Abstract
Induced pluripotent stem cell-derived mesenchymal stem cells (iPSC-MSCs) represent a promising cell source for patient-specific cell therapy. We previously demonstrated that they display an immunomodulatory effect on allergic airway inflammation. Glucocorticoids are powerful anti-inflammatory compounds and widely used in the therapy of allergic diseases. However, the effect of glucocorticoids on the immunomodulatory function of iPSC-MSCs remains unknown. This study aimed to determine the effect of dexamethasone (Dex) on the immunomodulatory function of iPSC-MSCs in vitro and in vivo. A total of three human iPSC-MSC clones were generated from amniocyte-derived iPSCs. Anti-CD3/CD28-induced peripheral blood mononuclear cell (PBMC) proliferation was used to assess the effect of Dex on the immunoinhibitory function of iPSC-MSCs in vitro. Mouse models of contact hypersensitivity (CHS) and allergic airway inflammation were induced, and the levels of inflammation in mice were analyzed with the treatments of iPSC-MSCs and Dex, alone and combined. The results showed that Dex did not interfere with the immunoinhibitory effect of iPSC-MSCs on PBMC proliferation. In CHS mice, simultaneous treatment with Dex did not affect the effect of iPSC-MSCs on the inflammation, both in regional draining lymph nodes and in inflamed ear tissue. In addition, co-administration of iPSC-MSCs with Dex decreased the local expression of interferon (IFN)-γ and tumor necrosis factor (TNF)-α in the ears of CHS mice. In the mouse model of allergic airway inflammation, iPSC-MSC treatment combined with Dex resulted in a similar extent of reduction in pulmonary inflammation as iPSC-MSCs or Dex treatment alone. In conclusion, Dex does not significantly affect the immunomodulatory function of iPSC-MSCs both in vitro and in vivo. These findings may have implications when iPSC-MSCs and glucocorticoids are co-administered.
Keywords: Induced pluripotent stem cell-derived mesenchymal stem cells (iPSC-MSCs), dexamethasone (Dex), immunoinhibitory, contact hypersensitivity (CHS), allergic airway inflammation
Introduction
Mesenchymal stem cells (MSCs) are multipotent cells with immunomodulatory functions on immune cells1, which have been being clinically explored as a new therapeutic treatment for various immune-related diseases, including graft-versus-host disease (GvHD)2, systemic lupus erythematosus (SLE)3, and Crohn’s disease4. Most preclinical and clinical studies are performed using MSCs derived from adult bone marrow (BM) and adipose tissue5–8. However, several disadvantages may restrict these adult MSCs for clinical applications, including the requirement of invasive techniques for their isolation, a limited number of cells obtained initially from a single donor, limited capacity to proliferate when cultured in vitro, and varying cell qualities from different donors9–12.
In the last decade, several reprogramming techniques generating human pluripotent stem cells (iPSCs) from adult somatic cells were successfully developed13. These iPSCs hold enormous promise for patient-personalized cell therapies14 and for research into various human diseases15,16, and importantly, represent an important alternative source of functional MSCs17,18. Recently, we and others have successfully induced MSCs from iPSCs (iPSC-MSCs)19–24, which are similar to BM-MSCs in terms of morphology, multipotent differentiation potential, and expression of common MSC surface markers. The iPSC-MSCs display a strong immunomodulatory effect on natural killer (NK) cells22, dendritic cells and T-cells20,25. The induced iPSC-MSCs are potent in the protection of limb ischemia and doxorubicin-induced cardiomyopathy19,26. We have previously demonstrated that, similar to BM-MSCs, iPSC-MSCs can inhibit the Th2 phenotype both in vitro and in vivo20,21. Given the advantages of iPSC-MSCs over adult MSCs, there are large expectations for the use of iPSC-MSCs for the clinical application of the treatment of allergic diseases. MSCs in combination with glucocorticoids may provide further potential benefits in the treatment of several disorders, such as ulcerative colitis, kidney transplantation, and multiple sclerosis27–31. Therefore, it is foreseen that iPSC-MSCs may be used for clinical purposes in combination with glucocorticoids, which is currently the gold standard for controlling inflammation in allergic diseases. Then, the effects of glucocorticoids on the function of iPSC-MSCs should be well studied in the case of combined administration.
Few studies have investigated the interaction between BM-MSCs and glucocorticoids. Human BM-MSCs have been reported to inhibit allogeneic lymphocyte proliferation in the presence of dexamethasone (Dex) in vitro32. Conversely, a recent study demonstrated that Dex can eliminate the immunomodulatory effect of BM-MSCs on lymphocyte proliferation in vitro and in a murine model of liver fibrosis33. In addition, a clinical trial using combined therapy with BM-MSCs and glucocorticoids in GvHD patients performed no better overall than a placebo34. However, whether glucocorticoids interrupt the immunomodulatory function of iPSC-MSCs remains unknown.
In this study, we investigated the effect of Dex on the immunosuppression of iPSC-MSCs on the proliferation of anti-CD3/CD28-activated human peripheral blood mononuclear cells (PBMCs) in vitro, and examined the interactions between Dex and iPSC-MSCs in mouse models of contact hypersensitivity (CHS) and allergic airway inflammation. We found that Dex (at tested concentrations) had no significant effects on the immunomodulatory function of iPSC-MSCs, both in vitro and in vivo. It may be important for the potential clinical use of iPSC-MSCs in combination with glucocorticoids.
Materials and Methods
Generation of Human iPSC-MSCs
Amniocyte-derived iPSCs were purchased from Cell Inspire Bio (IPSN-0008, Shenzhen, China). Prior to generating the iPSC-MSCs, iPSCs were plated into a Matrigel-coated six-well plate, and cultured in mTeSR1 basal medium containing 20% mTeSR1 supplement (Stem Cell, MA, USA), 1% penicillin/streptomycin (Gibco, CA, USA). Once 60% confluence was reached, the mTeSR1 medium was replaced with induction medium containing minimum essential medium eagle-α modified (α-MEM), 10% fetal bovine serum (FBS), 1% penicillin/ streptomycin, 200 mM l-glutamine and 10 mM non-essential amino acids (Gibco, CA, USA), sodium pyruvate, 10 mM l-ascorbate-2-phosphate (Sigma, MO, USA) and changed every other day. At 2 weeks later, cells were split with a 1:2 ratio using 0.25% trypsin-ethylenediaminetetraacetic acid (Gibco, CA, USA), plated in a gelatin-coated six-well plate as passage one (P1). After two passages, cells were seeded in an uncoated six-well plate and cultured in iPSC-MSCs growth medium containing high glucose Dulbecco’s modified eagle’s medium (Hyclone, UT, USA), 10% FBS, 5 ng/ml fibroblast growth factor (bFGF), 10 ng/ml epidermal growth factor (Gibco, CA, USA), 1% penicillin/streptomycin. Overall, three human iPSC-MSC clones were generated. Passage 9 to passage 17 iPSC-MSCs were used in this study.
Differentiation of Human iPSC-MSCs
Functional differentiation of iPSC-MSCs, including adipogenesis, osteogenesis and chondrogenesis, were carried out at passage 9. For adipocytic differentiation, cells were incubated in growth medium in six-well plates and changed to adipogenic differentiation medium (medium A for 3 days and medium B for 1 day; Cyagen Biosciences, CA, USA) when confluence was reached. After six cycles, cells were cultured in medium B for an additional 7 days, then stained with Oil Red O working solution (Cyagen Biosciences, CA, USA) and visualized under a light microscope. For osteogenic differentiation, cells were re-plated in growth medium at 3×104 cells/cm2 in six-well plates pre-coated with gelatin solution. After 24 hours, the medium was changed to osteogenic differentiation medium (Cyagen Biosciences, CA, USA). After 3 weeks in culture, cells were fixed and stained with Alizarin red. For chondrogenic differentiation, 2.5×105 iPSC-MSCs were transferred to a 15-ml centrifugation tube to wash twice with incomplete chondrogenic medium (Cyagen Biosciences, CA, USA), then resuspended in complete chondrogenic medium to a density of 5.0×105 cells per ml. Cell suspension was transferred into a 15-ml polypropylene culture tube and centrifuged at 150×g for 5 minutes. The tube was incubated at 5% CO2, 37°C, avoiding aspirating the supernatant or resuspending the pellet. After 24 hours, cell pellets were fed with fresh complete chondrogenic medium every 2–3 days. Chondrogenic pellets were harvested after 28 days in culture, formalin fixed and paraffin embedded for Alcian blue stain.
PBMC Proliferation Assay
The buffy coats from anonymous healthy donors provided by Guangzhou Blood Center were used for human PBMC collecting as described previously20. The study protocol was approved by the Ethics Committee of the First Affiliated Hospital, Sun Yat-sen University, China (No. 2014-C-053), and exemption of written informed consent for using human buffy coats was approved. Cells were suspended in 500 µl of phosphate-buffered saline (PBS) containing 10% FBS and stained by 2 mM carboxyfluoresceinsuccinimidyl amino ester (CFSE; Sigma, MO, USA). After 10 minutes, cells were washed twice with 10 ml RPMI 1640 medium (Hyclone, UT, USA) with 10% FBS, 1% penicillin/streptomycin, and 1% L-glutamine. Cells were resuspended and dispensed in 24-well plates at a density of 2 ×105 cells/well. Then PBMCs with a stimulation of 1 µg/ml anti-CD3 and 1µg/ml anti-CD28 (BD Biosciences, NJ, USA) were cultured alone or co-cultured with iPSC-MSCs in a ratio of 10:1, which was determined in our previous study20, in the absence or presence of Dex at concentrations ranging from 10 ng/ml to 100 µg /ml for 3 days.
Flow Cytometry of PBMCs and iPSC-MSCs
CFSE-stained PBMCs were harvested after 3 days of co-culture with iPSC-MSCs or Dex, and then the PBMC proliferation was assessed by flow cytometry (Beckman Gallios, IN, USA) using standard techniques. Cell surface antigens and human indoleamine 2,3-dioxygenase (IDO) expression in human iPSC-MSCs (passage 9) were also analyzed by flow cytometry. Antibodies against human antigens CD166, CD146, CD34, CD44, CD45, CD73, CD90, CD105 were purchased from BD Bioscience. Antibody against IDO (# P14902) was purchased from R&D systems (MN, USA). Data were analyzed by Kaluza Analysis Software (Beckman Coulter Life Sciences, IN, USA).
Enzyme-linked Immunosorbent Assay
Interleukin (IL)-6 and IL-10 levels in serum were determined using the ELISA Kit (KeyGEN BioTECH, Shanghai, China).
Animals
Female BALB/c mice (6–8 weeks) were purchased from Experimental Animal Center, Sun Yat-sen University (Guangzhou, China) and housed under specific pathogen-free conditions, maintained on a 12 h light/dark cycle, and provided food and water ad libitum.
All procedures were performed according to protocols approved by the Institutional Animal Care and Use Committee, Sun Yat-sen University.
Mouse Contact Hypersensitivity Model
Mice were sensitized to oxazolone (Sigma, MO, USA) by the application of 20 µl of 1% oxazolone in an acetone/sesame seed oil vehicle (4:1 v/v) to both ears on day 1 and day 735. iPSC-MSCs (1×106 per mice, intravenous injection) or/with Dex (5 mg/kg, intraperitoneal injection) were injected into mice at the same time on day 6. Control mice received PBS. Ears and draining auricular lymph nodes at the base of the ear were photographed on day 8 and 9 respectively and harvested on day 9. The biggest lymph node was weighed immediately after excision. Serum samples were collected on day 9 and serum IL-6 and IL-10 levels were determined by ELISA assay.
Mouse Model of Allergic Airway Inflammation
Ovalbumin (OVA)-induced mouse model of allergic airway inflammation was established as our previous study21. Briefly, mice were sensitized by intraperitoneal injection of 40 µg of OVA (grade V; Sigma, MO, USA) and 4 mg of aluminum hydroxide (Sigma, MO, USA) in 200 µl PBS on day 1 and day 14. From day 21 to 24, mice were challenged daily with aerosolized 5% OVA in a plexiglass chamber and through an air-compressing nebulizer for 30 minutes. iPSC-MSCs (1×106 per mice) was injected via the tail vein on day 20. Mice were injected intraperitoneally with Dex (1.25 mg/kg/day) from day 21 to 24. The animals were sacrificed after the last challenge on day 24.
Histology and Microscopic Measurement of Ear Thickness
Ear biopsy specimens were fixed with 4% paraformaldehyde and embedded in paraffin. Deparaffinized 5-µm sections were stained with hematoxylin and eosin (H&E) and analyzed by Leica DM2500 microscope and Leica Application Suite (Leica, IL, USA) software. Ear thickness (including epidermis, dermis and subcutaneous layer) was determined by taking measurements on six individual sections.
Evaluation of the Inflammatory Cells in the Bronchoalveolar Lavage Fluids
On day 24, bronchoalveolar lavage fluids (BALFs) were collected with 1 ml of cold PBS via a 22-gauge needle inserted into the trachea. After centrifugation, cells in the BALFs were counted using a hemocytometer and then resuspended in 5 µl of PBS. The cell suspension was streaked in a thin film over a glass slide using the second slide as a spreader and was stained with Diff-Quik kit (D030, Nanjing Jiancheng Bioengineering Institute, China, http://www.njjcbio.com). A total of 250 cells per slide were evaluated for inflammatory cell counting.
Lung Histology and Inflammation Scoring
Lung sections were prepared at a thickness of 4-µm and were stained with H&E. For the lung inflammation quantification, five sections across the main bronchus and vascular per mouse were randomly selected. The inflammation levels were scored from 0 to 3 according to the following criteria: 0 = no inflammation; 1 = occasional inflammatory cells; 2 = most bronchus or vascular were surrounded by 1–5 layers of inflammatory cells; 3 = most bronchus or vascular were surrounded by more than five layers of inflammatory cells.
Western Blotting
Western blotting analysis of the homogenized mouse ears was performed following standard procedures. Antibodies against TNF-α (Cell Signaling Technology, MA, USA) and IFN-γ (Abcam, Cambridge, UK) were used.
Statistical Analysis
All data shown are expressed as means ± SEMs. Data were evaluated by one-way analysis of variance and further evaluated with the Bonferroni test for multiple comparisons using GraphPad Prism Software (CA, USA). A Student’s t test was also used to determine significance when applicable. P-values lower than 0.05 were considered to be significant.
Results
Characterization of iPSC-MSCs
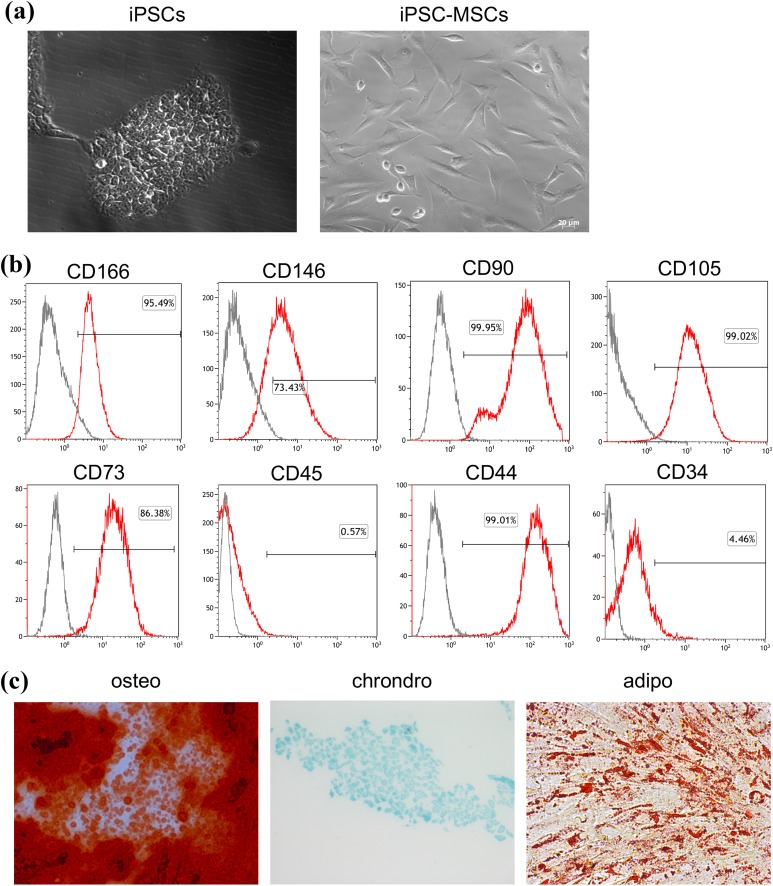
Using a modification of previously described protocols22,24, three human iPSC-MSC clones were successfully generated from iPSCs reprogramed from amniocytes. The iPSC-MSCs displayed a fibroblastic morphology, which was distinct from their parent iPSCs but similar to BM-MSCs (Fig. 1(a)). Flow cytometry analysis showed that iPSC-MSCs expressed surface markers typical of adult MSCs36, including CD105, CD73, CD90, CD166, CD146 and CD44, but did not express CD34 and CD45 (Fig. 1(b)). Trilineage differentiation assays demonstrated that iPSC-MSCs had the ability to differentiate along the chondrogenic, osteoblastic or adipocytic pathways when cultured in the appropriate conditions (Fig. 1(c)).
Fig. 1.
Characterization of iPSC-MSCs. (a) Morphology of iPSCs and iPSC-MSCs (P4). Scale bar = 20μm (b) Surface antigen profiling by FACS in iPSC-MSCs (P4) cultures for CD166, CD146, CD90, CD105, CD73, CD45, CD44 and CD34. Data shown are representative of three independent experiments. Gray lines indicate isotype controls, and red lines represent the marker indicated. (c) Multipotent differentiation potential of iPSC-MSCs. Alizarin red staining for osteogenesis (left); Alcian blue staining for chondrogenesis (middle); and Oil Red O staining for adipogenesis (right). Images are representative of 3–4 independent experiments. (magnification, ×200).
FACS: fluorescent-activated cell sorting; iPSC: induced pluripotent stem cell; iPSC-MSC: induced pluripotent stem cell-derived mesenchymal stem cell.
Dex had no Effects on the Immunoinhibitory of iPSC-MSCs on PBMC Proliferation
Previously, we have demonstrated that iPSC-MSCs have a potent ability to inhibit lymphocytes proliferation20. However, whether Dex affects this ability remains unclear.
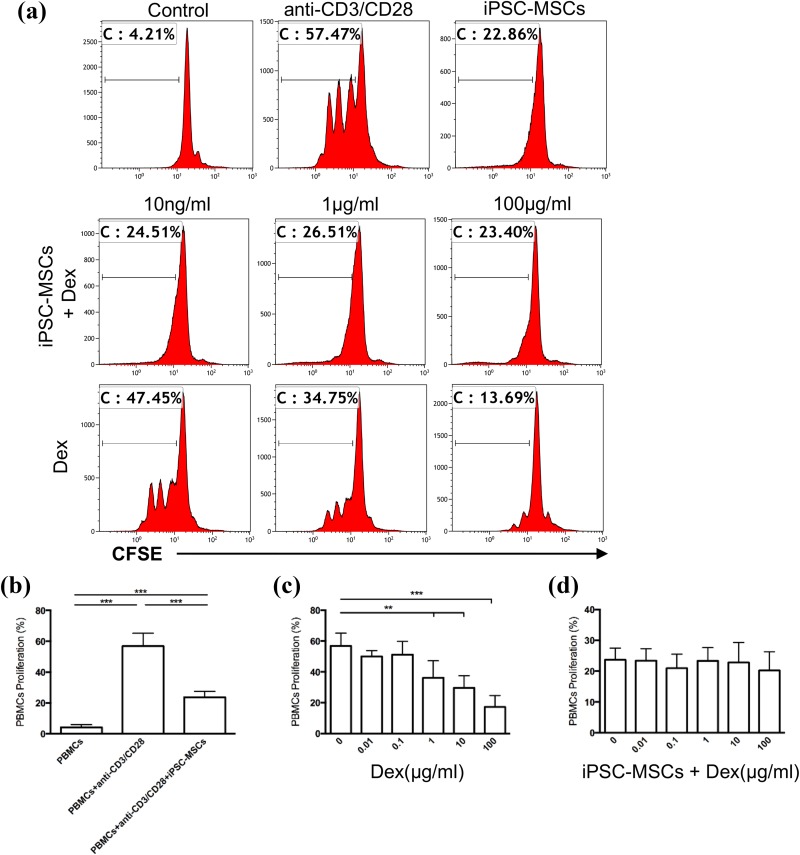
Therefore, we employed a co-culture system of lymphocyte proliferation induced by anti-CD3/CD28 stimulation to examine the effect of Dex on the immunoinhibitory function of iPSC-MSCs. PBMCs were separated from healthy volunteers’ blood samples and stimulated with anti-CD3/CD28 in the absence or presence of iPSC-MSCs and Dex. Consistent with our previous study20, iPSC-MSCs alone significantly inhibited PBMC proliferation at a ratio of 1:10 (Fig. 2(a), upper panel, and Fig. 2(b), P < 0.001). As expected, Dex alone showed a dose-dependent inhibition of PBMCs proliferation (Fig. 2(a), lower panel, and Fig. 2(c), P < 0.01 or 0.001). After iPSC-MSCs were added with Dex with a graded concentration from 10 ng/ml to 100 µg/ml, the inhibition of PBMC proliferation was almost invariably similar to those when iPSC-MSCs were added alone (Fig. 2(a), upper and middle panel, and Fig. 2(d), P > 0.05), suggesting Dex does not affect the immunoinhibitory effects of iPSC-MSCs on PBMC proliferation.
Fig. 2.
Dex had no effect on the immunoinhibition of iPSC-MSCs on PBMC proliferation in vitro. PBMCs were labelled with CFSE prior to culture and stimulated by anti-CD3/CD28 with graded concentrations of Dex in the presence or absence of iPSC-MSCs. (a) The immunoinhibitory effect of iPSC-MSCs (upper panel), the combination of iPSC-MSCs and Dex (middle panel) and Dex (lower panel) on the proliferation of PBMCs. Histograms are representative of three independent experiments. The numbers in the plots indicate the percentage of CFSE-positive cells. (b–d) Quantitative analysis for the proliferation of PBMCs. Results are expressed as means ± SEMs for n = 3 measured in duplicate. **P < 0.01, ***P < 0.001.
CFSE: carboxyfluorescein succinimidyl ester; Dex, dexamethasone; iPSC-MSC: induced pluripotent stem cell-derived mesenchymal stem cell; PBMC: peripheral blood mononuclear cell.
Additionally, we analyzed whether IDO was involved in the inhibitory effects of iPSC-MSCs on PBMC proliferation. However, we did not observe any difference in IDO expression in iPSC-MSCs after co-culture with PBMCs in the presence or absence of Dex (Supplementary Fig. 1).
Dex had no Effects on the Immunomodulation of iPSC-MSCs on the Local Inflammation in CHS Model
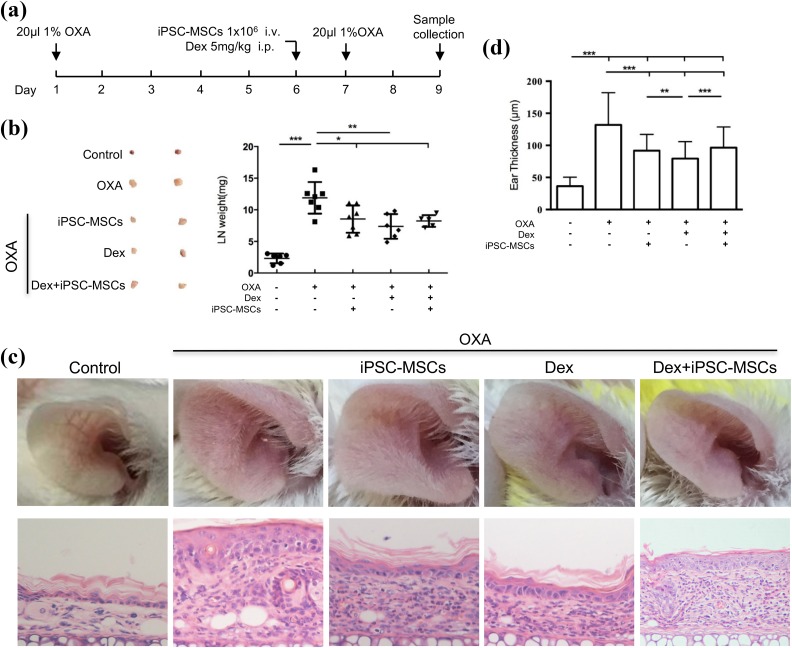
We previously found that iPSC-MSCs could suppress Th2 response in a mouse model of allergic inflammation21. To determine whether Dex influences the immunomodulatory function of iPSC-MSCs in allergic inflammation, a mouse model of CHS, in which Th2-like reaction is involved, was developed (Fig. 3(a))37. Firstly, we investigated whether Dex treatment affects the iPSC-MSC inhibition of the lymph node’s weight. As expected, there was a significant enhancement of lymph node’s weight in CHS models compared with control mice (Fig. 3(b)). Mice with the treatment of iPSC-MSCs or Dex alone had a marked reduction of lymph node weight (Fig. 3(b)). Interestingly, there were no differences in lymph node weight between mice with the treatment of iPSC-MSCs alone and iPSC-MSCs combined with Dex (Fig. 4(b)). These data suggested that simultaneous treatment with Dex does not affect the immunomodulation of iPSC-MSCs on the inflammation in regional draining lymph nodes in the CHS model.
Fig. 3.
Dex had no effect on the inhibition of iPSC-MSCs on the local inflammation in the CHS model. (a) The experimental protocol used to examine the effect of iPSC-MSCs and Dex. (b) The effect of iPSC-MSCs and Dex on the weight of draining lymph nodes. Representative results (left) and quantification of the size of draining lymph nodes (right). (c) Representative photomicrographs of H&E stained ear sections. (magnification, ×400). (d) Statistical analysis for ear thickness. Data are expressed as means ± SEMs. For each experiment, 5–8 animals were evaluated for each group. *P < 0.05, **P < 0.01, ***P < 0.001.
CHS: contact hypersensitivity; Dex: dexamethasone; H&E: hematoxylin and eosin; i.p.: intraperitoneal injection; i.v.: intravenous injection; iPSC-MSC: induced pluripotent stem cell-derived mesenchymal stem cell; LN: lymph node; OXA: oxazolone.
Fig. 4.
Co-administration of iPSC-MSCs with Dex decreased local levels of IFN-γ and TNF-α in a CHS model. The effect of iPSC-MSCs and Dex on the levels of IFN-γ and TNF-α in the ears was determined by western blot. The results represent three independent experiments, 5–8 animals were evaluated for each group. Data are expressed as means ± SEMs. *P < 0.05, **P < 0.01, ***P < 0.001.
CHS: contact hypersensitivity; Dex: dexamethasone; IFN: interferon; iPSC-MSC: induced pluripotent stem cell-derived mesenchymal stem cell; OXA: oxazolone; TNF: tumor necrosis factor.
Next, we qualitatively and quantitatively compared the effects of iPSC-MSCs with or without Dex treatment on the histopathology of the external ear. The results showed that there was a significant infiltration of inflammatory cells in the external ear of CHS mice, and the appearance of the ear was redder and more swollen than the control mice (Fig. 3(c–d)). As expected, both iPSC-MSCs and Dex treatment alone showed significant attenuation of the external ear inflammation, including swelling, inflammatory infiltration and thickness of the external ear compared with CHS mice (Fig. 3(c–d)). Interestingly, Dex treatment alone showed a stronger immunoinhibitory effect on the ear thickness of CHS mice compared with those of iPSC-MSCs alone or combined with Dex (Fig. 3(d)). However, there was no significant difference between iPSC-MSCs alone and iPSC-MSCs combined with Dex (Fig. 3(d)), suggesting that Dex had no effects on the immunomodulation of iPSC-MSCs on the inflammation in CHS mice. Next, we measured the IL-6 and IL-10 levels in the serum of CHS mice. Unexpectedly, the results showed that there was no significant difference in IL-6 and IL-10 levels among the five groups (Supplementary Fig. 2). Taken together, these findings suggested that Dex did not affect the immunomodulation of iPSC-MSCs on the local inflammation in the CHS model.
Co-administration of iPSC-MSCs with Dex Decreased Local Expression of IFN-γ and TNF-α in the CHS Model
After 2 days from challenge in CHS mice, the ear tissues were harvested to determine the expressions of IFN-γ and TNF-α by western blot analysis. CHS mice showed increased expression of IFN-γ and TNF-α compared with those of control mice (Fig. 4). Dex alone decreased the expression of IFN-γ and TNF-α in the ears of CHS mice. Treatment of iPSC-MSCs alone showed no significant effect on both IFN-γ and TNF-α expression. The levels of IFN-γ and TNF-α in the ears of CHS mice co-administered with iPSC-MSCs and Dex were lower than those of iPSC-MSCs administered alone, but similar to Dex alone, suggesting Dex could inhibit the production of IFN-γ and TNF-α in the CHS model independent of iPSC-MSC transplantation.
Dex did Not Affect the Immunomodulatory Effect of iPSC-MSCs on Allergic Airway Inflammation in Mice
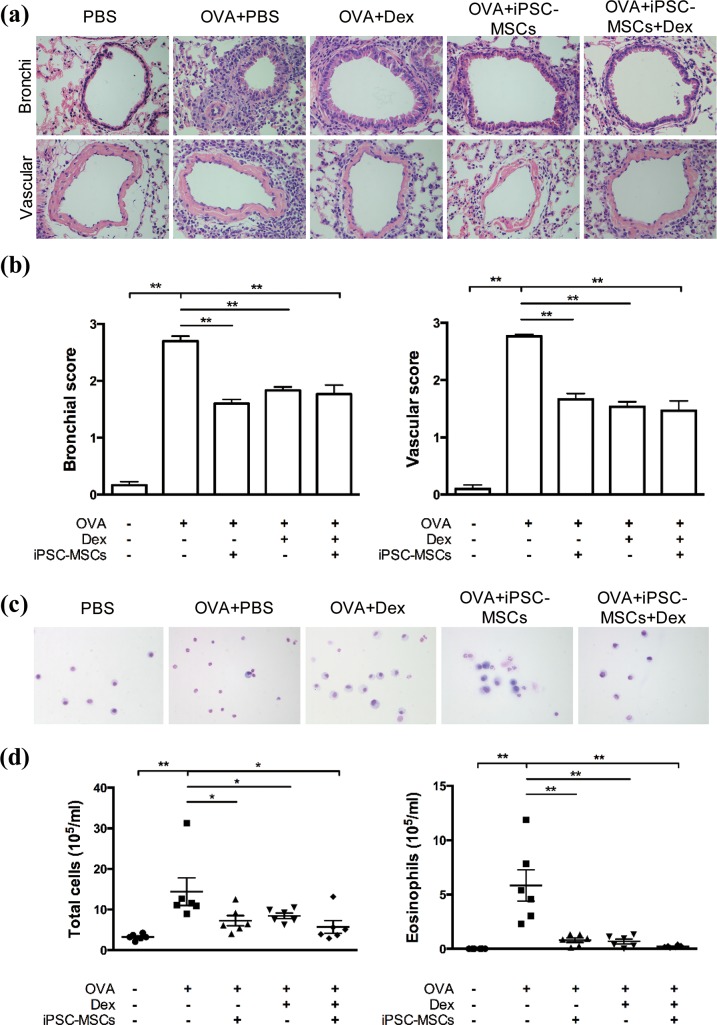
To further confirm the effects of Dex on the immunomodulatory effect of iPSC-MSCs in vivo, we developed a mouse model of OVA-induced allergic airway inflammation. OVA-sensitized mice developed a significant pulmonary inflammation as evidenced by higher bronchial and vascular scores in lung histology (Fig. 5(a) and (b)), and increased numbers of total inflammatory cells and eosinophils in BALF (Fig. 5(c) and (d)) compared with those in PBS-sensitized mice. In contrast, iPSC-MSC and Dex treatment, alone and combined, exhibited a significant reduction in pulmonary inflammation compared with those in OVA-sensitized mice treated with PBS (Fig. 5(a–d)). Furthermore, the mice treated with iPSC-MSCs combined with Dex showed a similar extent of reduction in pulmonary inflammation as those treated with iPSC-MSCs or Dex alone. This suggested that Dex had no effects on the immunomodulation of iPSC-MSCs on allergic airway inflammation.
Fig. 5.
Dex did not affect the immunomodulatory effect of iPSC-MSCs on allergic airway inflammation in mice. (a) Representative photomicrographs of H&E stained lung sections from each group (magnification, ×400). (b) Statistical analysis for inflammation score in the lungs. (c) Representative diff-quick staining of the inflammatory cells present in the BALF (magnification, ×400). (d) Total cells and eosinophils counts in the BALF. The results represent three independent experiments, 4–6 animals were evaluated for each group. Data are expressed as means ± SEMs. *P < 0.05, **P < 0.01, ***P < 0.001.
BALF: bronchoalveolar lavage fluid, Dex: dexamethasone; H&E: hematoxylin and eosin; iPSC-MSC: induced pluripotent stem cell-derived mesenchymal stem cell; OVA: ovalbumin; PBS: phosphate-buffered saline.
Discussion
The iPSC-MSCs have advantages over BM-MSCs, including longer lifespan, various sources and easier in vitro expansion, which makes it an important source of MSCs17,38. We have successfully derived multipotent MSCs from human iPSCs and demonstrated that human iPSC-MSCs are capable of immunomodulation, such as inhibiting lymphocyte proliferation and suppressing Th2 cytokines including IL-4, IL-5, or IL-13 in a mouse model of allergic airway inflammation20,21, suggesting the potential clinical application of iPSC-MSCs for the treatment of allergic diseases. Glucocorticoids are used commonly to treat allergic diseases. Therefore, the interaction between glucocorticoids and iPSC-MSCs need to be determined before clinical application39,40. In the present study, we have observed that simultaneous treatment with iPSC-MSCs and Dex showed a comparable immunoinhibitory effects on PBMCs proliferation induced by anti-CD3/CD28 in vitro, and local inflammation of the ear in a mouse model of CHS, as well as OVA-induced allergic airway inflammation in mice, which were similar to those treated with iPSC-MSCs alone, suggesting that Dex does not affect the immunoinhibitory effects of iPSC-MSCs.
In line with our previous studies20,21, iPSC-MSCs were successfully induced from amniocyte-derived iPSCs and were phenotypically and functionally characterized. iPSC-MSCs had the ability to differentiate into adipocytes, osteoblasts and chondrocytes. We also identified that iPSC-MSCs inhibited anti-CD3/CD28-induced lymphocyte proliferation in vitro, reduced local inflammation in the ears of CHS mice, as indicated by reduction of gross lymph node weight, inflammatory infiltration and the thickness of the external ear, and decreased allergic airway inflammation in mice as evidenced by lower inflammation scores in the lung and fewer inflammatory cells and eosinophils in BALF. In terms of the mechanisms underlying the action of iPSC-MSCs, we recently reported that iPSC-MSCs expressed a high level of IL-10, an important anti-inflammatory cytokine, to inhibit monocyte differentiation into dendritic cells in vitro41. Here we did not observe a significant difference in IL-10 levels in blood between mice treated with and without iPSC-MSCs. Additionally, no difference was observed for IDO expression in iPSC-MSCs after co-culture with PBMCs in vitro. These results suggested that there may be other mechanisms involved in the iPSC-MSC action in the present study.
There have been several studies investigating the effects of immunosuppressive drugs on the viability, proliferation, differentiation and function of MSCs. Mycophenolic acid, a cell cycle inhibitor, and rapamycin, an mTOR inhibitor, have been shown to suppress MSC proliferation at therapeutic doses42. High-dose tacrolimus, a calcineurin inhibitor, induced toxicity in adult MSCs42, whereas MSCs combined with low-dose tacrolimus was shown to be as effective as standard dose tacrolimus in maintaining graft survival after kidney transplantation43, supporting the simultaneous application of MSCs and low-dose tacrolimus.
Only a few studies have investigated the effects of steroids on the immunomodulatory function of MSCs. One study reported that Dex did not antagonize the inhibitory effect of human BM-MSCs in a mixed lymphocyte response32. However, a recent study showed that Dex significantly eliminated the immunosuppressive effects of BM-MSCs on anti-CD3-activated T-cell proliferation in vitro and in a mouse model of liver fibrosis33, in which Dex inhibited the expression of inducible nitric oxide synthase by preventing STAT1 phosphorylation, thus suggesting that concurrent application of MSCs with steroids should be avoided in clinical settings. However, our findings have shown that Dex had no effects on the immunosuppressive effects of iPSC-MSCs on lymphocyte proliferation, allergic diseases of CHS or airway inflammation. It supported the feasibility of concomitant application of iPSC-MSCs with steroids in some clinical settings. Or sometimes, concomitant application of iPSC-MSCs with a low dosage of steroid may have better results compared with a high dosage of steroid. Of course, the discrepancy underlying the interaction between steroids and adult MSCs or iPSC-MSCs should be further investigated.
We observed that the combination of iPSC-MSCs and Dex had less effect on decreasing ear thickness compared with Dex treatment alone in CHS mice. However, no significant difference was found for the IFN-γ and TNF-α levels in the ears of CHS mice, or the inflammation in the lungs in allergic mice between the combination of iPSC-MSCs and Dex and Dex alone. Anyway, we are unable to exclude a possibility that iPSC-MSCs might have a disruptive effect on the anti-inflammatory effects of Dex in some settings. Their interaction should be carefully addressed in specific settings. Fortunately, we found that although Dex was able to inhibit PBMC proliferation in a concentration-dependent manner (from 0.01 to 100 µg/ml), it showed a consistent suppressive effect on PBMC proliferation when co-cultured with iPSC-MSCs, which was equivalent to that of iPSC-MSCs in the absence of Dex. It suggested good application of iPSC-MSCs combined with an even low dosage of steroid.
We acknowledge some limitations in our current study. Although we determined the effect of iPSC-MSCs combined with Dex on local inflammation in CHS mice, we did not evaluate the differential subsets of inflammatory cells, which should be further assessed by flow cytometry analysis. Secondly, we acknowledge the possibility that iPSC-MSCs might interrupt the inhibitory effect of Dex on lymphocyte proliferation and in CHS mice in our study, which should be further studied in glucocorticoid receptor knockout mice in the future.
In conclusion, to our knowledge, we provide the first preliminary evidence that Dex does not significantly affect the immunomodulatory function of iPSC-MSCs both in vitro and in vivo. It supports the feasibility of concomitant application of iPSC-MSCs with steroids in clinical settings.
Supplemental Material
Supplementary_Material for An in Vitro and in Vivo Study of the Effect of Dexamethasone on Immunoinhibitory Function of Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cells by Dan Wang, Yue-Qi Sun, Wen-Xiang Gao, Xing-Liang Fan, Jian-Bo Shi, and Qing-Ling Fu in Cell Transplantation
Acknowledgments
Dan Wang and Yue-Qi Sun contributed equally for this article. Dan Wang and Yue-Qi Sun performed the cell culture, collection and assembly of data, data analysis, and prepared the manuscript; Wen-Xiang Gao and Dan Wang performed flow cytometry, western blot, and preparation of stem cells; Xing-Liang Fan edited the manuscript. Jian-Bo Shi and Qing-Ling Fu contributed to the conception and design of the study. Qing-Ling Fu wrote and revised the manuscript. All authors reviewed and approved the final manuscript.
Footnotes
Ethical Approval: The study protocol was approved by the Ethics Committee of the First Affiliated Hospital, Sun Yat-sen University (No. 2014-C-053 for human) and Institutional Animal Care and Use Committee Sun Yat-sen University, China (No. DB-16-0404 for animals).
Statement of Human and Animal Rights: All procedures were performed according to protocols approved by the Institutional Animal Care and Use Committee, Sun Yat-sen University.
Statement of Informed Consent: Human blood buffy coats from ‘anonymous donors’ were obtained from Guangzhou Blood and an exemption of the written informed consent was approved.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was supported by grants from the National Natural Science Foundation of China (No. 81500768, 81322012, 81373174, 81273272, 81470069, 81670902, 81770984) and the Science and Technology Foundation of Guangdong Province of China (No. 2015B020225001, 2016A020215049, 2016A030308017).
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Singer NG, Caplan AI. Mesenchymal stem cells: Mechanisms of inflammation. Annu Rev Pathol. 2011;6:457–478. [DOI] [PubMed] [Google Scholar]
- 2. Lazarus HM, Koc ON, Devine SM, Curtin P, Maziarz RT, Holland HK, Shpall EJ, McCarthy P, Atkinson K, Cooper BW, Gerson SL, Laughlin MJ, Loberiza FR, Jr., Moseley AB, Bacigalupo A. Cotransplantation of HLA-identical sibling culture-expanded mesenchymal stem cells and hematopoietic stem cells in hematologic malignancy patients. Biology of blood and marrow transplantation: J Am Soc Blood Marrow Transplantat. 2005;11(5):389–398. [DOI] [PubMed] [Google Scholar]
- 3. Sun LY, Wang DD, Liang J, Zhang HY, Feng XB, Wang H, Hua BZ, Liu BJ, Ye SQ, Hu XA, Xu WR, Zeng XF, Hou YY, Gilkeson GS, Silver RM, Lu LW, Shi ST. Umbilical cord mesenchymal stem cell transplantation in severe and refractory systemic lupus erythematosus. Arthritis Rheum. 2010;62(8):2467–2475. [DOI] [PubMed] [Google Scholar]
- 4. Garcia-Olmo D, Garcia-Arranz M, Herreros D, Pascual I, Peiro C, Rodriguez-Montes JA. A phase I clinical trial of the treatment of Crohn’s fistula by adipose mesenchymal stem cell transplantation. Dis Colon Rectum. 2005;48(7):1416–1423. [DOI] [PubMed] [Google Scholar]
- 5. Squillaro T, Peluso G, Galderisi U. Clinical trials with mesenchymal stem cells: An update. Cell Transplant. 2016;25(5):829–848. [DOI] [PubMed] [Google Scholar]
- 6. Faiella W, Atoui R. Immunotolerant properties of mesenchymal stem cells: Updated review. Stem Cells Int. 2016;2016:1859567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Uccelli A, Pistoia V, Moretta L. Mesenchymal stem cells: A new strategy for immunosuppression? Trends Immunol. 2007;28(5):219–226. [DOI] [PubMed] [Google Scholar]
- 8. Bonfield TL, Nolan Koloze MT, Lennon DP, Caplan AI. Defining human mesenchymal stem cell efficacy in vivo. J Inflamm (Lond). 2010;7:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang Y, Liang X, Lian Q, Tse HF. Perspective and challenges of mesenchymal stem cells for cardiovascular regeneration. Expert Rev Cardiovasc Ther. 2013;11(4):505–517. [DOI] [PubMed] [Google Scholar]
- 10. Xin Y, Wang YM, Zhang H, Li J, Wang W, Wei YJ, Hu SS. Aging adversely impacts biological properties of human bone marrow-derived mesenchymal stem cells: Implications for tissue engineering heart valve construction. Artif Organs. 2010;34(3):215–222. [DOI] [PubMed] [Google Scholar]
- 11. Zaim M, Karaman S, Cetin G, Isik S. Donor age and long-term culture affect differentiation and proliferation of human bone marrow mesenchymal stem cells. Ann Hematol. 2012;91(8):1175–1186. [DOI] [PubMed] [Google Scholar]
- 12. Sepulveda JC, Tome M, Fernandez ME, Delgado M, Campisi J, Bernad A, Gonzalez MA. Cell senescence abrogates the therapeutic potential of human mesenchymal stem cells in the lethal endotoxemia model. Stem Cell. 2014;32(7):1865–1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Takahashi K, Yamanaka S. A decade of transcription factor-mediated reprogramming to pluripotency. Nat Rev Mol Cell Biol. 2016;17(3):183–193. [DOI] [PubMed] [Google Scholar]
- 14. Amabile G, Meissner A. Induced pluripotent stem cells: current progress and potential for regenerative medicine. Trends Mol Med. 2009;15(2):59–68. [DOI] [PubMed] [Google Scholar]
- 15. Nishikawa S, Goldstein RA, Nierras CR. The promise of human induced pluripotent stem cells for research and therapy. Nat Rev Mol Cell Biol. 2008;9(9):725–729. [DOI] [PubMed] [Google Scholar]
- 16. Yamanaka S. Strategies and new developments in the generation of patient-specific pluripotent stem cells. Cell Stem Cell. 2007;1(1):39–49. [DOI] [PubMed] [Google Scholar]
- 17. Lin L, Bolund L, Luo Y. 2016. Towards personalized regenerative cell therapy: Mesenchymal stem cells derived from human induced pluripotent stem cells. Curr Stem Cell Res Ther. 11(2):122–130. [DOI] [PubMed] [Google Scholar]
- 18. Jung Y, Bauer G, Nolta JA. Concise review: Induced pluripotent stem cell-derived mesenchymal stem cells: Progress toward safe clinical products. Stem Cell. 2012;30(1):42–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lian Q, Zhang Y, Zhang J, Zhang HK, Wu X, Zhang Y, Lam FF, Kang S, Xia JC, Lai WH, Au KW, Chow YY, Siu CW, Lee CN, Tse HF. Functional mesenchymal stem cells derived from human induced pluripotent stem cells attenuate limb ischemia in mice. Circulation. 2010;121(9):1113–1123. [DOI] [PubMed] [Google Scholar]
- 20. Fu QL, Chow YY, Sun SJ, Zeng QX, Li HB, Shi JB, Sun YQ, Wen W, Tse HF, Lian Q, Xu G. Mesenchymal stem cells derived from human induced pluripotent stem cells modulate T-cell phenotypes in allergic rhinitis. Allergy. 2012;67(10):1215–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sun YQ, Deng MX, He J, Zeng QX, Wen W, Wong DS, Tse HF, Xu G, Lian Q, Shi J, Fu QL. Human pluripotent stem cell-derived mesenchymal stem cells prevent allergic airway inflammation in mice. Stem Cell. 2012;30(12):2692–2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Giuliani M, Oudrhiri N, Noman ZM, Vernochet A, Chouaib S, Azzarone B, Durrbach A, Bennaceur-Griscelli A. Human mesenchymal stem cells derived from induced pluripotent stem cells down-regulate NK-cell cytolytic machinery. Blood. 2011;118(12):3254–3262. [DOI] [PubMed] [Google Scholar]
- 23. Chen YS, Pelekanos RA, Ellis RL, Horne R, Wolvetang EJ, Fisk NM. Small molecule mesengenic induction of human induced pluripotent stem cells to generate mesenchymal stem/stromal cells. Stem Cell Transl Med. 2012;1(2):83–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hynes K, Menicanin D, Mrozik K, Gronthos S, Bartold PM. Generation of functional mesenchymal stem cells from different induced pluripotent stem cell lines. Stem Cell Develop. 2014;23(10):1084–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kimbrel EA, Kouris NA, Yavanian GJ, Chu J, Qin Y, Chan A, Singh RP, McCurdy D, Gordon L, Levinson RD, Lanza R. Mesenchymal stem cell population derived from human pluripotent stem cells displays potent immunomodulatory and therapeutic properties. Stem Cell Develop. 2014;23(14):1611–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang Y, Liang X, Liao S, Wang W, Wang J, Li X, Ding Y, Liang Y, Gao F, Yang M, Fu Q, Xu A, Chai YH, He J, Tse HF, Lian Q. Potent paracrine effects of human induced pluripotent stem cell-derived mesenchymal stem cells attenuate doxorubicin-induced cardiomyopathy. Sci Rep. 2015;5:11235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Knyazev OV, Parfenov AI, Konoplyannikov AG, Boldyreva ON. [Use of mesenchymal stem cells in the combination therapy of ulcerative colitis]. Ter Arkh. 2016;88(2):44–48. [DOI] [PubMed] [Google Scholar]
- 28. Peng Y, Ke M, Xu L, Liu L, Chen X, Xia W, Li X, Chen Z, Ma J, Liao D, Li G, Fang J, Pan G, Xiang AP. Donor-derived mesenchymal stem cells combined with low-dose tacrolimus prevent acute rejection after renal transplantation: A clinical pilot study. Transplantation. 2013;95(1):161–168. [DOI] [PubMed] [Google Scholar]
- 29. Perico N, Casiraghi F, Introna M, Gotti E, Todeschini M, Cavinato RA, Capelli C, Rambaldi A, Cassis P, Rizzo P, Cortinovis M, Marasa M, Golay J, Noris M, Remuzzi G. Autologous mesenchymal stromal cells and kidney transplantation: A pilot study of safety and clinical feasibility. Clin J Am Soc Nephrol. 2011;6(2):412–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yin F, Battiwalla M, Ito S, Feng X, Chinian F, Melenhorst JJ, Koklanaris E, Sabatino M, Stroncek D, Samsel L, Klotz J, Hensel NF, Robey PG, Barrett AJ. Bone marrow mesenchymal stromal cells to treat tissue damage in allogeneic stem cell transplant recipients: Correlation of biological markers with clinical responses. Stem Cell. 2014;32(5):1278–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li JF, Zhang DJ, Geng T, Chen L, Huang H, Yin HL, Zhang YZ, Lou JY, Cao B, Wang YL. The potential of human umbilical cord-derived mesenchymal stem cells as a novel cellular therapy for multiple sclerosis. Cell Transplant. 2014;23(Suppl. 1):S113–S122. [DOI] [PubMed] [Google Scholar]
- 32. Buron F, Perrin H, Malcus C, Hequet O, Thaunat O, Kholopp-Sarda MN, Moulin FT, Morelon E. Human mesenchymal stem cells and immunosuppressive drug interactions in allogeneic responses: An in vitro study using human cells. Transplant Proc. 2009;41(8):3347–3352. [DOI] [PubMed] [Google Scholar]
- 33. Chen X, Gan Y, Li W, Su J, Zhang Y, Huang Y, Roberts AI, Han Y, Li J, Wang Y, Shi Y. The interaction between mesenchymal stem cells and steroids during inflammation. Cell Death Dis. 2014;5:e1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, Lanino E, Sundberg B, Bernardo ME, Remberger M, Dini G, Egeler RM, Bacigalupo A, Fibbe W, Ringden O, Developmental Committee of the European Group for B, Marrow T. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: A phase II study. Lancet. 2008;371(9624):1579–1586. [DOI] [PubMed] [Google Scholar]
- 35. Su WR, Zhang QZ, Shi SH, Nguyen AL, Le AD. Human gingiva-derived mesenchymal stromal cells attenuate contact hypersensitivity via prostaglandin e2-dependent mechanisms. Stem Cell. 2011;29(11):1849–1860. [DOI] [PubMed] [Google Scholar]
- 36. Keating A. Mesenchymal stromal cells: New directions. Cell Stem Cell. 2012;10(6):709–716. [DOI] [PubMed] [Google Scholar]
- 37. Hayashi T, Hara S, Hasegawa K. Enhanced contact hypersensitivity by delayed T-helper 2 response in BALB/C mice. Allergy Asthma Proc. 2009;30(4):449–457. [DOI] [PubMed] [Google Scholar]
- 38. Whitt J, Vallabhaneni KC, Penfornis P, Pochampally R. Induced pluripotent stem cell-derived mesenchymal stem cells: a leap toward personalized therapies. Curr Stem Cell Res Ther. 2016;11(2):141–148. [DOI] [PubMed] [Google Scholar]
- 39. LeBlanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, Lanino E, Sundberg B, Bernardo ME, Remberger M, Dini G, Egeler RM, Bacigalupo A, Fibbe W, Ringden O. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: A phase II study. Lancet. 2008;371(9624):1579–1586. [DOI] [PubMed] [Google Scholar]
- 40. Wu KH, Chan CK, Tsai C, Chang YH, Sieber M, Chiu TH, Ho M, Peng CT, Wu HP, Huang JL. Effective treatment of severe steroid-resistant acute graft-versus-host disease with umbilical cord-derived mesenchymal stem cells. Transplantation. 2011;91(12):1412–1416. [DOI] [PubMed] [Google Scholar]
- 41. Gao WX, Sun YQ, Shi J, Li CL, Fang SB, Wang D, Deng XQ, Wen W, Fu QL. Effects of mesenchymal stem cells from human induced pluripotent stem cells on differentiation, maturation, and function of dendritic cells. Stem Cell Res Ther. 2017;8(1):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hoogduijn MJ, Crop MJ, Korevaar SS, Peeters AM, Eijken M, Maat LP, Balk AH, Weimar W, Baan CC. Susceptibility of human mesenchymal stem cells to tacrolimus, mycophenolic acid, and rapamycin. Transplantation. 2008;86(9):1283–1291. [DOI] [PubMed] [Google Scholar]
- 43. Pan GH, Chen Z, Xu L, Zhu JH, Xiang P, Ma JJ, Peng YW, Li GH, Chen XY, Fang JL, Guo YH, Zhang L, Liu LS. Low-dose tacrolimus combined with donor-derived mesenchymal stem cells after renal transplantation: A prospective, non-randomized study. Oncotarget. 2016;7(11):12089–12101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary_Material for An in Vitro and in Vivo Study of the Effect of Dexamethasone on Immunoinhibitory Function of Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cells by Dan Wang, Yue-Qi Sun, Wen-Xiang Gao, Xing-Liang Fan, Jian-Bo Shi, and Qing-Ling Fu in Cell Transplantation