The interplay between inflammation and tissue homeostasis is a critical balance in the oral cavity, ranging from a transitory “constructive” inflammation during bone turnover that can mediate orthodontic tooth movement until chronic “destructive” osteolytic inflammation-associated alveolar bone loss during periodontitis or apical bone resorption occurs. Although inflammatory events are commonly identified as key elements of healing processes, the mechanistic basis of this “constructive” inflammation remains unclear, especially given that the inflammation/bone connection is usually explored from the disease viewpoint.
In the chronic inflammation scenario, such as in periodontitis or endodontic pathology, studies converge to a CD4–T-cell centered process, where T-helper subsets with distinct/opposing functions are presumed to ultimately determine the inflammation/bone interaction outcome. In this setting, Th1 and Th17 subsets have been described to independently drive disease progression, boosting inflammation and increasing proteolytic and osteoclastogenic pathways, while Th2 cells and Tregs suppress disease progression by counteracting such processes (Garlet 2010). In this “T-centric” framework, other cellular cell types, such as neutrophils and macrophages, are considered principal elements in the inflammatory immune response but have a putative minor role acting under T-cell commands.
The evolution of the better understanding of macrophage biology has been undergoing redefining concepts in recent years. The dual roles of macrophages in tissue repair and turnover (or destruction) are likely due to the high plasticity of macrophages. These macrophages exhibit a wide range of phenotypes and functions depending on the wound-healing microenvironment (Chauzad 2014). Essentially, under the influence of local influencers, macrophages can acquire M1 (proinflammatory type) or M2 (proresolving) phenotypes, with opposing pro- and anti-inflammatory functions (Dutzan et al. 2016). In this month’s issue of the Journal, Sima and coworkers demonstrate that M1 and M2 are temporally associated with differing stages of experimentally induced periodontitis activity and inactivity, respectively (Viniegra et al. 2018). Such recognition fits well in the current Th paradigm of periodontitis, since Th1-type cytokines can instruct M1 polarization being both leukocyte subsets associated with periodontal destruction (Garlet 2010; Shapouri-Moghaddam et al. 2018). Indeed, in their study, Sima et al. directly implicate macrophages in periodontitis progression since macrophage depletion prevented bone resorption.
Conversely, the natural development of the M2 profile at a later disease state is temporally associated with the skew of the Th response transitioning from Th1 to Th2/Tregs, with the Th2/Tregs axis mechanistically implicated in the arrest of disease progression (Garlet 2010; Araujo-Pires et al. 2015). Interestingly, Th2/Tregs-related cytokines can skew macrophages toward an M2 profile (Weirather et al. 2014), and therefore, M2 macrophages may comprise an additional component to the immunoregulatory axis that suppresses periodontal disease progression. In addition, M2-related cytokines can boost Tregs activity (Haribhai et al. 2016), suggesting that an active Tregs/M2 cooperation may take place during healing processes.
However, despite the temporal association of Th2, Tregs, and M2 subsets, the factors involved in an M1/M2 switch in inflamed periodontium remains elusive. In this context, the Sima et al. (2018) study points to a broader role of interleukin (IL)–4 in local immunoregulation. In addition to triggering Treg chemoattraction by inducing CCL22 expression in the periodontium (Araujo-Pires et al. 2015), IL-4 can mediate the M2 phenotype acquisition, characterized not only by anti-inflammatory cytokine production but also by anabolic properties in vitro.
In this setting, a sequential and cooperative activity of T (Th2/Tregs) and M2 cells would be responsible not only for shutting down the injurious (chronic inflammatory) osteolytic stimuli but also for triggering tissue repair. A major concept change driven by Sima et al. (2018) comes from the experimental demonstration that the induction of an M2 phenotype (with rosiglitazone, a peroxisome proliferator-activated receptor (PPAR)–γ agonist) not only arrested bone resorption but also increased bone formation. The proreparative effect of M2 cells has been usually regarded as the production of pleiotropic anabolic mediators such as transforming growth factor (TGF) β and IL-10, the same mediators alleged to mediate tissue regeneration after chemoattraction of Tregs, which reinforce the existence of a possible Treg/M2 cooperative action. However, the Sima et al. study also underscores some specificity to the role of M2 cells since it adds a new proregenerative element in the system, cystatin C. While M1 conditions inhibit cystatin C production by macrophages, the findings demonstrate that the cystatin C produced by M2 cells boosts osteoblastic function in vitro.
This interplay between macrophages and osteoblastic precursors or upstream mesenchymal stem cells (MSCs) has been demonstrated in a variety of contexts for resolution and regeneration. For example, greater bone regeneration after tooth extraction or sinus floor elevation is augmented by the cotransplantation of CD90+ and CD14+ (monocytic or macrophage precursor) stem cells (Kaigler et al. 2013, 2015). The approach of codelivering such combinations of immune cells and more typical regenerative cells appears to be supported by the concept that endogenous activation of the local microenvironment machinery supports a natural wound-healing response in inflammation-driven contexts.
The demonstration that the M2 cells can promote tissue regeneration instead of simply downregulating tissue destructive pathways highlights a significant advance in the understanding of the mechanistic basis of “constructive” inflammation. In this evolving concept, the proreparative environment seems to tie the resolution of destructive inflammation with regeneration via elements (such as IL-4) that can attract and instruct cells (such as Tregs, M2, and MSCs) toward constructive phenotypes and elements (such as cystatin C) that mediate specific anabolic functions (Fig.). Therefore, the elucidation of such specific elements that serve as a bridge between inflammation resolution and tissue repair can provide interesting opportunities for regenerative medicine approaches in the oral cavity.
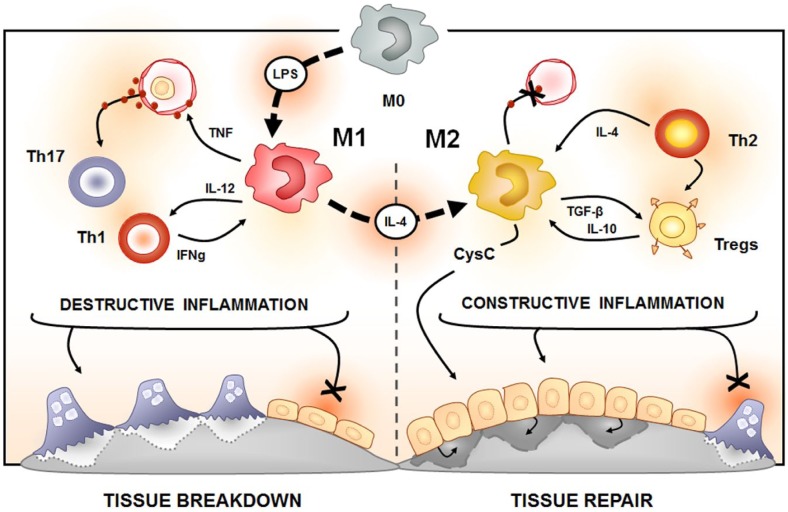
Figure.
Schematic representation of the potential role of macrophages as a bridge between inflammation resolution and tissue repair. In the periodontal environment, the presence of periodontopathogens and its products (such as lipopolysaccharide) is assumed to drive an initial polarization of M0 macrophages toward the M1 phenotype. M1 macrophages are a characteristic source of proinflammatory cytokines, such as tumor necrosis factor (TNF), which present a key role in the development of inflammatory immune reaction in periodontal tissues. The subsequent migration of certain Th subsets, such as Th1 and Th17 cells, can independently boost the inflammatory process. Th1 cells are described to have an interesting interplay with the M1 subset, since M1 cells can produce interleukin (IL)–12, enhancing Th1 polarization, and Th1 cells are the prototypic source of interferon (IFN) γ, which contribute to M1 phenotype acquisition. Taken together, such cellular elements contribute to a sustained and exacerbated host response, resulting in a local milieu characterized by increased proteolytic and osteoclastogenic pathways, linking the tissue breakdown outcome as a result of the “destructive inflammation.” On the other hand, the conversion of M1 macrophages toward a M2 phenotype, which can be mediated by IL-4, can result in a switch of the overall environment. M2 macrophages can produce anabolic and anti-inflammatory factors such as transforming growth factor (TGF) β and IL-10; coincidentally, the same cytokines are characteristically produced by Tregs. The production of such cytokines can boost both M2 and Treg activity and consequently counteract the “destructive inflammation.” Th2 cells can also contribute to the local immunoregulation by mediating Treg infiltration via the IL-4/CCL22/CCR4 axis. Interestingly, the putative cooperative activity of Th2, Treg, and M2 cells would be responsible for shutting down the chronic inflammatory osteolytic stimuli and also for triggering tissue repair via elements such as CysC, characterizing a constructive inflammation environment.
Author Contributions
G.P. Garlet, W.V. Giannobile, contributed to conception and design, drafted and critically revised the manuscript. Both authors gave final approval and agree to be accountable for all aspects of the work.
Footnotes
G.P.G. was supported in part by the Sao Paulo Research Foundation (FAPESP) 2015/24637-3 and the National Council for Scientific and Technological Development (CNPq). W.V.G. was supported in part by National Institutes of Health/National Institute of Dental and Craniofacial Research U24 DE026915.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
ORCID iDs: G.P. Garlet  https://orcid.org/0000-0002-5071-8382
https://orcid.org/0000-0002-5071-8382
W.V. Giannobile  https://orcid.org/0000-0002-7102-9746
https://orcid.org/0000-0002-7102-9746
References
- Araujo-Pires AC, Vieira AE, Francisconi CF, Biguetti CC, Glowacki A, Yoshizawa S, Campanelli AP, Trombone AP, Sfeir CS, Little SR, et al. 2015. IL-4/CCL22/CCR4 axis controls regulatory T-cell migration that suppresses inflammatory bone loss in murine experimental periodontitis. J Bone Miner Res. 30(3):412–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chazaud B. 2014. Macrophages: supportive cells for tissue repair and regeneration. Immunobiology. 219(3):172–178. [DOI] [PubMed] [Google Scholar]
- Dutzan N, Konkel JE, Greenwell-Wild T, Moutsopoulos NM. 2016. Characterization of the human immune cell network at the gingival barrier. Mucosal Immunol. 9(5):1163–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garlet GP. 2010. Destructive and protective roles of cytokines in periodontitis: a re-appraisal from host defense and tissue destruction viewpoints. J Dent Res. 89(12):1349–1363. [DOI] [PubMed] [Google Scholar]
- Haribhai D, Ziegelbauer J, Jia S, Upchurch K, Yan K, Schmitt EG, Salzman NH, Simpson P, Hessner MJ, Chatila TA, et al. 2016. Alternatively activated macrophages boost induced regulatory T and Th17 cell responses during immunotherapy for colitis. J Immunol. 196(8):3305–3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaigler D, Avila-Ortiz G, Travan S, Taut AD, Padial-Molina M, Rudek I, Wang F, Lanis A, Giannobile WV. 2015. Bone engineering of maxillary sinus bone deficiencies using enriched CD90+ stem cell therapy: a randomized clinical trial. J Bone Miner Res. 30(7):1206–1216. [DOI] [PubMed] [Google Scholar]
- Kaigler D, Pagni G, Park CH, Braun T, Holman LA, Yi E, Tarle SA, Bartel RL, Giannobile WV. 2013. Stem cell therapy for craniofacial bone regeneration: a randomized, controlled, and feasibility trial. Cell Transplant. 22(5):779–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani A, Biswas SK, Galdiero MR, Sica A, Locati M. 2013. Macrophage plasticity and polarization in tissue repair and remodeling. J Pathol. 229(2):176–185. [DOI] [PubMed] [Google Scholar]
- Serhan CN. 2014. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 510(7503):92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapouri-Moghaddam A, Mohammadian S, Vazini H, Taghadosi M, Esmaeili SA, Mardani F, Seifi B, Mohammadi A, Afshari JT, Sahebkar A. 2018. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol. 233(9):6425–6440. [DOI] [PubMed] [Google Scholar]
- Viniegra A, Goldberg H, Çil Ç, Fine N, Sheikh Z, Galli M, Freire M, Wang Y, Van Dyke TE, Glogauer M, Sima C. 2018. Resolving macrophages counter osteolysis by anabolic actions on bone cells. J Dent Res. 97(10):1160–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weirather J, Hofmann UD, Beyersdorf N, Ramos GC, Vogel B, Frey A, Ertl G, Kerkau T, Frantz S. 2014. Foxp3+ CD4+ T cells improve healing after myocardial infarction by modulating monocyte/macrophage differentiation. Circ Res. 115(1):55–67. [DOI] [PubMed] [Google Scholar]
- Wynn TA, Vannella KM. 2016. Macrophages in tissue repair, regeneration, and fibrosis. Immunity. 44(3):450–462. [DOI] [PMC free article] [PubMed] [Google Scholar]