Abstract
Trauma causes spinal cord injury, and the devastating consequences of the injury are due to the failure of the damaged central nervous system (CNS) axons to regenerate. Previous studies have shown that olfactory ensheathing cells (OECs) are a unique type of glial cell and they can promote regeneration of CNS axons to aid recovery after spinal cord injury. Transplantation of OECs, in particular from the olfactory bulb (OB), is considered one of the most promising therapeutic strategies for the repair of CNS injuries, including spinal cord injury. Transplantation of OECs can be autologous or allogenic. Here we focused on the less invasive and more error-proof allograft approach which needs a collection of donor OB tissue for OEC production. In this study, we investigated the effects on the yield and proportions of OECs and olfactory nerve fibroblasts (ONFs) from storing OB tissue in various media for periods of 24 and 48 hours. The OEC yield contributes to the viability of a successful cell transplant. We concluded that storing OB tissue for a period longer than 24 hours negatively impacted the total cell number and subsequently the OEC population. This study provides useful information for future clinical applications.
Keywords: Glia, translation, olfactory ensheathing cells, cell therapy, CNS, spinal injury
Introduction
Nerve fibres in the adult central nervous system (CNS) lack the capacity to regenerate after injury. Spinal cord injury is a result of trauma which can lead to loss of voluntary movement, sensation and or dysfunction of the autonomic functions. The spinal cord is unable to repair itself after trauma and needs therapeutic intervention to aid regeneration and restore the loss of function.
The sensory neurons in the olfactory system are replaced throughout adult life, and the newly formed axons elongating in the peripheral nerve region enter the CNS and terminate at the olfactory bulb (OB)1,2. The ability of olfactory axons to enter the OB is thought to be assisted by specialized glial cells known as olfactory ensheathing cells (OECs)3–6. Transplantation of OECs has been used in animal models and recent clinical application. OECs can promote anatomical repair and functional restoration7–13. It is one of the most promising current candidates for treating spinal cord injuries.
For clinical application, OECs can be sourced from the patient’s olfactory tissue for autologous transplantation or donor banks for allogeneic transplantation13,14. The autologous approach has the advantage of avoiding an immune response that rejects the graft and Graft-versus-Host Disease (GVHD)15,16. However, this approach requires a second surgery and also presents the risk of poor cell production due to tissue variation from patient to patient. Although an allograft presents the risk of immune rejection and GVHD, on balance, the advantage over an autograft lies in (1) having only one surgery for the patient and (2) ensuring the quality and quantity of the cells.
The present study examined whether storing OB tissue before culturing can be achieved without compromising the viability of the cell culture. We used the established OEC culturing protocol that has been well documented5,6,17,18 but we prepared the cell culture either immediately after OB was collected, or cells were stored in Hank’s balanced salt solution (HBBS: Thermo Fisher, 14175-053, Paisley, Scotland, UK), Dulbecco’s modified Eagle’s medium F/12 nutrient (DMEM/F12: Thermo Fisher, 31331-028, Paisley, Scotland, UK) and DMEM with 10% fetal bovine serum FBS: Thermo Fisher, 10500064, EU Approved [South American] (DMEMF); all medium commonly used in some capacity when culturing OB tissue19,20. HBSS is commonly used to keep OB tissue cold and balanced once harvested before digestion. DMEM is the basal medium universally used for culturing OB tissue, and the addition of 10% fetal bovine serum (DMEMF) is used to maintain the culture.
Serum is an essential source for stimulating growth and proliferation as it contains growth and adhesion factors. In addition to this, it contains hormones, lipids, and minerals which play a part in regulating cell membrane permeability21,22. The use of serum in cell culture can be advantageous for cell growth however it presents disadvantages including high costs, problems with standardization and variability21–23. Fetal bovine serum is used to supplement DMEM in culturing OB tissue and has been shown to promote growth and proliferation of OECs21,22,24. Studies have shown that serum-free culturing of OB affect the OEC population and morphology5,25,26 and so we were interested in determining if there would be a need for serum in storing store OB tissue as is needed in culturing OB tissue.
To characterize the cell cultures, we analyzed total cell numbers, subpopulations of OECs, and olfactory nerve fibroblasts (ONFs) while observing the morphological properties.
Materials and Methods
The use of the rats and the protocols employed for culturing were performed in accordance with the United Kingdom (UK) Home Office regulations and guidelines. For this study, male adult Sprague–Dawley rats ∼250 g (Charles River, UK) were used for all cultures.
Tissue Collection for Storage
Rats were terminally anesthetized, and the heads were removed from the body for tissue collection in sterile condition. OBs were isolated through dorsal craniotomy and collected in pairs, with an average bulb weighing 71±5 mg. The OBs were culture immediately (0 hour) or stored in HBSS (Thermo Fisher, 14175-053, Paisley, Scotland, UK), DMEM/F12 (Thermo Fisher, 31331-028, Paisley, Scotland, UK) and DMEM/F12 with 10% fetal bovine serum (Thermo Fisher, 10500064, EU Approved [South American]) for 24 hours and 48 hours at 4°C before culturing. All media were supplemented with penicillin/streptomycin (100U/ml, 100μg/ml) (PS: Thermo Fisher, 15140148, Paisley, Scotland, UK) and herein are characterized respectively as HBSS, DMEM, and DMEMF. All the media were chilled to 4°C. Experiments were performed four times for each condition n = 64.
Preparation of OEC Culture
After the meninges were peeled off the OBs, the tissue was then cut into 2 mm2 fragments and digested with 0.1mg/µl trypsin (Thermo Fisher, 15400054, Grand Island, New York) in HBSS/PS and incubated at 37°C for 15 min. After terminating trypsinization with the addition of serum-containing medium, the tissue fragments were triturated with a 1 ml plastic pipette and collected through a 0.70 µm cell strainer (Falcon, 352350, VWR UK) as a single cell suspension. The cell suspension was centrifuged for 5 min at 250 g, and the pellet resuspended in DMEMF. After counting on a cell counter (Countess, Invitrogen, Thermo Fisher, UK), the cells were seeded onto poly-D-lysine (Sigma Aldrich, P6407, Saint Louis, MO, USA) coated 2D 35 mm Nunc dishes at a density of 4.5 × 106/ml–7.25 × 106/ml. The cells were left for 5 days before changing the media for the first time due to the OEC’s weak adhering property. The cells were cultured in DMEMF for the duration of culturing, and the medium was replaced every other day. All cultures were maintained in a humidified incubator enriched with 5% CO2 at 37° for 14 days when the culture became 100% confluent.
Immunohistochemistry
The cells were fixed for 30 minutes in ice-cold 4% paraformaldehyde solution in 0.1 M phosphate buffer (PB). The dishes were washed 4× with 0.01 M phosphate-buffered saline (PBS), incubated in a blocking buffer consisting of 2% skimmed milk (Oxoid LP6031) and 1% Triton X-100 (Fisher Bioreagents, BP151-500, Fairlawn, NJ, USA) in 0.01 M PBS for 60 min. The cells were then incubated at 4°C overnight, using a cocktail of primary antibodies: mouse monoclonal anti-nerve growth factor receptor (MAB365 p75NGFR; 1:500, Millipore, Temecula, CA, USA) and rabbit polyclonal anti-human fibronectin (1:1000, A0245: Dako, Denmark). Subsequently, fluorescent conjugated secondary antibodies (goat anti-mouse Alexa-488, and goat anti-rabbit Alexa-546, both 1:500, Molecular Probes, Invitrogen) were applied to the dishes for 1 h at room temperature. Following this, all dishes were counterstained using Prolong Gold antifade reagent containing the nuclear dye 40, 6-diamidino-2-phenylindole (DAPI; 1mg/ml; Invitrogen, P36935).
Data Collection and Analysis
Images were captured with a Nikon Eclipse 55i microscope at 100× image sample size calibrated as 0.86 mm × 0.64 mm. The study included three storage conditions HBSS, DMEM, and DMEMF at two time points, 24 and 48 hours, in addition to the control of 0 hour. Each storage condition at a time point was regarded as one repeat procedure. For this study, there were four repeats for all storage condition at a time point including control. A total of 16 sample images were taken from each repeat, hence the total sample number was n = 448.
The total cell number was counted manually using the Fiji software-cell counter plugin. All DAPI-stained nuclei were counted including those cut off by the edges of the area of interest.
OEC and ONF cell numbers were identified by calculating the number of p75NGFR-positive cells and fibronectin-positive cells respectively as a proportion of the total cell count using the colocalization plugin in Fiji software. Images were analysed as an 8 bit; compositing and masking DAPI-stained nuclei onto p75NGFR stained OEC cells and fibronectin stained ONFs. The nuclei of OECs and ONFs were different sizes and parameters were predetermined and set at a range of 3.03 × 10-6 - 1mm2 for OECs and 2 x 10-5 - 1mm2 for ONF. The analysed particles plugin was used to count the numbers of OECs and ONFs after colocalization compositing and masking.
Statistical Analysis
All data are presented as mean ± SEM from the indicated number of experiments. Statistical analysis was performed on SPPS Statistics 24 using one-way multivariate analysis of variance to determine F-ratio significance followed by Tukey’s post-hoc test. Statistical significance was accepted at p < 0.05.
Results
Cell cultures from OBs that were immediately prepared after harvesting are termed 0 hour. Cell preparation after storing OBs in HBSS, DMEM and DMEMF for 24 and 48 hours are termed respectively as 24 HBSS, 48 HBSS, 24 DMEM, 48 DMEM, 24 DMEMF, and 48 DMEMF.
Effect on Total Cell Number by Storing OB Tissue
Total cell numbers for each condition was an average of all 16 samples taken from each repeat of the condition.
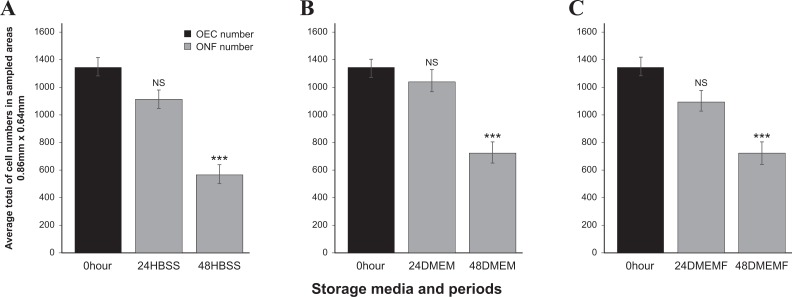
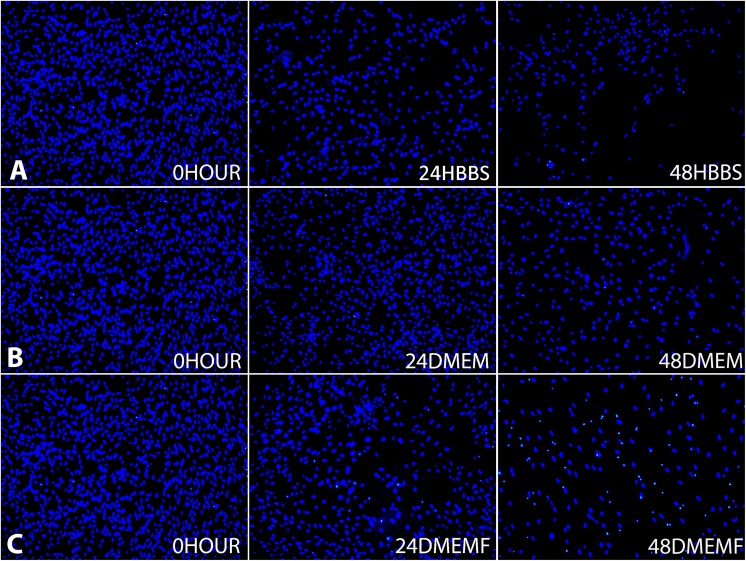
The total cell number for the 0 hour culture (1351±65) was the highest obtained. The storage of OBs in HBSS showed that the decline of the average cell number to 1114±68 in 24 HBSS was not statistically significant, on the other hand, the apparent reduction to 570±67 in 48 HBSS was (Fig. 1a). The storage of OBs in DMEM showed that although at 24 DMEM there was no significant loss, but there was in 48 DMEM, 737±80 (Fig. 1b). For the storage of OBs in DMEMF, the average total cell number showed no significant loss at 24 DMEMF. However, the numbers significantly declined to 724±80 at 48 DMEMF (Fig. 1c). The fluorescent micrograph shows the trend of average total cell numbers dropping drastically at 48 hours (Fig. 2).
Fig. 1.
Comparison of the total cell numbers in different storage media and time (a) 0 hour versus 24 HBSS and 48 HBSS; (b) 0 hour versus 24 DMEM and 48 DMEM (c) 0 hour versus 24 DMEMF and 48 DMEMF.
*p < 0.05, **p < 0.01 and ***p < 0.001, NS, not significant, n = 448.
Fig. 2.
Representative fluorescence micrographs of cells stained with DAPI at DIV14 show changes of cell density in different storage media and time: (a), 0 hour versus 24 HBSS and 48 HBSS; (b), 0 hour versus 24 DMEM and 48 DMEM and (c), 0 hour versus 24 DMEMF and 48 DMEMF. DAPI, 6-diamidino-2-phenylindole; DMEM, Dulbecco’s modified Eagle’s medium F/12 nutrient; DMEMF, DMEM with 10% fetal bovine serum; HBSS, Hank’s balanced salt solution.
Effects of Storage Conditions and Time on OEC and ONF Cell Number and Morphology
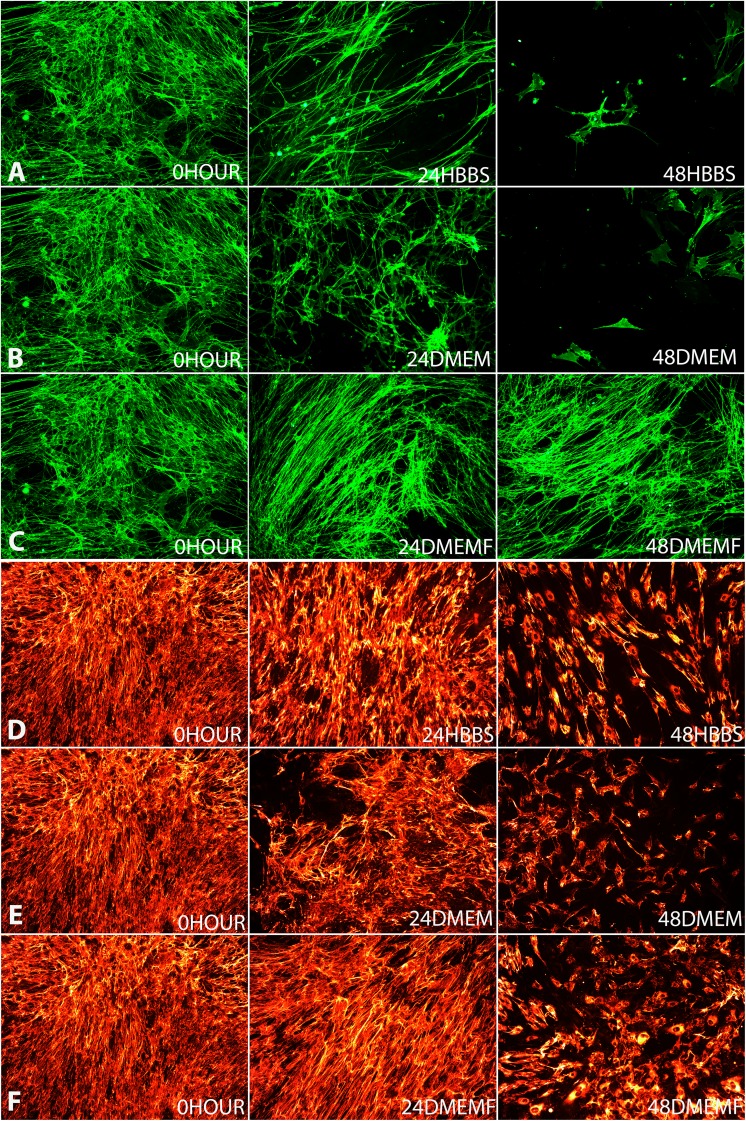
The populations of OECs and ONFs were also presented as average cell number obtained from the 16 samples taken from each repeat of the condition. The morphology of OECs and ONFs are shown in fluorescent micrographs (Fig. 4) to reveal the differences in morphology after storage.
Fig. 4.
Representative fluorescence micrographs of cells stained with anti-p75 NGF receptor antibody at DIV14 show changes of cell morphology in different storage media and time: Row A, 0hours vs 24HBBS and 48HBSS; Row B, 0hour vs 24DMEM and 48DMEM and Row C, 0hour vs 24DMEMF and 48DMEMF and representative fluorescence micrographs of cells stained with anti-fibronectin antibody at DIV14 show changes of cell morphology in different storage media and time: Row D, 0hours vs. 24HBBS and 48HBSS; Row E, 0hour vs. 24DMEM and 48DMEM and Row F, 0hour vs. 24DMEMF and 48DMEMF.
OECs
Population
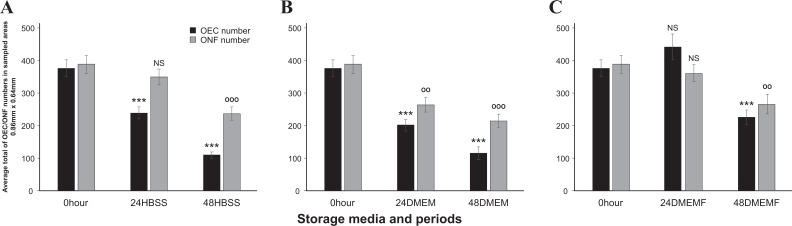
The average OEC population counted in the 0 hour culture was 375±25, and all but 24 DMEMF showed significant decline from that of the 0 hour culture. The storage of OBs in HBSS caused a considerable reduction in the OEC population with the average decreasing to 237±19 in 24 HBSS and then further decreasing more than 50% to 108±11 in 48 HBSS (Fig. 3a). The storage of OBs in DMEM caused the OEC average population to decrease to 200±16 at 24 DMEM and decreasing further to more than 50% to 115±19 at 48 DMEM (Fig. 3b). The storage of OBs in DMEMF caused a decline of the OEC population but only at 48 hours with the average population falling to 224±22 at 48 DMEMF (Fig. 3c).
Fig. 3.
Comparison of OEC and ONF cell numbers in different storage media and time (a) 0 hour versus 24 HBSS and 48 HBSS; (b) 0 hour versus 24 DMEM and 48 DMEM (c) 0 hour versus 24 DMEMF and 48 DMEMF. *p < 0.05, **p < 0.01 and ***p < 0.001 for OECs, p < 0.05, p < 0.01 and p < 0.001 for ONF and NS, not significant, n = 448. DMEM, Dulbecco’s modified Eagle’s medium F/12 nutrient; DMEMF, DMEM with 10% fetal bovine serum; HBSS, Hank’s balanced salt solution; OEC, olfactory ensheathing cells; ONF, olfactory nerve fibroblast.
Morphology
Morphological changes to OECs showed that at 24 hours there were fewer cells, albeit the morphology remained mostly unchanged from multipolar and bipolar (Fig. 4a–c). However, at 48 hours the three conditions did deviate from 0 hour with 48 HBSS and 48 DMEM showing less more cells that were flat and stellate (Fig. 4a–c). On the other hand, the cells in 48 DMEMF remained bipolar although also with fewer cells (Fig. 4c).
ONF
Population
The average ONF population obtained from the 0 hour culture was 388±28 where 24 HBSS and 24 DMEMF showed no sign of decline in cell numbers. The ONF population in HBSS significantly declined at 48 HBSS to 235±25 (Fig. 3a). In DMEM the ONF population decreased significantly from 0 hour with 24 DMEM at 263±22 and dropped even further to 213±20 for 48 DMEM (Fig. 4b). The average ONF population decreased only at 48hours in DMEMF, falling to 265±30 (Fig. 3c).
Morphology
Morphological changes for ONF showed less variation compared with the OECs at 24 hours. There was not much change in the distribution of fibronectin (Fig. 4d–f). However, at 48 hours the three conditions deviated from the morphology of 0 hour. There were more cells in 48 DMEMF than 48 HBSS and 48 DMEM which can be seen by the presence of the cell bodies (Fig. 4d–f).
Discussion
In this study, we used rat OBs to establish if there is a relationship between the OB storage condition at two time points and the cell culture quality. These data provide useful information for the future use of autologous, particularly allogenic OECs transplants to repair CNS injuries including spinal cord. Allogenic OEC transplantation is possible by establishing donor banks from cadavers therefore eliminating the risk of low yield and or failure of cell preparation27. It is vital to establish to what extent the quality of the cells produced are compromised and whether the surgical intervention can be justified after such compromise.
Storage Conditions and the Time Delay Before Culture Affects Total Cell Number
Storing OBs rather than immediate culture (0 hour) causes a loss to the cell population (Fig. 2). HBSS, DMEM and DMEMF at 24 hours showed no significant loss in average cell number, falling by 18%, 7% and 18% respectively. However, at 48 hours the average cell number fell significantly for HBSS, DMEM and DMEMF with a loss of more than 50% for HBSS, a 45% cell number loss for DMEM and 46% for DMEMF. At 48 hours, all storages in any media showed a considerable loss in cell numbers compared with 0 hour; however, that of HBSS was the most severe with a loss in cell numbers of 58%. Further to this, the fall in cell numbers in 48 DMEM and 48 DMEMF were very similar while 48 HBSS shows a difference of more than 10% from them. The lower cell numbers in HBSS suggest that the lack of any kind vitamins, amino acids and glucose in HBSS may be the cause for this28.
Storage Conditions and the Time Delay Before Culture Affects OECs and ONFs Cell Numbers and Morphologies
The morphology of subpopulations of OECs and ONFs are affected differently by the time and the storage media. The morphological changes are visible (see Fig. 4a–f). Overall the ONF population is more robust than that of the OEC population (Fig. 3) also showing less morphological variation than that of the OEC population (Fig. 4a–f).
OEC number and morphological changes
The established protocol of culturing OBs makes use of DMEMF mainly because of the serum. HBSS and DMEM may be used in the process of harvesting to culture but not for feeding the cells, as is the use of DMEMF19,20. At 24 and 48 hours in HBSS and DMEM, the OEC population declines which suggests that HBSS and DMEM are not ideal for storage in preserving the OEC population. OECs are better able to form elongated spindled bipolar and multipolar structures contributing to a dense network when they are highly populated25,26. In HBSS the OEC population drops by 37% at 24 hours and then by 71% at 48 hours; a large cell loss is reflected by the morphological changes seen in Fig. 4a. As expected at 0 hour (control) the cells are elongated bipolar and multipolar structures, interconnecting and overlapping to form a dense cellular network5 whereas in 24 HBSS there is no such overlapping or interconnecting and hence no dense cellular network and furthermore in 48 HBSS cells appear flat, stellate and singular forming no cellular network. In DMEM there is a similar loss to that of HBSS where the OEC population drops by 47% at 24 hours and then by 69% at 48 hours. Again, this loss is reflected by the morphological changes with 24 DMEM showing a less dense cell network than 0 hour but still with visible overlapping and interconnecting bipolar cells whereas 48 DMEM showed a more significant loss with cells becoming singular and flat unable to form a network (Fig. 4b). In DMEMF at 24hours, there was an increase in OECs; data analysis indicates that this increase was not significant; however, it can be still be concluded that it is the only storage condition where there was no decline in OEC population. Nevertheless, at 48 hours DMEMF there was a loss of OEC cell numbers, falling significantly by 40%. DMEMF presented the smallest reduction in the OEC population at 48 hours which indicates that OECs are best preserved in DMEMF, which is illustrated by Fig. 4c where the morphological changes are minimal, cells staying multipolar and bipolar from 0 hour through to 48 hours. Overall, DMEMF allows for the OEC population and morphology to be maintained for longer periods than in HBSS and DMEM. It appears that the serum contributes to the stability of OECs5,25.
ONF cell number and morphological changes
The ONF population overall is more stable than that of OECs which is also indicated by the morphology changes. In HBSS the considerable fall of ONFs at 48 hours is reflected in the morphology. At 24 HBSS fibronectin expression changes minimally from 0 hour, where it is hard to notice the difference but at 48 HBSS there is a clear difference with the coverage of fibronectin now limited to cell bodies (Fig. 4d), a reoccurring trend with all 48 hour conditions; suggesting that ONFs are more robust in HBSS than OECs. In DMEM ONF numbers fell considerably by 32% at 24 hours and then by 45%, suggesting that ONFs are better maintained in HBSS than DMEM; however, still more robust than OECs are in DMEM. Again, the morphology effects are not seen until 48 hours where the least cell bodies are stained for fibronectin. In DMEMF ONF are similar to OECs in that they are better conserved with an insignificant loss of 7% cell loss at 24 hours and 32% at 48 hours. Although morphology representation shows that there is no visible difference between 0 hour and 24 DMEMF similarly to 24 HBSS and DMEM, it does show that at 48 hour DMEMF has the most cell bodies and fibronectin coverage (Fig. 4d–F). Overall, it can be suggested that the serum also contributes to the stability of ONF.
In conclusion, it would be ideal to culture immediately after harvesting OB tissues to obtain higher numbers of valued axon growth promoting OECs. However, this may not be clinically applicable, and so it is imperative to establish and standardize the optimal conditions required to store OB tissues. In this study, we have established that the period of 24 hours should be the practical limit and that although there is cell loss overall, the OEC and ONF population morphologically remains mostly unaffected at this time period. Furthermore, it would seem that DMEMF is the best protective storage condition to keep OEC loss at its lowest while also consequentially protecting the ONF population. We did not investigate the functional capabilities of the cultures in the present study, and further investigation would involve using the cultured cells in animal models to assess the effects of storage conditions.
Supplementary Material
Footnotes
Ethical Approval: Animals were used with the approval of the University College London (UCL) Institute of Neurology.
Statement of Human and Animal Rights: Animals were used according to the UK Home Office regulations for the care and use of laboratory animals, the UK Animals (Scientific Procedures) Act 1986.
Statement of Informed Consent: Statement of Informed Consent is not applicable for this article.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by UK Stem cell foundation, and Nicholls Spinal injury foundation: nsif
References
- 1. Graziadei GA, Graziadei PP. Neurogenesis and neuron regeneration in the olfactory system of mammals. II. Degeneration and reconstitution of the olfactory sensory neurons after axotomy. J Neurocytol. 1979;8(2):197–213. [DOI] [PubMed] [Google Scholar]
- 2. Blanes T. Sobre algunes puntos dudosos de la estructura del bulbo olfactorio. Revista Trimestral Micrográfica. 3:99–127. [Google Scholar]
- 3. Raisman G. Specialized neuroglial arrangement may explain the capacity of vomeronasal axons to reinnervate central neurons. Neuroscience. 1985;14(1):237–254. [DOI] [PubMed] [Google Scholar]
- 4. Doucette R. PNS-CNS transitional zone of the first cranial nerve. J Comp Neurol. 1991;312(3):451–466. [DOI] [PubMed] [Google Scholar]
- 5. Ramon-Cueto A, Avila J. Olfactory ensheathing glia: properties and function. Brain Res Bull. 1998;46(3):175–187. [DOI] [PubMed] [Google Scholar]
- 6. Barnett SC. Olfactory ensheathing cells: unique glial cell types? J Neurotrauma. 2004;21(4):375–382. [DOI] [PubMed] [Google Scholar]
- 7. Plant GW, Harvey AR, Leaver SG, Lee SV. Olfactory ensheathing glia: repairing injury to the mammalian visual system. Exp Neurol. 2011;229(1):99–108. [DOI] [PubMed] [Google Scholar]
- 8. Raisman G. Repair of spinal cord injury by transplantation of olfactory ensheathing cells. C R Biol. 2007;330(6–7):557–560. [DOI] [PubMed] [Google Scholar]
- 9. Roet KC, Verhaagen J. Understanding the neural repair-promoting properties of olfactory ensheathing cells. Exp Neurol. 2014;261:594–609. [DOI] [PubMed] [Google Scholar]
- 10. Granger N, Blamires H, Franklin RJ, Jeffery ND. Autologous olfactory mucosal cell transplants in clinical spinal cord injury: a randomized double-blinded trial in a canine translational model. Brain. 2012;135(Pt 11):3227–3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lima C, Escada P, Pratas-Vital J, Branco C, Arcangeli CA, Lazzeri G, Maia CA, Capucho C, Hasse-Ferreira A, Peduzzi JD. Olfactory mucosal autografts and rehabilitation for chronic traumatic spinal cord injury. Neurorehabil Neural Repair. 2010;24(1):10–22. [DOI] [PubMed] [Google Scholar]
- 12. Lima C, Pratas-Vital J, Escada P, Hasse-Ferreira A, Capucho C, Peduzzi JD. Olfactory mucosa autografts in human spinal cord injury: a pilot clinical study. J Spinal Cord Med. 2006;29(3):191–203; discussion 204–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tabakow P, Jarmundowicz W, Czapiga B, Fortuna W, Miedzybrodzki R, Czyz M, Huber J, Szarek D, Okurowski S, Szewczyk P, Gorski A, Raisman G. Transplantation of autologous olfactory ensheathing cells in complete human spinal cord injury. Cell Transplant. 2013;22(9):1591–1612. [DOI] [PubMed] [Google Scholar]
- 14. Gorrie CA, Hayward I, Cameron N, Kailainathan G, Nandapalan N, Sutharsan R, Wang J, Mackay-Sim A, Waite PM. Effects of human OEC-derived cell transplants in rodent spinal cord contusion injury. Brain Res. 2010;1337:8–20. [DOI] [PubMed] [Google Scholar]
- 15. Shlomchik WD. Graft-versus-host disease. Nat Rev Immunol. 2007;7(5):340–352. [DOI] [PubMed] [Google Scholar]
- 16. Mason C, Dunnill P. Assessing the value of autologous and allogeneic cells for regenerative medicine. Regen Med. 2009;4(6):835–853. [DOI] [PubMed] [Google Scholar]
- 17. Li Y, Field PM, Raisman G. Regeneration of adult rat corticospinal axons induced by transplanted olfactory ensheathing cells. J Neurosci. 1998;18(24):10514–10524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Barber PC, Lindsay RM. Schwann cells of the olfactory nerves contain glial fibrillary acidic protein and resemble astrocytes. Neuroscience. 1982;7(12):3077–3090. [DOI] [PubMed] [Google Scholar]
- 19. Mayeur A, Duclos C, Honoré A, Gauberti M, Drouot L, do Rego JC, Bon-Mardion N, Jean L, Vérin E, Emery E, Lemarchant S, Vivien D, Boyer O, Marie JP, Guérout N. Potential of olfactory ensheathing cells from different sources for spinal cord repair. PLoS One. 2013;8(4):e62860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jiao Y, Novozhilova E, Karlen A, Muhr J, Olivius P. Olfactory ensheathing cells promote neurite outgrowth from co-cultured brain stem slice. Exp Neurol. 2011;229(1):65–71. [DOI] [PubMed] [Google Scholar]
- 21. Gstraunthaler G. Alternatives to the use of fetal bovine serum: serum-free cell culture. ALTEX. 2003;20(4):275–281. [PubMed] [Google Scholar]
- 22. Fang CY, Wu CC, Fang CL, Chen WY, Chen CL. Long-term growth comparison studies of FBS and FBS alternatives in six head and neck cell lines. PLoS One. 2017;12(6):e0178960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ito D, Fujita N, Ibanez C, Sasaki N, Franklin RJ, Jeffery ND. Serum-free medium provides a clinically relevant method to increase olfactory ensheathing cell numbers in olfactory mucosa cell culture. Cell Transplant. 2008;16(10):1021–1027. [PubMed] [Google Scholar]
- 24. Higginson JR, Barnett SC. The culture of olfactory ensheathing cells (OECs)--a distinct glial cell type. Exp Neurol. 2011;229(1):2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pellitteri R, Catania MV, Bonaccorso CM, Ranno E, Dell’Albani P, Zaccheo D. Viability of olfactory ensheathing cells after hypoxia and serum deprivation: implication for therapeutic transplantation. J Neurosci Res. 2014;92(12):1757–1766. [DOI] [PubMed] [Google Scholar]
- 26. Pellitteri R, Cova L, Zaccheo D, Silani V, Bossolasco P. Phenotypic Modulation and neuroprotective effects of olfactory ensheathing cells: a promising tool for cell therapy. Stem Cell Rev. 2016;12(2):224–234. [DOI] [PubMed] [Google Scholar]
- 27. Ryszard M, Pawel T, Wojciech F, Bogdan C, Wlodzimierz J. The olfactory bulb and olfactory mucosa obtained from human cadaver donors as a source of olfactory ensheathing cells. Glia. 2006;54(6):557–565. [DOI] [PubMed] [Google Scholar]
- 28. Arora M. Cell culture media: a review. Mater Methods. 2013;3:175. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.