Abstract
Progression of inflammatory osteolytic diseases, including rheumatoid arthritis and periodontitis, is characterized by increased production of proinflammatory mediators and matrix-degrading enzymes by macrophages and increased osteoclastic activity. Phenotypic changes in macrophages are central to the healing process in virtually all tissues. Using a murine model of periodontitis, we assessed the timing of macrophage phenotypic changes and the impact of proresolving activation during inflammatory osteolysis and healing. Proinflammatory macrophage activation and TNF-α overproduction within 3 wk after induction of periodontitis was associated with progressing bone loss. Proresolving activation within 1 wk of stimulus removal and markers of resolving macrophages (IL-10, TGF-β, and CD206) correlated strongly with bone levels. In vivo macrophage depletion with clodronate liposomes prevented bone resorption but impaired regeneration. Induction of resolving macrophages with rosiglitazone, a PPAR-γ agonist, led to reduced bone resorption during inflammatory stimulation and increased bone formation during healing. In vitro assessment of primary bone marrow–derived macrophages activated with either IFN-γ and LPS (proinflammatory activation) or IL-4 (proresolving activation) showed that IL-4-activated cells have enhanced resolving functions (production of anti-inflammatory cytokines; migration and phagocytosis of aged neutrophils) and exert direct anabolic actions on bone cells. Cystatin C secreted by resolving but not inflammatory macrophages explained, in part, the macrophage actions on osteoblasts and osteoclasts. This study supports the concept that therapeutic induction of proresolving functions in macrophages can recouple bone resorption and formation in inflammatory osteolytic diseases.
Keywords: periodontitis, osteoblast, osteoclasts, bone regeneration, inflammation resolution, innate immunity
Introduction
Macrophages coordinate inflammation resolution and healing by limiting proinflammatory stimuli, removing dead cells through efferocytosis, and facilitating neovascularization required for tissue regeneration (Murray and Wynn 2011). Despite their low numbers in periodontal tissues, macrophages exhibit functional plasticity by switching between proinflammatory (M1-like) and anti-inflammatory and proresolving (M2-like) phenotypes, making them amenable to therapeutic modulation (Dutzan et al. 2016; Yu et al. 2016). Disease progression in periodontitis and rheumatoid arthritis is associated with high levels of macrophage-derived proinflammatory cytokines interleukin 1β (IL-1β) and tumor necrosis factor α (TNFα) at bone resorption sites. Inhibition of these pathways reduces disease progression (Delima et al. 2001; Mayer et al. 2009). However, since these cytokines play divergent roles, modulating innate and adaptive responses to pathogens and bone healing, this antagonism poses risks for altered competence in fighting infections and delayed tissue regeneration (Mountziaris and Mikos 2008). The anti-inflammatory cytokines IL-10 and IL-4, which skew macrophages to a resolving phenotype, are associated with disease remission and protection against bone resorption in rheumatoid arthritis and periodontitis (Lubberts et al. 2000; Garlet 2010). The overreaching impact of IL-10 and IL-4 on the healing process seems to relate to proinflammatory cytokine and matrix metalloprotease downregulation and osteoblast stimulation (Garlet 2010), indicating resolving macrophage roles in coupling bone resorption and formation.
The timing and relative contribution of macrophage activation states in osseous regeneration are important to delineate for drug development. In vitro and in vivo experiments with macrophage-depleted osteoblast cultures and mouse models demonstrate that reducing macrophages produces low bone mass phenotypes through reduced bone formation (Xing et al. 2010; Raggatt et al. 2014). In addition to temporally regulated macrophage recruitment and activation during bone healing, the location of macrophages relative to the remodeling bone surface seems to relate to efficient coupling of resorption and formation. In recent years, osteal macrophage—or osteomac—characterization in the canopy structures surrounding osteoblasts has indicated an important role for these cells in bone mass maintenance (Alexander et al. 2011; Cho et al. 2014). Phenotypically, osteomacs resemble resolving macrophages by sustaining anabolic processes through osteoblast stimulation and control of proinflammatory environments around bone (Chang et al. 2008; Wu et al. 2013). Failure to resolve inflammatory bone loss in chronic osteolytic diseases depends in part on continuous MCP-1 (CCL2)–mediated recruitment and activation of inflammatory macrophages (Koch et al. 1992; Graves 1999).
Using a murine periodontitis model based on endogenous biofilms matured on silk ligatures placed in the gingival sulci of molars, we tested the hypothesis that proresolving macrophage activation reduces bone resorption and accelerates bone gain during progression and healing of periodontitis, respectively. We performed 1) in vivo macrophage depletion and proresolving (M2-like) activation in mice to assess their impact on bone changes in periodontitis and 2) in vitro anti-inflammatory and proresolving (M2) activation to assess its impact on bone cells.
Materials and Methods
Animals
All procedures were carried out in accordance with the Guide for the Humane Use and Care of Laboratory Animals (National Research Council). were approved by the University of Toronto Animal Care Committee, and conformed to the ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines. C57BL/6 male mice (Charles River), 8 to 12 wk of age, were housed in the animal facility at the University of Toronto with 12-h dark/light cycles at 21 °C to 23 °C and 45% to 55% relative humidity. Whenever possible, samples collected from different treatment groups were processed and analyzed by investigators/personnel blinded to treatment received.
Bone Loss and Regeneration
Bone loss was induced by placing 9.0 silk sutures (Ethicon) in the gingival sulci of maxillary left second molars as previously described (Sima et al. 2014; Sima et al. 2016). Biofilm-retentive subgingival silk ligatures stimulate local inflammation that results in osteoclast-mediated bone resorption. To evaluate the pattern of bone loss with this model, ligatures were placed in healthy male mice, and groups of 4 mice were sacrificed on days 1, 6, 11, 16, and 21. In a subset of mice, ligatures were removed on day 21 under brief isoflurane anesthesia and lesions left to heal for 42 d. Groups of 4 mice were sacrificed at days 28, 35, 42, and 49 to evaluate bone regeneration during healing.
In Vivo Macrophage Depletion and Proresolving Activation
Macrophage depletion was accomplished with clodronate liposomes (Vrije University) as previously described (Rooijen et al. 2003), administered at 1 µg/g of body weight, or with an equal volume of phosphate-buffered saline liposomes by intraperitoneal injections every 2 d. In one experiment, mice received clodronate liposomes from day 4 prior to ligature placement (periodontitis induction), which continued for 3 wk after induction. In another experiment, mice received clodronate liposomes from day 18 after induction; ligatures were removed on day 21; and treatment continued for 1 wk during healing (day 28). To induce M2-like macrophage activation, mice were administered oral rosiglitazone (30 mg/kg; Avandia) or sodium starch glycolate (15 g of feed per 100 g of body weight) daily with the clodronate study design.
Skull Morphometry and Micro–computed tomography
Dry skulls were stained with methylene blue and imaged for assessment of bone loss. Bone level was measured from the cementoenamel junction to the alveolar bone crest on the buccal aspect of ligated and contralateral nonligated second molars. The bone-level ratio was calculated as the ratio between day 28 and day 21. To assess 3-dimensional bone changes, skulls were scanned by micro–computed tomography at 11-μm resolution in all spatial dimensions (Scanco Medical AG). Reconstructed 3-dimensional maxillary regions were cropped from consecutive slice images as the volume of interest with Amira software (FEI Visualization Sciences Group). Volumetric analysis was performed after global thresholding of bone tissue (3,400 to 11,000 AU). Bone volume around second molars was measured in a digital volume of interest (apical, occlusal, buccal, lingual) and tooth volume excluded by thresholding and segmentation of tooth structures.
Histology and Quantitative Reverse Transcription Polymerase Chain Reaction
Fixed maxillae (10% formaldehyde) were decalcified (10% ethylenediaminetetraacetic acid) and paraffin embedded. Coronal sections (5 μm thick) were then 1) stained for tartrate-resistant acid phosphatase (TRAP) with the Leukocyte Acid Phosphatase Kit (Sigma-Aldrich; hematoxylin counterstain), 2) stained with Masson’s trichrome, or 3) probed with anti-mouse antibodies for F4/80 (1:100; Vector Labs), TNF-α (1:100), CD206 (1:100; LifeSpan Biosciences), osterix (1:300; Abcam), and cystatin C (1:200; R&D Systems). Nonspecific binding was blocked with Protein Block Serum-Free (Dako) or Normal Horse Serum (Vector Labs). Gene expression was quantified with the LightCycler 480 SYBR Select Master Mix (Applied Biosystems). mRNA was measured at ligated sites relative to nonligated sites and normalized to the HPRT housekeeping gene (hypoxanthine-guanine phosphoribosyltransferase) via the ΔΔCq method. Correlations between gene expression and bone-level ratios (days 21 vs. 28) were calculated for ligated and nonligated sites. Protocols are detailed in the Appendix.
Macrophage Activation and Cystatin C Depletion
Primary bone marrow monocytes were collected from tibia and femora of 8- to 12-wk-old C57BL/6J mice. Isolated cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin. Cells to be activated to M1-like phenotype were stimulated with 10 ng/mL of GM-CSF, and those to be activated to M2-like phenotype were stimulated with 20 ng/mL of M-CSF for 2 d. On day 3, cells were incubated with either of the following for 2 d (Peprotech):
M1 activation: 10 ng/mL of GM-CSF plus 1 ng/mL of IFN-γ and 10 ng/mL of LPS (purified from Escherichia coli)
M2 activation: 20 ng/mL of M-CSF plus 10 ng/mL of IL-4
The conditioned media of M2 cells (5 × 106) was depleted with anti-cystatin C antibody (7 μM; R&D Systems) for 16 h at 4 °C, followed by incubation with Pierce Protein A/G Agarose Beads (Thermo Fisher Scientific) for 2 h and supernatant added to primary osteoblast and osteoclast cultures.
Functional Assays
The assays used to characterize macrophage activation states (flow cytometry, mass spectrometry, Western blotting, phagocytosis, and migration) or osteoclast and osteoblast activity are detailed in the Appendix.
Statistical Analyses
All statistical analyses were carried out with SPSS v24 (IBM) and GraphPad Prism 7. One-way analysis of variance, followed by Tukey’s post hoc test or Student’s t test, was used as indicated. Statistical significance was considered at P = 0.05. For in vivo testing, a minimum of 4 mice per group was needed to detect 1) a difference of 25% ± 10% in bone levels between healthy and diseased sites or between treatment groups at diseased sites and 2) a 2- ± 0.5-fold difference in gene expression between inflammatory and resolution phases, with a power of 0.8 at an alpha level of 0.05.
Results
Macrophage Activation Is Differentially Regulated in Inflammation and Healing of Periodontal Osteolytic Lesions
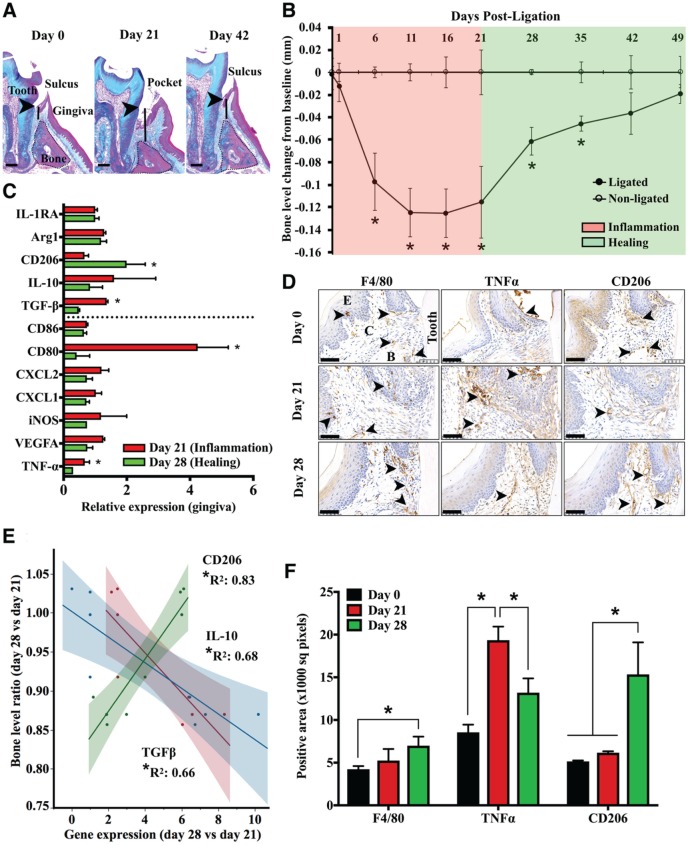
To investigate macrophage activation in the healing versus inflammatory phase of periodontitis, we used a murine model of silk biofilm–induced bone loss and regeneration. With this approach, moderate bone loss is achieved in 21 d of induction (25% bone loss) and almost complete regeneration in 21 d after stimuli removal (Fig. 1A). Most of the bone resorption occurs within 7 d of induction (P < 0.05 vs. baseline) and regeneration within 7 d of stimulus removal (P < 0.05 vs. day 21; Fig. 1B). Bone resorption is associated with inflammatory cell infiltration and pocket formation by day 11 (Appendix Fig. 1). To interrogate macrophage activation changes in the early healing phase, we assessed mRNA levels for genes coding for M1 (TNF-α, VEGFA, iNOS, CD80, CD86) and M2 (TGF-β, IL-10, CD206, Arg1, IL1-RA) macrophage markers and for chemokines (CXCL2, CXCL1) in gingival tissues collected on days 21 and 28 from ligated and nonligated molars. Expression of TGF-β, CD80, and TNF-α significantly increased in inflammation (day 21), while CD206 significantly increased in healing (day 28; P < 0.05, day 21 vs. day 28; Fig. 1C). Tissue expression of TNF-α in inflamed lesions concentrated around the ligature, in perivascular areas of lamina propria, and at the bone crest, whereas CD206 was mainly expressed along bone crest and perivascular areas of lamina propria in healing sites (Fig. 1D). The gingival expression of IL-10 and TGF-β inversely correlated with, and CD206 correlated strongly with, bone-level ratio between days 28 and 21 at ligated and nonligated molars (P < 0.05; Fig. 1E). Macrophage numbers (F4/80+) and CD206 expression in periodontal lesions significantly increased by day 28, while TNF-α expression increased on day 21 and decreased on day 28 (Fig. 1F). Thus, healing of periodontal lesions relates to an inflammatory-to-resolving macrophage phenotype shift that correlates with bone levels.
Figure 1.
Resolving macrophage markers correlate strongly with bone levels during progression and healing of periodontitis lesions. (A) Periodontitis was induced with a biofilm-retentive silk suture (ligature) placed inside the gingival sulcus of maxillary left molars for 21 d. In a subset of mice, ligatures were removed and lesions left to heal for another 21 d. Bone loss and gain in inflammation and healing (solid line) were measured as the distance between the cementoenamel junction (arrowheads) and alveolar bone (dotted line) crest on Masson’s trichrome–stained frontal sections. Scale bars, 0.2 mm. (B) Bone level changes during the inflammatory and healing phases of periodontitis were calculated relative to nonligated contralateral internal control molars (n = 4 mice/time point, *P < 0.05 vs. nonligated, paired t test). (C) mRNA levels for macrophage activation markers relative to the HPRT housekeeping gene in periodontal explants from diseased sites during peak inflammation (day 21) and healing (day 28), normalized to contralateral internal control (n = 4 mice/group, *P < 0.05 healing vs. inflammation, paired t tests). Dashed line separates proinflammatory (top) from anti-inflammatory (bottom) markers. (D) Representative immunohistochemistry micrographs of healthy (day 0), inflamed (day 21), and healing (day 28) periodontal tissues of maxillary second molars identifying macrophage markers F4/80 (pan-macrophage), TNFα (proinflammatory), and CD206 (proresolving). Arrows indicate areas of high expression in the superficial layer of lamina propria (F4/80) and bone crest (F4/80, TNFα, CD206), around the ligature (TNFα), and in perivascular areas of lamina propria (TNF-α, CD206). Magnification: 40×. Scale bar, 50 μm. (E) Correlations between bone level and gene expression of IL-10, TGF-β, and CD206, measured as the ratio between day 28 and day 21 at ligated and nonligated sites (*P < 0.05, Pearson). (F) Tissue expression of F4/80, TNFα, and CD206 in periodontal tissues surrounding diseased and healing second molars (n = 3 samples/group, 6 fields/sample *P < 0.05 vs. day 0, 1-way analysis of variance with multiple comparisons as unpaired t tests). Values are presented as mean ± SEM.
Resolving Macrophage Activation Enhances Alveolar Bone Regeneration
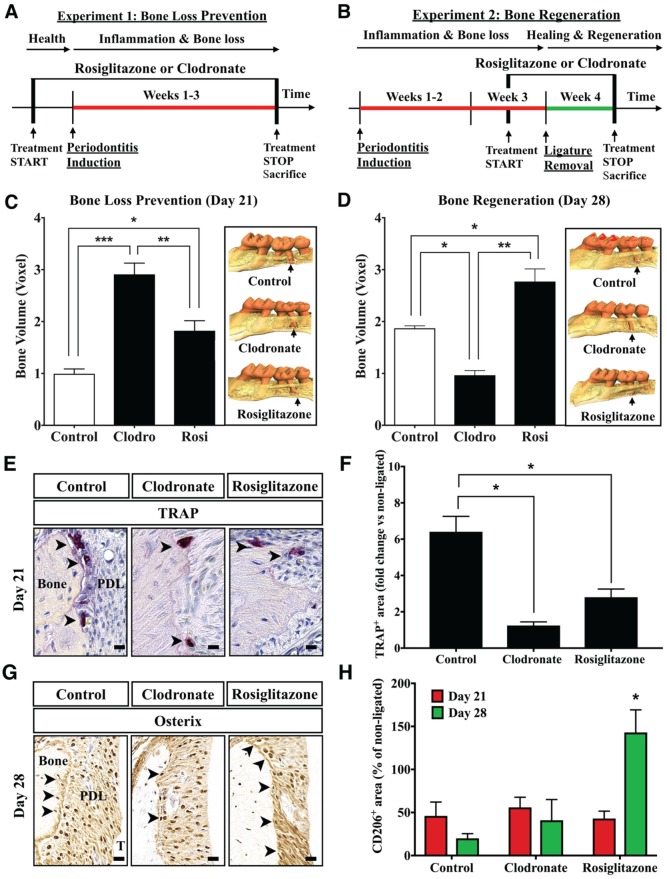
We next investigated the contribution of macrophages to bone changes in periodontitis by testing the impact of in vivo macrophage depletion with clodronate liposomes and proresolving activation with rosiglitazone on bone loss and regeneration in experimental periodontitis. Clodronate liposome treatment results in macrophage suicidal death upon ingestion by phagocytosis and intracellular clodronate release, which results in almost complete depletion of gingival macrophages (Appendix Fig. 2). Rosiglitazone has agonistic actions on macrophages via PPAR-γ, leading to anti-inflammatory and proresolving activation in peripheral blood monocytes and tissue macrophages (Bouhlel et al. 2007). We conducted 2 experiments to assess the impact of macrophage depletion and proresolving activation on bone loss and regeneration (Fig. 2A, B). Macrophage depletion and proresolving activation significantly reduced the bone loss during the 3-wk experimental periodontitis (clodronate vs. control, P < 0.001; rosiglitazone vs. control, P < 0.05; Fig. 2C). Rosiglitazone treatment significantly enhanced, and clodronate impaired, bone regeneration during healing of periodontitis lesions (P < 0.05; Fig. 2D). Rosiglitazone was associated with reduced osteoclastic coverage (TRAP+) of the inner cortex of alveolar bone during inflammation (day 21, Fig. 2E, F) and with increased expression of bone formation marker osterix during healing (day 28; Fig. 2G). CD206 expression in periodontal tissues was significantly increased by rosiglitazone during healing (P < 0.05; Fig. 2H). These findings indicate that resolving macrophage activation has a positive impact on alveolar bone level in ligature-induced periodontitis.
Figure 2.
Proresolving macrophage activation reduces in vivo bone resorption and enhances regeneration. (A) Bone loss prevention study: To test the impact of macrophage depletion and M2 activation on inflammatory bone loss, mice were treated with clodronate (macrophage depletion) or rosiglitazone (M2 activation) starting at 4 d prior to ligature placement and continuing for 3 wk. (B) Bone regeneration study: To test the impact of clodronate and rosiglitazone on bone regeneration following inflammatory resorption, mice were treated with the same regimen as in panel A starting at 4 d prior to ligature removal (day 21) and throughout the first week of healing. Micro–computed tomography volumetric analysis of bone levels in the (C) prevention and (D) regeneration studies (n = 3 mice/group, *P < 0.05, **P < 0.01, ***P < 0.001, 1-way analysis of variance with multiple comparisons as unpaired t tests). Data represent 1 of 2 similar experiments. Insets: Representative 3-dimensional renderings of micro–computed tomography scans of maxillae on day 21 (prevention study) and day 28 (regeneration study) indicating root exposure at the second molar (arrows). (E) Representative coronal sections of diseased maxillary second molars on day 21 stained for TRAP (arrowheads, positive cells). PDL, periodontal ligament; T, tooth. Magnification: 60×. Scale bars: 10 μm. (F) Quantification of TRAP-positive area in the area of ligated versus nonligated molars on day 21 (n = 3 mice/group, 10 micrographs/sample, *P < 0.05, 1-way analysis of variance with Tukey’s honestly significant difference post hoc). (G) Representative coronal sections of healing maxillary second molars on day 28 stained for osterix (arrowheads, positive cells along inner cortex). PDL, periodontal ligament; T, tooth. Magnification: 60×. Scale bars: 10 μm. (H) Quantification of CD206-positive tissue area on coronal sections of ligated versus nonligated second molars on days 21 and 28 (n = 3 mice/group, 10 micrographs/sample, *P < 0.05, day 21 vs. 28, unpaired t test). Values are presented as mean ± SEM.
IL-4-Activated Macrophages Have Enhanced Inflammation Resolving and Bone Anabolic Functions
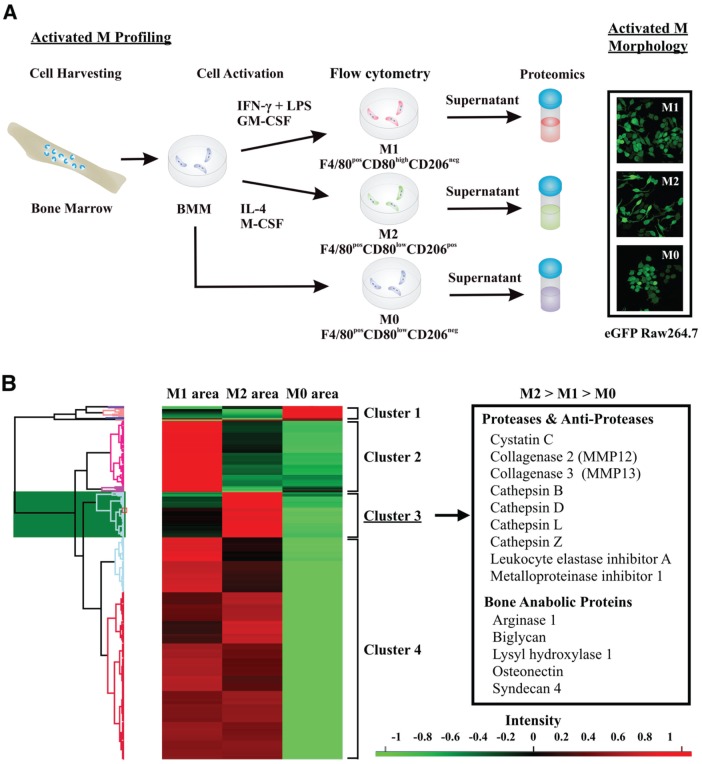
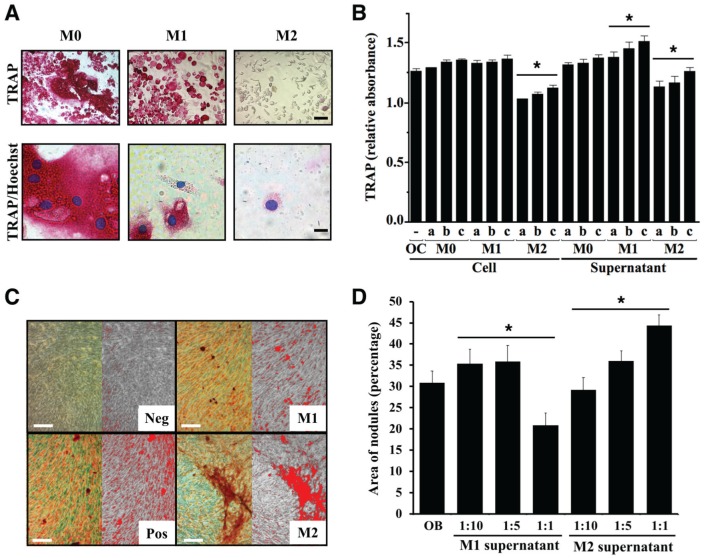
We next assessed functional differences between 2 opposing activation states across the macrophage phenotypic spectrum, induced by IFN-γ plus LPS (M1, predominantly proinflammatory) and by IL-4 (M2, predominantly resolving), focusing on inflammation resolution and bone resorption and formation. Primary murine bone marrow–derived M1-activated macrophages increased production of IL-12 and expression of CD80 but not CD206, whereas M2 increased production of IL-1RA and expression of CD206 but not CD80 (Appendix Fig. 3A, C). M2 activation significantly increased the apoptotic neutrophil uptake and cell spreading and migration in response to M-CSF as compared with M1 activation (Fig. 3A; Appendix Fig. 3B, D). Proteomic analysis of supernatants from M2, M1, and nonactivated (M0) macrophages identified a specific protease/antiprotease and bone anabolic protein secretory profile of M2 cells, with cystatin C being the most abundant protease inhibitor secreted exclusively by M2 (Fig. 3B; Appendix Table). We next assessed the impact of M2 activation on osteoclast and osteoblast function in vitro. M2-activated cells reduced the numbers of active osteoclasts (TRAP+) and inhibited TRAP activity in a dose-dependent manner, whereas M1 cells increased mononuclear osteoclast numbers and TRAP activity (P < 0.01; Fig. 4A, B). Supernatants of M2 cells stimulated mineral deposition by MC3T3-E1 osteoblastic cells in a dose-dependent manner, while those of M1 cells inhibited mineral deposition at the highest concentration (Fig. 4C, D). Thus, macrophage activation by IL-4 induces a proresolving phenotype characterized by tissue clearing and secretion of bone anabolic factors.
Figure 3.
Profiling of differentially activated macrophages. (A) Primary bone marrow macrophages—nonactivated (M0) and activated (M1, IFNγ and LPS; M2, IL-4)—were profiled by flow cytometry and mass spectrometry. M1 cells were F4/80posCD80highCD026neg and M2 cells F4/80posCD80lowCD026pos. Inset: Morphologic characteristics of M1 and M2 cells assessed by confocal microscopy of activated eGFP Raw264.7 cells. (B) Heat map representing the relative abundance (expressed as Z scores) in supernatants of M0, M1, and M2 macrophages. The area provided by PEAKS label-free quantification search from samples separately collected, prepared, and analyzed by mass spectrometry was log transformed for Z-score calculation. The Z-score values were hierarchically clustered. Cluster 3 includes 77 proteins highly expressed in M2-activated cells relative to M0 and M1. Inset: Proteases, antiproteases, and bone anabolic factors identified in cluster 3.
Figure 4.
IL-4-activated macrophages inhibit osteoclast differentiation and stimulate mineralization by osteoblasts. (A) Representative micrographs of osteoclasts differentiated in the presence of factors secreted by differentially activated macrophages. Bone marrow–derived osteoclast precursors were plated on glass chamber plates (2 × 105 cells/well) and incubated with M-CSF and RANKL for 8 d. On day 4, supernatants of primary activated macrophages (M0, nonactivated; M1, IFN-γ and LPS activation; M2, IL-4 activation) were added to differentiating osteoclasts and trained for TRAP/Hoechst. Scale bars: top, 50 μm; bottom, 10 μm. (B) Primary osteoclast precursors were incubated with different ratios (a, 1:1; b, 1:5; c, 1:10) of activated macrophages or their supernatants for 4 d. Relative TRAP absorbance was measured as optical density at 405 nm (n = 3 separate experiments run in triplicate, *P < 0.05, 1-way analysis of variance with Tukey’s honestly significant difference post hoc). (C) MC3T3-E1 cells (7 × 105 cells/well) were cultured to induce osteoblast differentiation (ascorbic acid, β-glycerophosphate) and then incubated with different ratios (1:10, 1:5, 1:1) of activated macrophage supernatant every 3 d for 21 d. Representative micrographs of calcium deposition by primary osteoblasts incubated at 1:10 ratio with supernatants of activated macrophages assessed by alizarin red S staining. Positive and negative controls: MC3T3-E1 with and without differentiation factors, respectively. Left: alizarin red–stained calcification; right: thresholding for positive area of calcification. (D) ImageJ quantification of mineral coverage area of the culture dish (n = 3, *P < 0.05, 1-way analysis of variance with Tukey’s honestly significant difference post hoc). Values are presented as mean ± SEM.
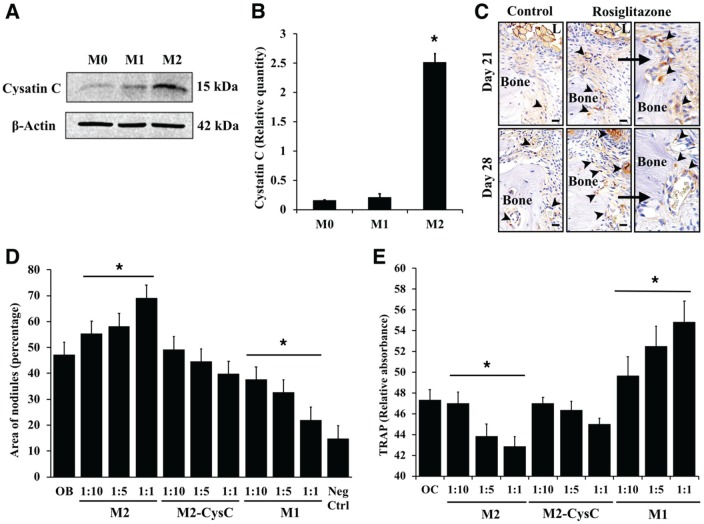
Cystatin C Mediates the Actions of Resolving Macrophages on Osteoclasts and Osteoblasts
Next, we asked whether M2-derived cystatin C mediates the observed bone anabolic actions. Primary bone marrow–derived M2-activated macrophages had a 2-fold higher expression as compared with nonactivated (M0) and M1-activated cells (Figure 5A, B). Staining for cystatin C at ligated molar sites in mice treated with rosiglitazone showed increased expression on the inner cortex of alveolar bone in the treatment group versus control and during healing versus inflammation (Fig. 5C). Depletion of cystatin C in M2 supernatant abolished the promineralization action on primary calvarial osteoblasts (Fig. 5D) and reduced the inhibition of TRAP activity in osteoclasts (Fig. 5E). These findings indicate that cystatin C is required for bone anabolic actions of IL-4-stimulated resolving macrophages.
Figure 5.
Cystatin C mediates the regulatory actions of resolving macrophages on bone cells. (A) Western blot analysis of cystatin C in cell lysates of primary bone marrow cells activated to M1- and M2-like phenotype. (B) Band intensity was measured in ImageJ and normalized with β-actin (n = 3 experiments, *P < 0.05, unpaired t test vs. M0). (C) Representative images of cystatin C staining in inflamed (day 21) and healing (day 28) periodontitis lesions of mice treated with rosiglitazone, indicating increased expression with treatment at inner cortex and periodontal blood vessels (arrowheads). L, ligature. Magnification: 60×; rosiglitazone (right): 80×. Scale bars: 10 μm. (D) Primary mouse calvarial stromal cells were differentiated to osteoblasts (50 μg/mL of ascorbic acid, 10mM β-glycerophosphate) and incubated with different ratios (1:10, 1:5, 1:1) of supernatants from M1, M2, or cystatin C–immunodepleted M2 cells for 10 d. Mineral coverage area was assessed by alizarin red S staining. Negative control: osteoblast precursors without differentiation factors (n = 3 separate experiments run in triplicate, *P < 0.05, 1-way analysis of variance with Tukey’s honestly significant difference post hoc). (E) Bone marrow mononuclear osteoclast precursors cells were plated with different ratios (1:10, 1:5, 1:1) of supernatant from M1, M2, or cystatin C–immunodepleted M2 cells activated for 8 d. TRAP activity was measured as optical density at 405 nm (n = 3 separate experiments run in triplicate, *P < 0.05, 1-way analysis of variance with Tukey’s honestly significant difference post hoc). Values are presented as mean ± SEM.
Discussion
Increasing evidence shows that macrophages play essential roles in regulation of bone cells. We addressed the contribution of inflammatory and resolving macrophages to bone resorption and regeneration in experimental periodontitis. Resolving macrophages reduced bone resorption in periodontitis and enhanced bone formation during healing. These actions were attributed to inflammation resolution and osteoblast and osteoclast regulation.
Macrophages in resolving inflammation exhibit a phenotype that shares M2 features but also carries functions specific to M1, such as production of reactive nitrogen species (Bystrom et al. 2008). These resolution-phase macrophages proliferate, produce chemoattractants for lymphocytes, present antigens, and produce proresolving lipid mediators, including lipoxins, resolvins, and protectins that mediate the active inflammation resolution process (Bystrom et al. 2008; Stables et al. 2011). These studies are consistent with the current general view that the conventional M1-M2 macrophage nomenclature does not predict in vivo behavior, and they indicate that macrophages activated during inflammation resolution are more akin to M2b cells, a subtype that is tolerant to bacterial inflammatory stimuli but produces anti-inflammatory cytokines such as IL-10 (Mantovani et al. 2004). Periodontitis progression in the subgingival ligature model used is associated with biofilm maturation around the ligature, containing 50% aerobic and 50% anaerobic bacteria, and with oral dysbiosis (Sima et al. 2016). This sustains unresolved local inflammation that results in 25% alveolar bone resorption that regenerates upon stimulus removal in nonsusceptible animals, as demonstrated in the present study. Bone levels correlate strongly with resolving macrophage marker expression, indicating close relationships between proresolving activation and bone anabolic functions.
Although tissue macrophage roles in wound healing are well documented, its actions in bone remodeling and regeneration are now being unveiled (Wu et al. 2013; Loi et al. 2016). Resident macrophages present throughout human and murine osseous tissues, named osteomacs, exert potent stimulating actions on osteoblasts and control proinflammatory environments around bone, therefore being functionally bone-associated resolving macrophages (Chang et al. 2008; Wu et al. 2013). Systemic mature macrophage depletion with clodronate liposomes was protective against inflammatory osteolysis but detrimental to bone regeneration. Clodronate liposomes seems to deplete mature M1-like and stimulate M2-like macrophages with enhanced proresolving activities, which partially explains our observations (Winkler et al. 2010). However, M2-like activation with rosiglitazone was particularly beneficial during healing. Rosiglitazone activates peripheral blood monocytes to an M2-like phenotype, as opposed to switching tissue from M1- to M2-like cells, with a net positive impact on local inflammation (Bouhlel et al. 2007). However, a well-documented side effect of thiazolidinediones in long-term treatment is reduction in bone quality and mass, particularly in women, primarily to induce adipogenesis in bone marrow stromal cells and decrease osteoblast and aromatase activities, thus altering estrogen production and bone formation (Loke et al. 2009). In the present study, short-term rosiglitazone treatment in male mice upregulated expression of M2 marker CD206 in healing, with a net positive impact on regaining periodontitis-associated lost bone mass. It is therefore likely that long-term treatment affects monocytic lineage cells in tissue, including newly recruited macrophages and osteoclasts, in addition to affecting bone marrow adipocytes. We used rosiglitazone for <4 wk and therefore expect most impact to be on circulating and recruiting monocytes at inflamed sites. This further suggests that targeting monocytes for commitment to a specific macrophage type upon recruitment may be an effective strategy for chronic osteolytic diseases.
Cystatin C, a cysteine proteinase inhibitor constitutively present in virtually all tissues and body fluids, is used as biomarker for kidney function (Koenig et al. 2005). In saliva, cystatin C was found to increase in patients with gingivitis and periodontitis, while gingival levels were reported to decrease proportional to disease severity (Henskens et al. 1994; Lie et al. 2001). Cystatin C is produced by osteoblasts in addition to other cell types, and it inhibits osteoclast-mediated bone resorption (Lerner et al. 1997; Johansson et al. 2000; Brand et al. 2004). Earlier reports showed that cystatin C is constitutively secreted by monocytes/macrophages and that secretion is inhibited by IFN-γ, LPS, or zymosan (Warfel et al. 1987; Chapman et al. 2012). Monocyte/macrophage-derived cystatin C exhibits immune modulatory actions on neutrophils through chemotaxis and superoxide inhibition (Leung-Tack, Tavera, Gensac, et al. 1990; Leung-Tack, Tavera, Martinez, and Colle 1990). We found that cystatin C secreted by M2-activated macrophages enhances mineral deposition by osteoblasts and reduces osteoclast TRAP activity. To our knowledge, this is the first report of cystatin C requirement for resolving macrophage-mediated regulation of bone cells in favor of bone formation. Cystatin C likely reduces osteoclastic activity through inhibition of cathepsins, as it binds to cathepsins B, H, L, and S with Ki < 1nM (Mussap and Plebani 2008). However, cystatin C was found to stimulate osteoblasts through bone morphogenetic protein signaling pathways (Danjo et al. 2007). Cystatin C actions in controlling extracellular proteolysis are likely operating in concert with other regenerative systems involved in bone resorption and formation coupling and macrophage-mediated biofilm control mechanisms that reduce the local inflammatory burden. Although counterintuitive, macrophage depletion may improve the host response to pathogenic microbes. Importantly, with the Porphyromonas gingivalis oral challenge model, 2 groups showed that monocyte/macrophage depletion with clodronate liposomes is protective and that macrophage depletion improves clearance of P. gingivalis (Lam et al. 2014; Steinmetz et al. 2016).
In conclusion, the present study demonstrates that resolving macrophage activation enhances bone regeneration during healing of periodontitis lesions, in part through direct actions on bone cells. Resolving macrophages inhibit osteoclast activity and stimulate mineral deposition by osteoblasts via cystatin C. These findings indicate that immune modulation of macrophages to gain proresolving functions can promote bone formation in osteolytic diseases.
Author Contributions
A. Viniegra, contributed to design, data acquisition, analysis, and interpretation, drafted and critically revised manuscript; H. Goldberg, contributed to design, data acquisition, and analysis, drafted the manuscript; C. Çil, N. Fine, Z. Sheikh, M. Freire, contributed to data acquisition and analysis, critically revised the manuscript; M. Galli, contributed to data acquisition, drafted the manuscript; Y. Wang, contributed to data acquisition, analysis, and interpretation, critically revised the manuscript; T.E. Van Dyke, contributed to data interpretation, critically revised the manuscript; M. Glogauer, contributed to conception, design, and data interpretation, critically revised the manuscript; C. Sima, contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplemental Material
Supplemental material, DS_10.1177_0022034518777973 for Resolving Macrophages Counter Osteolysis by Anabolic Actions on Bone Cells by A. Viniegra, H. Goldberg, Ç. Çil, N. Fine, Z. Sheikh, M. Galli, M. Freire, Y. Wang, T.E. Van Dyke, M. Glogauer and C. Sima in Journal of Dental Research
Acknowledgments
We thank Jonathan Krieger at the SPARC Biocentre, Hospital for Sick Children, for assistance with mass spectrometry analyses and Milan Ganguly at the Pathology Core, Centre for Phenogenomics, Mount Sinai Hospital, for assistance with histology.
Footnotes
A supplemental appendix to this article is available online.
This work was supported by the Canadian Institutes of Health Research (grant TBO-122068 to M.G.) and the National Institutes of Health / National Institute of Dental and Craniofacial Research through the U.S. Public Health Service (grant K99DE024575 to C.S.).
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
ORCID iDs: M. Freire  https://orcid.org/0000-0003-4906-7698
https://orcid.org/0000-0003-4906-7698
T. E. Van Dyke  https://orcid.org/0000-0003-0856-3396
https://orcid.org/0000-0003-0856-3396
References
- Alexander KA, Chang MK, Maylin ER, Kohler T, Müller R, Wu AC, van Rooijen N, Sweet MJ, Hume DA, Raggatt LJ, et al. 2011. Osteal macrophages promote in vivo intramembranous bone healing in a mouse tibial injury model. J Bone Miner Res. 26(7):1517–1532. [DOI] [PubMed] [Google Scholar]
- Bouhlel MA, Derudas B, Rigamonti E, Dièvart R, Brozek J, Haulon S, Zawadzki C, Jude B, Torpier G, Marx N, et al. 2007. PPARgamma activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metab. 6(2):137–143. [DOI] [PubMed] [Google Scholar]
- Brand HS, Lerner UH, Grubb A, Beertsen W, Amerongen AVN, Everts V. 2004. Family 2 cystatins inhibit osteoclast-mediated bone resorption in calvarial bone explants. Bone. 35(3):689–696. [DOI] [PubMed] [Google Scholar]
- Bystrom J, Evans I, Newson J, Stables M, Toor I, van Rooijen N, Crawford M, Colville-Nash P, Farrow S, Gilroy DW. 2008. Resolution-phase macrophages possess a unique inflammatory phenotype that is controlled by cAMP. Blood. 112(10):4117–4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang MK, Raggatt LJ, Alexander KA, Kuliwaba JS, Fazzalari NL, Schroder K, Maylin ER, Ripoll VM, Hume DA, Pettit AR. 2008. Osteal tissue macrophages are intercalated throughout human and mouse bone lining tissues and regulate osteoblast function in vitro and in vivo. J Immunol. 181(2):1232–1244. [DOI] [PubMed] [Google Scholar]
- Chapman HA, Jr, Reilly JJ, Jr, Yee R, Grubb A. 2012. Identification of cystatin C, a cysteine proteinase inhibitor, as a major secretory product of human alveolar macrophages in vitro. Am Rev Respir Dis. 141(3):698–705. [DOI] [PubMed] [Google Scholar]
- Cho SW, Soki FN, Koh AJ, Eber MR, Entezami P, Park SI, van Rooijen N, McCauley LK. 2014. Osteal macrophages support physiologic skeletal remodeling and anabolic actions of parathyroid hormone in bone. Proc Natl Acad Sci U S A. 111(4):1545–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danjo A, Yamaza T, Kido MA, Shimohira D, Tsukuba T, Kagiya T, Yamashita Y, Nishijima K, Masuko S, Goto M, et al. 2007. Cystatin C stimulates the differentiation of mouse osteoblastic cells and bone formation. Biochem Biophys Res Commun. 360(1):199–204. [DOI] [PubMed] [Google Scholar]
- Delima AJ, Oates T, Assuma R, Schwartz Z, Cochran D, Amar S, Graves DT. 2001. Soluble antagonists to interleukin-1 (IL-1) and tumor necrosis factor (TNF) inhibits loss of tissue attachment in experimental periodontitis. J Clin Periodontol. 28(3):233–240. [DOI] [PubMed] [Google Scholar]
- Dutzan N, Konkel JE, Greenwell-Wild T, Moutsopoulos NM. 2016. Characterization of the human immune cell network at the gingival barrier. Mucosal Immunol. 9(5):1163–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlet GP. 2010. Destructive and protective roles of cytokines in periodontitis: a re-appraisal from host defense and tissue destruction viewpoints. J Dent Res. 89(12):1349–1363. [DOI] [PubMed] [Google Scholar]
- Graves DT. 1999. The potential role of chemokines and inflammatory cytokines in periodontal disease progression. Clin Infect Dis. 28(3):482–490. [DOI] [PubMed] [Google Scholar]
- Henskens YM, Veerman EC, Mantel MS, van der Velden U, Nieuw Amerongen AV. 1994. Cystatins S and C in human whole saliva and in glandular salivas in periodontal health and disease. J Dent Res. 73(10):1606–1614. [DOI] [PubMed] [Google Scholar]
- Johansson L, Grubb A, Abrahamson M, Kasprzykowski F, Kasprzykowska R, Grzonka Z, Lerner UH. 2000. A peptidyl derivative structurally based on the inhibitory center of cystatin C inhibits bone resorption in vitro. Bone. 26(5):451–459. [DOI] [PubMed] [Google Scholar]
- Koch AE, Kunkel SL, Harlow LA, Johnson B, Evanoff HL, Haines GK, Burdick MD, Pope RM, Strieter RM. 1992. Enhanced production of monocyte chemoattractant protein-1 in rheumatoid arthritis. J Clin Invest. 90(3):772–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenig W, Twardella D, Brenner H, Rothenbacher D. 2005. Plasma concentrations of cystatin C in patients with coronary heart disease and risk for secondary cardiovascular events: more than simply a marker of glomerular filtration rate. Clin Chem. 51(2):321–327. [DOI] [PubMed] [Google Scholar]
- Lam RS, O’Brien-Simpson NM, Lenzo JC, Holden JA, Brammar GC, Walsh KA, McNaughtan JE, Rowler DK, van Rooijen N, Reynolds EC. 2014. Macrophage depletion abates Porphyromonas gingivalis–induced alveolar bone resorption in mice. J Immunol. 193(5):2349–2362. [DOI] [PubMed] [Google Scholar]
- Lerner UH, Johansson L, Ransjö M, Rosenquist JB, Reinholt FP, Grubb A. 1997. Cystatin C, an inhibitor of bone resorption produced by osteoblasts. Acta Physiol Scand. 161(1):81–92. [DOI] [PubMed] [Google Scholar]
- Leung-Tack J, Tavera C, Gensac MC, Martinez J, Colle A. 1990. Modulation of phagocytosis-associated respiratory burst by human cystatin C: role of the N-terminal tetrapeptide Lys-Pro-Pro-Arg. Exp Cell Res. 188(1):16–22. [DOI] [PubMed] [Google Scholar]
- Leung-Tack J, Tavera C, Martinez J, Colle A. 1990. Neutrophil chemotactic activity is modulated by human cystatin C, an inhibitor of cysteine proteases. Inflammation. 14(3):247–258. [DOI] [PubMed] [Google Scholar]
- Lie MA, Loos BG, Henskens YM, Timmerman MF, Veerman EC, van der Velden U, van der Weijden GA. 2001. Salivary cystatin activity and cystatin C in natural and experimental gingivitis in smokers and non-smokers. J Clin Periodontol. 28(10):979–984. [DOI] [PubMed] [Google Scholar]
- Loi F, Córdova LA, Pajarinen J, Lin TH, Yao Z, Goodman SB. 2016. Inflammation, fracture and bone repair. Bone. 86:119–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loke YK, Singh S, Furberg CD. 2009. Long-term use of thiazolidinediones and fractures in type 2 diabetes: a meta-analysis. CMAJ. 180(1):32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubberts E, Joosten LA, Chabaud M, van Den Bersselaar L, Oppers B, Coenen-De Roo CJ, Richards CD, Miossec P, van Den Berg WB. 2000. IL-4 gene therapy for collagen arthritis suppresses synovial IL-17 and osteoprotegerin ligand and prevents bone erosion. J Clin Invest. 105(12):1697–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. 2004. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 25(12):677–686. [DOI] [PubMed] [Google Scholar]
- Mayer Y, Balbir-Gurman A, Machtei EE. 2009. Anti-tumor necrosis factor-alpha therapy and periodontal parameters in patients with rheumatoid arthritis. J Periodontol. 80(9):1414–1420. [DOI] [PubMed] [Google Scholar]
- Mountziaris PM, Mikos AG. 2008. Modulation of the inflammatory response for enhanced bone tissue regeneration. Tissue Eng Part B Rev. 14(2):179–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray PJ, Wynn TA. 2011. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 11(11):723–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mussap M, Plebani M. 2008. Biochemistry and clinical role of human cystatin C. Crit Rev Clin Lab Sci. 41(5–6):467–550. [DOI] [PubMed] [Google Scholar]
- Raggatt LJ, Wullschleger ME, Alexander KA, Wu AC, Millard SM, Kaur S, Maugham ML, Gregory LS, Steck R, Pettit AR. 2014. Fracture healing via periosteal callus formation requires macrophages for both initiation and progression of early endochondral ossification. Am J Pathol. 184(12):3192–3204. [DOI] [PubMed] [Google Scholar]
- Rooijen NV, Kesteren-Hendrikx EV. 2003. “In vivo” depletion of macrophages by liposome-mediated “suicide.” In: Methods in enzymology, part C, liposomes. Vol. 373 New York (NY): Elsevier; p. 3–16. [DOI] [PubMed] [Google Scholar]
- Sima C, Cheng Q, Rautava J, Levesque C, Sherman P, Glogauer M. 2016. Identification of quantitative trait loci influencing inflammation-mediated alveolar bone loss: insights into polygenic inheritance of host-biofilm disequilibria in periodontitis. J Periodont Res. 51(2):237–249. [DOI] [PubMed] [Google Scholar]
- Sima C, Gastfreund S, Sun C, Glogauer M. 2014. Rac-null leukocytes are associated with increased inflammation-mediated alveolar bone loss. Am J Pathol. 184(2):472–482. [DOI] [PubMed] [Google Scholar]
- Stables MJ, Shah S, Camon EB, Lovering RC, Newson J, Bystrom J, Farrow S, Gilroy DW. 2011. Transcriptomic analyses of murine resolution-phase macrophages. Blood. 118(26):e192–e208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz O, Hoch S, Avniel-Polak S, Gavish K, Eli-Berchoer L, Wilensky A, Nussbaum G. 2016. CX3CR1hi monocyte/macrophages support bacterial survival and experimental infection-driven bone resorption. J Infect Dis. 213(9):1505–1515. [DOI] [PubMed] [Google Scholar]
- Warfel AH, Zucker-Franklin D, Frangione B, Ghiso J. 1987. Constitutive secretion of cystatin C (gamma-trace) by monocytes and macrophages and its downregulation after stimulation. J Exp Med. 166(6):1912–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler IG, Sims NA, Pettit AR, Barbier V, Nowlan B, Helwani F, Poulton IJ, van Rooijen N, Alexander KA, Raggatt LJ, et al. 2010. Bone marrow macrophages maintain hematopoietic stem cell (HSC) niches and their depletion mobilizes HSCs. Blood. 116(23):4815–4828. [DOI] [PubMed] [Google Scholar]
- Wu AC, Raggatt LJ, Alexander KA, Pettit AR. 2013. Unraveling macrophage contributions to bone repair. Bonekey Rep. 2:373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Z, Lu C, Hu D, Yu Y-Y, Wang X, Colnot C, Nakamura M, Wu Y, Miclau T, Marcucio RS. 2010. Multiple roles for CCR2 during fracture healing. Dis Model Mech. 3(7–8):451–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu T, Zhao L, Huang X, Ma C, Wang Y, Zhang J, Xuan D. 2016. Enhanced activity of the macrophage M1/M2 phenotypes and phenotypic switch to M1 in periodontal infection. J Periodontol. 87(9):1092–1102. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, DS_10.1177_0022034518777973 for Resolving Macrophages Counter Osteolysis by Anabolic Actions on Bone Cells by A. Viniegra, H. Goldberg, Ç. Çil, N. Fine, Z. Sheikh, M. Galli, M. Freire, Y. Wang, T.E. Van Dyke, M. Glogauer and C. Sima in Journal of Dental Research