Abstract
Induced pluripotent stem cells (iPSCs), which are generated through reprogramming adult somatic cells by expressing specific transcription factors, can differentiate into derivatives of the three embryonic germ layers and accelerate rapid advances in stem cell research. Neurological diseases such as amyotrophic lateral sclerosis (ALS) have benefited enormously from iPSC technology. This approach can be particularly important for creating iPSCs from patients with familial or sporadic forms of ALS. Motor neurons differentiated from the ALS-patient-derived iPSC can help to determine the relationship between cellular phenotype and genotype. Patient-derived iPSCs facilitate the development of new drugs and/or drug screening for ALS treatment and allow the exploration of the possible mechanism of ALS disease. In this article, we reviewed ALS-patient-specific iPSCs with various genetic mutations, progress in drug development for ALS disease, functional assays showing the differentiation of iPSCs into mature motor neurons, and promising biomarkers in ALS patients for the evaluation of drug candidates.
Keywords: ALS, biomarker, drug screening, iPSC, motor neuron, neurodegenerative disease
Introduction
Amyotrophic lateral sclerosis (ALS), also known as Lou Gehrig’s disease, is a fatal disease with a survival time of less than 5 years and prevalence of 3–4 per 100,000 persons in the United States1,2. The neurodegenerative disorder was first described in the late 19th century and is characterized by the death of motor neurons in the brain, brainstem, and spinal cord, leading to paralysis3,4. Although ALS has been studied for many decades, scientific and clinical results have only partially revealed that genetic mutation may play a role in the onset of disease5–15. In addition to hereditary factors, an unregulated neural transmitter system and environmental factors are the major factors influencing the progression of ALS16. These broad and general causes have confounded the investigation of therapies.
Although most cases of ALS are the sporadic form (sALS) of unknown etiology, ∼25% of ALS cases are the familial form (fALS), which is clinically and pathologically indistinguishable from sALS17. More detailed studies have been conducted on fALS than on sALS. Dominantly inherited gene mutations associated with fALS such as cytosolic copper-zinc superoxide dismutase 1 (SOD1), hexanucleotide repeat (chromosome 9 open reading frame 72, C9ORF72), tar DNA-binding protein 43 (TDP-43), fused in sarcoma (FUS), and other less frequent genes contribute to considerable genetic heterogeneity6,17–23. Among the ALS-related gene mutations, SOD1 mutants are responsible for 20% of fALS24 and the synergistic effect of the mutant Alsin (also known as ALS2) and SOD1 are linked to the early onset of ALS7,25. Genetically engineered cell lines or animal models for these genetic mutations are used to investigate the development of new drugs and to verify drug efficacy.
ALS modeling rodents or embryonic stem cells (ESCs) expressing ALS-causing genes have been generated to represent a specific subset of the syndrome in an effort to understand the onset and progression of ALS disease22,26–32. However, the transgene method alone cannot be used for mimicking the real physiology of motor neurons in sALS patients because it cannot completely correlate the genotype with the neuronal phenotype26,33. Because animal and stem cell models for elucidating ALS pathogenesis have demonstrated shortcomings in recapitulating sALS patients, their capacity and application for the evaluation of therapeutic efficacy remain unmet needs. As an alternative, researchers have obtained induced pluripotent stem cells (iPSCs) from patients with neurodegenerative diseases, which have allowed the study of these cells with specific cell differentiation2,34–36.
Human iPSCs can be produced by reprogramming the somatic cells for a state of embryonic-like stem cell via delivering the required transcription factors (Fig. 1A and B)37–39. Such iPSCs can be directed to generate different kinds of neural cell types. iPSCs obtained from patients with ALS symptoms can be investigated at the molecular level to probe the changes in degeneration/death of motor neurons that trigger destruction of the neuromuscular junction40–42. Therefore, differentiated cells from ALS patients, such as those of the motor neuron type, can be used to identify drug candidates for ALS treatment. The present review focuses on existing medication and medical needs of ALS patients, advantages of using patients’ iPSCs, scope for improvement in the approaches of motor neuron differentiation and functional assays, and strategies for the evaluation of drug efficacy in ALS disease.
Fig. 1.
Schematic illustration showing that iPSCs generated from ALS patients differentiate into motor neurons for functional and morphological analysis. (A) ALS patients’ somatic cells are collected with donors’ informed consent under institutional review board monitoring (A) and the cells can be reprogrammed to the induced pluripotent state (B). After motor neuron differentiation of ALS patient’s iPSCs (C), further studies of the disease can be performed to assess physiological properties (D), providing a link to the level of maturation. The examination of morphological changes such as neurite length (E) subcellular aggregate (F), and patient-specific samples can be used to characterize the pathogenesis of ALS in patient-derived iPSCs. Nu: nucleus.
Currently Available Treatments and Potential Small Molecules
Drugs Approved by the U.S. Food and Drug Administration for ALS Treatment
Riluzole is the first available compound known to attenuate the progression and partly relieve the symptoms of ALS via depressing N-methyl-D-aspartate (NMDA) receptor- and α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptor-mediated signaling43,44. Riluzole has several side effects and extends survival only by an average of 2–3 months, so more effective ALS therapy is urgently needed45,46. The recently approved molecule edaravone is a small-radical scavenger that can inhibit oxidative stress. Edaravone was initially used to treat acute ischemic stroke47. It quenches the hydroxyl radical functional group and downregulates both radical-dependent and radical-independent lipid peroxidation48. Previous results showed that edaravone could delay the degeneration of motor neurons in a murine model with mutated SOD1 expression49 and data from a phase 2 trial in ALS patients consistently reported the effect of the drug in reducing oxidative stress50. Although evidence supported these positive outcomes in a phase 3 trial of edaravone, the inconvenience of intravenous administration and the extremely restricted population of ALS patients caused physicians not to prescribe the drug51. In addition to the inconvenience and high treatment cost, the long-term efficacy and toxicity profile of edaravone have not yet been established. Therefore, the clinical needs and challenges in clinical trials for ALS patients still exist and much more efficient protocols for drug screening are required.
Small-Molecule Development for Clinical Application Targeting ALS
Because of extensive studies investigating small molecules, exploring mechanisms, and synthesizing new compounds, the scope of drug discovery is changing and scientists can combine the power of synthetic chemistry with advanced screening technologies for small-molecule development. These small molecules can be covalent bonding inhibitors, protein–protein interaction modulators, protein conformational allosteric regulators, or epigenetic controllers and they sometimes have effects on multiple targets at one time52–54. For example, olaparib, an ADP-ribose polymerase inhibitor, and imatinib, a tyrosine kinase inhibitor, can significantly reduce ovarian cancer and chronic myeloid leukemia cells, respectively55,56. Furthermore, small molecules can potentially penetrate the blood–brain barrier into the central nervous system to treat patients with brain tumors or neurological diseases57. Creating small molecules that target mutant proteins associated with ALS may be promising candidates for the treatment of ALS.
Drug development based on neuroprotective properties such as reducing oxidative stress and excitotoxicity and eliminating toxic aggregates/fragments may benefit ALS patients. Recently, several groups have shown that autophagy plays an important role in ALS pathogenesis58–60. The autophagy–lysosome machinery is the major system for removing abnormal aggregates through cytoplasmic protein degradation61. One of these molecules that has been preferentially examined using in vitro and in vivo ALS models and shows promise is a purified compound from a plant used in traditional Chinese medicine (Angelica sinensis), n-butylidenephthalide (n-BP). Evidence has suggested that accumulated mutant SOD1 can initiate autophagy in an animal model59 and n-BP can extend the lifespans of the SOD1 murine model through modulating cellular autophagy and apoptosis in the spinal cord60. Furthermore, findings also indicated that n-BP could reduce the death of motor neurons, relieve muscular atrophy, suppress neuroinflammation, and recover gastrocnemius muscle59. Although the functions of n-BP in anti-apoptosis, anti-inflammation, anti-oxidation, and autophagy modulation have been revealed, the data were collected from mutated SOD1 in in vitro and in vivo models. Investigations based on ALS patients’ iPSCs may provide further information for the development of this drug candidate.
iPSCs Differentiate into Functional Motor Neuron Units
Differentiation into Motor Neurons
iPSCs can generate different subtypes of neural cells, such as neurons, motor neurons, astrocytes, and other cell types35,62–64; therefore, the characterization of these in vitro cells recapitulating in vivo ALS progression can be achieved. Previous studies reported that human ESCs have been differentiated successfully into motor neurons by exogenously expressing the mutated genes of ALS. The approaches of motor neuron differentiation have also been applied to iPSCs. fALS/sALS-derived motor neurons can be harvested and investigated for the development of new drugs (Fig. 1C). Crucial secreted signaling factors, such as bone morphogenetic protein (BMP), fibroblast growth factor (FGF), retinoic acid (RA), Sonic hedgehog (Shh), and Wnts and transforming growth factor-beta (TGFβ) signaling, which are involved in the patterning neural axis during brain development, have been used to direct iPSCs toward motor neurons65–68.
Motor neurons are classified as corticospinal upper motor neurons and spinal cord lower motor neurons (LMNs). The degeneration and death of motor neurons lead to the onset of neurodegenerative diseases such as ALS69–71. According to results obtained in different species67,72–75, signaling in the development of neuroectoderm has been shown to specify LMN fate66,68. The process of motor neuron differentiation is time consuming, expensive, and inefficient in the acquisition of cells. After making efforts to fine-tune the protocols, two small molecules, LDN193189 and SB431542, were found to accelerate the differentiation of motor neurons through the inhibition of both BMP and TGFβ signaling and blocking mesoderm and endoderm fates76. Therefore, more abundant motor neurons can be obtained from iPSCs in a shorter time period.
Functional Assay and Morphological Marker for Motor Neurons
The most extensive functional examination of differentiated motor neurons is detection of the electrophysiological action potential for evaluating the maturation of motor neurons in vitro 76 (Fig. 1D). During the early stages of neural differentiation, voltage-gated ion channels need to be induced to generate an action potential. In addition, spontaneous action potentials require time to develop after the maturation of motor neurons77. To achieve a high level of mature motor neurons, the transition between induced and spontaneously occurred action potentials requires the involvement of various factors such as synapse formation78,79. To determine the propagation of electrical signals, protocols to direct the differentiation of motor neurons from iPSCs with the capability of synapse formation is critical.
Because ALS pathogenesis presents heterogeneous causes accompanied by distinct mechanisms linked to the onset and progression of ALS, imaging-based approaches are required to determine cellular/molecular changes and drug effects on ALS disease80. For evaluation, information on cellular morphology can be obtained by automated data acquisition, thereby lowering the cost of drug screening in terms of labor. Both the fragmentation of neurites with reduced length and morphological swelling, the so-called bead-like structure resulting from abnormal protein aggregation (Fig. 1E and F)22,81–83, can be analyzed under the microscope. Such findings suggest that specific antibodies can be also used to label mutants for understanding the disease progression of ALS84. These protein aggregates are expressed in vivo in native form, so the antibodies may not be available to recognize targets correctly and represent the real distribution or localization.
Use of ALS Patient-Derived iPSCs for Drug Development
Drug-Screening Platform via iPSCs from ALS Patients
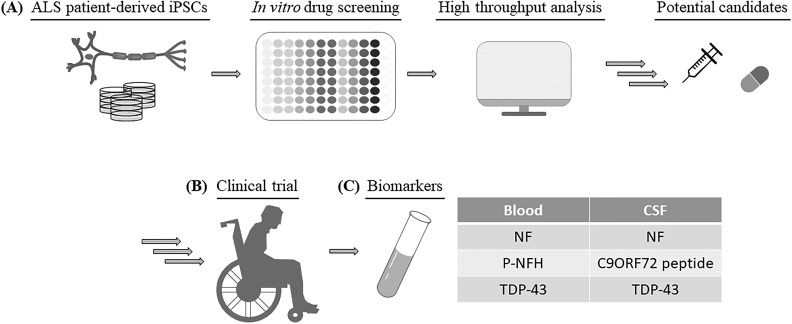
During the process of novel drug development, safety and efficacy testing are costly and time consuming. Many clinical trials of ALS have been withdrawn or failed due to unexpected toxicity or lack of efficacy85. The potential toxicities challenging clinical translation of drug candidates suggest that in vitro and in vivo nonhuman models might not be sufficient. To select effective therapies for ALS patients and to improve the success rate of drug selection, iPSCs have been used as models in many outstanding studies (Fig. 2A and Table 1)2,23,34,35,52,58,83,86–90. Egawa et al. recently reported an iPSC drug-screening assay in which they used several compounds targeting the epigenetic/transcriptional level of iPSC-derived motor neurons from ALS patients with the TDP-43 mutation2. One of the compounds, anacardic acid, was shown to reverse part of the ALS phenotype found in motor neurons. This work can be a stepping stone to toward using ALS-specific motor neurons to verify candidate drugs.
Fig. 2.
Combination of human iPSC approach, high-throughput drug screening, and biomarker application can potentiate clinical candidate selection. (A) iPSCs derived from patients with ALS can be used for high-content drug screening to identify pathways and targets of small molecules with therapeutic potential. After preclinical studies, these small molecules may enter clinical trials with suitable administration routes (B) and the use of biomarkers can be used to calculate and determine drug efficacy (C). P-NFH: phosphorylated heavy chain of NF.
Table 1.
ALS Cell Models from Disease-Derived iPSCs.
| ALS patient (responsible gene) | Neural cell type (differentiation marker) | Functional assay | Potential candidate | Author and year [reference number] |
|---|---|---|---|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
WB: Western blotting analysis; IF: immunofluorescence assay; FISH: fluorescence in situ hybridization; IHC: immunohistochemistry; FRAP: fluorescence recovery after photobleaching; Isl-1: Islet-1; HB9: Homeobox 9; Tuj1: beta III tubulin; SMI32: neurofilament H non-phosphorylated; CHAT: choline acetyltransferase; VGLUT1: vesicular glutamate transporter 1; NMDAR1: NMDA receptor 1; SYT1: synaptotagmin 1, SYP: synaptophysin; ALDH1: aldehyde dehydrogenase 1; GFAP: glial fibrillary acidic protein; ASO: anti-sense oligo-nucleotide; CDK: cyclin-dependent kinase; JNK: c-Jun N-terminal kinase
Motor neurons derived from patients with fALS or sALS were used to screen various kinase inhibitors, epigenetic modulators, anti-sense oligo-nucleotides (ASOs), or approved molecules for any other indication (Table 1). Furthermore, Wainger et al. used a high-throughput electrode array to detect the action potentials of variant ALS patients’ motor neurons (SOD1, C9ORF72, and FUS mutation) and revealed that retigabine could reduce the death of motor neurons by blocking hyperexcitability89. The drug candidates identified by such methods can confirm the toxicity and efficacy of drugs in humans and retigabine has entered a phase 2 trial for efficacy evaluation (NCT02450552; estimated completion date: February 2018). Importantly, ALS-patient-specific iPSCs present many benefits, including the correct genetic background and pluripotency. The results from the above-mentioned drug-screening studies have shown that high-throughput approaches can produce abundant and useful information that can expedite and increase the probability of success for drug candidates entering clinical trials91 (Fig. 2B).
Although iPSCs are mainly generated from skin fibroblasts or peripheral blood from ALS patients, the pathogenic phenotypes may disappear. These iPSCs may be reverted to the early stage pluripotent cells. Thus, the recapitulation of a late onset of neurodegenerative disease cannot be fully achieved92. To generate iPSCs associated with the characterization of aging, oxidative stress, DNA-damaging and proteasome modulator agents are being tested to produce high-level maturation of patient-specific iPSC derivatives93–95. Previous reports indicated that AMPA receptor, a glutamate receptor, could mediate excitatory transmission in the brain; therefore, glutamate insult accelerates the aging process by allowing high level of calcium ions to enter the neurons15,96. Such induction can be used to change the phenotypes of patient-derived iPSCs to mimic neurodegeneration. In addition, downregulation of phosphatase and tensin homolog deleted on chromosome 10 (PTEN) can attenuate neuron death by manipulating the trafficking of the GluR2 subunit of the AMPA receptor within the cell membrane97. Many small molecules modulating PTEN have been synthesized, so it may be a potential therapeutic strategy for medical intervention in ALS patients.
To verify the use of screening approaches and to develop reliable platforms for exploring the molecular underpinnings of ALS, multiple iPSC clones from different ALS patients and several markers are urgently required. Using at least two iPSC lines from patients with ALS in combination with automated and imaging-based techniques would acquire considerable information on cell morphology, formation of protein aggregates, and distribution/patterns of specific molecules by immunocytochemistry/immunocytochemistry or immunofluorescence staining. Moreover, high-throughput equipment has been used for examining small-molecule compounds in several studies. Nevertheless, there is no clear mechanism of neuron death that can clearly distinguish sALS from fALS. Large sample sizes are necessary to reveal the detailed and unique ALS pathogenesis, but the cost of reprogramming ALS patients’ fibroblasts for iPSCs remains high.
Biomarkers of Motor Neurons
Throughout the drug development process, biologically validated biomarkers are used to assess the subject’s response to a specific treatment. Biomarker analysis demonstrating the effect of the drug candidates on ALS targets is also important (Fig. 2C). Previous studies showed that CD40L, which is expressed by activated T cells and required for induced immune response, could be a treatment target for ALS patients. Moreover, inhibiting CD40L-mediated inflammatory responses can improve survival in animal studies98,99. In addition to altered inflammatory gene expression, monocyte-expressed, ALS-specific, inflammation-related microRNA or the unregulated complement system can be treated with specific antibodies or inhibitors to delay ALS progression, extend survival, and improve motor function in animal models100,101.
Abundant neurofilament (NF) proteins have been detected in the cerebral spinal fluid (CSF) or blood from SOD1 G93A mice102–104 (Fig. 2C). Among them, plasma containing the phosphorylated heavy chain of NF was thought to be associated with motor neuron death105,106 (Fig. 2C). Moreover, NF aggregates (bead-like structure) can also be observed in ALS-patient-specific iPSCs with mutation of SOD1 A4V or SOD1 D90A 81 (Fig. 1F).
Several studies have revealed that translational products of C9ORF72 repeats resulted in aggregation and were localized in the neurons of the central nervous system. This phenotype was seen in iPSC models107,108. When small molecules bind to the GGGGCC repeat sequence, they can inhibit the formation of translational aggregates in the differentiated motor neurons derived from iPSCs (Table 1). In addition, peptide aggregation has been found in CSF from ALS patients with the C9ORF72 mutation108 (Fig. 2C); therefore, this measurement is available for evaluating therapeutic efficacy.
Other reports about genetic mutations also found the formation of TDP-43 inclusion bodies, which was used as one of the biomarkers2,34,109. Using enzyme-linked immunosorbent assay-based approaches, the TDP-43 level was found to be increased significantly in plasma and CSF from patients with ALS compared with healthy individuals. Although the discovery of biomarkers to distinguish ALS patients and healthy people are highly challenging, much more effective therapies may be investigated and developed with more biomarkers
Conclusion
Neurodegenerative diseases have a potentially disastrous impact on quality of life, so ongoing efforts to develop novel therapeutic drug candidates are of utmost importance99,110–113. The use of genetic manipulation in cell systems and nonhuman animal models may not predict the potential toxicity and lack of efficacy accurately in humans85. Recent achievements in differentiation of patient-specific iPSCs into motor neurons have provided the basis to establish a platform to screen drug candidates, test toxicity, and confirm the efficacy of drugs in complicated fALS/sALS populations (Table 1). Although some constraints, such as the labor-intensive and high-cost procedure, protocols for generating fully mature motor neurons, detection methods of protein aggregates, applicable biomarkers, and basic mechanism of motor neuron death in sALS, need to be improved, the use of iPSC-dependent screening may accelerate the process of drug development. Following a systemic assessment (Fig. 2), studies of ALS may shed light on causes of disease and help to improve drug development in the future.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Everfront Biotech Inc.
References
- 1. Rothstein JD. Current hypotheses for the underlying biology of amyotrophic lateral sclerosis. Ann Neurol. 2009;65(suppl 1):S3–S9. [DOI] [PubMed] [Google Scholar]
- 2. Egawa N, Kitaoka S, Tsukita K, Naitoh M, Takahashi K, Yamamoto T, Adachi F, Kondo T, Okita K, Asaka I, Aoi T, Watanabe A, Yamada Y, Morizane A, Takahashi J, Ayaki T, Ito H, Yoshikawa K, Yamawaki S, Suzuki S, Watanabe D, Hioki H, et al. Drug screening for ALS using patient-specific induced pluripotent stem cells. Sci Transl Med. 2012;4(145):145ra104. [DOI] [PubMed] [Google Scholar]
- 3. Charcot JM. Deux cas d’atrophie musculaire progressive avec lesions de la substance grise et des faisceaux anterolaterax de la moelle epiniere. Pathol. 1869;2:354–367. [Google Scholar]
- 4. Dimos JT, Rodolfa KT, Niakan KK, Weisenthal LM, Mitsumoto H, Chung W, Croft GF, Saphier G, Leibel R, Goland R, Wichterle H, Henderson CE, Eggan K. Induced pluripotent stem cells generated from patients with ALS can be differentiated into motor neurons. Science. 2008;321(5893):1218–1221. [DOI] [PubMed] [Google Scholar]
- 5. Maruyama H, Morino H, Ito H, Izumi Y, Kato H, Watanabe Y, Kinoshita Y, Kamada M, Nodera H, Suzuki H, Komure O, Matsuura S, Kobatake K, Morimoto N, Abe K, Suzuki N, Aoki M, Kawata A, Hirai T, Kato T, Ogasawara K, Hirano A, et al. Mutations of optineurin in amyotrophic lateral sclerosis. Nature. 2010;465(7295):223–226. [DOI] [PubMed] [Google Scholar]
- 6. Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, Donaldson D, Goto J, O’Regan JP, Deng HX, Rahmani Z, Krizus A, McKenna-Yasek D, Cayabyab A, Gaston SM, Berger R, Tanzi RE, Halperin JJ, Herzfeldt B, Van den Bergh R, Hung WY, Bird T, et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362(6415):59–62. [DOI] [PubMed] [Google Scholar]
- 7. Yang Y, Hentati A, Deng HX, Dabbagh O, Sasaki T, Hirano M, Hung WY, Ouahchi K, Yan J, Azim AC, Cole N, Gascon G, Yagmour A, Ben-Hamida M, Pericak-Vance M, Hentati F, Siddique T. The gene encoding alsin, a protein with three guanine-nucleotide exchange factor domains, is mutated in a form of recessive amyotrophic lateral sclerosis. Nat Genet. 2001;29(2):160–165. [DOI] [PubMed] [Google Scholar]
- 8. Chen YZ, Bennett CL, Huynh HM, Blair IP, Puls I, Irobi J, Dierick I, Abel A, Kennerson ML, Rabin BA, Nicholson GA, Auer-Grumbach M, Wagner K, De Jonghe P, Griffin JW, Fischbeck KH, Timmerman V, Cornblath DR, Chance PF. DNA/RNA helicase gene mutations in a form of juvenile amyotrophic lateral sclerosis (ALS4). Am J Hum Genet. 2004;74(6):1128–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nishimura AL, Mitne-Neto M, Silva HC, Richieri-Costa A, Middleton S, Cascio D, Kok F, Oliveira JR, Gillingwater T, Webb J, Skehel P, Zatz M. A mutation in the vesicle-trafficking protein VAPB causes late-onset spinal muscular atrophy and amyotrophic lateral sclerosis. Am J Hum Genet. 2004;75(5):822–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Coyle JT, Puttfarcken P. Oxidative stress, glutamate, and neurodegenerative disorders. Science. 1993;262(5134):689–695. [DOI] [PubMed] [Google Scholar]
- 11. Lipton SA, Rosenberg PA. Excitatory amino acids as a final common pathway for neurologic disorders. New Engl J Med. 1994;330(9):613–622. [DOI] [PubMed] [Google Scholar]
- 12. Benveniste H, Drejer J, Schousboe A, Diemer NH. Elevation of the extracellular concentrations of glutamate and aspartate in rat hippocampus during transient cerebral ischemia monitored by intracerebral microdialysis. J Neurochem. 1984;43(5):1369–1374. [DOI] [PubMed] [Google Scholar]
- 13. Rosenberg PA, Amin S, Leitner M. Glutamate uptake disguises neurotoxic potency of glutamate agonists in cerebral cortex in dissociated cell culture. J Neurosci. 1992;12(1):56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hollmann M, O’Shea-Greenfield A, Rogers SW, Heinemann S. Cloning by functional expression of a member of the glutamate receptor family. Nature. 1989;342(6250):643–648. [DOI] [PubMed] [Google Scholar]
- 15. Keinanen K, Wisden W, Sommer B, Werner P, Herb A, Verdoorn TA, Sakmann B, Seeburg PH. A family of AMPA-selective glutamate receptors. Science. 1990;249(4968):556–560. [DOI] [PubMed] [Google Scholar]
- 16. Sutedja NA, Veldink JH, Fischer K, Kromhout H, Heederik D, Huisman MH, Wokke JH, van den Berg LH. Exposure to chemicals and metals and risk of amyotrophic lateral sclerosis: a systematic review. Amyotroph Lateral Scler. 2009;10(5–6):302–309. [DOI] [PubMed] [Google Scholar]
- 17. Wiedau-Pazos M, Goto JJ, Rabizadeh S, Gralla EB, Roe JA, Lee MK, Valentine JS, Bredesen DE. Altered reactivity of superoxide dismutase in familial amyotrophic lateral sclerosis. Science. 1996;271(5248):515–518. [DOI] [PubMed] [Google Scholar]
- 18. Majounie E, Renton AE, Mok K, Dopper EG, Waite A, Rollinson S, Chiò A, Restagno G, Nicolaou N, Simon-Sanchez J, van Swieten JC, Abramzon Y, Johnson JO, Sendtner M, Pamphlett R, Orrell RW, Mead S, Sidle KC, Houlden H, Rohrer JD, Morrison KE, Pall H, et al. Frequency of the C9orf72 hexanucleotide repeat expansion in patients with amyotrophic lateral sclerosis and frontotemporal dementia: a cross-sectional study. Lancet Neurol. 2012;11(4):323–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Barmada SJ, Skibinski G, Korb E, Rao EJ, Wu JY, Finkbeiner S. Cytoplasmic mislocalization of TDP-43 is toxic to neurons and enhanced by a mutation associated with familial amyotrophic lateral sclerosis. J Neurosci. 2010;30(2):639–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Corrado L, Del Bo R, Castellotti B, Ratti A, Cereda C, Penco S, Sorarù G, Carlomagno Y, Ghezzi S, Pensato V, Colombrita C, Gagliardi S, Cozzi L, Orsetti V, Mancuso M, Siciliano G, Mazzini L, Comi GP, Gellera C, Ceroni M, D’Alfonso S, Silani V. Mutations of FUS gene in sporadic amyotrophic lateral sclerosis. J Med Genet. 2010;47(3):190–194. [DOI] [PubMed] [Google Scholar]
- 21. Millecamps S, Salachas F, Cazeneuve C, Gordon P, Bricka B, Camuzat A, Guillot-Noël L, Russaouen O, Bruneteau G, Pradat PF, Le Forestier N, Vandenberghe N, Danel-Brunaud V, Guy N, Thauvin-Robinet C, Lacomblez L, Couratier P, Hannequin D, Seilhean D, Le Ber I, Corcia P, Camu W, et al. SOD1, ANG, VAPB, TARDBP, and FUS mutations in familial amyotrophic lateral sclerosis: genotype–phenotype correlations. J Med Genet. 2010;47(8):554–560. [DOI] [PubMed] [Google Scholar]
- 22. Wegorzewska I, Bell S, Cairns NJ, Miller TM, Baloh RH. TDP-43 mutant transgenic mice develop features of ALS and frontotemporal lobar degeneration. Proc Natl Acad Sci USA. 2009;106(44):18809–18814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Guo W, Naujock M, Fumagalli L, Vandoorne T, Baatsen P, Boon R, Ordovás L, Patel A, Welters M, Vanwelden T, Geens N, Tricot T, Benoy V, Steyaert J, Lefebvre-Omar C, Boesmans W, Jarpe M, Sterneckert J, Wegner F, Petri S, Bohl D, Vanden Berghe P, et al. HDAC6 inhibition reverses axonal transport defects in motor neurons derived from FUS-ALS patients. Nat Commun. 2017;8(1):861–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hart PJ. Pathogenic superoxide dismutase structure, folding, aggregation and turnover. Curr Opin Chem Biol. 2006;10(2):131–138. [DOI] [PubMed] [Google Scholar]
- 25. Kanekura K, Hashimoto Y, Niikura T, Aiso S, Matsuoka M, Nishimoto I. Alsin, the product of ALS2 gene, suppresses SOD1 mutant neurotoxicity through RhoGEF domain by interacting with SOD1 mutants. J Biol Chem. 2004;279(18):19247–19256. [DOI] [PubMed] [Google Scholar]
- 26. Gurney ME. The use of transgenic mouse models of amyotrophic lateral sclerosis in preclinical drug studies. J Neurol Sci. 1997;152(suppl 1):S67–S73. [DOI] [PubMed] [Google Scholar]
- 27. Azzouz M, Leclerc N, Gurney M, Warter J-M, Poindron P, Borg J. Progressive motor neuron impairment in an animal model of familial amyotrophic lateral sclerosis. Muscle Nerve. 1997;20(1):45–51. [DOI] [PubMed] [Google Scholar]
- 28. Dal Canto MC, Gurney ME. Development of central nervous system pathology in a murine transgenic model of human amyotrophic lateral sclerosis. Am J Pathol. 1994;145(6):1271–1279. [PMC free article] [PubMed] [Google Scholar]
- 29. Collard J-F, Côté F, Julien J-P. Defective axonal transport in a transgenic mouse model of amyotrophic lateral sclerosis. Nature. 1995;375(6526):61–64. [DOI] [PubMed] [Google Scholar]
- 30. Karumbayaram S, Kelly TK, Paucar AA, Roe AJ, Umbach JA, Charles A, Goldman SA, Kornblum HI, Wiedau-Pazos M. Human embryonic stem cell-derived motor neurons expressing SOD1 mutants exhibit typical signs of motor neuron degeneration linked to ALS. Dis Model Mech. 2009;2(3–4):189–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Di Giorgio FP, Carrasco MA, Siao MC, Maniatis T, Eggan K. Non–cell autonomous effect of glia on motor neurons in an embryonic stem cell–based ALS model. Nat Neurosci. 2007;10(5):608–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Di Giorgio FP, Boulting GL, Bobrowicz S, Eggan KC. Human embryonic stem cell-derived motor neurons are sensitive to the toxic effect of glial cells carrying an ALS-causing mutation. Cell Stem Cell. 2008;3(6):637–648. [DOI] [PubMed] [Google Scholar]
- 33. Zwiegers P, Lee G, Shaw CA. Reduction in hSOD1 copy number significantly impacts ALS phenotype presentation in G37 R (line 29) mice: implications for the assessment of putative therapeutic agents. J Negat Results Biomed. 2014;13(1):14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Burkhardt MF, Martinez FJ, Wright S, Ramos C, Volfson D, Mason M, Garnes J, Dang V, Lievers J, Shoukat-Mumtaz U, Martinez R, Gai H, Blake R, Vaisberg E, Grskovic M, Johnson C, Irion S, Bright J, Cooper B, Nguyen L, Griswold-Prenner I, Javaherian A. A cellular model for sporadic ALS using patient-derived induced pluripotent stem cells. Mol Cell Neurosci. 2013;56:355–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sareen D, O’Rourke JG, Meera P, Muhammad AK, Grant S, Simpkinson M, Bell S, Carmona S, Ornelas L, Sahabian A, Gendron T, Petrucelli L, Baughn M, Ravits J, Harms MB, Rigo F, Bennett CF, Otis TS, Svendsen CN, Baloh RH. Targeting RNA foci in iPSC-derived motor neurons from ALS patients with a C9ORF72 repeat expansion. Sci Transl Med. 2013;5(208):208ra149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Alves CJ, Dariolli R, Jorge FM, Monteiro MR, Maximino JR, Martins RS, Strauss BE, Krieger JE, Callegaro D, Chadi G. Gene expression profiling for human iPS-derived motor neurons from sporadic ALS patients reveals a strong association between mitochondrial functions and neurodegeneration. Front Cell Neurosci. 2015;9:289–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. [DOI] [PubMed] [Google Scholar]
- 38. Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. [DOI] [PubMed] [Google Scholar]
- 39. Kwon D, Kim J-S, Cha B-H, Park K-S, Han I, Park K-S, Bae H, Han M-K, Kim K-S, Lee S-H. The effect of fetal bovine serum (FBS) on efficacy of cellular reprogramming for induced pluripotent stem cell (iPSC) generation. Cell Transplant. 2016;25(6):1025–1042. [DOI] [PubMed] [Google Scholar]
- 40. Li M, Ona VO, Guégan C, Chen M, Jackson-Lewis V, Andrews LJ, Olszewski AJ, Stieg PE, Lee J-P, Przedborski S, Friedlander RM. Functional role of caspase-1 and caspase-3 in an ALS transgenic mouse model. Science. 2000;288(5464):335–339. [DOI] [PubMed] [Google Scholar]
- 41. Pasinelli P, Houseweart MK, Brown RH, Cleveland DW. Caspase-1 and-3 are sequentially activated in motor neuron death in Cu, Zn superoxide dismutase-mediated familial amyotrophic lateral sclerosis. Proc Natl Acad Sci USA. 2000;97(25):13901–13906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nagai M, Re DB, Nagata T, Chalazonitis A, Jessell TM, Wichterle H, Przedborski S. Astrocytes expressing ALS-linked mutated SOD1 release factors selectively toxic to motor neurons. Nat Neurosci. 2007;10(5):615–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. De Sarro G, Siniscalchi A, Ferreri G, Gallelli L, De Sarro A. NMDA and AMPA/kainate receptors are involved in the anticonvulsant activity of riluzole in DBA/2 mice. Eur J Pharmacol. 2000;408(1):25–34. [DOI] [PubMed] [Google Scholar]
- 44. Lamanauskas N, Nistri A. Riluzole blocks persistent Na+ and Ca2+ currents and modulates release of glutamate via presynaptic NMDA receptors on neonatal rat hypoglossal motoneurons in vitro. Eur J Neurosci. 2008;27(10):2501–2514. [DOI] [PubMed] [Google Scholar]
- 45. Groeneveld G, Van Kan H, Kalmijn S, Veldink J, Guchelaar H-J, Wokke J, Van den Berg L. Riluzole serum concentrations in patients with ALS Associations with side effects and symptoms. Neurology. 2003;61(8):1141–1143. [DOI] [PubMed] [Google Scholar]
- 46. Bensimon G, Lacomblez L, Meininger VF; ALS/Riluzole Study Group. A controlled trial of riluzole in amyotrophic lateral sclerosis. N Engl J Med. 1994;330(9):585–591. [DOI] [PubMed] [Google Scholar]
- 47. Edaravone Acute Infarction Study Group. Effect of a novel free radical scavenger, edaravone (MCI-186), on acute brain infarction. Randomized, placebo-controlled, double-blind study at multicenters. Cerebrovasc Dis. 2003;15(3):222–229. [DOI] [PubMed] [Google Scholar]
- 48. Watanabe T, Tahara M, Todo S. The novel antioxidant edaravone: from bench to bedside. Cardiovasc Ther. 2008;26(2):101–114. [DOI] [PubMed] [Google Scholar]
- 49. Ito H, Wate R, Zhang J, Ohnishi S, Kaneko S, Ito H, Nakano S, Kusaka H. Treatment with edaravone, initiated at symptom onset, slows motor decline and decreases SOD1 deposition in ALS mice. Exp Neurol. 2008;213(2):448–455. [DOI] [PubMed] [Google Scholar]
- 50. Yoshino H, Kimura A. Investigation of the therapeutic effects of edaravone, a free radical scavenger, on amyotrophic lateral sclerosis (Phase II study). Amyotroph Lateral Sc. 2006;7(4):247–251. [DOI] [PubMed] [Google Scholar]
- 51. Tanaka M, Sakata T, Palumbo J, Akimoto M. A 24-week, phase III, double-blind, parallel-group study of edaravone (MCI-186) for treatment of amyotrophic lateral sclerosis (ALS). Neurology. 2016;86(suppl 16):P3.189. [Google Scholar]
- 52. Yang YM, Gupta SK, Kim KJ, Powers BE, Cerqueira A, Wainger BJ, Ngo HD, Rosowski KA, Schein PA, Ackeifi CA, Arvanites AC, Davidow LS, Woolf CJ, Rubin LL. A small molecule screen in stem-cell-derived motor neurons identifies a kinase inhibitor as a candidate therapeutic for ALS. Cell Stem Cell. 2013;12(6):713–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ray SS, Nowak RJ, Brown RH, Lansbury PT. Small-molecule-mediated stabilization of familial amyotrophic lateral sclerosis-linked superoxide dismutase mutants against unfolding and aggregation. Proc Natl Acad Sci USA. 2005;102(10):3639–3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Belzil VV, Bauer PO, Prudencio M, Gendron TF, Stetler CT, Yan IK, Pregent L, Daughrity L, Baker MC, Rademakers R, Boylan K, Patel TC, Dickson DW, Petrucelli L. Reduced C9orf72 gene expression in c9FTD/ALS is caused by histone trimethylation, an epigenetic event detectable in blood. Acta Neuropathol. 2013;126(6):895–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kim G, Ison G, McKee AE, Zhang H, Tang S, Gwise T, Sridhara R, Lee E, Tzou A, Philip R, Chiu HJ, Ricks TK, Palmby T, Russell AM, Ladouceur G, Pfuma E, Li H, Zhao L, Liu Q, Venugopal R, Ibrahim A, Pazdur R. FDA approval summary: olaparib monotherapy in patients with deleterious germline BRCA-mutated advanced ovarian cancer treated with three or more lines of chemotherapy. Clin Cancer Res. 2015;21(19):4257–4261. [DOI] [PubMed] [Google Scholar]
- 56. Druker BJ, Guilhot F, O’Brien SG, Gathmann I, Kantarjian H, Gattermann N, Deininger MW, Silver RT, Goldman JM, Stone RM, Cervantes F, Hochhaus A, Powell BL, Gabrilove JL, Rousselot P, Reiffers J, Cornelissen JJ, Hughes T, Agis H, Fischer T, Verhoef G, et al. ; IRIS Investigators. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355(23):2408–2417. [DOI] [PubMed] [Google Scholar]
- 57. Pardridge WM. Blood–brain barrier delivery. Drug Discov Today. 2007;12(1):54–61. [DOI] [PubMed] [Google Scholar]
- 58. Imamura K, Izumi Y, Watanabe A, Tsukita K, Woltjen K, Yamamoto T, Hotta A, Kondo T, Kitaoka S, Ohta A, Tanaka A, Watanabe D, Morita M, Takuma H, Tamaoka A, Kunath T, Wray S, Furuya H, Era T, Makioka K, Okamoto K, Fujisawa T, et al. The Src/c-Abl pathway is a potential therapeutic target in amyotrophic lateral sclerosis. Sci Transl Med. 2017;9(391):eaaf3962. [DOI] [PubMed] [Google Scholar]
- 59. Zhou QM, Zhang JJ, Li S, Chen S, Le WD. n-butylidenephthalide treatment prolongs life span and attenuates motor neuron loss in SOD1G93A mouse model of amyotrophic lateral sclerosis. CNS Neurosci Ther. 2017;23(5):375–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hsueh K-W, Chiou T-W, Chiang S-F, Yamashita T, Abe K, Borlongan CV, Sanberg PR, Lin S-Z, Harn H-J. Autophagic down-regulation in motor neurons remarkably prolongs the survival of ALS mice. Neuropharmacology. 2016;108:152–160. [DOI] [PubMed] [Google Scholar]
- 61. Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y, Yoshimori T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000;19(21):5720–5728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Serio A, Bilican B, Barmada SJ, Ando DM, Zhao C, Siller R, Burr K, Haghi G, Story D, Nishimura AL, Carrasco MA, Phatnani HP, Shum C, Wilmut I, Maniatis T, Shaw CE, Finkbeiner S, Chandran S. Astrocyte pathology and the absence of non-cell autonomy in an induced pluripotent stem cell model of TDP-43 proteinopathy. Proc Natl Acad Sci USA. 2013;110(12):4697–4702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Ogawa S-i, Tokumoto Y, Miyake J, Nagamune T. Induction of oligodendrocyte differentiation from adult human fibroblast-derived induced pluripotent stem cells. In Vitro Cell Dev-An. 2011;47(7):464–469. [DOI] [PubMed] [Google Scholar]
- 64. Liu Q, Spusta SC, Mi R, Lassiter RN, Stark MR, Höke A, Rao MS, Zeng X. Human neural crest stem cells derived from human ESCs and induced pluripotent stem cells: induction, maintenance, and differentiation into functional schwann cells. Stem Cell Transl Med. 2012;1(4):266–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hu B-Y, Weick JP, Yu J, Ma L-X, Zhang X-Q, Thomson JA, Zhang S-C. Neural differentiation of human induced pluripotent stem cells follows developmental principles but with variable potency. Proc Natl Acad Sci USA. 2010;107(9):4335–4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. LaVaute TM, Yoo YD, Pankratz MT, Weick JP, Gerstner JR, Zhang SC. Regulation of neural specification from human embryonic stem cells by BMP and FGF. Stem Cells. 2009;27(8):1741–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Li XJ, Hu BY, Jones SA, Zhang YS, LaVaute T, Du ZW, Zhang SC. Directed differentiation of ventral spinal progenitors and motor neurons from human embryonic stem cells by small molecules. Stem Cells. 2008;26(4):886–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Nordström U, Jessell TM, Edlund T. Progressive induction of caudal neural character by graded Wnt signaling. Nat Neurosci. 2002;5(6):525–532. [DOI] [PubMed] [Google Scholar]
- 69. Gurney ME, Pu H, Chiu AY, Dal Canto MC, Polchow CY, Alexander DD, Caliendo J, Hentati A, Kwon YW, Deng HX. Motor neuron degeneration in mice that express a human Cu, Zn superoxide dismutase mutation. Science. 1994;264(5166):1772–1775. [DOI] [PubMed] [Google Scholar]
- 70. Ravits J, Paul P, Jorg C. Focality of upper and lower motor neuron degeneration at the clinical onset of ALS. Neurology. 2007;68(19):1571–1575. [DOI] [PubMed] [Google Scholar]
- 71. Mitsumoto H, Ulug AM, Pullman SL, Gooch CL, Chan S, Tang MX, Mao X, Hays AP, Floyd AG, Battista V, Montes J, Hayes S, Dashnaw S, Kaufmann P, Gordon PH, Hirsch J, Levin B, Rowland LP, Shungu DC. Quantitative objective markers for upper and lower motor neuron dysfunction in ALS. Neurology. 2007;68(17):1402–1410. [DOI] [PubMed] [Google Scholar]
- 72. Wichterle H, Lieberam I, Porter JA, Jessell TM. Directed differentiation of embryonic stem cells into motor neurons. Cell. 2002;110(3):385–397. [DOI] [PubMed] [Google Scholar]
- 73. Lindvall O, Kokaia Z, Martinez-Serrano A. Stem cell therapy for human neurodegenerative disorders–how to make it work. Nat Med. 2004;10(suppl):S42–S50. [DOI] [PubMed] [Google Scholar]
- 74. Harper JM, Krishnan C, Darman JS, Deshpande DM, Peck S, Shats I, Backovic S, Rothstein JD, Kerr DA. Axonal growth of embryonic stem cell-derived motoneurons in vitro and in motoneuron-injured adult rats. Proc Natl Acad Sci USA. 2004;101(18):7123–7128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Karumbayaram S, Novitch BG, Patterson M, Umbach JA, Richter L, Lindgren A, Conway AE, Clark AT, Goldman SA, Plath K, Wiedau-Pazos M, Kornblum HI, Lowry WE. Directed differentiation of human-induced pluripotent stem cells generates active motor neurons. Stem Cells. 2009;27(4):806–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Maury Y, Côme J, Piskorowski RA, Salah-Mohellibi N, Chevaleyre V, Peschanski M, Martinat C, Nedelec S. Combinatorial analysis of developmental cues efficiently converts human pluripotent stem cells into multiple neuronal subtypes. Nat Biotechnol. 2015;33(1):89–96. [DOI] [PubMed] [Google Scholar]
- 77. Johnson MA, Weick JP, Pearce RA, Zhang S-C. Functional neural development from human embryonic stem cells: accelerated synaptic activity via astrocyte coculture. J Neurosci. 2007;27(12):3069–3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Suzuki M, McHugh J, Tork C, Shelley B, Klein SM, Aebischer P, Svendsen CN. GDNF secreting human neural progenitor cells protect dying motor neurons, but not their projection to muscle, in a rat model of familial ALS. PLoS One. 2007;2(8):e689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Krakora D, Mulcrone P, Meyer M, Lewis C, Bernau K, Gowing G, Zimprich C, Aebischer P, Svendsen CN, Suzuki M. Synergistic effects of GDNF and VEGF on lifespan and disease progression in a familial ALS rat model. Mol Ther. 2013;21(8):1602–1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Wang J, Farr GW, Zeiss CJ, Rodriguez-Gil DJ, Wilson JH, Furtak K, Rutkowski DT, Kaufman RJ, Ruse CI, Yates JR, Perrin S, Feany MB, Horwich AL. Progressive aggregation despite chaperone associations of a mutant SOD1-YFP in transgenic mice that develop ALS. Proc Natl Acad Sci USA. 2009;106(5):1392–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Chen H, Qian K, Du Z, Cao J, Petersen A, Liu H, Blackbourn LW, Huang CL, Errigo A, Yin Y, Lu J, Ayala M, Zhang SC. Modeling ALS with iPSCs reveals that mutant SOD1 misregulates neurofilament balance in motor neurons. Cell Stem Cell. 2014;14(6):796–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Mori K, Weng SM, Arzberger T, May S, Rentzsch K, Kremmer E, Schmid B, Kretzschmar HA, Cruts M, Van Broeckhoven C, Haass C, Edbauer D. The C9orf72 GGGGCC repeat is translated into aggregating dipeptide-repeat proteins in FTLD/ALS. Science. 2013;339(6125):1335–1338. [DOI] [PubMed] [Google Scholar]
- 83. Du Z-W, Chen H, Liu H, Lu J, Qian K, Huang CT-L, Zhong X, Fan F, Zhang S-C. Generation and expansion of highly-pure motor neuron progenitors from human pluripotent stem cells. Nat Commun. 2015;6:6626–6634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Chou SM, Wang HS, Taniguchi A. Role of SOD-1 and nitric oxide/cyclic GMP cascade on neurofilament aggregation in ALS/MND. J Neurol Sci. 1996;139(suppl):16–26. [DOI] [PubMed] [Google Scholar]
- 85. Mitsumoto H, Brooks BR, Silani V. Clinical trials in amyotrophic lateral sclerosis: why so many negative trials and how can trials be improved? Lancet Neurol. 2014;13(11):1127–1138. [DOI] [PubMed] [Google Scholar]
- 86. Heemskerk J. High throughput drug screening. Amyotroph Lateral Scler Other Motor Neuron Disord. 2004;5(suppl 1):19–21. [DOI] [PubMed] [Google Scholar]
- 87. Aiken CT, Tobin AJ, Schweitzer ES. A cell-based screen for drugs to treat Huntington’s disease. Neurobiol Dis. 2004;16(3):546–555. [DOI] [PubMed] [Google Scholar]
- 88. Donnelly CJ, Zhang PW, Pham JT, Haeusler AR, Mistry NA, Vidensky S, Daley EL, Poth EM, Hoover B, Fines DM, Maragakis N, Tienari PJ, Petrucelli L, Traynor BJ, Wang J, Rigo F, Bennett CF, Blackshaw S, Sattler R, Rothstein JD. RNA toxicity from the ALS/FTD C9ORF72 expansion is mitigated by antisense intervention. Neuron. 2013;80(2):415–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Wainger BJ, Kiskinis E, Mellin C, Wiskow O, Han SS, Sandoe J, Perez NP, Williams LA, Lee S, Boulting G, Berry JD, Brown RH, Cudkowicz ME, Bean BP, Eggan K, Woolf CJ. Intrinsic membrane hyperexcitability of amyotrophic lateral sclerosis patient-derived motor neurons. Cell Rep. 2014;7(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Zhang K, Donnelly CJ, Haeusler AR, Grima JC, Machamer JB, Steinwald P, Daley EL, Miller SJ, Cunningham KM, Vidensky S, Gupta S, Thomas MA, Hong I, Chiu SL, Huganir RL, Ostrow LW, Matunis MJ, Wang J, Sattler R, Lloyd TE, Rothstein JD. The C9orf72 repeat expansion disrupts nucleocytoplasmic transport. Nature. 2015;525(7567):56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Madill M, McDonagh K, Ma J, Vajda A, McLoughlin P, O’Brien T, Hardiman O, Shen S. Amyotrophic lateral sclerosis patient iPSC-derived astrocytes impair autophagy via non-cell autonomous mechanisms. Mol Brain. 2017;10(1):22–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Liu G-H, Ding Z, Belmonte JCI. iPSC technology to study human aging and aging-related disorders. Curr Opin Cell Biol. 2012;24(6):765–774. [DOI] [PubMed] [Google Scholar]
- 93. Lopez-Gonzalez R, Lu Y, Gendron TF, Karydas A, Tran H, Yang D, Petrucelli L, Miller BL, Almeida S, Gao F-B. Poly (GR) in C9ORF72-related ALS/FTD compromises mitochondrial function and increases oxidative stress and DNA damage in iPSC-derived motor neurons. Neuron. 2016;92(2):383–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Hyun DH, Lee M, Halliwell B, Jenner P. Proteasomal inhibition causes the formation of protein aggregates containing a wide range of proteins, including nitrated proteins. J Neurochem. 2003;86(2):363–373. [DOI] [PubMed] [Google Scholar]
- 95. Zhang YJ, Jansen-West K, Xu YF, Gendron TF, Bieniek KF, Lin WL, Sasaguri H, Caulfield T, Hubbard J, Daughrity L, Chew J, Belzil VV, Prudencio M, Stankowski JN, Castanedes-Casey M, Whitelaw E, Ash PE, DeTure M, Rademakers R, Boylan KB, Dickson DW, Petrucelli L. Aggregation-prone c9FTD/ALS poly (GA) RAN-translated proteins cause neurotoxicity by inducing ER stress. Acta Neuropathol. 2014;128(4):505–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Yang DJ, Wang XL, Ismail A, Ashman CJ, Valori CF, Wang G, Gao S, Higginbottom A, Ince PG, Azzouz M, Xu J, Shaw PJ, Ning K. PTEN regulates AMPA receptor-mediated cell viability in iPS-derived motor neurons. Cell Death Dis. 2014;5(2):e1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Liu Y, Wang L, Long ZY, Wu YM, Wan Q, Jiang JX, Wang ZG. Inhibiting PTEN protects hippocampal neurons against stretch injury by decreasing membrane translocation of AMPA receptor GluR2 subunit. PLoS One. 2013;8(6):e65431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Lincecum JM, Vieira FG, Wang MZ, Thompson K, De Zutter GS, Kidd J, Moreno A, Sanchez R, Carrion IJ, Levine BA, Al-Nakhala BM, Sullivan SM, Gill A, Perrin S. From transcriptome analysis to therapeutic anti-CD40 L treatment in the SOD1 model of amyotrophic lateral sclerosis. Nat Genet. 2010;42(5):392–399. [DOI] [PubMed] [Google Scholar]
- 99. Moviglia GA, Moviglia-Brandolino MT, Varela GS, Albanese G, Piccone S, Echegaray G, Martinez G, Blasseti N, Farias J, Farina P, Perusso A, Gaeta CA. Feasibility, safety, and preliminary proof of principles of autologous neural stem cell treatment combined with T-cell vaccination for ALS patients. Cell Transplant. 2012;21(suppl 1):S57–S63. [DOI] [PubMed] [Google Scholar]
- 100. Butovsky O, Siddiqui S, Gabriely G, Lanser AJ, Dake B, Murugaiyan G, Doykan CE, Wu PM, Gali RR, Iyer LK, Lawson R, Berry J, Krichevsky AM, Cudkowicz ME, Weiner HL. Modulating inflammatory monocytes with a unique microRNA gene signature ameliorates murine ALS. J Clin Invest. 2012;122(9):3063–3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. El Idrissi NB, Bosch S, Ramaglia V, Aronica E, Baas F, Troost D. Complement activation at the motor end-plates in amyotrophic lateral sclerosis. J Neuroinflamm. 2016;13(1):72–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Pasinetti GM, Ungar LH, Lange DJ, Yemul S, Deng H, Yuan X, Brown RH, Cudkowicz ME, Newhall K, Peskind E, Marcus S, Ho L. Identification of potential CSF biomarkers in ALS. Neurology. 2006;66(8):1218–1222. [DOI] [PubMed] [Google Scholar]
- 103. Noto Y, Shibuya K, Sato Y, Kanai K, Misawa S, Sawai S, Mori M, Uchiyama T, Isose S, Nasu S, Sekiguchi Y, Fujimaki Y, Kasai T, Tokuda T, Nakagawa M, Kuwabara S. Elevated CSF TDP-43 levels in amyotrophic lateral sclerosis: specificity, sensitivity, and a possible prognostic value. Amyotroph Lateral Scler. 2011;12(2):140–143. [DOI] [PubMed] [Google Scholar]
- 104. Verstraete E, Kuiperij HB, van Blitterswijk MM, Veldink JH, Schelhaas HJ, van den Berg LH, Verbeek MM. TDP-43 plasma levels are higher in amyotrophic lateral sclerosis. Amyotroph Lateral Scler. 2012;13(5):446–451. [DOI] [PubMed] [Google Scholar]
- 105. Lehnert S, Costa J, de Carvalho M, Kirby J, Kuzma-Kozakiewicz M, Morelli C, Robberecht W, Shaw P, Silani V, Steinacker P, Tumani H, Van Damme P, Ludolph A, Otto M. Multicentre quality control evaluation of different biomarker candidates for amyotrophic lateral sclerosis. Amyotroph Lateral Scler Frontotemporal Degener. 2014;15(5–6):344–350. [DOI] [PubMed] [Google Scholar]
- 106. Boylan K, Yang C, Crook J, Overstreet K, Heckman M, Wang Y, Borchelt D, Shaw G. Immunoreactivity of the phosphorylated axonal neurofilament H subunit (pNF-H) in blood of ALS model rodents and ALS patients: evaluation of blood pNF-H as a potential ALS biomarker. J Neurochem. 2009;111(5):1182–1191. [DOI] [PubMed] [Google Scholar]
- 107. Almeida S, Gascon E, Tran H, Chou HJ, Gendron TF, Degroot S, Tapper AR, Sellier C, Charlet-Berguerand N, Karydas A, Seeley WW, Boxer AL, Petrucelli L, Miller BL, Gao FB. Modeling key pathological features of frontotemporal dementia with C9ORF72 repeat expansion in iPSC-derived human neurons. Acta Neuropathol. 2013;126(3):385–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Su Z, Zhang Y, Gendron TF, Bauer PO, Chew J, Yang WY, Fostvedt E, Jansen-West K, Belzil VV, Desaro P, Johnston A, Overstreet K, Oh SY, Todd PK, Berry JD, Cudkowicz ME, Boeve BF, Dickson D, Floeter MK, Traynor BJ, Morelli C, Ratti A, et al. Discovery of a biomarker and lead small molecules to target r (GGGGCC)-associated defects in c9FTD/ALS. Neuron. 2014;83(5):1043–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Junttila A, Kuvaja M, Hartikainen P, Siloaho M, Helisalmi S, Moilanen V, Kiviharju A, Jansson L, Tienari PJ, Remes AM, Herukka SK. Cerebrospinal fluid TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis patients with and without the C9ORF72 hexanucleotide expansion. Dement Geriatr Cogn Dis Extra. 2016;6(1):142–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Silva SA, Sousa AL, Haddad AF, Azevedo JC, Soares VE, Peixoto CM, Soares AJ, Issa AF, Felipe LR, Branco RV, Addad JA, Moreira RC, Tuche FA, Mesquita CT, Drumond CC, Junior AO, Rochitte CE, Luz JH, Rabischoffisky A, Nogueira FB, Vieira RB, Junior HS, et al. Autologous bone-marrow mononuclear cell transplantation after acute myocardial infarction: comparison of two delivery techniques. Cell Transplant. 2009;18(3):343–352. [DOI] [PubMed] [Google Scholar]
- 111. Kim KS, Lee HJ, An J, Kim YB, Ra JC, Lim I, Kim SU. Transplantation of human adipose tissue-derived stem cells delays clinical onset and prolongs life span in ALS mouse model. Cell Transplant. 2014;23(12):1585–1597. [DOI] [PubMed] [Google Scholar]
- 112. Mejía-Toiber J, Castillo CG, Giordano M. Strategies for the development of cell lines for ex vivo gene therapy in the central nervous system. Cell Transplant. 2011;20(7):983–1001. [DOI] [PubMed] [Google Scholar]
- 113. Lin S-Z. Translational cell therapies in regenerative medicine and cancers. Cell Transplant. 2016;25(5):781–782. [DOI] [PubMed] [Google Scholar]