Abstract
Current human papillomavirus (HPV)16 DNA testing has high sensitivity but low specificity, while mRNA testing (qualitative) improves the specificity. However, both techniques are not able to discriminate between transient and persistent infections. To overcome the disadvantages, we quantitatively detected E6 and E7 mRNAs by quantitative real-time polymerase chain reaction (qRT-PCR) in cervical brushing cells from 87 HPV16+ and 31 HPV16− patients. Our results showed that the expression levels of E6 mRNA or E7 mRNA were significantly increased in HPV16-positive cases than that in the negative cases. Furthermore, in HPV16+ cases, the expression levels of E6 mRNA were significantly increased in invasive cancer compared with high-grade squamous intraepithelial lesion (HSIL; p < 0.01), and HSIL compared with low-grade squamous intraepithelial lesion (LSIL; p < 0.01). There were no significant changes between LSIL and benign lesions. The expression levels of E7 mRNA presented no significant difference among the above-mentioned four groups. To test whether qRT-PCR can discriminate between transient and persistent infections, 57 HPV16+ patients were followed up for 1 year, and our results demonstrated that the expression levels of both E6 mRNA and E7 mRNA in the persistent infection group were significantly increased relative to the transient infection group (p < 0.01 or 0.05). Thus, a quantitative detection of the expression levels of E6 mRNA in cervical brushing cells may not only be used as an ancillary tool to cytological diagnosis of cervical neoplasia, but may also help to determine the severity of the lesions and the triage of transient infection.
Keywords: Human papillomaviruses, cervical lesions, E6 mRNA, E7 mRNA, cytological diagnosis
Introduction
It is well known that persistent infection of the high-risk type human papillomavirus (HPV)16 is closely related to the occurrence of cervical cancer1–3, and the introduction of HPV DNA testing into the cervical lesion diagnostic programs has improved the effectiveness of cervical screening. However, the current DNA testing has high sensitivity (97.6%) but low specificity (17%). The low specificity of HPV DNA testing is not able to discriminate transient infections, which usually clear within 2 years, from persistent infections. Numerous studies demonstrated that E6 and E7 proteins were key factors in the processing of HPV due to the above genomes being integrated into the human genome, which results in persistent infections4. The expression levels of E6 and E7mRNA are low during transient infections, but increase once the viral genome integrates into the host. Furthermore, as major oncogenes, E6 and E7 proteins, are correlated with modifying the expression of cell cycle controllers and DNA repair regulations in the development and progression of cervical cancer5. Thus, it is necessary to introduce the detection of E6 and E7 mRNA expression in primary cervical screening programs in order to enhance the overall diagnostic accuracy and provide better long-term protection.
Recently, it has been reported that the detection of E6 and E7 mRNAs in high-risk HPV is superior to the detection of HPV DNA6–9. However, the mRNA testing methods are only qualitative and will not be able to detect the severities of the lesions. Also, both current DNA and mRNA techniques cannot discriminate between transient and persistent infections. Quantitative real-time polymerase chain reaction (qRT-PCR) is the most sensitive and reliable method for detection and quantification of nucleic acids, including RNA10. There are numerous applications for qRT-PCR in our studies and it is commonly used for both diagnostic and basic research11,12.
In previous research, we used the qRT-PCR method to detect the mRNAs of both vascular endothelial growth factor (VEGF) and endostatin in cervical epithelial cells. Our results showed that the combined detection of VEGF and endostatin had important clinical application value, and the sensitivity and accuracy were significantly increased compared with cytological diagnosis alone13. The primary objective of this study was to apply qRT-PCR to quantitatively detect the mRNAs of E6 and E7 in HPV16-positive and negative cervical epithelial cells and determine the possibility of this technique to overcome the disadvantages of current HPV DNA and HPV mRNA testing. Our E6 mRNA qRT-PCR results showed that qRT-PCR was not only able to identify transient from persistent infections in HPV-positive patients, but also to detect the severities of cervical intraepithelial lesions. Thus, quantitative detection of the expression levels of E6 mRNA in cervical brushing cells may not only be used as an ancillary tool to cytological diagnosis of cervical neoplasia, but may also help to determine the severity of the lesions and the triage of transient infection.
Materials and Methods
Patients Recruitment and Sample Collection
The study was conducted according to the guidelines of the institutional review boards at the First Affiliated Hospital of China Medical University; we obtained internal review board approval and/or patients informed consent for this study. The cervical brushing cells from 87 patients with HPV16 who attended the laboratory of cytopathology at the First Affiliated Hospital of China Medical University during the period June to October 2015 were included in the study. Their ages ranged from 23 to 77 years, with an average age of 42.4 years. An additional 31 randomly selected patients without HPV16 were included in the study and used as a control group. All patients had cytological diagnoses and biopsy histological diagnoses, and the detailed diagnostic results are shown in Table 1. A total of 57 HPV16+ patients with LSIL and benign by histological diagnoses were followed up for 1 year.
Table 1.
Comparison of Histological and Cytological Diagnoses in HPV16-Positive Cases.
| Histological diagnosis | n | Cytological diagnosis | |||||
|---|---|---|---|---|---|---|---|
| Cancer | HSIL | ASC-H | LSIL | ASC-US | NILM | ||
| Cancer | 10 | 8 | 2 | ||||
| HSIL | 20 | 12 | 4 | 4 | |||
| LSIL | 18 | 11 | 5 | 2 | |||
| Benign | 39 | 39 | |||||
| Total | 87 | 8 | 14 | 4 | 15 | 5 | 41 |
ASC-H: atypical squamous cells, cannot exclude high-grade squamous intraepithelial lesion; ASC-US: atypical squamous cells of undetermined significance; benign: no intraepithelial lesion; carcinoma: invasive carcinoma; HPV: human papillomaviruses; HSIL: high-grade squamous intraepithelial lesion; LSIL: low-grade squamous intraepithelial lesion; NILM: negative for intraepithelial lesion or malignancy.
HPV16 DNA Testing
HPV16 DNA was detected using an HPV GenoArray DNA Test Kit (HybriBro Ltd., Hong Kong, China) and the protocol was performed according to the manufacturer’s instructions. The detailed procedure of HPV16 DNA testing was described in reference with pubmed identification (PMID) 1832206310.
Cytology Test
Liquid-based cytology was used for the cytological preparation. All slides were automatically prepared and stained with Papanicolaou method for all 118 cases (BD Tripath, Burlington, NC, USA). The cytological diagnosis was assessed by two independent cytologists. The results were interpreted according to the 2015 Bethesda System. Residual materials were available for E6 and E7 mRNA qRT-PCR analysis.
qRT-PCR
Total RNA was extracted from cells using TRIzol Reagent (Life Technologies, Carlsbad, CA, USA) and RNeasy RNA isolation kit (QIAGEN, Hilden, Germany). First-strand cDNA was synthesized from 500 ng total RNA using a high-capacity cDNA RT Kit (Applied Biosystems, Carlsbad, CA, USA). The qRT-PCR was performed using SYBER GreenMaster Mix on a 7900HT Fast Real-Time PCR system (Applied Biosystems). Non-template controls were prepared every time for each paired primer to detect non-specific amplification. Average threshold cycle (Ct) values for GAPDH (housekeeping gene) were used to normalize average Ct values of the genes of interest. These values were used to calculate the average Ct values between groups, and the relative quantity (power of –ΔΔCt) was used to calculate fold change between each group. The detail information of the primers is listed in Table 2.
Table 2.
Sequences and Features of Primers Used for qRT-PCR.
| Gene | Forward/ reverse | Sequence | Size (bp) | mRNA |
|---|---|---|---|---|
| E6 | 270 | GTATGGAACAACATTAGAACAGCAA | 79 | KX545363 |
| 349 | GTGGCTTTTGACAGTTAATACACC | |||
| E7 | 482 | GCATGGAGATACACCTACATTG | 273 | KX545363 |
| 754 | TGGTTTCTGAGAACAGATGG | |||
| GAPDH | 50 | TTCTTTTGCGTCGCCAGCCGAG | 71 | XM_019023188.1 |
| 120 | CCAGGCGCCCAATACGACCAAA |
qRT-PCR: quantitative real-time polymerase chain reaction
The amplified products of E6, E7, and GAPDH were confirmed by DNA gel with correct sizes. The products were extracted, purified from the gel, and sent for DNA sequencing. The sequencing results were 100% correct.
Colposcopy and Histological Diagnoses
All HPV16-positive women were examined by colposcopy and underwent cervical biopsy. Biopsy samples were obtained within 4 weeks after the initial HPV DNA tests. Histological diagnosis was made by two experienced pathologists. The histological biopsy results were categorized into four general groups: benign (including no pathologic alteration and benign or reactive changes), LSIL (CIN1), and HSIL (including CIN2/CIN3, squamous cell carcinoma in situ, and/or involving glands). CIN2 lesions were confirmed by immunohistochemical staining for p16 and Ki-67. In patients who had more than one tissue sample, the highest grade of diagnosis was recorded.
Statistical Analysis
The SPSS 16.0 statistical software package (SPSS, Inc. Chicago, IL, USA) was used for all analyses. A Student’s t-test was used to compare data from the densitometry analysis. The McNemar’s test was used to compare the expression levels of both E6 and E7 mRNA in cervical brushing cells from HPV16+ and HPV16− patients. Analysis of variance and the least significant difference test were employed for statistical analysis. The level of statistical significance was set at p < 0.05.
Results
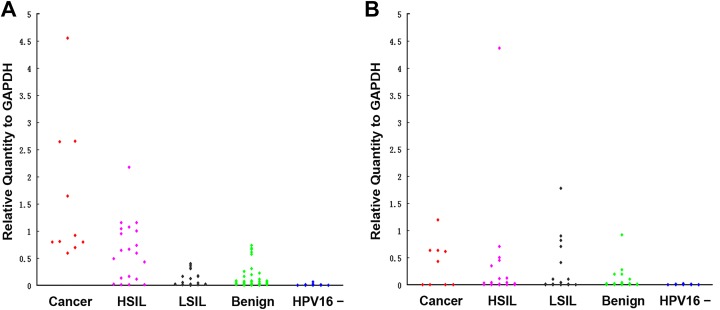
The qRT-PCR results of cervical cells from all 118 patients are presented in Table 3. For the mean E6 mRNA expression in 87 HPV16-positive patients was 0.480 ± 0.099; and in 31 HPV16-negative patients was 0.005 ± 0.002. The mean E7 mRNA expression in 87 HPV16-positive patients was 0.210 ± 0.06; and in 31 HPV16-negative patients was 0.003 ± 0.001. The expression levels of both E6 and E7 mRNA were significantly increased in HPV16-positive patients relative to HPV16-negative patients with p < 0.01 respectively (Fig.1A and B).
Table 3.
The mRNA of E6, and E7 in Cervical Brushing Cells of Patients With Cervical Dysplasia and Carcinoma l (mean ± SEM).
| Histology | n | E6 | E7 |
|---|---|---|---|
| HPV16 (+) | 87 | 0.484 ± 0.099 | 0.210 ± 0.06 |
| Carcinoma | 10 | 1.610 ± 0.410 | 0.470 ± 0.149* |
| HSIL | 20 | 0.628 ± 0.124 | 0.339 ± 0.217* |
| LSIL | 18 | 0.143 ± 0.346 | 0.272 ± 0.115 |
| Benign | 39 | 0.141 ± 0.034 | 0.048 ± 0.025 |
| HPV16 (−) | 31 | 0.005 ± 0.002 | 0.003 ± 0.001 |
Benign: no intraepithelial lesion; carcinoma: invasive carcinoma; E6: HPV16 E6; E7: HPV16 E7; HPV: human papillomavirus; HSIL: high-grade squamous intraepithelial lesion; LSIL: low-grade squamous intraepithelial lesion.
* p < 0.05 as compared with benign.
Fig. 1.
A and B represent the distribution of E6 mRNA and E7 mRNA in HPV16-positive and negative patients, respectively.
Benign: no intraepithelial lesion; carcinoma: invasive carcinoma; HPV16: human papillomavirus 16; HSIL: high-grade squamous intraepithelial lesion; LSIL: low-grade squamous intraepithelial lesion.
The 87 HPV16-positive patients were further divided into four groups: invasive carcinoma; HSIL; LSIL, and benign according the histological diagnosis. The mean and individual E6 and E7 mRNA expression levels are presented in Table 3 and Fig. 1 separately. The expression levels of E6 mRNA were significantly increased in invasive carcinoma compared with HSIL (p < 0.01), and in HSIL compared with LSIL (p < 0.01). There were no significant changes between LSIL and benign groups (p = 0.97). The expression levels of E7 mRNA were not significantly different among invasive cancer, HSIL, and LSIL groups, but there was a significant increase in the invasive cancer and HSIL groups when they were compared with the benign group individually (p < 0.05).
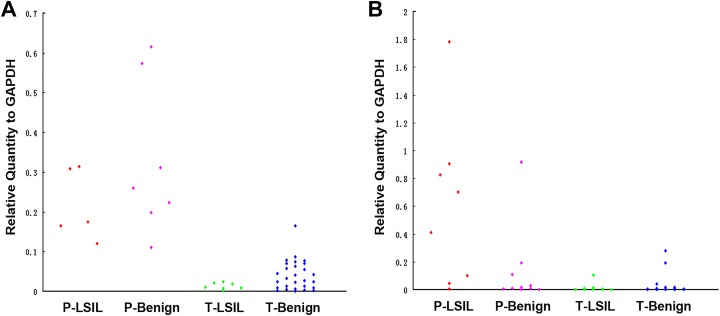
We further tried to differentiate transient from persistent infections in LSIL and benign patients. We followed up a total of 57 patients for 1 year and divided them into two groups: the persistent infection group (18 patients) and transient infection group (39 patients). Each group was further divided into LSIL and benign groups according to histological diagnosis. Our results are shown in Table 4 and Fig. 2. The expression levels of both E6 and E7 mRNA were significantly increased in the persistent infection group relative to the transient infection group (p < 0.01). The follow-up results showed that 10 patients with LSIL and 29 patients with benign lesions had a natural outcome in transient infection group after 1 year.
Table 4.
The mRNA of E6, and E7 in Cervical Brushing Cells of HPV16 (+) Patients with LSIL and Benign (mean ± SEM).
| Histology | n | E6 | E7 |
|---|---|---|---|
| Persistent infection | 18 | 0.367 ± 0.048* | 0.336 ± 0.117* |
| LSIL | 8 | 0.278 ± 0.038* | 0.596 ± 0.211* |
| Benign | 10 | 0.438 ± 0.075* | 0.127 ± 0.090** |
| Transient infection | 39 | 0.037 ± 0.006 | 0.018 ± 0.009 |
| LSIL | 10 | 0.034 ± 0.015 | 0.013 ± 0.010 |
| Benign | 29 | 0.038 ± 0.007 | 0.020 ± 0.011 |
E6: HPV16 E6; E7: HPV16 E7; HPV: human papillomavirus; LSIL: low-grade squamous intraepithelial lesion; benign: no intraepithelial lesion.
*p < 0.01 as compared with transient infection; **p < 0.05 as compared with transient infection.
Fig. 2.
A and B represent the distribution of E6 mRNA and E7 mRNA in HPV16-persistent infection and transient infection patients, respectively.
Benign: no intraepithelial lesion; LSIL: low-grade squamous intraepithelial lesion; P: persistent infection; T: transient infection.
Discussion
HPV is a cyclic double stranded DNA virus and it is well known that the high-risk type HPV is closely related to the occurrence of cervical cancer1. Because of its important causal role, HPV is used as a biomarker for the detection of cervical intraepithelial neoplasia and invasive cervical cancers14–16. Accumulated evidence had shown that HPV DNA testing provides higher sensitivity (97.6%) than cytology. Thus, it has been recommended as the primary cervical cancer screening in many countries17. However, DNA testing is not able to discriminate the transient from the persistent infection with a low specificity (17%). Furthermore, more than 90% of women have been infected with HPV infection in their lifetime, while most of the infections are transient infections and result in spontaneous recovery within 2 years18. Therefore, HPV DNA testing alone may increase the psychological burden for the positive patients and may also lead to excessive colposcopies.
HPV16 E6 mRNA and E7 mRNA are two critical oncogenic transcriptional factors in the early stages of the viral life cycle19. Persistent HPV infection is a consequence of increased E6 and E7 mRNA expression in host cells to promote cellular proliferation and malignant transformation20,21. HPV mRNA tests have higher specificity (76%) than DNA tests (17%) with comparable sensitivity22. It was approved by the United States Food and Drug Administration (US FDA) for the screening of women older than 21 years old with cytology results as atypical squamous cells of undetermined significance, or for women older than 30 years old23. However, these testing are qualitative tests only and E6 and E7 mRNA are cocktailed together. E6 plays an important role in the degradation of p53 and abrogates cell growth arrest, while E7 binds and deactivates retinoblastomas protein (pRb) to interfere with cell cycle regulation. In the current study, qRT-PCR was applied to quantitatively detect the mRNA expression of E6 and E7 individually in HPV16-positive and negative cervical epithelial cells. Our results demonstrated that quantitative detection of E6 mRNA and E7mRNA expression levels was able to identify the transient from persistent infections in HPV-positive patients. Only E6 mRNA expression levels were able to be used for interpreting the severity of cervical intraepithelial lesions. Our small sample size study of E6 and E7 biomarker expression results were promising, and these results need be used to predict the severities of cervical intraepithelial lesions and identify transient from persistent infections in HPV-positive patients in large sample size studies. Additional clinical trials are needed to determine the true clinical value of this assay.
Conclusion
In summary, the current DNA testing of cervical brushing cells has high sensitivity but low specificity, while the mRNA testing (qualitative) improves specificity. However, both techniques cannot discriminate between transient and persistent infections. In the current study, qRT-PCR was applied to quantitatively detect the mRNA of E6 and E7 individually in HPV16-positive and negative cervical epithelial cells. Our results demonstrated that quantitative detection of the expression levels of E6 mRNA in cervical brushing cells may not only be used as an ancillary tool to cytological diagnosis of cervical neoplasia, but also help to determine the severity of the lesions and the triage of transient infection.
Footnotes
Ethical Approval: This study was approved by our institutional review board.
Statement of Human and Animal Rights: Human specimens were tested in accordance with our institutional review board guidelines [2018]2018-10-2.
Statement of Informed Consent: We had obtained the patient informed consent for this study.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from the National Natural Science Foundation of China to Guang-Ping Wu, Grant No.81171650 and 81672082.
References
- 1. Guo Y, Meng X, Ma J, Zheng Y, Wang Q, Wang Y, Shang H. Human papillomavirus 16 E6 contributes HIF-1α induced Warburg effect by attenuating the VHL-HIF-1α interaction. Int J Mol Sci. 2014;15(5):7974–7986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bouvard V, Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, Benbrahim-Tallaa L, Guha N, Freeman C, Galichet L, Cogliano V; WHO International Agency for Research on Cancer Monograph Working Group. A review of human carcinogens--Part B: biological agents. Lancet Oncol. 2009;10(4):321–322. [DOI] [PubMed] [Google Scholar]
- 3. Menon S, van den Broeck D, Rossi R, Ogbe E, Mabeya H. Multiple HPV infections in female sex workers in western Kenya: implications for prophylactic vaccines within this sub population. Infect Agent Cancer. 2017;12:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhang L, Yang B, Zhang A, Zhou A, Yuan J, Wang Y, Sun L, Cao H, Wang J, Zheng W. Association between human papillomavirus type 16 E6 and E7 variants with subsequent persistent infection and recurrence of cervical high-grade squamous intraepithelial lesion after conization. J Med Virol. 2016;88(11):1982–1988. [DOI] [PubMed] [Google Scholar]
- 5. Padilla-Quirarte HO, Trejo-Moreno C, Fierros-Zarate G, Castañeda JC, Palma-Irizarry M, Hernández-Márquez E, Burguete-Garcia AI, Peralta-Zaragoza O, Madrid-Marina V, Torres-Poveda K, Bermúdez-Morales VH. Interferon-Tau has antiproliferative effects, represses the expression of E6 and E7 oncogenes, induces apoptosis in cell Lines transformed with HPV16 and inhibits tumor growth in vivo. J Cancer. 2016;7(15):2231–2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liu TY, Xie R, Luo L, Reilly KH, He C, Lin YZ, Chen G, Zheng XW, Zhang LL, Wang HB. Diagnostic validity of human papillomavirus E6/E7 mRNA test in cervical cytological samples. J Virol Methods. 2014;196:120–125. [DOI] [PubMed] [Google Scholar]
- 7. Wang HY, Lee D, Park S, Kim G, Kim S, Han L, Yubo R, Li Y, Park KH, Lee H. Diagnostic performance of HPV E6/E7 mRNA and HPV DNA assays for the detection and screening of oncogenic human papillomavirus infection among woman with cervical lesions in China. Asian Pac J Cancer Prev. 2015;16(17):7633–7640. [DOI] [PubMed] [Google Scholar]
- 8. Ge Y, Christensen P, Luna E, Armylagos D, Schwartz MR, Mody DR. Performance of Aptima and Cobas HPV testing platforms in detecting high-grade cervical dysplasia and cancer. Cancer. 2017;125(8):652–657. [DOI] [PubMed] [Google Scholar]
- 9. Yao YL, Tian QF, Cheng B, Cheng YF, Ye J, Lu WG. Human papillomavirus (HPV) E6/E7 mRNA detection in cervical exfoliated cells: a potential triage for HPV-positive women. J Zhejiang Univ Sci B. 2017;18(3):256–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pang Z, Li A, Li J, Qu J, He C, Zhang S, Li C, Zhang Q, Liang M, Li D. Comprehensive multiplex one-step real-time TaqMan qRT-PCR assays for detection and quantification of hemorrhagic fever viruses. PLoS One. 2014;9(4):e95635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shao JS, Sun J, Wang S, Chung K, Du JT, Wang J, Qiu XS, Wang EH, Wu GP. HPV16 E6/E7 upregulates HIF-2α and VEGF by inhibiting LKB1 in lung cancer cells. Tumour Biol. 2017;39(7):1010428317717137. [DOI] [PubMed] [Google Scholar]
- 12. Yang JH, Li XY, Wang X, Hou WJ, Qiu XS, Wang EH, Wu GP. Long-term persistent infection of HPV 16 E6 up-regulate SP1 and hTERT by inhibiting LKB1 in lung cancer cells. PLoS One. 2017;12(8):e0182775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wu MZ, Zhang Y, Wu X, Fu ZM, Wu GP, Guo KJ. Transcription expression and clinical significance of mRNA of vascular endothelial growth factor and endostatin in liquid-based preparation specimens from patients with cervical dysplasia and carcinoma. Acta Cytol. 2013;57(5):522–527. [DOI] [PubMed] [Google Scholar]
- 14. Bulkmans NW, Berkhof J, Rozendaal L, van Kemenade FJ, Boeke AJ, Bulk S, Voorhorst FJ, Verheijen RH, van Groningen K, Boon ME, Ruitinga W, van Ballegooijen M, Snijders PJ, Meijer CJ. Human papillomavirus DNA testing for the detection of cervical intraepithelial neoplasia grade 3 and cancer: 5-year follow-up of a randomised controlled implementation trial. Lancet. 2007;370(9601):1764–1772. [DOI] [PubMed] [Google Scholar]
- 15. Naucler P, Ryd W, Törnberg S, Strand A, Wadell G, Elfgren K, Rådberg T, Strander B, Johansson B, Forslund O, Hansson BG, Rylander E, Dillner J. Human papillomavirus and Papanicolaou tests to screen for cervical cancer. N Engl J Med. 2007;357(16):1589–1597. [DOI] [PubMed] [Google Scholar]
- 16. Ronco G, Giorgi-Rossi P, Carozzi F, Confortini M, Dalla Palma P, Del Mistro A, Ghiringhello B, Girlando S, Gillio-Tos A, De Marco L, Naldoni C, Pierotti P, Rizzolo R, Schincaglia P, Zorzi M, Zappa M, Segnan N, Cuzick J; New Technologies for Cervical Cancer screening (NTCC) Working Group. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: a randomised controlled trial. Lancet Oncol. 2010;11(3):249–257. [DOI] [PubMed] [Google Scholar]
- 17. Franceschi S, Denny L, Irwin KL, Jeronimo J, Lopalco PL, Monsonego J, Peto J, Ronco G, Sasieni P, Wheeler CM. Eurogin 2010 roadmap on cervical cancer prevention. Int J Cancer. 2011;128(12):2765–2774. [DOI] [PubMed] [Google Scholar]
- 18. De Sanjosé S, Diaz M, Castellsagué X, Clifford G, Bruni L, Muñoz N, Bosch FX. Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: a meta-analysis. Lancet Infect Dis. 2007;7(7):453–459. [DOI] [PubMed] [Google Scholar]
- 19. Kahla S, Kochbati L, Chanoufi MB, Maalej M, Oueslati R. HPV-16 E2 physical status and molecular evolution in vivo in cervical carcinomas. Int J Biol Markers. 2014;29(1):e78–e85. [DOI] [PubMed] [Google Scholar]
- 20. Scheffner M, Werness BA, Huibregtse JM, Levine AJ, Howley PM. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990;63(6):1129–1136. [DOI] [PubMed] [Google Scholar]
- 21. Scheurer ME, Tortolero-Luna G, Adler-Storthz K. Human papillomavirus infection: biology, epidemiology, and prevention. Int J Gynecol Cancer. 2005;15(5):727–746. [DOI] [PubMed] [Google Scholar]
- 22. Burger EA, Kornør H, Klemp M, Lauvrak V, Kristiansen IS. HPV mRNA tests for the detection of cervical intraepithelial neoplasia: a systematic review. Gynecol Oncol. 2011;120(3):430–438. [DOI] [PubMed] [Google Scholar]
- 23. Monsonego J, Zerat L, Syrjänen K, Zerat JC, Smith JS, Halfon P. Prevalence of type-specific human papillomavirus infection among women in France: implications for screening, vaccination, and a future generation of multivalent HPV vaccines. Vaccine. 2012;30(35):5215–5221. [DOI] [PubMed] [Google Scholar]