Abstract
Periodontal disease (PD) shares common risk factors with cardiovascular disease. Our hypothesis was that having a family history of myocardial infarction (FamHxMI) may be a novel risk factor for PD. Risk assessment based on FamHxMI, conditional on smoking status, was examined given the strong influence of smoking on PD. Exploratory analysis with inflammatory biomarkers and genetic determinants was conducted to understand potential mechanistic links. The Women’s Genome Health Study (WGHS) is a prospective cohort of US female health care professionals who provided blood samples at baseline in the Women’s Health Study, a 2 × 2 factorial clinical trial investigating vitamin E and aspirin in the prevention of cardiovascular disease and cancer. PD was ascertained via self-report over 12 y of follow-up. Prevalence (3,442 cases), incidence (1,365 cases), and survival analysis of PD were investigated for associations of FamHxMI as well as in strata of FamHxMI by smoking. Kruskal-Wallis, chi-square tests, multivariate regression, and Cox proportional hazard models were used for the analyses. In the WGHS, women with FamHxMI showed higher risk of ever having PD. A particularly high-risk group of having both FamHxMI and smoking at baseline was highlighted in the prevalence and risk of developing PD. PD risk increased according to the following strata: no FamHxMI and nonsmokers (reference), FamHxMI and nonsmokers (hazard ratio [HR] = 1.2, 95% CI = 1.0 to 1.5), smokers without FamHxMI (HR = 1.3, 95% CI = 1.2 to 1.5), and smokers with FamHxMI (HR = 1.5, 95% CI = 1.2 to 1.8). An independent analysis by the dental Atherosclerosis Risk in Communities study (N = 5,552) identified more severe periodontitis cases among participants in the high-risk group (smokers with FamHxMI). Further examination of interactions among inflammatory biomarkers or genetic exploration with FamHxMI did not explain the risk increase of PD associated with FamHxMI in the WGHS. Future efforts based on an integrative-omics approach may facilitate validation of these findings and suggest a mechanistic link between PD and FamHxMI.
Keywords: cardiovascular disease, epidemiology, genetics, inflammation, periodontal disease/periodontitis, risk factors
Introduction
The prevalence of periodontal disease (PD) is estimated at about 46% of the US adult population, with 8.9% having the severe form (Eke et al. 2015). Systemic conditions—including cardiovascular disease (CVD), diabetes, osteoporosis, hormonal factors, and psychological stress—socioeconomic status, and smoking behavior are known risk factors for PD (Cullinan and Seymour 2013). Importantly, smoking alone accounts for 52.8% periodontitis cases in the United States (Tomar and Asma 2000). The current consensus regarding the pathogenesis of PD is that it is a microbial-induced chronic inflammatory disease with variations of clinical expression primarily determined by multifaceted dynamic interactions due to innate and adaptive immune responses, adverse environmental events, and genetic susceptibility factors (Meyle and Chapple 2015).
PD and CVD share common risk factors, including genetic susceptibility, age, smoking, low socioeconomic status, and systemic conditions (e.g., diabetes; Lockhart et al. 2012). Abundant epidemiologic evidence supports the link between PD and cardiovascular events, although a causal inference has not been confirmed (Lockhart et al. 2012; Dietrich et al. 2013; Yu et al. 2015). A Swedish study investigating 15,273 twins suggested that presence of tooth loss or PD at baseline was correlated with future risk for total CVD. A common pathogenetic mechanism between PD and CVD (Mucci et al. 2009) was suggested, with genetic factors estimated to account for 50% to 75% of the phenotypic correlations.
It has been established that family history of myocardial infarction (FamHxMI) is a predictive variable that summarizes the causal contribution of diet, genetics, and lifestyle to risk of myocardial infarction (Benjamin et al. 2017). Among women, having 1 parental FamHxMI will increase the risk of cardiovascular events by 70% (Sesso et al. 2001; Lloyd-Jones et al. 2004). However, genetic studies of twins demonstrated that about 50% of PD was contributed by heritability (Michalowicz et al. 2000)—specifically, at 0.55 for alveolar bone height (Michalowicz, Aeppli, Kuba, et al. 1991) and 0.51 for probing depth (Michalowicz, Aeppli, Virag, et al. 1991). Other common genetic loci, such as 9p21.3 or the plasminogen gene associated with CVD and the aggressive form of PD, further suggested a shared genetic component (Schaefer et al. 2009; Schaefer et al. 2015; Aarabi et al. 2017).
The Women’s Genome Health Study (WGHS; Ridker et al. 2008) is an ongoing prospective cohort with whole-genome genetic data originating from the Women’s Health Study (WHS; Buring 1992). Follow-up of incident health events, such as CVD and PD, as well as extensive annual data by questionnaire survey among these women has been ongoing for >20 y. In our prior report, women having PD at baseline or during the prospective follow-up had higher risks of cardiovascular events (Yu et al. 2015). Here, we hypothesized that FamHxMI could serve as a novel risk factor for PD. We further examined risk of PD in terms of FamHxMI and smoking status, as well as from the perspective of genetics and inflammatory biomarkers.
Methods
Study Population of the WHS/WGHS
Participants in the WGHS were initially healthy female health care professionals at least 45 y old at baseline and represented participants in the WHS who provided a blood sample at baseline. The WHS was conducted as a 2 × 2 clinical trial from 1992 to 1994 that investigated vitamin E and low-dose aspirin in the prevention of cancer and CVD with 10 y of follow-up. Since the end of the trial, follow-up has continued in observational mode. Additional information related to health and lifestyle were collected by questionnaire throughout the WHS trial and continuing observational follow-up. The study was approved by the Institutional Review Board of Brigham and Women’s Hospital and conforms to the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines.
Self-reported PD
Participants in the WHS/WGHS were asked about whether they had prevalent PD at the study entry (Yu et al. 2015). Incident PD cases were then assessed at 36, 48, 60, 72, 84, 96, 108, and 120 mo during the randomized trial and subsequently at the first follow-up that occurred during the observational extension study (about 1 y after trial completion). The month and year of the periodontal diagnosis during the study interval were requested for participants reporting incident PD. Prevalent PD was defined as that having occurred at or before baseline and incident PD as that having occurred during the follow-up. Ever having had PD status was combined from the baseline prevalent PD as well as the incident PD.
Assessment of Covariates
Details of the study protocol are described in the primary endpoint report of the randomized controlled trial (Ridker, Cook, et al. 2005). Covariates of interest were collected at the baseline by validated questionnaires and used in the analysis—age, body mass index, education, self-reported, verified incident type 2 diabetes (Liu et al. 2006), smoking, and FamHxMI. Plasma lipid fractions, serum levels of intercellular adhesion molecule 1 (ICAM), fibrinogen, and high-sensitivity C-reactive protein (CRP) were measured for the WGHS participants who provided a blood sample at baseline, as described (Ridker, Rifai, et al. 2005).
WGHS Genotype Data
Genotyping in the WGHS was performed with the Human-Hap300 Duo and iSelect chips (Illumina) with the Infinium II protocol. We used BeadStudio 3.3 software (Illumina) for quality control. The analysis comprised samples with at least 98% successful genotyping and single-nucleotide polymorphisms (SNPs) with at least 90% of samples successfully genotyped. A total of 339,596 SNPs were retained with minor allele frequency >1% and deviations from Hardy-Weinberg equilibrium not exceeding P = 10−6 in significance. Among the final 23,294 individuals of European ancestry verified by multidimensional scaling of ancestry-informative markers in PLINK 1.06, genotypes for a total of 30,052,423 (autosomes) + 1,264,493 (X) SNPs were imputed from the experimental genotypes and phase information from the 1000G ALL panel (phase I, version 3; release: March 2012) with MaCH 1.0.16 and Minimac (release: May 29, 2012). A total of 332,927 genotyped SNPs that were selected by Hardy-Weinberg equilibrium P value >10−6 but unrestricted by minor allele frequency could be reconciled with the 1000G ALL panel and were used for imputation. This project is restricted to the 19,804 women of European ancestry with genotyping data and nonmissing FamHxMI status.
Replication Population of the Dental ARIC Study
The Atherosclerosis Risk in Communities (ARIC) cohort is an ongoing prospective cohort study of atherosclerosis. Dental evaluation was conducted at ARIC visit 4 from 1996 to 1998 for dentate participants not needing prophylactic antibiotics. Periodontal clinical measures (including probing) were collected by calibrated dental examiners from 6 sites of all teeth present. A total of 5,552 men and women participated in the dental ARIC; they were 52 to 74 y old and predominantly Whites and African Americans. Details of study methods are available in prior reports (Beck et al. 2001; Divaris et al. 2012; Rhodin et al. 2014; Offenbacher et al. 2016).
Statistical Analyses
Group comparisons were done with the Kruskal-Wallis test for continuous variables and the chi-square test for categorical variables (Tables 1 and 2). We used the Cox proportional hazard model for survival analysis. Three levels of models were developed to assess risk increase of PD by having FamHxMI: model 1, adjusted for age, smoking, education, and body mass index (BMI); model 2, further adjusted for incident diabetes and hormone replacement therapy; model 3, including covariates of model 2 and serum levels of inflammatory biomarkers of HDL, LDL, ICAM, fibrinogen, and CRP. All the covariates were collected at the baseline, except incident diabetes. Time-varying survival analysis (Therneau and Crowson 2014) was used to accommodate incident diabetes (Fig.; see also section Survival Analysis of New-Onset Self-reported PD). Logistic regression analyses were done for risk factors to examine the main effects and interactions among 4 groups of women defined by a combination of FamHxMI and smoking status. All analyses in the WGHS were performed with R software. Replication analyses in the dental ARIC study were independently performed with SAS software and the PROC REG or PROC LOGISTIC function.
Table 1.
Demographic and Biological Characteristics of Women at the Baseline.
| FamHxMI, n (%)a |
|||
|---|---|---|---|
| No |
Yes |
||
| Participants | 17,248 (87.1) | 2,556 (12.9) | P Value b |
| Age, yc | 54.8 ± 7.2 | 53.6 ± 6.5 | <0.001 |
| Body mass indexc | 25.1 ± 6.6 | 25.6 ± 6.7 | <0.001 |
| Bachelor degree | 7,651 (44.4) | 1,111 (43.5) | 0.4 |
| Income, $ | 0.1 | ||
| <20,000 | 98 (4.9) | 89 (3.7) | |
| 20,000 to 29,999 | 1,567 (9.6) | 224 (9.2) | |
| 30,000 to 39,999 | 2,259 (13.8) | 330 (13.6) | |
| 40,000 to 49,999 | 2,739 (16.8) | 400 (16.5) | |
| 50,000 to 99,999 | 6,835 (41.8) | 1,039 (42.9) | |
| ≥100,000 | 2,139 (13.1) | 342 (14.1) | |
| Smoker | 8,382 (48.6) | 1,277 (50.0) | 0.19 |
| Pack-years | 14.7 (13.7) | 14.5 (13.0) | 0.73 |
| HRT | 7,678 (44.5) | 1,114 (43.6) | 0.4 |
| Inflammatory biomarker | |||
| CRP >3, mg/L | 6,199 (35.9) | 971 (38.0) | 0.04 |
| HDL, mg/dLd | 52.4 (43.7 to 63.0) | 50.7 (42.3 to 60.9) | <0.001 |
| LDL, mg/dLd | 120.5 (99.7 to 143.3) | 124.0 (103.5 to 146.6) | <0.001 |
| ICAM, ng/mLd | 341.8 (301.1 to 392.8) | 342.7 (302.1 to 392.6) | 0.34 |
| Fibrinogen, mg/dLd | 348.4 (305.6 to 399.3) | 349.1 (305.8 to 398.1) | 0.98 |
CRP, C-reactive protein (natural logarithm transformed); FamHxMI, family history of myocardial infarction; HDL, high-density lipoprotein; HRT, hormone replacement therapy; ICAM, intercellular adhesion molecule 1; LDL, low-density lipoproteins.
Values are presented as n (%) unless noted otherwise.
P values are based on the Kruskal-Wallis test for continuous variables and the chi-square test for categorical variables. Bold indicates P < 0.05
Mean ± SD.
Median (Q25 to Q75).
Table 2.
Prevalence and Incidence of Periodontal Disease by FamHxMI vs. Smoking Status.
| No FamHxMI | FamHxMI | P Valuea | |||
|---|---|---|---|---|---|
| Participants | 17,248 | 2,556 | |||
| Prevalence of PD | 2,968 (17.2) | 474 (18.5) | 0.09 | ||
| Incidence of PD | 1,166 (6.8) | 199 (7.8) | 0.06 | ||
| Ever having PD | 4,134 (24.0) | 673 (26.3) | 0.01 | ||
| Nonsmoker |
Smoker |
||||
| Group 1: No FamHxMI | Group 2: FamHxMI | Group 3: No FamHxMI | Group 4: FamHxMI | ||
| Participants | 8,866 | 1,279 | 8,382 | 1,277 | |
| Prevalence of PD | 1,059 (11.9) | 169 (13.2) | 1,909 (22.8) | 305 (23.9) | <0.001 |
| Incidence of PD | 556 (6.3) | 96 (7.5) | 610 (7.3) | 103 (8.1) | 0.02 |
| Incidence rateb | 6.52 (5.98 to 7.06) | 8.00 (6.40 to 9.60) | 8.75 (8.06 to 9.45) | 9.86 (7.96 to 11.77) | |
| Ever-having PD | 1,615 (18.2) | 265 (20.7) | 2,519 (30.1) | 408 (31.9) | <0.001 |
Values are presented as n (%) unless noted otherwise.
FamHxMI, family history of myocardial infarction; PD, periodontal disease.
P values are based on the chi-square tests for categorical variables. Bold indicates P < 0.05.
Median (Q1 to Q3) per 1,000 person-years of follow-up.
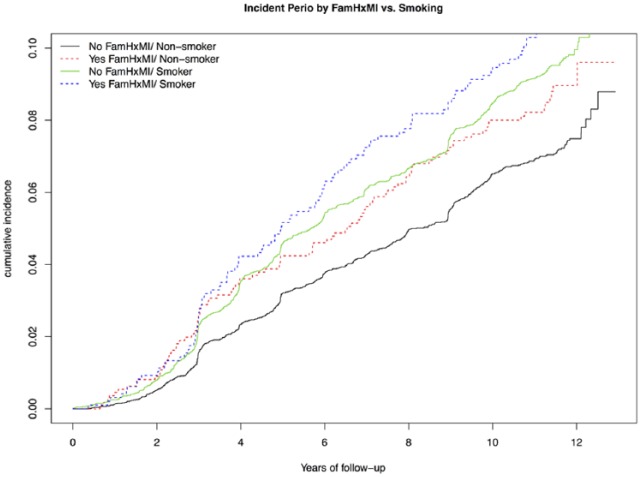
Figure.
Survival analysis of incidence of periodontal disease based on having a family history of myocardial infarction (FamHxMI) versus smoking status. Women without periodontal disease at the baseline were categorized in 4 groups based on FamHxMI versus smoking status. Survival analysis was adjusted for age, body mass index, education, income, incident diabetes, hormone replacement therapy, and all the inflammatory biomarkers.
Results
Baseline Characteristics
As shown in Table 1, the analysis included 2,556 women (12.9%) with self-reported FamHxMI. Compared with those without FamHxMI, women with FamHxMI were significantly younger (mean: 53.6 vs. 54.8 y, P < 0.001) and more obese (mean BMI: 25.6 vs. 25.1). Biomarker profiles were significantly less healthy among women with FamHxMI, reflecting lower levels of HDL, higher levels of LDL, and higher rates of having serum levels of CRP >3 mg/L; however, no difference was found for ICAM and fibrinogen. Although not statistically significant, women with FamHxMI were less likely to have a bachelor’s degree (43.5% vs. 44.4%) or use hormone replacement therapy (43.6% vs. 44.5%).
Prevalence and Incidence of PD by FamHxMI versus Smoking
At baseline, prevalence of PD among women with FamHxMI (Table 2) was not significantly different from those without FamHxMI (18.5% vs. 17.2%, P = 0.09). Similarly, no significant difference was found in incidence of new-onset PD (7.8% vs. 6.8%, P = 0.06). However, when prevalent PD and incident PD were combined, there was a significantly higher percentage of women ever having PD among those with FamHxMI (26.3% vs. 24.0%, P = 0.01).
We evaluated the combined effects of 2 risk factors: having FamHxMI and smoking (given the known strong influence of smoking on the risk of developing PD). Women were classified into 4 groups by smoking versus FamHxMI: group 1, never smoker and no FamHxMI (reference); group 2, never smoker but with FamHxMI; group 3, smoker but no FamHxMI; and group 4, smoker and with FamHxMI. During 12.6 y of follow-up of the incident PD cases, having FamHxMI posed as much risk of PD as smoking (group 2, 7.5%; group 3, 7.3%). Group 4 had 8.1% risk of incident PD. We identified incremental risk increase of PD across these 4 groups. Similar patterns were shown in prevalent PD (groups 1 to 4, respectively: 11.9%, 13.2%, 22.8%, and 23.9%) and ever having PD (18.2%, 20.7%, 30.1%, and 31.9%).
Survival Analysis of New-Onset Self-reported PD
In Table 3, with base model 1, having FamHxMI was associated an increased risk of developing PD (hazard ratio [HR] = 1.18, 95% CI = 1.01 to 1.38, P = 0.04), and smokers had an increased HR of 1.33. Results remained similar when incident diabetes and hormone replacement therapy were incorporated (model 2). In model 3, which accounted for inflammatory biomarkers, the effect of FamHxMI on PD was attenuated and became insignificant (HR = 1.16, 95% CI = 1.00 to 1.36, P = 0.056). Last, we examined but did not find significant multiplicative interactions between FamHxMI and smoking. The combined risk increment on PD by having FamHxMI and smoking was additive on the log HR scale (Table 3, Fig.). As illustrated, group 4 women (having FamHxMI and smokers) had the highest risks of PD throughout follow-up (HR = 1.52, 95% CI = 1.23 to 1.89, P < 0.001).
Table 3.
Cox Proportional Hazard Survival Analysis for New-Onset Self-reported Periodontal Disease.
| No FamHxMI | FamHxMI | |||
|---|---|---|---|---|
| Model 1 | Reference | 1.18 (1.01 to 1.38) | ||
| Model 2 | Reference | 1.18 (1.01 to 1.37) | ||
| Model 3 | Reference | 1.16 (1.00 to 1.36) | ||
| Nonsmoker |
Smoker |
|||
| Group 1: No FamHxMI | Group 2: FamHxMI | Group 3: No FamHxMI | Group 4: FamHxMI | |
| Model 1a | Reference | 1.23 (0.98 to 1.54) | 1.35 (1.20 to 1.52) | 1.52 (1.23 to 1.89) |
| Model 2a | Reference | 1.23 (0.98 to 1.54) | 1.35 (1.20 to 1.52) | 1.52 (1.23 to 1.89) |
| Model 3a | Reference | 1.22 (0.97 to 1.53) | 1.33 (1.18 to 1.49) | 1.48 (1.19 to 1.84) |
Values are presented as hazard ratio (95% CI). Bold indicates P < 0.05 (Cox proportional hazard model). All covariates in Model 1 to 3 were reported at the study baseline except for incident diabetes, which was recorded time varying by annual follow-up questionnaires and verified with medical records. Model 1: adjusted for age, education, income, BMI, smoking. Model 2 (time varying) = adjusted for age, education, income, BMI, smoking, incident DM, HRT. Model 3 (time varying) = adjusted for age, education, income, BMI, smoking, incident DM, HRT, standardized serum levels of 1-SD increase of HDL, LDL, ICAM, fibrinogen, and CRP.
BMI, body mass index; CRP, C-reactive protein (natural logarithm transformed); DM, diabetes; FamHxMI, family history of myocardial infarction; HDL, high-density lipoprotein; HRT, hormone replacement therapy; ICAM, intercellular adhesion molecule 1; LDL, low-density lipoprotein.
Same as Models 1 to 3 but without smoking.
Interactions of Inflammatory Markers and Other Risk Factors
To test whether there is differential role played by traditional risk factors or inflammatory biomarkers on risk of ever having PD across 4 groups of women based on FamHxMI and smoking status, we performed logistic regression analyses including an interaction term between each risk factor and individual dummy variables of groups (Table 4). Women without FamHxMI and never smoker served as the reference group. We noticed significant main effects on ever having PD for such risk factors as BMI, incident diabetes, LDL, CRP, and fibrinogen. There was a modest interaction of serum levels of ICAM among smokers (odds ratio [OR] = 1.08, 95% CI = 1.01 to 1.17, P = 0.03). The influence of the following risk factors was attenuated among group 4 women (FamHxMI and smoker), as indicated by the OR of the interaction terms: incident diabetes (OR = 0.55, 95% CI = 0.32 to 0.91, P = 0.02), high serum levels of CRP (OR = 0.83, 95% CI = 0.73 to 0.95, P = 0.006), and fibrinogen (OR = 0.88, 95% CI = 0.77 to 1.0, P = 0.04). Across these models, FamHxMI and smoking remained as significant risk factors. For a fully adjusted multivariate logistic regression analysis, see Appendix Table 2. Thus, the associated risk increase of PD by FamHxMI cannot be explained or mediated by these risk factors or biomarkers.
Table 4.
Risks of Ever Having Periodontal Disease by Inflammatory Biomarkers versus FamHxMI and Smoking Status.
| RFInteraction |
Group 2: FamHxMI, Nonsmoker | Group 3: No FamHxMI, Smoker | Group 4: FamHxMI, Smoker | |||
|---|---|---|---|---|---|---|
| RF | RFMain Effect | Group 3 | Group 4 | |||
| Bachelor degree | 1.08 (0.97 to 1.20) | 1.09 (0.94 to 1.26) | 1.07 (0.82 to 1.39) | 1.14 (0.93 to 1.39) | 1.87 (1.70 to 2.06) | 2.06 (1.73 to 2.44) |
| Income | 1.00 (0.96 to 1.04) | 0.99 (0.93 to 1.04) | 0.98 (0.89 to 1.08) | 1.10 (0.65 to 1.81) | 2.06 (1.62 to 2.61) | 2.30 (1.47 to 3.58) |
| BMI | 1.01 (1.00 to 1.02) | 1.00 (0.98 to 1.01) | 0.98 (0.96 to 1.00) | 1.83 (1.07 to 3.09) | 2.17 (1.63 to 2.91) | 3.75 (2.16 to 6.50) |
| HRT | 1.10 (0.99 to 1.22) | 0.93 (0.81 to 1.07) | 1.06 (0.82 to 1.38) | 1.31 (1.08 to 1.59) | 1.99 (1.81 to 2.20) | 2.05 (1.72 to 2.44) |
| Incident DM | 1.31 (1.06 to 1.63) | 0.84 (0.63 to 1.12) | 0.55 (0.32 to 0.91) | 1.19 (1.02 to 1.38) | 1.95 (1.81 to 2.10) | 2.19 (1.92 to 2.51) |
| HDLa | 0.98 (0.92 to 1.03) | 0.97 (0.91 to 1.05) | 1.08 (0.95 to 1.23) | 1.17 (1.01 to 1.35) | 1.93 (1.80 to 2.07) | 2.12 (1.86 to 2.41) |
| LDLa | 1.12 (1.06 to 1.18) | 1.01 (0.94 to 1.08) | 0.98 (0.86 to 1.11) | 1.17 (1.01 to 1.36) | 1.93 (1.80 to 2.07) | 2.09 (1.83 to 2.38) |
| CRPa | 1.10 (1.04 to 1.16) | 0.93 (0.87 to 1.00) | 0.83 (0.73 to 0.95) | 1.18 (1.01 to 1.36) | 1.93 (1.80 to 2.07) | 2.12 (1.86 to 2.41) |
| ICAMa | 1.06 (0.99 to 1.12) | 1.08 (1.01 to 1.17) | 1.10 (0.97 to 1.25) | 1.16 (1.00 to 1.35) | 1.87 (1.74 to 2.01) | 2.04 (1.79 to 2.33) |
| Fibrinogena | 1.13 (1.07 to 1.19) | 0.97 (0.91 to 1.04) | 0.88 (0.77 to 1.00) | 1.17 (1.01 to 1.35) | 1.92 (1.79 to 2.06) | 2.11 (1.85 to 2.40) |
Values are presented as odds ratio (95% CI). Bold indicates P < 0.05 (logistic regression). Each row presents an individual logistic regression analytical model: Group 1 women (no FamHxMI/nonsmoker) was the reference group. Ever having had PD ~ individual RF + group 2 (FamHxMI/nonsmoker) + group 3 (no FamHxMI/smoker) + group 4 (FamHxMI/smoker) + interaction of RF group 2 + interaction of RF group 3 + interaction of RF group 4. All results from interaction of RF group 2 were nonsignificant and thus not presented in the table.
BMI, body mass index; CRP, C-reactive protein (natural logarithm transformed); FamHxMI, family history of myocardial infarction; HDL, high-density lipoprotein; HRT, hormone replacement therapy; ICAM, intercellular adhesion molecule 1; DM, diabetes; LDL, low-density lipoprotein; RF, risk factor.
Standardized serum levels (1-SD increase).
FamHxMI and Smoking Behavior Associated with Severe Periodontitis
We attempted to validate our findings by evaluating data in the dental ARIC study. FamHxMI had no significant association with any of the periodontal measurements investigated, such as severity of periodontitis (as defined by the Centers for Disease Control and Prevention/American Academy of Periodontology), number of teeth, levels of IL-1b in the gingival crevicular fluid, or microbial composition (data not shown; unadjusted models). However, participants in the dental ARIC study who smoked and had FamHxMI demonstrated prevalence of severe periodontitis as high as smokers without FamHxMI (unadjusted; Appendix Table 1). The association remained significant and slightly enhanced after further adjustment for other covariates, such as age, race/center, sex, education, income, BMI, and diabetes.
Genetic Exploratory Analyses
We investigated whether there is a potential genetic link between FamHxMI and PD by examining candidate genetic variants derived from the genome-wide association study (GWAS) catalog (MacArthur et al. 2017).
Candidate SNPs Associated with Coronary Heart Disease
We examined 45 SNPs with genome-wide significance for CVD from the CARDIoGRAM consortium (CARDIoGRAM plusC4D Consortium et al. 2013; Benjamin et al. 2017). Notably, WGHS was not involved in the discovery of these candidate SNPs. We focused on determining whether there was a common association for PD as well as FamHxMI in the WGHS. Accounting for multiple testing, we did not find any significant associations between FamHxMI and PD among the 45 candidate CVD SNPs. However, at a nominal significance level (P < 0.05), rs2246833 was associated with FamHxMI (OR = 0.93, 95% CI = 0.88 to 0.99, P = 0.02) as well as incident PD (OR = 1.1, 95% CI = 1.02 to 1.18, P = 0.02), although these effects were discordant.
Candidate SNPs Associated with PD
There are 141 unique candidate SNPs reported by 9 published PD-related studies in the GWAS catalog (Feng et al. 2014; Shaffer et al. 2014; Divaris et al. 2012; Teumer et al. 2013; Schaefer et al. 2010; Hong et al. 2015; Shimizu et al. 2015; Offenbacher et al. 2016; Sanders et al. 2017; MacArthur et al. 2017). We tested whether the 141 SNPs were associated with having FamHxMI and PD in the WGHS. Although no SNP met a significance threshold consistent with multiple testing, rs149740259 was nominally associated with concordant effects for FamHxMI (OR = 0.76, 95% CI = 0.60 to 0.97, P = 0.03) and ever having PD (OR = 0.77, 95% CI = 0.64 to 0.94, P = 0.008).
Discussion
Our results demonstrated that having FamHxMI increased the risk of developing PD in the WGHS. In addition, a higher-risk profile was identified among women who had FamHxMI and smoked. Although the dental ARIC study did directly replicate the association of FamHxMI and risk of PD in this report, a strong association was found for severe periodontitis among participants who had FamHxMI and smoked, which will need to be examined in additional large independent cohorts. Given that PD and many correlated systemic comorbidities, such as CVD and diabetes, share risk factors, validation of the association might suggest increased awareness of FamHxMI as a PD risk factor and thus motivate studies addressing the potential impact of managing cardiovascular risk in PD prevention.
Second, effects based on FamHxMI may imply potential PD risk influences from genetic profiles and/or environmental exposure, such as lifestyle and dietary habits. It was of interest to determine whether our epidemiologic associations between FamHxMI and PD can be further explained by inflammatory biomarkers or genetic factors. We did not find traditional risk factors or inflammatory biomarkers mediating the association between FamHxMI and PD. However, we suggest that by categorizing smokers as never ever, residual confounding is likely. Next, we conducted candidate genetic analyses hoping to support such hypotheses of shared pathogenetic links. We examined the PD and CVD candidate SNPs in the published GWAS catalog. In spite of a substantial heritability of PD (Michalowicz et al. 2000) as well as the large sample size of WGHS, we did not find a strong genetic signal meeting significance thresholds accounting for multiple testing.
Several plausible reasons follow: 1) there might be imprecise disease definition or characterization; 2) effect-modifying factors, such as smoking and other conditions (e.g., CVD), might change the genetic susceptibility of PD; or 3) PD susceptibility may be polygenic, with each contributing genetic variant exerting a very weak effect on its own. Indeed, our study has the strength of a sufficiently large sample size but also the limitation of self-reported incidence of PD without information of severity of PD. The significance of the latter is not clear. Women in the WHS/WHGS are known to be accurate in reporting biometric measurements and medical diagnoses (Ridker, Cook, et al. 2005; Ridker et al. 2008). However, a validation study of self-reported PD has not been done in the WHS/WGHS. Self-reported PD status might lead to misclassification and potentially bias results, mostly likely toward the null. Additionally, recent evidence from the Hispanic Community Health Study / Study of Latinos did suggest a genetic component in chronic periodontitis, but the significant association was with the clinical interproximal attachment loss instead of PD diagnosis (Sanders et al. 2017).
Two specific SNPs that were suggestively associated with FamHxMI and PD merit discussion. First, rs149740259, an intron variant of cadherin 13 (CDH13), was associated with mean periodontal attachment loss in a GWAS of PD (Teumer et al. 2013). This gene was shown to protect vascular endothelial cells from apoptosis due to oxidative stress (Serbanovic-Canic et al. 2017), and it was associated with atherosclerosis in a Korean hypertensive population (Lee et al. 2013). In the WGHS, women with the same allele demonstrated nominal associations with ever having PD, prevalent PD, and FamHxMI. The other SNP, rs2246833, lysosomal acid lipase A (LIPA), was reported for its association with CVD (Vargas-Alarcon et al. 2013; Morris et al. 2017). Autophagy and lysosomal activities are frequently activated by microbial challenges and thus increase the inflammatory and immune responses. A possible role of autophagy was speculated in regulating the initiation and progression of PD (Bullon et al. 2012; Lee et al. 2015), although we found that the directions of effect estimates of rs2246833 were discordant for FamHxMI and PD.
In summary, this study is the first to examine FamHxMI as a potential risk factor for developing PD. Limitations of small effect estimate of FamHxMI and self-reported PD status were discussed. Additionally, we showed an additive combined risk profile of having FamHxMI and smoking but did not identify multiplicative interaction between these risk factors. Traditional risk factors, inflammatory biomarkers, and candidate genetic analyses did not explain the FamHxMI association. That said, we do not have a clear a priori hypothesis about the underlying mechanistic links between PD and CVD, which indeed may be highly complex. Regardless, future independent studies will be needed to further support or verify our findings in this population of female health care professionals.
Author Contributions
Y.H. Yu, L. Doucette-Stamm, J. Rogus, K. Kornman, contributed to conception, design, data analysis, and interpretation, drafted and critically revised the manuscript; K. Moss, contributed to design, data analysis, and interpretation, drafted and critically revised the manuscript; B. Steffensen, contributed to design and data interpretation, drafted and critically revised the manuscript; P.M. Ridker, J.E. Buring, R.Y.L. Zee, contributed to design, data acquisition, and interpretation, drafted and critically revised the manuscript; S. Offenbacher, D.I. Chasman, contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplemental Material
Supplemental material, DS_10.1177_0022034518782189 for Family History of MI, Smoking, and Risk of Periodontal Disease by Y.H. Yu, L. Doucette-Stamm, J. Rogus, K. Moss, R.Y.L. Zee, B. Steffensen, P.M. Ridker, J.E. Buring, S. Offenbacher, K. Kornman and D.I. Chasman in Journal of Dental Research
Acknowledgments
The authors thank the staff and participants of the ARIC study for their important contributions.
Footnotes
A supplemental appendix to this article is available online.
The WHS/WGHS study is supported by grants HL043851, HL080467, and HL099355 from the National Heart, Lung, and Blood Institute; by grant CA047988 and CA182913 from the National Cancer Institute; and by the Donald W. Reynolds Foundation. Dr. Yu was supported for the study by Interleukin Genetics (2015 to 2016) and was the 2016 recipient of the William B. Clark Research Fellowship from the American Association of Dental Research. Dr. Yu is currently supported by the National Institute of Dental and Craniofacial Research (1K23DE026804-01A1). The ARIC study is carried out as a collaborative study supported by the National Heart, Lung, and Blood Institute (contracts HHSN268201100005C, HHSN268201100006C, HHSN268201 100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C and HSN268 20110 0012C); grants R01HL087641, R01HL59367 and R01HL 086694), the National Human Genome Research Institute (contract U01HG 004402), the National Institutes of Health (contract HHSN2682 00625226C), the National Institute of Environmental Health Sciences (grant P30ES010126), and the National Institute of Dental and Craniofacial Research (grants R01DE11551, R01DE 021418). Infrastructure was partly supported by grant UL1RR0 25005, a component of the National Institutes of Health and NIH Roadmap for Medical Research.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
ORCID iD: Y.H. Yu  https://orcid.org/0000-0002-4723-5064
https://orcid.org/0000-0002-4723-5064
References
- Aarabi G, Zeller T, Seedorf H, Reissmann DR, Heydecke G, Schaefer AS, Seedorf U. 2017. Genetic susceptibility contributing to periodontal and cardiovascular disease. J Dent Res. 96(6):610–617. [DOI] [PubMed] [Google Scholar]
- Beck JD, Elter JR, Heiss G, Couper D, Mauriello SM, Offenbacher S. 2001. Relationship of periodontal disease to carotid artery intima-media wall thickness: the Atherosclerosis Risk in Communities (ARIC) study. Arterioscler Thromb Vasc Biol. 21(11):1816–1822. [DOI] [PubMed] [Google Scholar]
- Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, et al. 2017. Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation. 135(10):e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullon P, Cordero MD, Quiles JL, Ramirez-Tortosa Mdel C, Gonzalez-Alonso A, Alfonsi S, Garcia-Marin R, de Miguel M, Battino M. 2012. Autophagy in periodontitis patients and gingival fibroblasts: unraveling the link between chronic diseases and inflammation. BMC Med. 10:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buring JE, Hennekens CH; Women’s Health Study Research Group. 1992. The Women’s Health Study: summary of the study design. J Myocardial Ischemia. 4:27–29. [Google Scholar]
- CARDIoGRAMplusC4D Consortium;Deloukas P, Kanoni S, Willenborg C, Farrall M, Assimes TL, Thompson JR, Ingelsson E, Saleheen D, Erdmann J, Goldstein BA, et al. 2013. Large-scale association analysis identifies new risk loci for coronary artery disease. Nat Genet. 45(1):25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullinan MP, Seymour GJ. 2013. Periodontal disease and systemic illness: will the evidence ever be enough? Periodontol 2000. 62(1):271–286. [DOI] [PubMed] [Google Scholar]
- Dietrich T, Sharma P, Walter C, Weston P, Beck J. 2013. The epidemiological evidence behind the association between periodontitis and incident atherosclerotic cardiovascular disease. J Clin Periodontol. 40 Suppl 14:S70–S84. Erratum in: J Clin Periodontol. 2013;40 Suppl 14:S210–S215. [DOI] [PubMed] [Google Scholar]
- Divaris K, Monda KL, North KE, Olshan AF, Lange EM, Moss K, Barros SP, Beck JD, Offenbacher S. 2012. Genome-wide association study of periodontal pathogen colonization. J Dent Res. 91(7 Suppl):21S–28S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eke PI, Dye BA, Wei L, Slade GD, Thornton-Evans GO, Borgnakke WS, Taylor GW, Page RC, Beck JD, Genco RJ. 2015. Update on prevalence of periodontitis in adults in the United States: NHANES 2009 to 2012. J Periodontol. 86(5):611–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng P, Wang X, Casado PL, Küchler EC, Deeley K, Noel J, Kimm H, Kim JH, Haas AN, Quinelato V, et al. 2014. Genome wide association scan for chronic periodontitis implicates novel locus. BMC Oral Health. 14:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong KW, Shin MS, Ahn YB, Lee HJ, Kim HD. 2015. Genomewide association study on chronic periodontitis in Korean population: results from the Yangpyeong health cohort. J Clin Periodontol. 42(8):703–710. [DOI] [PubMed] [Google Scholar]
- Lee JH, Shin DJ, Park S, Kang SM, Jang Y, Lee SH. 2013. Association between CDH13 variants and cardiometabolic and vascular phenotypes in a Korean population. Yonsei Med J. 54(6):1305–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YH, Lee HY, Kim TG, Lee NH, Yu MK, Yi HK. 2015. PPARγ maintains homeostasis through autophagy regulation in dental pulp. J Dent Res. 94(5):729–737. [DOI] [PubMed] [Google Scholar]
- Liu S, Lee IM, Song Y, Van Denburgh M, Cook NR, Manson JE, Buring JE. 2006. Vitamin E and risk of type 2 diabetes in the Women’s Health Study randomized controlled trial. Diabetes. 55(10):2856–2862. [DOI] [PubMed] [Google Scholar]
- Lloyd-Jones DM, Nam BH, D’Agostino RB, Sr, Levy D, Murabito JM, Wang TJ, Wilson PW, O’Donnell CJ. 2004. Parental cardiovascular disease as a risk factor for cardiovascular disease in middle-aged adults: a prospective study of parents and offspring. JAMA. 291(18):2204–2211. [DOI] [PubMed] [Google Scholar]
- Lockhart PB, Bolger AF, Papapanou PN, Osinbowale O, Trevisan M, Levison ME, Taubert KA, Newburger JW, Gornik HL, Gewitz MH, et al. 2012. Periodontal disease and atherosclerotic vascular disease: does the evidence support an independent association? A scientific statement from the American Heart Association. Circulation. 125(20):2520–2544. [DOI] [PubMed] [Google Scholar]
- MacArthur J, Bowler E, Cerezo M, Gil L, Hall P, Hastings E, Junkins H, McMahon A, Milano A, Morales J, et al. 2017. The new NHGRI-EBI catalog of published genome-wide association studies (GWAS catalog). Nucleic Acids Res. 45(D1):D896–D901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyle J, Chapple I. 2015. Molecular aspects of the pathogenesis of periodontitis. Periodontol 2000. 69(1):7–17. [DOI] [PubMed] [Google Scholar]
- Michalowicz BS, Aeppli DP, Kuba RK, Bereuter JE, Conry JP, Segal NL, Bouchard TJ, Jr, Pihlstrom BL. 1991. A twin study of genetic variation in proportional radiographic alveolar bone height. J Dent Res. 70(11):1431–1435. [DOI] [PubMed] [Google Scholar]
- Michalowicz BS, Aeppli D, Virag JG, Klump DG, Hinrichs JE, Segal NL, Bouchard TJ, Jr, Pihlstrom BL. 1991. Periodontal findings in adult twins. J Periodontol. 62(5):293–299. [DOI] [PubMed] [Google Scholar]
- Michalowicz BS, Diehl SR, Gunsolley JC, Sparks BS, Brooks CN, Koertge TE, Califano JV, Burmeister JA, Schenkein HA. 2000. Evidence of a substantial genetic basis for risk of adult periodontitis. J Periodontol. 71(11):1699–1707. [DOI] [PubMed] [Google Scholar]
- Morris GE, Braund PS, Moore JS, Samani NJ, Codd V, Webb TR. 2017. Coronary artery disease-associated lipa coding variant rs1051338 reduces lysosomal acid lipase levels and activity in lysosomes. Arterioscler Thromb Vasc Biol. 37(6):1050–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucci LA, Hsieh CC, Williams PL, Arora M, Adami HO, de Faire U, Douglass CW, Pedersen NL. 2009. Do genetic factors explain the association between poor oral health and cardiovascular disease? A prospective study among swedish twins. Am J Epidemiol. 170(5):615–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offenbacher S, Divaris K, Barros SP, Moss KL, Marchesan JT, Morelli T, Zhang S, Kim S, Sun L, Beck JD, et al. 2016. Genome-wide association study of biologically informed periodontal complex traits offers novel insights into the genetic basis of periodontal disease. Hum Mol Genet. 25(10):2113–2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodin K, Divaris K, North KE, Barros SP, Moss K, Beck JD, Offenbacher S. 2014. Chronic periodontitis genome-wide association studies: gene-centric and gene set enrichment analyses. J Dent Res. 93(9):882–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridker PM, Chasman DI, Zee RY, Parker A, Rose L, Cook NR, Buring JE; Women’s Genome Health Study Working Group. 2008. Rationale, design, and methodology of the Women’s Genome Health Study: a genome-wide association study of more than 25,000 initially healthy American women. Clin Chem. 54(2):249–255. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Cook NR, Lee IM, Gordon D, Gaziano JM, Manson JE, Hennekens CH, Buring JE. 2005. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med. 352(13):1293–1304. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Rifai N, Cook NR, Bradwin G, Buring JE. 2005. Non-HDL cholesterol, apolipoproteins A-I and B100, standard lipid measures, lipid ratios, and CRP as risk factors for cardiovascular disease in women. JAMA. 294(3):326–333. [DOI] [PubMed] [Google Scholar]
- Sanders AE, Sofer T, Wong Q, Kerr KF, Agler C, Shaffer JR, Beck JD, Offenbacher S, Salazar CR, North KE, et al. 2017. Chronic periodontitis genome-wide association study in the Hispanic Community Health Study/Study of Latinos. J Dent Res. 96(1):64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer AS, Bochenek G, Jochens A, Ellinghaus D, Dommisch H, Guzeldemir-Akcakanat E, Graetz C, Harks I, Jockel-Schneider Y, Weinspach K, et al. 2015. Genetic evidence for plasminogen as a shared genetic risk factor of coronary artery disease and periodontitis. Circ Cardiovasc Genet. 8(1):159–167. [DOI] [PubMed] [Google Scholar]
- Schaefer AS, Richter GM, Groessner-Schreiber B, Noack B, Nothnagel M, El Mokhtari NE, Loos BG, Jepsen S, Schreiber S. 2009. Identification of a shared genetic susceptibility locus for coronary heart disease and periodontitis. PLoS Genet. 5(2):e1000378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer AS, Richter GM, Nothnagel M, Manke T, Dommisch H, Jacobs G, Arlt A, Rosenstiel P, Noack B, Groessner-Schreiber B, et al. 2010. A genome-wide association study identifies GLT6D1 as a susceptibility locus for periodontitis. Hum Mol Genet. 19(3):553–562. [DOI] [PubMed] [Google Scholar]
- Serbanovic-Canic J, de Luca A, Warboys C, Ferreira PF, Luong LA, Hsiao S, Gauci I, Mahmoud M, Feng S, Souilhol C, et al. 2017. Zebrafish model for functional screening of flow-responsive genes. Arterioscler Thromb Vasc Biol. 37(1):130–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesso HD, Lee IM, Gaziano JM, Rexrode KM, Glynn RJ, Buring JE. 2001. Maternal and paternal history of myocardial infarction and risk of cardiovascular disease in men and women. Circulation. 104(4):393–398. [DOI] [PubMed] [Google Scholar]
- Shaffer JR, Polk DE, Wang X, Feingold E, Weeks DE, Lee MK, Cuenco KT, Weyant RJ, Crout RJ, McNeil DW, et al. 2014. Genome-wide association study of periodontal health measured by probing depth in adults ages 18-49 years. G3 (Bethesda). 4(2):307–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu S, Momozawa Y, Takahashi A, Nagasawa T, Ashikawa K, Terada Y, Izumi Y, Kobayashi H, Tsuji M, Kubo M, et al. 2015. A genome-wide association study of periodontitis in a Japanese population. J Dent Res. 94(4):555–561. [DOI] [PubMed] [Google Scholar]
- Teumer A, Holtfreter B, Volker U, Petersmann A, Nauck M, Biffar R, Volzke H, Kroemer HK, Meisel P, Homuth G, et al. 2013. Genome-wide association study of chronic periodontitis in a general German population. J Clin Periodontol. 40(11):977–985. [DOI] [PubMed] [Google Scholar]
- Therneau T, Crowson C. 2014. Using time dependent covariates and time dependent coefficients in the Cox model. Rochester (MN): Mayo Clinic; [accessed 2018 May 21]. https://cran.r-project.org/web/packages/survival/vignettes/timedep.pdf [Google Scholar]
- Tomar SL, Asma S. 2000. Smoking-attributable periodontitis in the united states: findings from NHANES III. National Health and Nutrition Examination Survey. J Periodontol. 71(5):743–751. [DOI] [PubMed] [Google Scholar]
- Vargas-Alarcon G, Posadas-Romero C, Villarreal-Molina T, Alvarez-Leon E, Angeles J, Vallejo M, Posadas-Sanchez R, Cardoso G, Medina-Urrutia A, Kimura-Hayama E. 2013. Single nucleotide polymorphisms within LIPA (lysosomal acid lipase A) gene are associated with susceptibility to premature coronary artery disease: a replication in the Genetic of Atherosclerotic Disease (GEA) Mexican study. PLoS One. 8(9):e74703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu YH, Chasman DI, Buring JE, Rose L, Ridker PM. 2015. Cardiovascular risks associated with incident and prevalent periodontal disease. J Clin Periodontol. 42(1):21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, DS_10.1177_0022034518782189 for Family History of MI, Smoking, and Risk of Periodontal Disease by Y.H. Yu, L. Doucette-Stamm, J. Rogus, K. Moss, R.Y.L. Zee, B. Steffensen, P.M. Ridker, J.E. Buring, S. Offenbacher, K. Kornman and D.I. Chasman in Journal of Dental Research