrahepatic bile ducts were positive; and a +++ score was assigned when ≥61% of bile ducts were positive.
AE2, anion exchanger 2; BDL, bile duct ligated; CFTR, cystic fibrosis transmembrane conductance regulator; ND, not determined; WT, wild type.
Hepatic fibrosis was evaluated by Sirius Red staining in paraffin-embedded liver sections (4 to 5 μm thick, 10 fields analyzed from three samples from three animals) and immunofluorescence for Col1a1 (costained with CK-19) in frozen liver sections (8 μm thick, 10 fields analyzed from three samples from three animals). Liver sections stained with Sirius Red were evaluated with Leica Microsystems DM 4500 B Microscopy equipped with a JenoptikProg Res C10 Plus Videocam. Immunofluorescence staining was visualized using Leica AF 6000 Modular Systems (Leica Biosystems Newcastle Ltd, Newcastle upon Tyne, UK). Liver fibrosis was measured in total liver samples by the Hydroxyproline Assay Kit (MAK008; Sigma-Aldrich). The mRNA expression of TGF-β1, TGF-β1R, Col1a1, α-SMA (ACTA2), and Fn1 was measured by real-time PCR in total liver as well as isolated cholangiocytes and HSCs. The protein expression of collagen I and TGF-β1R was measured in isolated cholangiocytes by immunoblots, which was quantified by the LI-COR Odyssey Infrared Imaging System (LI-COR Bioscience, Lincoln, NE). The levels of TGF-β1 were measured by ELISA kits in serum and short-term (6-hour) cultures of isolated cholangiocytes.
Cellular senescence was assessed in frozen liver sections (10 μm thick) by staining for SA-β-galactosidase by commercially available kits (MilliporeSigma, Billerica, MA). Observations were performed in a blinded manner (T.Z.). Double immunofluorescence for p16 costained with CK-19 was performed in frozen serial liver sections (10 μm thick); 10 fields were analyzed from three different liver samples from three animals. Cellular senescence was measured by real-time PCR for p16 and p21 in cholangiocytes and HSCs as well as immunoblots for p16 in cholangiocytes.
Measurement of TGF-β1 Levels and Fibrosis and Cellular Senescence in WT Mice and Large IMCLs Treated with Secretin
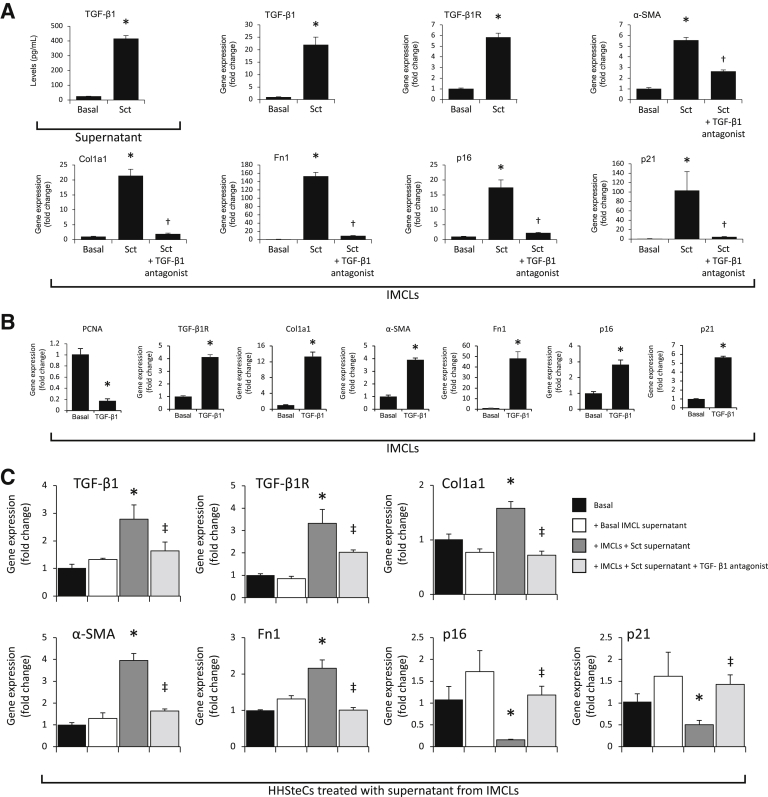
WT mice were treated for 1 week with saline or Sct (2.5 nmol/kg body weight per day)13 before collecting cholangiocytes, HSCs, and cholangiocyte supernatants. The levels of TGF-β1 were measured in short-term (6-hour) cultures of cholangiocytes by ELISA kits. Subsequently, the following were measured: liver fibrosis by immunofluorescence for Col1a1 in liver sections and real-time PCR for TGF-β1, TGF-β1R, Col1a1, α-SMA, and Fn1 in cholangiocytes and HSCs; and cellular senescence by immunofluorescence in liver sections for p16 and real-time PCR for p16 and p21 in cholangiocytes and HSCs. To determine whether secretin affects cholangiocyte senescence through the autocrine release of TGF-β1, large IMCLs were treated with 10 nmol/L secretin for 12 hours in the absence/presence of LY2109761, a small-molecule inhibitor selectively targeting both TGF-β receptor types I and II with Ki of 38 and 300 nmol/L, respectively28 (10 nmol/L; Cayman Chemical Company, Ann Arbor, MI), before measuring the expression of fibrosis and/or senescence genes by real-time PCR.
Paracrine Effect of Secretin-Stimulated Biliary TGF-β1 on the Expression of Fibrosis and Senescence Genes in HHSteCs
Experiments were performed to demonstrate that biliary TGF-β1 levels (modulated by the Sct/SR axis)13 alter fibrosis and cellular senescence of HHSteCs by a paracrine mechanism. Biliary supernatants were obtained from normal and BDL WT mice and Sct−/−, SR−/−, and Sct−/−/SR−/− BDL mice as well as normal WT mice treated with saline or secretin. HHSteCs were incubated with the aforementioned biliary supernatants (containing different levels of TGF-β1) for 24 hours (in the absence or presence of LY2109761) before measuring the expression of senescence and fibrosis genes by real-time PCR. HHSteCs were incubated with the supernatant of large IMCLs treated for 0.2% bovine serum albumin (basal) or secretin (10 nmol/L) with/without LY2109761 for 6 hours before measuring the mRNA expression of fibrosis and senescence genes. To provide direct evidence that TGF-β1 directly modulates fibrosis and senescence of IMCLs and HHSteCs, these cells were stimulated with 10 nmol/L TGF-β1 for 24 hours before measuring the expression of PCNA as well as fibrosis and senescence genes by real-time PCR. Although immortalized cholangiocyte lines are limiting by the fact that they have modified pathways of senescence, this is the only biliary cell line available in our laboratory. The goal of the experiments in IMCLs was to demonstrate that the effects of the Sct/SR TGF-β1 axis on cellular senescence are mediated by a direct interaction with cholangiocytes rather that in vivo nonspecific effects. Murine stellate cell lines are not available in our laboratory. HHSteCs are guaranteed to further expand for 15 population doublings under the conditions provided by ScienCell Research Laboratories. Thus, HHSteCs are suitable for in vitro studies to provide direct evidence for changes in fibrogenic activity and cellular senescence after incubation with the selected cholangiocyte supernatant. Furthermore, the use of human hepatic stellate cells increases the clinical relevance of the study.
Expression of the Sct-Dependent miR-125b/VEGF-A Axis
To begin to determine the signaling mechanisms by which the Sct/SR axis modulates liver fibrosis by a paracrine mechanism through changes in TGF-β1–mediated biliary senescence, the expression of the miR-125b/VEGF-A axis (ie, a key signaling pathway in secretin induction of biliary hyperplasia) was evaluated. This was accomplished by measuring the expression of miR-125b and VEGF-A by real-time PCR analysis in isolated cholangiocytes and immunoblots of VEGF-A in isolated cholangiocytes from the selected groups of animals.
Evaluation of TGF-β1 mRNA Expression in LCM-Isolated Kupffer Cells
Because macrophages, especially M2 polarized macrophages, are a main source of TGF-β1 secretion during chronic liver fibrosis,29 the expression of TGF-β1 mRNA was measured by real-time PCR in LCM-isolated Kupffer cells (using F4/80 as a marker of Kupffer cells)30 from normal and BDL mice. Also, in paraffin-embedded liver sections (4 to 5 μm thick), macrophage infiltration was evaluated by immunohistochemistry using the F4/80 antibody.
Statistical Analysis
Data are expressed as means ± SEM. Differences between groups were analyzed by unpaired t-test when two groups were analyzed and analysis of variance when more than two groups were analyzed, followed by an appropriate post hoc test.
Results
Immunoreactivity of Sct and SR in Liver Sections and Sct Serum and Bile Levels
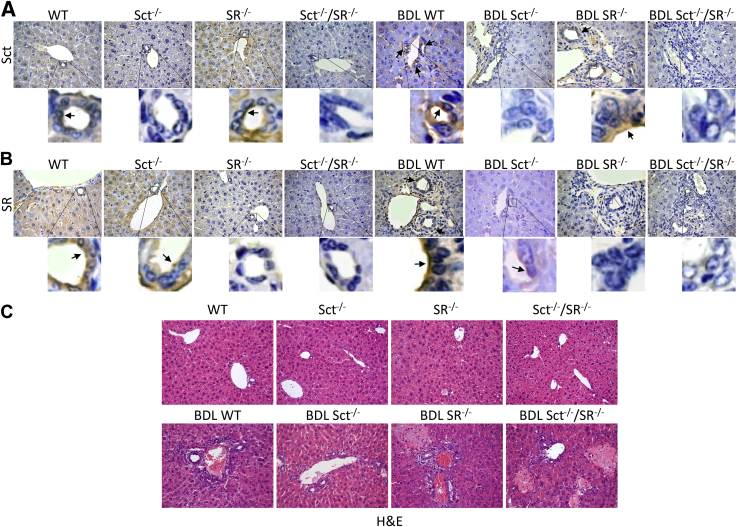
The immunoreactivity of Sct and SR was higher in liver sections from BDL WT compared with WT mice but was absent in their respective knockout mice (Figure 1, A and B, and Table 3). Secretin levels were higher in both serum and bile from BDL compared with WT mice but decreased significantly to background levels in both serum and bile from Sct−/−, SR−/−, and Sct−/−/SR−/− BDL mice compared with BDL WT mice (Table 2). The background Sct levels in serum and bile (observed in Sct−/− and Sct−/−/SR−/− mice) (Table 2) are likely attributable to cross-reactivity of the antibodies present in the ELISA kit with other peptides.
Figure 1.
A and B: The immunoreactivity of Sct and SR (arrows) is higher in liver sections from bile duct ligated (BDL) wild-type (WT) mice compared with WT mice but is absent in the respective knockout mice. Insets show cholangiocyte areas in higher magnification. C: No significant differences in the degree of necrosis, lobular damage, and portal inflammation among normal liver samples are observed by hematoxylin and eosin (H&E) staining, except for a low degree of lobular damage in Sct−/−/SR−/− normal mice. In BDL livers, the severity of necrosis, inflammation, and lobular damage is increased in all of the samples compared with the corresponding WT mice. Original magnification: ×40 (A and B, main images); ×25 (C).
Evaluation of Liver Histology/Fibrosis, Intrahepatic Bile Duct Mass, and Immunoreactivity for CFTR and AE2 in Liver Sections
Liver/body weight ratio increased in BDL WT compared with WT mice but decreased in Sct−/−, SR−/−, and Sct−/−/SR−/− BDL mice (Table 2); no significant difference was observed between normal WT and Sct−/−, SR−/−, and Sct−/−/SR−/− mice (Table 2). By hematoxylin and eosin staining, there were no significant differences in the degree of necrosis, lobular damage, and portal inflammation among the normal liver samples, except for a low degree of lobular damage in Sct−/−/SR−/− normal mice (Figure 1C). In BDL liver, there was an increase in necrosis, inflammation, and lobular damage in all of the samples compared with the corresponding WT mice (Figure 1C). The structure of stomach, small and large intestine, pancreas, lung, spleen, and kidney from WT and Sct−/−/SR−/− mice after sham and BDL appear comparable and did not show pathologic alterations (Supplemental Figure S1).
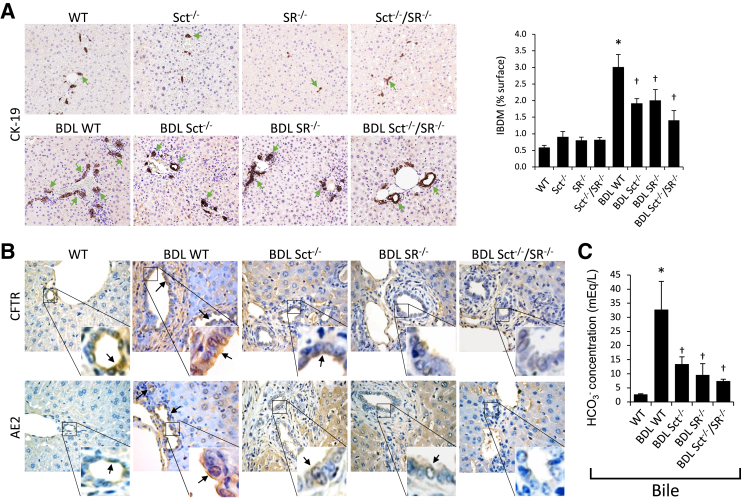
Intrahepatic bile duct mass was higher in BDL WT compared with WT mice but decreased in Sct−/−, SR−/−, and Sct−/−/SR−/− BDL mice compared with BDL WT mice (Figure 2A); no significant difference in bile duct mass was observed between normal WT and Sct−/−, SR−/−, and Sct−/−/SR−/− mice (Figure 2A). The immunoreactivity of CFTR and AE2 increased in liver sections from BDL WT compared with WT mice but decreased in Sct−/−, SR−/−, and Sct−/−/SR−/− BDL mice compared with BDL WT mice (Figure 2B and Table 3). Bicarbonate concentration was higher in BDL WT compared with normal WT mice, but significantly decreased in Sct−/−, SR−/−, and Sct−/−/SR−/− BDL mice compared with BDL WT mice (Figure 2C).
Figure 2.
A: Ductular reaction [intrahepatic bile duct mass (IBDM)] is higher in bile duct ligated (BDL) wild-type (WT) mice compared with normal WT mice but decreases in Sct−/−, SR−/−, and Sct−/−/SR−/− BDL compared with BDL WT mice; no significant differences in IBDM are observed between normal WT and Sct−/−, SR−/−, and Sct−/−/SR−/− mice. Green arrows indicate bile ducts positive for cytokeratin-19 (CK-19). B: The immunoreactivity of cystic fibrosis transmembrane conductance regulator (CFTR) and anion exchanger 2 (AE2; black arrows) increases in liver sections from BDL WT compared with WT mice but decreases in Sct−/−, SR−/−, and Sct−/−/SR−/− BDL mice compared with BDL WT mice. Insets show cholangiocyte areas in higher magnification. C: Bicarbonate concentration is higher in BDL WT mice compared with normal WT mice but decreases in Sct−/−, SR−/−, and Sct−/−/SR−/− BDL mice compared with BDL WT mice. Data are expressed as means ± SEM of three evaluations from three individual mice (A–C). ∗P < 0.05 versus normal WT mice; †P < 0.05 versus BDL WT mice. Original magnification: ×20 (A); ×40 (B).
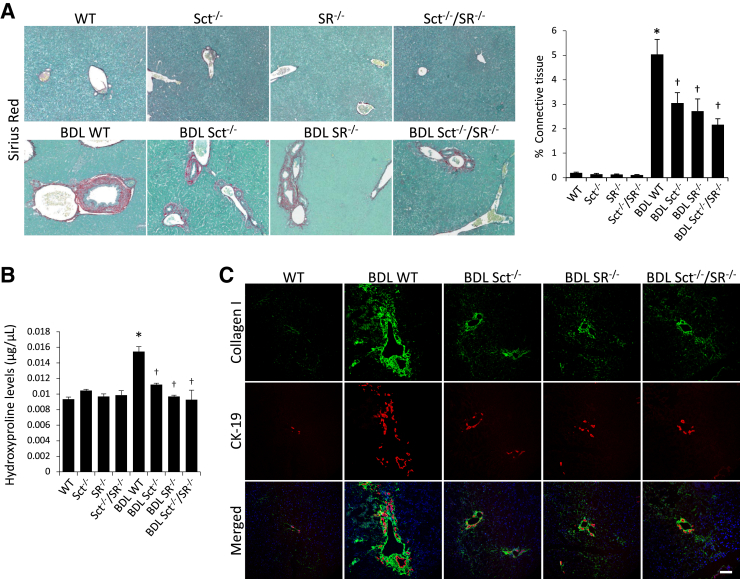
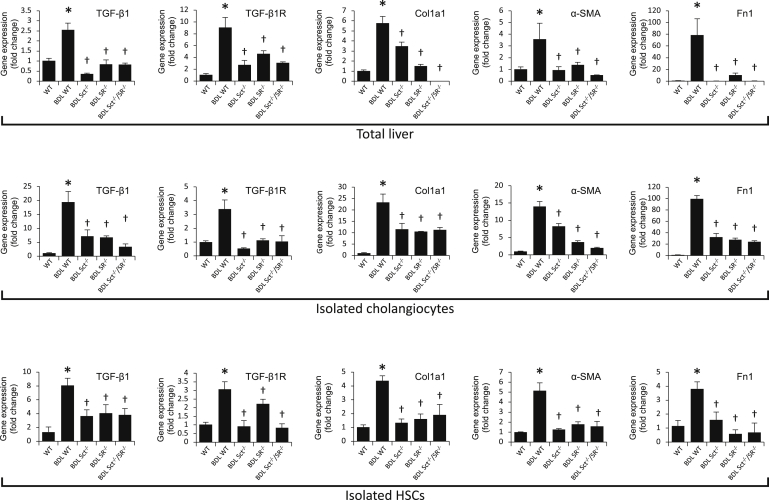
BDL WT mice displayed higher collagen deposition compared with WT mice (Figure 3A). There was reduced collagen deposition in Sct−/−, SR−/−, and Sct−/−/SR−/− BDL mice compared with BDL WT mice (Figure 3A); knockout of Sct or/and SR did not alter liver fibrosis in normal mice (Figure 3A). Similar changes in liver fibrosis were observed by measurement of hydroxyproline levels in liver samples (Figure 3B). BDL WT mice have increased collagen expression in bile ducts and the periductal region compared with WT mice, which was reduced in Sct−/−, SR−/−, and Sct−/−/SR−/− BDL mice compared with BDL WT mice (Figure 3C). Furthermore, there was enhanced expression of TGF-β1, TGF-β1R, Col1a1, α-SMA, and Fn1 in total liver, isolated cholangiocytes, and HSCs from BDL WT mice that was reduced in Sct−/−, SR−/−, and Sct−/−/SR−/− BDL compared with BDL WT mice (Figure 4). Similar trend of protein expression was observed for collagen I and TGF-β1R in isolated cholangiocytes by immunoblots (Supplemental Figure 2A). There were increased levels of TGF-β1 in serum as well as cholangiocyte supernatant from BDL WT compared with WT mice, whereas the increases were reduced in Sct−/−, SR−/−, and Sct−/−/SR−/− BDL compared with BDL WT mice (Table 2).
Figure 3.
A: Bile duct ligated (BDL) wild-type (WT) mice display higher collagen deposition compared with normal WT mice. There is reduced collagen deposition in Sct−/−, SR−/−, and Sct−/−/SR−/− BDL mice compared with BDL WT mice; knockout of Sct or/and SR does not alter fibrosis in normal mice. B: Similar changes in liver fibrosis are observed by measurement of hydroxyproline levels in liver samples. Three different liver samples from three different mice were used. C: BDL WT mice have increased immunoreactivity for collagen I (green) in bile ducts [costained for cytokeratin-19 (CK-19); red] and periductal region compared with normal WT mice, which returned to values similar to those of normal WT mice in Sct−/−, SR−/−, and Sct−/−/SR−/− BDL mice. ∗P < 0.05 versus normal WT mice; †P < 0.05 versus BDL WT mice. Scale bar = 100 μm (C). Original magnification, ×10 (A).
Figure 4.
There is increased mRNA expression of TGF-β1, TGF-β1R, Col1a1, α-smooth muscle actin (α-SMA; ACTA2), and Fn1 in total liver, isolated cholangiocytes, and hepatic stellate cells (HSCs) from bile duct ligated (BDL) wild-type (WT) mice (ie, reduced in Sct−/−, SR−/−, and Sct−/−/SR−/− BDL mice compared with BDL WT mice). Data are from the following: i) three evaluations from three total liver samples from three different animals; ii) three evaluations from three cumulative preparations of cholangiocytes from four mice; and iii) three evaluations from three preparations of laser capture microdissection–isolated HSCs from three mice. Data are expressed as means ± SEM. ∗P < 0.05 versus normal WT mice; †P < 0.05 versus BDL WT mice. Col1a1, collagen I; Fn1, fibronectin 1; TGF-β1, transforming growth factor-β1; TGF-β1R, TGF-β1 receptor.
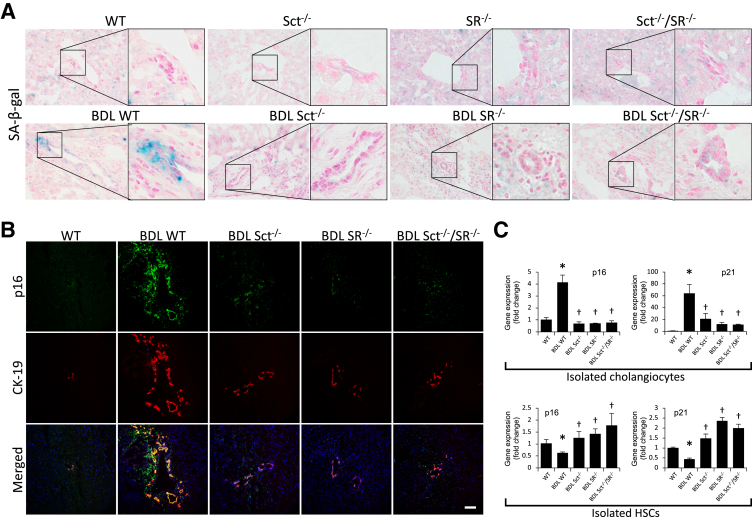
Measurement of Cellular Senescence in Liver Sections, Cholangiocytes, and HSCs
In BDL WT mice, there was increased biliary senescence (by SA-β-galactosidase staining) as well as immunoreactivity for p16 in cholangiocytes (stained with CK-19) compared with WT mice (Figure 5, A and B). The increased cholangiocyte senescence observed in BDL WT mice was reduced in Sct−/−, SR−/−, and Sct−/−/SR−/− BDL mice (Figure 5, A and B). A similar profile was observed in isolated cholangiocytes by PCR analysis for p16 and p21 (Figure 5C) as well as immunoblots for p16 (Supplemental Figure S2A). There was reduced expression of p16 and p21 in HSCs from BDL WT compared with WT mice (Figure 5C) but enhanced cellular senescence in HSCs from Sct−/−, SR−/−, and Sct−/−/SR−/− BDL mice compared with BDL WT mice (Figure 5C).
Figure 5.
A and B: There is increased cellular senescence [by SA-β-galactosidase (SA-β-gal) staining] and immunoreactivity for p16 (green) in bile ducts [costained for cytokeratin-19 (CK-19); red] from bile duct ligated (BDL) compared with normal wild-type (WT) mice. The increase in cell senescence observed in BDL WT mice is reduced in Sct−/−, SR−/−, and Sct−/−/SR−/− BDL mice. Insets show cholangiocyte areas in higher magnification. C: There is increased mRNA expression of p16 and p21 in cholangiocytes but decreased expression of p16 and p21 in hepatic stellate cells (HSCs) from BDL compared with normal WT mice; cellular senescence returns to values similar to those of normal WT mice in Sct−/−, SR−/−, and Sct−/−/SR−/− BDL mice compared with BDL WT mice. Data are from three evaluations from three cumulative preparations of cholangiocytes from four mice and three evaluations from three preparations of laser capture microdissection–isolated HSCs from three mice. Data are expressed as means ± SEM (C). n = 12 (C). ∗P < 0.05 versus normal WT mice; †P < 0.05 versus BDL WT mice. Scale bar = 100 μm (B). Original magnification, ×40 (A).
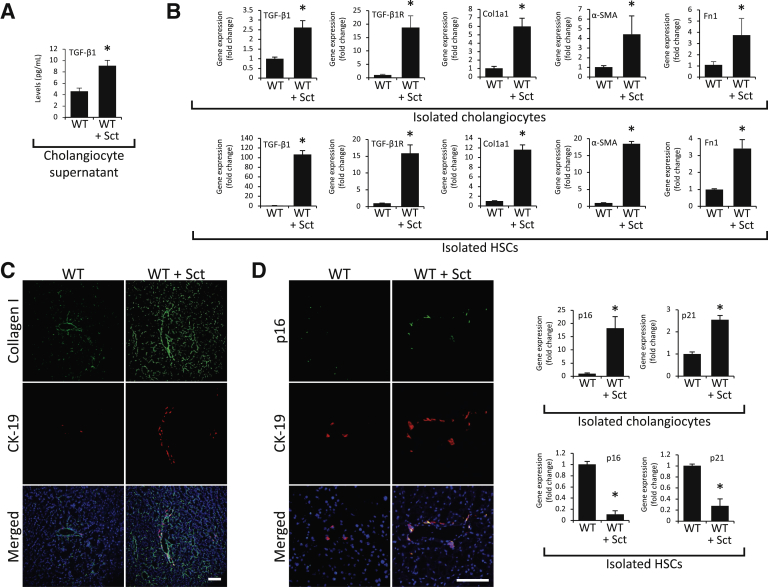
Measurement of TGF-β1 Levels and Fibrosis and Cellular Senescence in WT Mice and Large IMCLs Treated with Secretin
Parallel with a previous study,13 there were enhanced levels of TGF-β1 in cholangiocyte supernatant from WT mice treated in vivo with secretin compared with saline-treated mice (Figure 6A). Treatment of WT mice with secretin increased the biliary immunoreactivity of collagen I in liver sections as well as mRNA expression of TGF-β1, TGF-β1R Col1a1, α-SMA (ACTA2), and Fn1 in isolated cholangiocytes and HSCs (because of a paracrine interaction with cholangiocytes synthesizing more TGF-β1)13 compared with WT mice (Figure 6, B and C). The following were also observed: enhanced p16 immunoreactivity in liver sections as well as p16 and p21 expression in cholangiocytes from secretin-treated WT mice; the stimulatory effect of secretin on biliary senescence is likely attributable to enhanced release of TGF-β1 that induces cholangiocyte senescence by an autocrine loop; and reduced p16 and p21 mRNA expression in isolated HSCs (likely because of a paracrine interaction with cholangiocytes synthesizing more TGF-β1)13 from secretin-treated WT mice compared with saline-treated mice (Figure 6D).
Figure 6.
A: There is an enhanced level of transforming growth factor (TGF)-β1 in cholangiocyte supernatant from wild-type (WT) mice treated with Sct compared with saline-treated WT mice. B and C: Sct increases the immunoreactivity of collagen I in liver sections as well as the expression of TGF-β1, TGF-β1R, Col1a1, α-smooth muscle actin (α-SMA; ACTA2), and Fn1 in isolated cholangiocytes and hepatic stellate cells (HSCs) in WT mice treated with Sct compared with normal WT mice. D: There is increased expression of p16 and p21 in cholangiocytes from Sct-treated WT mice compared with saline-treated WT mice; and reduced p16 and p21 mRNA expression in HSCs from Sct-treated WT mice compared with saline-treated mice. Data are from three evaluations from three cumulative preparations of cholangiocytes from four mice and three evaluations from three preparations of laser capture microdissection–isolated HSCs from three mice. Data are expressed as means ± SEM (A, B, and D). n = 12 (A, B, and D). ∗P < 0.05 versus WT. Scale bars = 100 μm (C and D). Col1a1, collagen I; Fn1, fibronectin 1; TGF-β1R, TGF-β1 receptor.
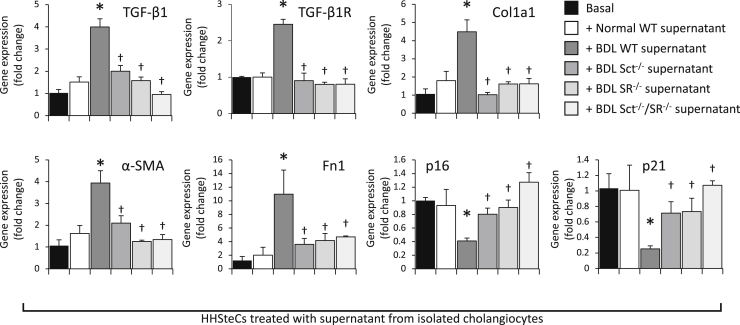
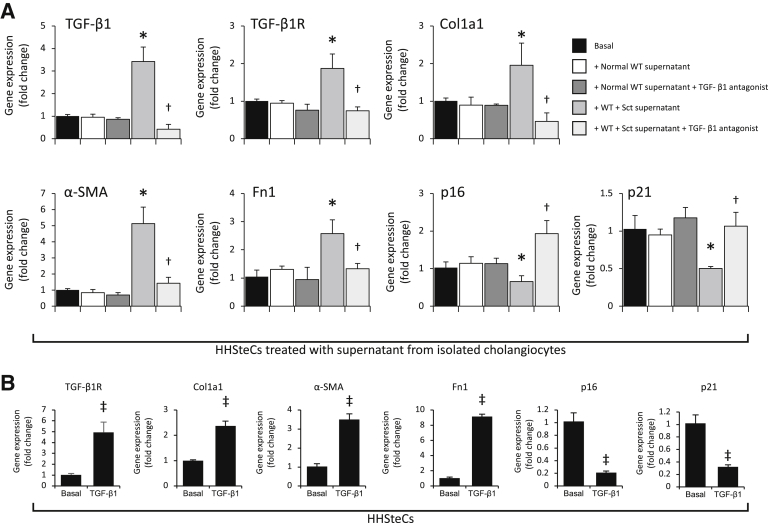
Paracrine Effect of Secretin-Stimulated Biliary TGF-β1 on the Expression of Fibrosis and Senescence Genes in HHSteCs
When HHSteCs were treated with cholangiocyte supernatants from BDL WT mice (containing higher levels of TGF-β1 compared with normal mice) (Table 2), there was enhanced expression of fibrosis genes and reduced expression of senescence genes compared with HHSteCs treated with normal cholangiocyte supernatant (Figure 7); the changes in fibrosis and senescence gene expression were reversed in HHSteCs that were treated with cholangiocyte supernatant from Sct−/−, SR−/−, and Sct−/−/SR−/− BDL mice (Figure 7). When HHSteCs were treated with cholangiocyte supernatant from secretin-treated WT mice (containing higher levels of TGF-β1) (Figure 6A), there was increased expression of fibrosis but reduced expression of senescence genes in HHSteCs (Figure 8A); these effects were reversed by preincubation of HHSteCs with LY2109761 before incubation with cholangiocyte supernatant from secretin-treated WT mice (Figure 8A). No effect was observed with normal cholangiocyte supernatant (Figures 7 and 8A). TGF-β1 stimulated expression of genes involved in fibrosis but reduced expression of genes involved in senescence in HHSteCs (Figure 8B). The next in vitro experiments demonstrated the following: i) secretin increased the secretion of TGF-β1 of large IMCLs and the expression of fibrosis and senescence genes, effects that were prevented by preincubation with LY2109761 (Figure 9A); ii) TGF-β1 decreased PCNA mRNA expression and increased the expression of fibrosis and senescence genes of large IMCLs (Figure 9B); and iii) the supernatant of large IMCLs (containing higher levels of TGF-β1 after secretin treatment) increases fibrosis gene expression but decreases the expression of senescence genes of HHSteCs, effects that were reversed by preincubation with LY2109761 (Figure 9C).
Figure 7.
In human hepatic stellate cell lines (HHSteCs) treated with cholangiocyte supernatant from bile duct ligated (BDL) wild-type (WT) mice, there is enhanced expression of genes relating to fibrosis and reduced expression of genes related to senescence compared with HHSteCs treated with normal cholangiocyte supernatant; the changes in fibrosis and senescence gene expression are reversed in HHSteCs that were treated with cholangiocyte supernatant from Sct−/−, SR−/−, and Sct−/−/SR−/− BDL mice. Data are from three experiments from three individual preparations of HHSteCs and are from three evaluations. Data are expressed as means ± SEM. ∗P < 0.05 versus HHSteCs treated with normal cholangiocyte supernatant; †P < 0.05 versus HHSteCs treated with BDL cholangiocyte supernatant. Col1a1, collagen I; Fn1, fibronectin 1; α-SMA, α-smooth muscle actin; TGF-β1, transforming growth factor-β1; TGF-β1R, TGF-β1 receptor.
Figure 8.
A: In human hepatic stellate cell lines (HHSteCs) treated with cholangiocyte supernatant from secretin-treated wild-type (WT) mice, there is increased expression of fibrosis but reduced expression of senescence genes, changes that are reversed by preincubation of HHSteCs with LY2109761; no effects were observed with normal cholangiocyte supernatant. Data are from three evaluations. B: Transforming growth factor (TGF)-β1 stimulates fibrosis gene expression but reduces senescence gene expression in HHSteCs. Data are from three experiments from three individual preparations of HHSteCs. Data are expressed as means ± SEM (A and B). ∗P < 0.05 versus HHSteCs treated with normal cholangiocyte supernatant; †P < 0.05 versus HHSteCs treated with bile duct ligated cholangiocyte supernatant; ‡P < 0.05 versus basal value. Col1a1, collagen I; Fn1, fibronectin 1; α-SMA, α-smooth muscle actin; TGF-β1R, TGF-β1 receptor.
Figure 9.
A and B: Although secretin increases the secretion of transforming growth factor (TGF)-β1 of large immortalized murine cholangiocyte lines (IMCLs) and the expression of fibrosis and senescence genes, these effects are partially blocked by preincubation with LY2109761. TGF-β1 decreases PCNA expression but increases the expression of fibrosis and senescence genes of large IMCLs. Data are from three experiments from three individual preparations of large IMCLs or human hepatic stellate cell lines (HHSteCs). C: The supernatant of large IMCLs (containing higher levels of TGF-β1 after secretin treatment) increases fibrosis gene expression but decreases the expression of senescence genes of HHSteCs, effects that are reversed by preincubation with LY2109761. Data are from three experiments from three individual preparations of HHSteCs. Data are expressed as means ± SEM (A–C). ∗P < 0.05 versus basal value; †P < 0.05 versus large IMCLs treated with Sct; ‡P < 0.05 versus HHSteCs treated with supernatant of large IMCLs containing higher levels of TGF-β1 after secretin treatment. Col1a1, collagen I; Fn1, fibronectin 1; PCNA, proliferating cell nuclear antigen; α-SMA, α-smooth muscle actin; TGF-β1R, TGF-β1 receptor.
Expression of the Sct-Dependent miR-125b/VEGF-A Axis
Parallel to a previous study,29 there was reduced expression of miR-125b and enhanced expression of VEGF-A (in isolated cholangiocytes) in BDL mice compared with normal mice, values that returned to values similar to that of normal mice in Sct−/−, SR−/−, and Sct−/−/SR−/− BDL mice (Supplemental Figure S2, A and B).
Evaluation of TGF-β1 mRNA Expression in LCM-Isolated Kupffer Cells and Macrophage Distribution in Liver Sections
By real-time PCR analysis, M2 macrophages from BDL WT mice displayed higher mRNA expression of TGF-β1 compared with WT mice, an increase that was reduced in M2 macrophages from Sct−/−, SR−/−, and Sct−/−/SR−/− BDL mice (Supplemental Figure S2C). By immunohistochemistry, in liver WT mice, a normal perisinusoidal distribution of macrophages (Kupffer cells) was observed. In BDL WT mice, the macrophages were highly concentrated in the connective tissue around the proliferating bile ducts and peribiliary plexus, whereas they seemed reduced in Sct−/−, SR−/−, and Sct−/−/SR−/− BDL mice (Supplemental Figure S2C).
Discussion
Ductular reaction, cellular senescence, and liver fibrosis are key hallmarks of chronic cholestatic liver diseases, such as PSC.2, 3, 4, 20, 21 The Sct/SR axis plays a key role in the enhancement of biliary mass and liver fibrosis in animal models of cholestasis, such as BDL and Mdr2−/−, and the Sct/SR axis is up-regulated in liver samples from Mdr2−/− mice as well as PSC patients.12, 13 This study extends our prior observations by elucidating a more in-depth evaluation of the coordinated factors by which the Sct/SR axis stimulates liver fibrosis by the following: increased cellular senescence in cholangiocytes through an autocrine loop involving decreased expression of miR-125b and enhanced TGF-β1 biliary secretion and VEGF-A expression after Sct stimulation; and secretin-mediated paracrine inhibition of HSC senescence (by secretin-mediated increase of TGF-β1 secretion from cholangiocytes) in BDL WT mice. In the present study, the following were found: i) both biliary mass and hepatic fibrosis were significantly reduced in Sct−/−, SR−/−, and Sct−/−/SR−/− BDL compared with BDL WT mice; ii) the reduction of biliary mass (ductular reaction) in Sct−/−, SR−/−, and Sct−/−/SR−/− BDL mice was coupled with decreased expression of functional markers of the secretory and proliferative processes, such as CFTR and AE2,14, 15, 27 and biliary bicarbonate concentration compared with BDL WT mice; iii) the reduction of hepatic fibrosis observed in Sct−/−, SR−/−, and Sct−/−/SR−/− BDL mice was associated with reduced TGF-β1 levels in both serum and cholangiocyte supernatant as well as decreased expression of biliary senescence in contrast to increased expression of HSC senescence compared with the expression levels observed in BDL WT mice; iv) concomitant with increased ductular reaction and liver fibrosis, secretin directly stimulated cellular senescence in a subset of large cholangiocytes (G.A., unpublished data) (Figure 9A) and decreased cellular senescence of HSCs by a paracrine TGF-β1–dependent mechanism; and v) the supernatants of cholangiocytes (containing higher levels of TGF-β1) activated HSCs through decreased cellular senescence.
Previous studies have shown that there is a close relationship between cholangiocyte proliferation/ductular reaction and the expression of the Sct/SR/CFTR/AE2 axis.1, 6, 9, 15, 18, 31 The expression of the Sct/SR axis (present only by cholangiocytes in the liver)9 is increased in animal models of cholestasis, such as BDL and Mdr2−/− mice, and in liver samples from patients with PSC.9, 11, 13, 14 Conversely, the damage of cholangiocytes and the decrease in ductular reaction (eg, after acute administration of carbon tetrachloride and chronic treatment with γ-aminobutyric acid) are associated with decreased expression of the Sct/SR/CFTR/AE2 axis.14, 31 Secretin stimulates biliary bicarbonate secretion via cAMP/protein kinase A–dependent activation of CFTR and AE2 exchanger activity, and bicarbonate secretion is enhanced in BDL mice.9, 10, 32, 33 In addition, to activate cAMP/protein kinase A/extracellular signal-regulated kinase 1/2 signaling,34 secretin increases biliary mass by down-regulation of the miR-125b that directly increases the expression of VEGF-A.12
Supporting these previous findings, herein it was shown that knockdown of the Sct/SR axis reduced bicarbonate levels in bile compared with BDL WT mice. Because the studies were performed in mice with BDL for 1 week, the levels of bicarbonate reduction in the mice lacking the Sct/SR axis were not completely reduced to normal basal levels but remain slightly elevated but significantly decreased compared with BDL WT mice. This may be because of the short time period and/or the remaining stimulus from extrahepatic cholestasis induced by BDL that activates alternative Ca2+-dependent Cl− efflux channels.35, 36, 37
The Sct/SR axis plays a key role in stimulating biliary damage, ductular reaction, and liver fibrosis during cholestasis through a paracrine interaction with HSCs mediated by secretin-induced increase in TGF-β1 biliary secretion.11, 12, 13 Cholestasis and hepatic fibrosis are hallmark features observed during the pathogenesis of PSC, which are mimicked in both BDL and Mdr2−/− mice.11, 12, 13 Recent evidence also implicates a role for cellular senescence in cholestatic liver injury and the pathogenesis of PSC.2, 20, 21, 38, 39 We demonstrated in the current study that the reduction of hepatic fibrosis observed in Sct−/−, SR−/−, and Sct−/−/SR−/− BDL mice was associated with reduced levels of TGF-β1 in serum and cholangiocyte supernatant and decreased expression of markers of cellular senescence in cholangiocytes, which was in contrast to increased expression of markers of cellular senescence in HSCs compared with those observed in BDL WT mice. Surprisingly, the decrease in biliary senescence observed in Sct−/−, SR−/−, and Sct−/−/SR−/− BDL mice was associated with reduced ductular reaction that may likely be because of lack of the proliferative stimulus of the Sct/SR axis that increases biliary senescence, likely through an autocrine TGF-β1 secretory loop. The unexpected enhanced biliary senescence observed in BDL WT mice (that are characterized by increased ductular reaction)1 is likely to because of enhanced biliary TGF-β1 secretion (mediated by secretin) that, in addition to inducing the activation of HSCs (by a paracrine pathway), reduces biliary proliferation while triggering cholangiocyte senescence as well. Because secretin modulates inflammatory pathways within cholangiocyte subpopulations40 and stimulates hepatic macrophage infiltration (G.A., unpublished data) and M2 polarized macrophages are a main source of TGF-β1 secretion during the progression of liver fibrosis,29 we speculate that Sct may participate in the paracrine activation of HSCs through release of TFG-β1 from macrophages. In preliminary experiments related to a different project, we demonstrated that miR-125b may be the link for Sct stimulation of TGF-β1 biliary secretion (G.A., unpublished data). This notion is also supported by a previous study showing that Sct increases biliary mass by down-regulation of the miR-125b that directly increases the expression of VEGF-A.12
The increase in biliary senescence observed after BDL may be limited to a subset of large cholangiocytes damaged in the BDL hyperplastic model.2, 41 This concept is also supported by our unpublished data showing that large cholangiocytes are more senescent compared with small, undifferentiated cholangiocytes42 as well by the findings that TGF-β1 increases senescence while decreasing proliferating cell nuclear antigen expression of large IMCLs (Figure 9A). Only large cholangiocytes express SR in the BDL model.41 However, further studies are necessary to pinpoint the specific subpopulations of large cholangiocytes undergoing senescence in the BDL model of biliary hyperplasia.1 In support of our findings, inhibition of the proliferative substance P/neurokinin-1 receptor axis in cholangiocytes during cholestasis has been shown to reduce biliary senescence and trigger senescence of HSCs, reducing hepatic fibrosis.2 This concept is further supported by a previous study showing that atorvastatin inhibits proliferation and apoptosis but induces senescence of rat hepatic myofibroblasts, decreasing liver fibrosis.43 Furthermore, another study demonstrated that cholangiocyte senescence is elevated in human PSC samples as well as in experimentally induced biliary senescence that was dependent on the activation of N-Ras.21 In fact, targeting senescent cholangiocytes with a Bcl-xL–specific inhibitor reduced hepatic fibrosis in cholestatic Mdr2−/− mice via a dual effect on activated HSCs and senescent cholangiocytes.22 In support of our findings and the association between enhanced biliary damage (eg, in the BDL model) and cellular senescence, a recent study has demonstrated the following: a link between biliary injury (observed during early chronic liver allograft rejection) and senescence; and the role of TGF-β1 in promoting senescence of bile ducts.44 Another recent study provides further support for the role of TGF-β1 in regulating cellular senescence of hepatic cells by demonstrating that sirtuin 6 promotes TGF-β1–mediated increases of hepatocellular carcinoma cell tumorigenicity by reducing cellular senescence of malignant hepatocytes.45 Furthermore, TGF-β1 has been shown to inhibit the proliferation of hepatocellular carcinoma cells by increased cellular senescence of hepatocellular carcinoma cells.46 Finally, several studies demonstrate that TGF-β phosphorylates Smad2/3 to mediate p16 or p21 to induce the senescence in several cell types, including fibroblasts,47 colon cancer cells,48 and hepatoma cells,46, 49 which supports the concept that TGF-β signaling regulates the cellular senescence of cholangiocytes and HSCs in this study. The finding that TGF-β1 increases the cellular senescence of cholangiocytes (while decreasing the proliferation but increasing fibrosis gene expression of ICMLs) may be attributable to the fact that TGF-β1 may induce cellular senescence of a subpopulation of large, senescent cholangiocytes (releasing senescence-associated secretory phenotypes) that, in turn, may further increase the cellular senescence of other normal, nonsenescent cholangiocyte subpopulations.
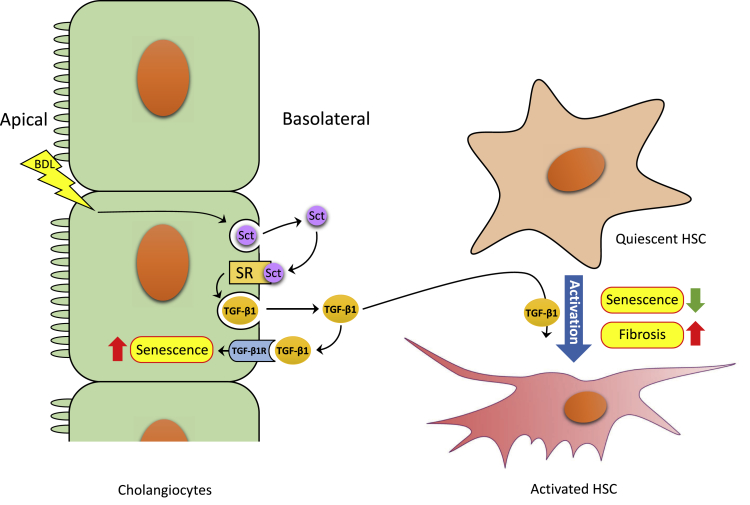
This study introduces the novel concept that secretin-induced biliary TGF-β1 secretion increases cellular senescence of cholangiocytes by an autocrine loop, which, in turn, releases proinflammatory factors (ie, senescence-associated secretory phenotypes), including TGF-β1, triggering liver fibrosis through reduced senescence of HSCs. In contrast, the knockdown of the Sct/SR axis limits ductular reaction and compensatory cholangiocyte senescence and reduces the levels of TGF-β1 that limit the activation of HSCs through increased senescence of HSCs (Figure 10). This balance between cholangiocyte and HSC senescence may play a critical role in the progression of fibrosis in cholestatic liver diseases.
Figure 10.
Working model of the role of the Sct/SR axis in the promotion of differential changes in senescence of cholangiocytes and hepatic stellate cells (HSCs) by transforming growth factor (TGF)-β1 during cholestasis induced by bile duct ligation (BDL). BDL-induced ductular reaction stimulates secretion of Sct and, in turn, via SR results in up-regulation of TGF-β1 expression in cholangiocytes. The increase of TGF-β1 secretion by cholangiocytes promotes senescence in cholangiocytes on the one hand and represses senescence and activates a profibrogenic phenotype in HSCs on the other, which promotes hepatic fibrosis. TGF-β1R, TGF-β1 receptor.
In summary, we demonstrated that the Sct/SR axis plays a key role in the pathogenesis of ductular reaction and hepatic fibrosis during cholestatic liver injury, which involved differential regulation of cellular senescence in cholangiocytes and HSCs. This was likely mediated by changes in biliary TGF-β1 secretion. Our findings, along with other studies, indicate that a balance between cholangiocyte and HSC senescence may play a key role in the regulation of ductular reaction and hepatic fibrosis and suggest that targeting senescent cholangiocytes by modification of the Sct/SR axis may provide a key pharmacologic approach for reducing hepatic fibrosis during the progression of cholestatic liver diseases.
Acknowledgment
We thank Dr. Robert Faris (Brown University, Providence, RI) for providing the monoclonal antibody used to collect cholangiocytes.
Footnotes
Supported in part by the Dr. Nicholas C. Hightower Centennial Chair of Gastroenterology from Scott & White, a Veterans Affairs (VA) Research Career Scientist Award, US Department of Veterans Affairs Biomedical Laboratory Research and Development Service VA Merit Awards 5I01BX000574 (G.A.), 5I01BX002192 (S.G.), 1I01BX001724 (F.M.), and 1I01BX003031 (H.F.), University of Rome La Sapienza (P.O.), NIH grants DK054811 (G.A., S.G., and F.M.), DK115184 (G.A., S.G., and F.M.), DK076898 (G.A., S.G., and F.M.), DK107310 (G.A., S.G., and F.M.), DK062975 (G.A., S.G., and F.M.), DK108959 (H.F.), and DK095862 (C.W.), American Diabetes Association grant 1-17-IBS-145 (C.W.), and the Central Texas Veterans Health Care System.
N.W., F.M., and T.Z. contributed equally to this work.
G.A., S.G., and A.F. contributed equally as senior authors to this work.
Disclosures: None declared.
The content is the responsibility of the author(s) alone and does not necessarily reflect the views or policies of the Department of Veterans Affairs or the US government.
Supplemental material for this article can be found at https://doi.org/10.1016/j.ajpath.2018.06.015.
Supplemental Data
By hematoxylin and eosin staining, the structure of stomach, small intestine, large intestine, pancreas, lung, spleen, and kidney organs from wild-type (WT) and Sct−/−/SR−/− mice after sham and bile duct ligation (BDL) appear comparable and do not show pathologic alterations. Original magnification, ×25.
A: There is enhanced protein expression of collagen I, transforming growth factor-β1 receptor (TGF-β1R), p16, and vascular endothelial growth factor-A (VEGF-A) in isolated cholangiocytes from bile duct ligated (BDL) wild-type (WT) mice that is reduced in Sct−/−, SR−/−, and Sct−/−/SR−/− BDL compared with BDL WT mice. Immunoblots in cholangiocytes from three separate animals. B: There is reduced expression of miR-125b and enhanced mRNA expression of VEGF-A in isolated cholangiocytes from BDL mice compared with WT mice; values returned to values similar to those of WT mice in Sct−/−, SR−/−, and Sct−/−/SR−/− BDL mice. C: There is increased mRNA expression of TGF-β1 in laser capture microdissection–isolated Kupffer cells from BDL WT compared with WT mice, which is reduced in Sct−/−, SR−/−, and Sct−/−/SR−/− BDL mice. PCR data (of three evaluations) from three cumulative cell preparations from three mice. By immunohistochemistry, there is an increase of macrophages in the connective tissue around the proliferating bile ducts and peribiliary plexus in BDL WT mice that is reduced in Sct−/−, SR−/−, and Sct−/−/SR−/− BDL mice (black arrows depict bile ducts). Data are expressed as means ± SEM (A–C). n = 3 (A); n = 9 (C). ∗P < 0.05 versus WT mice; †P < 0.05 versus BDL WT mice. Original magnification, ×20 (C).
References
- 1.Alpini G., Lenzi R., Sarkozi L., Tavoloni N. Biliary physiology in rats with bile ductular cell hyperplasia: evidence for a secretory function of proliferated bile ductules. J Clin Invest. 1988;81:569–578. doi: 10.1172/JCI113355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wan Y., Meng F., Wu N., Zhou T., Venter J., Francis H., Kennedy L., Glaser T., Bernuzzi F., Invernizzi P., Glaser S., Huang Q., Alpini G. Substance P increases liver fibrosis by differential changes in senescence of cholangiocytes and hepatic stellate cells. Hepatology. 2017;66:528–541. doi: 10.1002/hep.29138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lazaridis K.N., Strazzabosco M., LaRusso N.F. The cholangiopathies: disorders of biliary epithelia. Gastroenterology. 2004;127:1565–1577. doi: 10.1053/j.gastro.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 4.Lazaridis K.N., LaRusso N.F. Primary sclerosing cholangitis. N Engl J Med. 2016;375:2501–2502. doi: 10.1056/NEJMc1613273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alpini G., Glaser S., Robertson W., Rodgers R.E., Phinizy J.L., Lasater J., LeSage G. Large but not small intrahepatic bile ducts are involved in secretin-regulated ductal bile secretion. Am J Physiol Gastrointest Liver Physiol. 1997;272:G1064–G1074. doi: 10.1152/ajpgi.1997.272.5.G1064. [DOI] [PubMed] [Google Scholar]
- 6.Alpini G., Roberts S., Kuntz S.M., Ueno Y., Gubba S., Podila P.V., LeSage G., LaRusso N.F. Morphological, molecular, and functional heterogeneity of cholangiocytes from normal rat liver. Gastroenterology. 1996;110:1636–1643. doi: 10.1053/gast.1996.v110.pm8613073. [DOI] [PubMed] [Google Scholar]
- 7.Korner M., Hayes G.M., Rehmann R., Zimmermann A., Scholz A., Wiedenmann B., Miller L.J., Reubi J.C. Secretin receptors in the human liver: expression in biliary tract and cholangiocarcinoma, but not in hepatocytes or hepatocellular carcinoma. J Hepatol. 2006;45:825–835. doi: 10.1016/j.jhep.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 8.Glaser S., Gaudio E., Rao A., Pierce L.M., Onori P., Franchitto A., Francis H.L., Dostal D.E., Venter J.K., DeMorrow S., Mancinelli R., Carpino G., Alvaro D., Kopriva S.E., Savage J.M., Alpini G. Morphological and functional heterogeneity of the mouse intrahepatic biliary epithelium. Lab Invest. 2009;89:456–469. doi: 10.1038/labinvest.2009.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alpini G., Ulrich C.D., 2nd, Phillips J.O., Pham L.D., Miller L.J., LaRusso N.F. Upregulation of secretin receptor gene expression in rat cholangiocytes after bile duct ligation. Am J Physiol Gastrointest Liver Physiol. 1994;266:G922–G928. doi: 10.1152/ajpgi.1994.266.5.G922. [DOI] [PubMed] [Google Scholar]
- 10.Alvaro D., Cho W.K., Mennone A., Boyer J.L. Effect of secretion on intracellular pH regulation in isolated rat bile duct epithelial cells. J Clin Invest. 1993;92:1314–1325. doi: 10.1172/JCI116705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glaser S., Lam I.P., Franchitto A., Gaudio E., Onori P., Chow B.K., Wise C., Kopriva S., Venter J., White M., Ueno Y., Dostal D., Carpino G., Mancinelli R., Butler W., Chiasson V., DeMorrow S., Francis H., Alpini G. Knockout of secretin receptor reduces large cholangiocyte hyperplasia in mice with extrahepatic cholestasis induced by bile duct ligation. Hepatology. 2010;52:204–214. doi: 10.1002/hep.23657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glaser S., Meng F., Han Y., Onori P., Chow B.K., Francis H., Venter J., McDaniel K., Marzioni M., Invernizzi P., Ueno Y., Lai J.M., Huang L., Standeford H., Alvaro D., Gaudio E., Franchitto A., Alpini G. Secretin stimulates biliary cell proliferation by regulating expression of microRNA 125b and microRNA let7a in mice. Gastroenterology. 2014;146:1795–1808.e12. doi: 10.1053/j.gastro.2014.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu N., Meng F., Invernizzi P., Bernuzzi F., Venter J., Standeford H., Onori P., Marzioni M., Alvaro D., Franchitto A., Gaudio E., Glaser S., Alpini G. The secretin/secretin receptor axis modulates liver fibrosis through changes in transforming growth factor-b1 biliary secretion in mice. Hepatology. 2016;64:865–879. doi: 10.1002/hep.28622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.LeSage G., Glaser S., Marucci L., Benedetti A., Phinizy J.L., Rodgers R., Caligiuri A., Papa E., Tretjak Z., Jezequel A.M., Holcomb L.A., Alpini G. Acute carbon tetrachloride feeding induces damage of large but not small cholangiocytes from BDL rat liver. Am J Physiol Gastrointest Liver Physiol. 1999;276:G1289–G1301. doi: 10.1152/ajpgi.1999.276.5.G1289. [DOI] [PubMed] [Google Scholar]
- 15.Alpini G., Ulrich C., Roberts S., Phillips J.O., Ueno Y., Podila P.V., Colegio O., LeSage G., Miller L.J., LaRusso N.F. Molecular and functional heterogeneity of cholangiocytes from rat liver after bile duct ligation. Am J Physiol Gastrointest Liver Physiol. 1997;272:G289–G297. doi: 10.1152/ajpgi.1997.272.2.G289. [DOI] [PubMed] [Google Scholar]
- 16.Martinez-Anso E., Castillo J.E., Diez J., Medina J.F., Prieto J. Immunohistochemical detection of chloride/bicarbonate anion exchangers in human liver. Hepatology. 1994;19:1400–1406. [PubMed] [Google Scholar]
- 17.McGill J.M., Basavappa S., Gettys T.W., Fitz J.G. Secretin activates Cl- channels in bile duct epithelial cells through a cAMP-dependent mechanism. Am J Physiol Gastrointest Liver Physiol. 1994;266:G731–G736. doi: 10.1152/ajpgi.1994.266.4.G731. [DOI] [PubMed] [Google Scholar]
- 18.Fiorotto R., Scirpo R., Trauner M., Fabris L., Hoque R., Spirli C., Strazzabosco M. Loss of CFTR affects biliary epithelium innate immunity and causes TLR4-NF-kappaB-mediated inflammatory response in mice. Gastroenterology. 2011;141:1498–1508.e5. doi: 10.1053/j.gastro.2011.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guerrier M., Attili F., Alpini G., Glaser S. Prolonged administration of secretin to normal rats increases biliary proliferation and secretin-induced ductal secretory activity. Hepatobiliary Surg Nutr. 2014;3:118–125. doi: 10.3978/j.issn.2304-3881.2014.04.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meng L., Quezada M., Levine P., Han Y., McDaniel K., Zhou T., Lin E., Glaser S., Meng F., Francis H., Alpini G. Functional role of cellular senescence in biliary injury. Am J Pathol. 2015;185:602–609. doi: 10.1016/j.ajpath.2014.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tabibian J.H., O'Hara S.P., Splinter P.L., Trussoni C.E., LaRusso N.F. Cholangiocyte senescence by way of N-ras activation is a characteristic of primary sclerosing cholangitis. Hepatology. 2014;59:2263–2275. doi: 10.1002/hep.26993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moncsek A., Al-Suraih M.S., Trussoni C.E., O'Hara S.P., Splinter P.L., Zuber C., Patsenker E., Valli P.V., Fingas C.D., Weber A., Zhu Y., Tchkonia T., Kirkland J.L., Gores G.J., Mullhaupt B., LaRusso N.F., Mertens J.C. Targeting senescent cholangiocytes and activated fibroblasts with Bcl-xL inhibitors ameliorates fibrosis in Mdr2-/- mice. Hepatology. 2018;67:247–259. doi: 10.1002/hep.29464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ishhi M., Vroman B., LaRusso N.F. Isolation and morphologic characterization of bile duct epithelial cells from normal rat liver. Gastroenterology. 1989;97:1236–1247. doi: 10.1016/0016-5085(89)91695-8. [DOI] [PubMed] [Google Scholar]
- 24.Puche J.E., Lee Y.A., Jiao J., Aloman C., Fiel M.I., Munoz U., Kraus T., Lee T., Yee H.F., Jr., Friedman S.L. A novel murine model to deplete hepatic stellate cells uncovers their role in amplifying liver damage in mice. Hepatology. 2013;57:339–350. doi: 10.1002/hep.26053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ueno Y., Alpini G., Yahagi K., Kanno N., Moritoki Y., Fukushima K., Glaser S., LeSage G., Shimosegawa T. Evaluation of differential gene expression by microarray analysis in small and large cholangiocytes isolated from normal mice. Liver Int. 2003;23:449–459. doi: 10.1111/j.1478-3231.2003.00876.x. [DOI] [PubMed] [Google Scholar]
- 26.Jones H., Hargrove L., Kennedy L., Meng F., Graf-Eaton A., Owens J., Alpini G., Johnson C., Bernuzzi F., Demieville J., DeMorrow S., Invernizzi P., Francis H. Inhibition of mast cell-secreted histamine decreases biliary proliferation and fibrosis in primary sclerosing cholangitis Mdr2(-/-) mice. Hepatology. 2016;64:1202–1216. doi: 10.1002/hep.28704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Francis H., LeSage G., DeMorrow S., Alvaro D., Ueno Y., Venter J., Glaser S., Mancino M.G., Marucci L., Benedetti A., Alpini G. The alpha2-adrenergic receptor agonist UK 14,304 inhibits secretin-stimulated ductal secretion by downregulation of the cAMP system in bile duct-ligated rats. Am J Physiol Cell Physiol. 2007;293:C1252–C1262. doi: 10.1152/ajpcell.00031.2007. [DOI] [PubMed] [Google Scholar]
- 28.Yang H., Li W., Zhang Y., Li M., Gao Y., Lao C., Shi B. Regulatory role of miR-18a to CCN2 by TGF-beta1 signaling pathway in pulmonary injury induced by nano-SiO2. Environ Sci Pollut Res Int. 2018;25:867–876. doi: 10.1007/s11356-017-0344-0. [DOI] [PubMed] [Google Scholar]
- 29.Braga T.T., Agudelo J.S., Camara N.O. Macrophages during the fibrotic process: M2 as friend and foe. Front Immunol. 2015;6:602. doi: 10.3389/fimmu.2015.00602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lan T., Li C., Yang G., Sun Y., Zhuang L., Ou Y., Li H., Wang G., Kisseleva T., Brenner D., Guo J. Sphingosine kinase 1 promotes liver fibrosis by preventing miR-19b-3p-mediated inhibition of CCR2. Hepatology. 2018 doi: 10.1002/hep.29885. [Epub ahead of print] doi: 10.1002/hep.29885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mancinelli R., Franchitto A., Gaudio E., Onori P., Glaser S., Francis H., Venter J., Demorrow S., Carpino G., Kopriva S., White M., Fava G., Alvaro D., Alpini G. After damage of large bile ducts by gamma-aminobutyric acid, small ducts replenish the biliary tree by amplification of calcium-dependent signaling and de novo acquisition of large cholangiocyte phenotypes. Am J Pathol. 2010;176:1790–1800. doi: 10.2353/ajpath.2010.090677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spirli C., Nathanson M.H., Fiorotto R., Duner E., Denson L.A., Sanz J.M., Di Virgilio F., Okolicsanyi L., Casagrande F., Strazzabosco M. Proinflammatory cytokines inhibit secretion in rat bile duct epithelium. Gastroenterology. 2001;121:156–169. doi: 10.1053/gast.2001.25516. [DOI] [PubMed] [Google Scholar]
- 33.Alvaro D., Mennone A., Boyer J.L. Role of kinases and phosphatases in the regulation of fluid secretion and Cl-/HCO3- exchange in cholangiocytes. Am J Physiol Gastrointest Liver Physiol. 1997;273:G303–G313. doi: 10.1152/ajpgi.1997.273.2.G303. [DOI] [PubMed] [Google Scholar]
- 34.Alvaro D., Mancino M.G., Glaser S., Gaudio E., Marzioni M., Francis H., Alpini G. Proliferating cholangiocytes: a neuroendocrine compartment in the diseased liver. Gastroenterology. 2007;132:415–431. doi: 10.1053/j.gastro.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 35.Chen B., Jefferson D.M., Cho W.K. Characterization of volume-activated chloride currents in regulatory volume decrease of human cholangiocyte. J Membr Biol. 2010;235:17–26. doi: 10.1007/s00232-010-9252-7. [DOI] [PubMed] [Google Scholar]
- 36.Dutta A.K., Khimji A.K., Kresge C., Bugde A., Dougherty M., Esser V., Ueno Y., Glaser S.S., Alpini G., Rockey D.C., Feranchak A.P. Identification and functional characterization of TMEM16A, a Ca2+-activated Cl- channel activated by extracellular nucleotides, in biliary epithelium. J Biol Chem. 2011;286:766–776. doi: 10.1074/jbc.M110.164970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dutta A.K., Woo K., Khimji A.K., Kresge C., Feranchak A.P. Mechanosensitive Cl- secretion in biliary epithelium mediated through TMEM16A. Am J Physiol Gastrointest Liver Physiol. 2013;304:G87–G98. doi: 10.1152/ajpgi.00154.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakanuma Y., Sasaki M., Harada K. Autophagy and senescence in fibrosing cholangiopathies. J Hepatol. 2015;62:934–945. doi: 10.1016/j.jhep.2014.11.027. [DOI] [PubMed] [Google Scholar]
- 39.McDaniel K., Meng F., Wu N., Sato K., Venter J., Bernuzzi F., Invernizzi P., Zhou T., Kyritsi K., Wan Y., Huang Q., Onori P., Francis H., Gaudio E., Glaser S., Alpini G. Forkhead box A2 regulates biliary heterogeneity and senescence during cholestatic liver injury in mice‡. Hepatology. 2017;65:544–559. doi: 10.1002/hep.28831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sato K., Meng F., Venter J., Giang T., Glaser S., Alpini G. The role of the secretin/secretin receptor axis in inflammatory cholangiocyte communication via extracellular vesicles. Sci Rep. 2017;7:11183. doi: 10.1038/s41598-017-10694-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alpini G., Glaser S.S., Ueno Y., Pham L., Podila P.V., Caligiuri A., LeSage G., LaRusso N.F. Heterogeneity of the proliferative capacity of rat cholangiocytes after bile duct ligation. Am J Physiol Gastrointest Liver Physiol. 1998;274:G767–G775. doi: 10.1152/ajpgi.1998.274.4.G767. [DOI] [PubMed] [Google Scholar]
- 42.Han Y., Glaser S., Meng F., Francis H., Marzioni M., McDaniel K., Alvaro D., Venter J., Carpino G., Onori P., Gaudio E., Alpini G., Franchitto A. Recent advances in the morphological and functional heterogeneity of the biliary epithelium. Exp Biol Med (Maywood) 2013;238:549–565. doi: 10.1177/1535370213489926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klein S., Klosel J., Schierwagen R., Korner C., Granzow M., Huss S., Mazar I.G., Weber S., van den Ven P.F., Pieper-Furst U., Furst D.O., Nattermann J., Lammert F., Sauerbruch T., Trebicka J. Atorvastatin inhibits proliferation and apoptosis, but induces senescence in hepatic myofibroblasts and thereby attenuates hepatic fibrosis in rats. Lab Invest. 2012;92:1440–1450. doi: 10.1038/labinvest.2012.106. [DOI] [PubMed] [Google Scholar]
- 44.Lunz J.G., 3rd, Contrucci S., Ruppert K., Murase N., Fung J.J., Starzl T.E., Demetris A.J. Replicative senescence of biliary epithelial cells precedes bile duct loss in chronic liver allograft rejection: increased expression of p21(WAF1/Cip1) as a disease marker and the influence of immunosuppressive drugs. Am J Pathol. 2001;158:1379–1390. doi: 10.1016/S0002-9440(10)64089-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Feng X.X., Luo J., Liu M., Yan W., Zhou Z.Z., Xia Y.J., Tu W., Li P.Y., Feng Z.H., Tian D.A. Sirtuin 6 promotes transforming growth factor-beta1/H2O2/HOCl-mediated enhancement of hepatocellular carcinoma cell tumorigenicity by suppressing cellular senescence. Cancer Sci. 2015;106:559–566. doi: 10.1111/cas.12632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Senturk S., Mumcuoglu M., Gursoy-Yuzugullu O., Cingoz B., Akcali K.C., Ozturk M. Transforming growth factor-beta induces senescence in hepatocellular carcinoma cells and inhibits tumor growth. Hepatology. 2010;52:966–974. doi: 10.1002/hep.23769. [DOI] [PubMed] [Google Scholar]
- 47.Zerlanko B.J., Bartholin L., Melhuish T.A., Wotton D. Premature senescence and increased TGFbeta signaling in the absence of Tgif1. PLoS One. 2012;7:e35460. doi: 10.1371/journal.pone.0035460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li C.Y., Suardet L., Little J.B. Potential role of WAF1/Cip1/p21 as a mediator of TGF-beta cytoinhibitory effect. J Biol Chem. 1995;270:4971–4974. doi: 10.1074/jbc.270.10.4971. [DOI] [PubMed] [Google Scholar]
- 49.Moustakas A., Kardassis D. Regulation of the human p21/WAF1/Cip1 promoter in hepatic cells by functional interactions between Sp1 and Smad family members. Proc Natl Acad Sci U S A. 1998;95:6733–6738. doi: 10.1073/pnas.95.12.6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
By hematoxylin and eosin staining, the structure of stomach, small intestine, large intestine, pancreas, lung, spleen, and kidney organs from wild-type (WT) and Sct−/−/SR−/− mice after sham and bile duct ligation (BDL) appear comparable and do not show pathologic alterations. Original magnification, ×25.
A: There is enhanced protein expression of collagen I, transforming growth factor-β1 receptor (TGF-β1R), p16, and vascular endothelial growth factor-A (VEGF-A) in isolated cholangiocytes from bile duct ligated (BDL) wild-type (WT) mice that is reduced in Sct−/−, SR−/−, and Sct−/−/SR−/− BDL compared with BDL WT mice. Immunoblots in cholangiocytes from three separate animals. B: There is reduced expression of miR-125b and enhanced mRNA expression of VEGF-A in isolated cholangiocytes from BDL mice compared with WT mice; values returned to values similar to those of WT mice in Sct−/−, SR−/−, and Sct−/−/SR−/− BDL mice. C: There is increased mRNA expression of TGF-β1 in laser capture microdissection–isolated Kupffer cells from BDL WT compared with WT mice, which is reduced in Sct−/−, SR−/−, and Sct−/−/SR−/− BDL mice. PCR data (of three evaluations) from three cumulative cell preparations from three mice. By immunohistochemistry, there is an increase of macrophages in the connective tissue around the proliferating bile ducts and peribiliary plexus in BDL WT mice that is reduced in Sct−/−, SR−/−, and Sct−/−/SR−/− BDL mice (black arrows depict bile ducts). Data are expressed as means ± SEM (A–C). n = 3 (A); n = 9 (C). ∗P < 0.05 versus WT mice; †P < 0.05 versus BDL WT mice. Original magnification, ×20 (C).