Abstract
Background
Despite many publications regarding the role of faecal calprotectin (FC) in inflammatory bowel disease (IBD), clear recommendations for its use in clinical practice are currently lacking in the literature.
Aim
The aim of this article is to provide practical guidance for clinicians for the use of FC in the detection and management of patients with IBD.
Methods
All relevant publications were analysed and practical statements were proposed based on a Delphi consensus approach.
Results
Different commercial assays have been developed but international standardisation is lacking. FC can help in the diagnosis process of IBD. In IBD, FC can predict response to therapy, detect subclinical inflammation and help to drive treatment decisions to achieve better endoscopic and clinical outcomes. After Crohn’s surgery FC can identify patients with early endoscopic recurrence.
Conclusion
Although major therapeutic changes should not be based on FC alone, FC is a valuable tool to optimise the care for IBD patients.
Keywords: Faecal calprotectin, noninvasive biomarker, mucosal healing, subclinical inflammation, tight monitoring, Crohn’s disease, ulcerative colitis
Introduction
Overall the sensitivity and specificity of classical biochemical parameters of inflammation is low in inflammatory bowel disease (IBD). Emerging evidence shows that faecal markers can be used to detect and monitor intestinal inflammation selectively. Despite this growing body of literature, recommendations for the use of faecal calprotectin (FC) are currently lacking. Current guidelines on diagnosis and treatment of IBD mention FC as useful makers but do not include values or practical recommendations in the statements.1,2 The Belgian IBD Research and Development (BIRD) group performed a literature review on the potential use of FC in several situations in IBD. This manuscript can serve as practical guidance for clinicians but it is not a comprehensive or systematic review of the literature.
Methodology
We searched for relevant publications in PubMed/MEDLINE, EMBASE and the Cochrane Central Register of Controlled Trials from their inception until October 2017. Relevant articles (in English) were critically reviewed and discussed among the authors of this manuscript. Priority was given to randomised controlled trials and meta-analyses published in the last five years. Relevant abstracts from major meetings were also considered.
The evidence-based recommendations for the use of FC in clinical practice were drawn up through an electronic Delphi process, which is a group facilitation technique including an iterative multistage process to transform opinion into group consensus.3
A first draft of recommendations and statements was submitted to an expert panel of gastroenterologists and gastro-paediatricians within the BIRD. Thirty-two gastroenterologists (66%) answered the survey (47% from academic centres, 69% with ≥ 50% of medical activity in IBD, 91% involved in clinical or basic IBD research). No paediatrician participated. Final decisions on the statements were made if 80% agreement was achieved among the experts to come up with practical recommendations. A second round was necessary to validate two statements. The concordance rate within the expert panel is mentioned in brackets for each statement.
Results
Faecal sampling in practice
Stool sampling and dosage in clinical practice
– Use the morning sample or the first sample of the day. (91%)
– Avoid highly liquid or too solid stools. (84%)
– The samples can be stored up to 72 hours at room temperature. (94%)
– Owing to variability among tests, it is recommended to use the same test in the follow-up of a single patient. (97%)
It has been recommended to collect the first stool sample of the day as this is the most concentrated.4 The samples can be stored for 72 hours at room temperature and up to seven days at a temperature of 2℃–8℃.4 Approximately 50 mg to 100 mg is required. After extraction, samples can be kept permanently when frozen.
Technical and methodological aspects of FC measurement
Enzyme-linked immunosorbent assay (ELISA) tests using both monoclonal and polyclonal antibody reagents remained the reference technique till 2011.5 Recently, point-of-care tests have been developed allowing the immediate management of the stool sample.6 A good agreement with the ELISA technique was demonstrated mainly in cases of FC values under 500 µg/ml (kappa statistic of 86%, sensitivity of 96.2%, specificity of 90.1%).7 Later, different methods were developed for automated FC measurement.8 The characteristics of the different assays are illustrated in Table 1. More recently, smartphone applications have been developed enabling monitoring of FC levels at home.9 A recent prospective Dutch study comparing the home-testing IBDoc with conventional techniques demonstrated a good correlation with the ELISA (Spearman rank correlation coefficient = 0.85) and Quantum Blue testing (Spearman rank correlation coefficient = 0.94), particularly in cases of FC values under 500 µg/ml.9 The FC follow-up of a specific patient requires a similar FC technique measurement for the consecutive tests because results from different methods are not interchangeable. This highlights the lack of international standardisation for FC measurement.10
Table 1.
Faecal calprotectin assay characteristics.
| Manufacturer | Antibody |
Measuring principle | Proposed cut-off (µg/g) | Measuring range (µg/g) | Number of tests per kit | |
|---|---|---|---|---|---|---|
| Capture | Detection | |||||
| CALPRO Calprolab ELISA | monoclonal | NR | ELISA | 50 | 25–2500 | 96 |
| Eurospital Calprest ELISA | polyclonal | polyclonal | ELISA | <70: N >100:P | 15,6–500 62,5–2000 (dil) | 96 |
| Eurospital Calfast | monoclonal and polyclonal | Quantitative immunochromatography (automated reading) | <70: N >100:P | 50–300 | 20 | |
| Immundiagnostik PhiCal ELISA | monoclonal | NR | ELISA | 50 | 5,3–840 | 96 |
| Ridascreen ELISA | Monoclonal | Monoclonal | ELISA | 50 | 19,5–800 (1:5 redilution if >800) | 96 |
| Biotec CerTest Calprotectin | Monoclonal, mouse | Monoclonal, mouse | Semi-quantitative immunochromatography (visual reading) | 50 | 50–200 | NR |
| EliA Calprotectin 2 | Monoclonal, native | Monoclonal, mouse | FEIA | 50 | 3.8–6000 | 64 |
| Diasorin Calprotectin | Monoclonal, recombinant | Monoclonal, recombinant | CLIA | 50 | 5.0–8000.a | 100 |
| Inova Quanta Flash® | Polyclonal, native | Monoclonal, native | CLIA | 50 | 16.1–3500 | 100 |
| Bühlmann Quantum Blue | monoclonal | monoclonal | Quantitative immunochromatography (automated reading) | 50 | 30–300 100–1800 | 25 |
| Bühlmann ELISA | monoclonal | monoclonaal | ELISA | 50 | 10–600 30–1800 | 192 |
| Bühlmann fCAL Turbo | NA | Polyclonal avian | PETIA | 50 | 20.0–8000a | 200 |
| Euroimmun Calprotectin | Monoclonal, native | Monoclonal, native | ELISA | 50 | 6.5–2100 | 24 |
| Orgentec Calprotectin | Polyclonalb | Monoclonalb | ELISA | 50 | 5.2–1000.0 | 24 |
after 1:10 (Diasorin) and 1:4 (Bühlmann) dilution, concentrations up to 8000.0 µg/g faecal calprotectin can be obtained
Origin not reported
Abbreviations: FEIA: Fluoro Enzyme Immuno Assay; CLIA: Chemiluminescence Immunoassay, PETIA: Particle Enhanced Turbidimetric Immunoassay; ELISA: Enzyme Linked Immunosorbent assay; NA: Not Applicable; NR: Not Reported.
Use of FC in the detection of IBD
Use of FC as a diagnostic tool in clinical practice
– FC > 250 µg/g identifies patients who are most likely to have intestinal inflammation and justifies further endoscopic examination. (91%)
– FC between 100 and 250 µg/g could require a second measurement within three months. (97%)
– FC < 100 µg/g has a very high negative predictive value for IBD, justifying its use as a screening test to reduce the number of endoscopies and thereby the costs of health care management. This strategy delays the diagnosis in only a small proportion of patients. (97%)
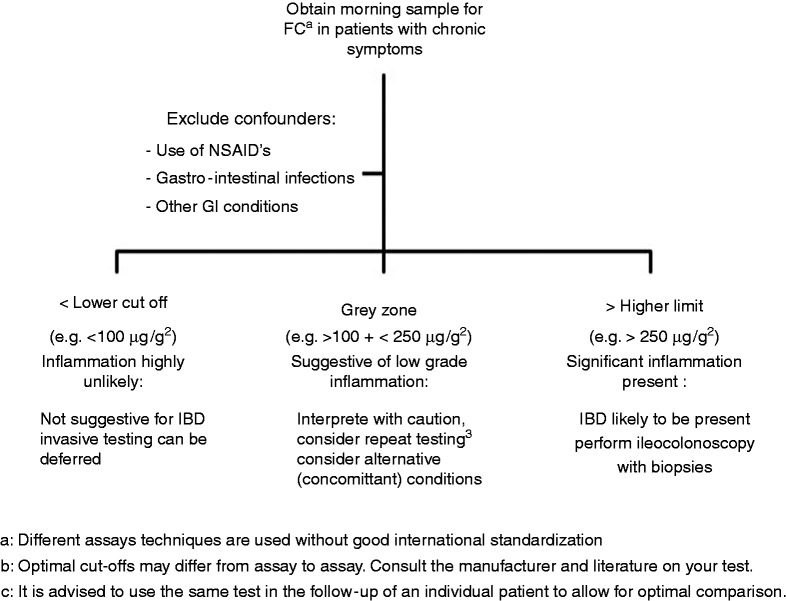
The value of FC as a diagnostic marker for IBD has been extensively studied. Several meta-analyses and systematic reviews have summarised the results of individual studies and report an overall sensitivity of 80%–98% and a specificity of 68%–96% for cut-offs ranging from 30 to 100 µg/g.11,12 The specificity of the test tends to be lower in children.11–13 A cut-off of 50 µg/g is recommended by most test suppliers.11,12 Values below 100 µg/g have a very high negative predictive value for IBD, and this can be particularly helpful to guide the need for further investigations in patients with nonspecific gastrointestinal symptoms and a low pre-test probability of IBD. The use of FC can reduce the number of negative colonoscopies by about two-thirds in this setting and results in a cost savings of $417 per patient.12 This strategy delayed diagnosis in only 7% of patients with actual IBD.14 It is not recommended to use FC in the acute setting of diarrhoea or in symptomatic patients with a high pre-test probability of IBD because of the small but still existent risk of false-negative test results. In high-risk patients (age above 40 years old and with symptoms suggestive of malignancies), an immediate endoscopic work-up remains the best and most cost-effective approach. A positive FC test should always be interpreted in the global clinical context as FC may be elevated in many other gastrointestinal conditions (infections, malignancies, nonsteroidal anti-inflammatory drugs (NSAIDs)-related mucosal injury).12 An algorithm illustrating the use of FC for the diagnosis of IBD is proposed in Figure 1.
Figure 1.
Algorithm for the use of FC in the detection of IBD.
aDifferent assay techniques are used without good international standardisation.
bOptimal cut-offs may differ from assay to assay; consult your test’s manufacturer and literature.
cIt is advised to use the same test in the follow-up of an individual patient to allow for optimal comparison.
FC: faecal calprotectin; GI: gastrointestinal; IBD: inflammatory bowel disease; NSAIDs: nonsteroidal anti-inflammatory drugs.
Use of FC for the clinical follow-up of symptomatic IBD patients
Clinical use of FC in symptomatic IBD patients
– FC should be measured at diagnosis of IBD or prior to major treatment changes for future comparison. (97%)
– In symptomatic IBD patients FC > 250 µg/g can discriminate an IBD flare from noninflammatory complications or underlying associated irritable bowel syndrome. (91%)
– FC decrease can predict clinical and endoscopic response to treatment. (94%)
– FC levels before initiating IBD therapy and serial FC measurements three and six months following treatment initiation are recommended to evaluate response to therapy and to predict long-term remission. (84%)
– In symptomatic IBD patients, endoscopy remains the gold standard to assess disease activity and major therapeutic changes are not recommended based on FC alone. (94%)
Objective assessment of the presence and degree of intestinal inflammation in symptomatic IBD patients is an essential part of disease management. Endoscopy remains the gold standard to detect and quantify mucosal inflammation in IBD patients, especially since the optimal therapeutic target to modify disease course should also be mucosal healing, which is associated with long-term improved outcomes.14,15 However, endoscopy is an expensive, invasive and time-consuming procedure that is, therefore, not ideal for repeated regular assessment of disease activity.
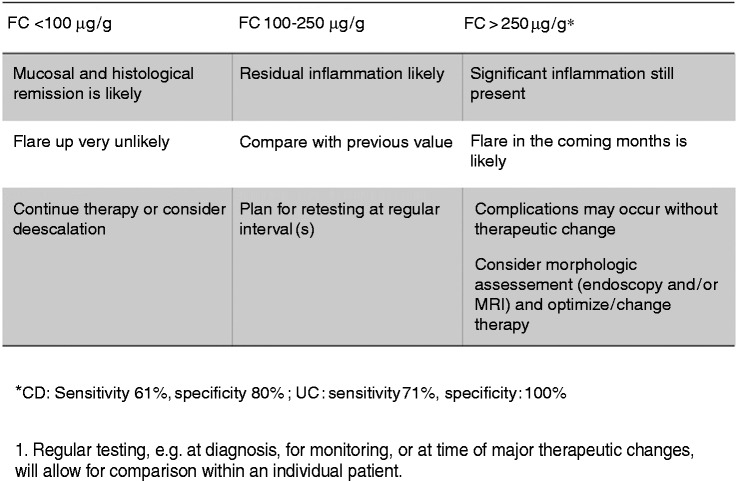
In current practice, FC can be used as an objective reliable marker of inflammation since it has been demonstrated to strongly correlate with endoscopic disease activity with high sensitivity and specificity (respectively 88% and 73% in Crohn’s disease (CD) and ulcerative colitis (UC)).14–17 FC correlates better with endoscopic activity than clinical activity17 and better than C-reactive protein (CRP) with endoscopic activity.17–20 However, FC appears to reflect disease activity better in UC compared to CD and ileocolonic/colonic CD is associated with significantly higher FC compared with isolated ileal CD.19 A practical algorithm is suggested in Figure 2. Optimal FC cut-off values for the detection of endoscopic active disease vary from 50 to 250 µg/g depending on the study and the test used as well as the type and location of the disease and intra-individual patient variability.4,21 To improve the reliability of FC testing, a baseline FC level during a period of known active inflammation should be obtained. In addition determining an FC value at the time of endoscopy allows the correlation of a patient’s individual value with endoscopic activity. Recent studies have shown that even in the absence of endoscopic signs of disease activity, levels of FC are predictive of long-term outcomes.22
Figure 2.
Algorithm for the use of FC in symptomatic IBD patients.
aUse prior FC value of a particular patient for comparison and for correlation if available with endoscopic disease activity. Using different tests from different manufacturers in one patient is not advisable because of the lack of international standardisation for FC measurement (Table 1).
FC: faecal calprotectin; IBD: inflammatory bowel disease.
In the follow-up of symptomatic patients, FC also plays an important role in the assessment of response to IBD treatment that is generally based on symptoms, which may not accurately reflect the underlying inflammatory process, while endoscopic evaluations are not frequently performed. Mucosal healing is increasingly advocated as a therapeutic target in IBD and can be noninvasively identified by normalisation of FC.23 A fast and significant fall in FC concentrations occurs in CD and UC patients treated with either high-dose corticosteroids,24 infliximab,25 adalimumab26 or vedolizumab27 and a normalisation or decrease in FC concentrations predicts clinical response and sustained remission at one year.28 An 80% decrease at week 2 of FC levels as compared to pre-treatment levels predicts endoscopic remission at week 10 after infliximab induction with a specificity of 67% and sensitivity of 54%.29 Looking at these data, a systematic endoscopy to assess mucosal healing after the initiation of new medications could be postponed when a normalisation or a decrease of 80% in FC concentrations is observed. Failure to reduce FC sufficiently may be a marker of nonresponse. In CALM,28 a recently published multicentre randomised controlled study, higher rates of mucosal healing (46% vs 30%, p = 0.01), steroid-free remission (60 vs 39%, p < 0.001) and biologic remission (30% vs 16%, p = 0.006) at one year were achieved when the escalation of the treatment strategy with adalimumab was based on symptoms and biomarkers (CRP and FC) compared to symptoms alone. A persistent raised FC (>250 µg/g) was the main driver of escalating treatment in the first group. FC can therefore be measured before initiating or changing IBD therapy, and serial measurements could be recommended in the weeks following treatment initiation to evaluate response to therapy.
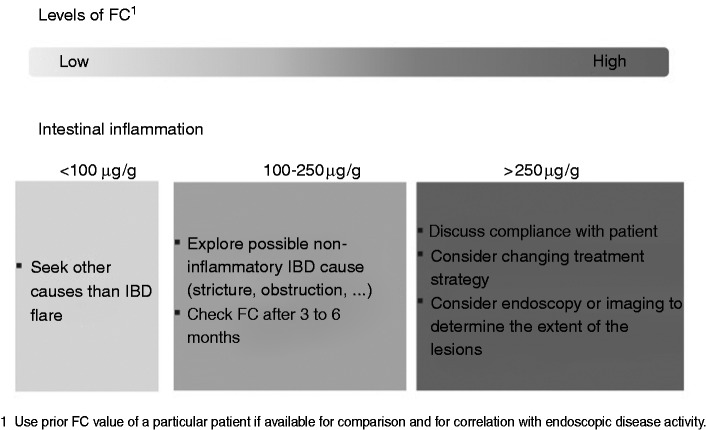
Role of FC in asymptomatic IBD patients (see Figure 3)
Clinical use of FC in asymptomatic IBD patients
– FC can identify patients in clinical remission with subclinical inflammation and high risk of short-term clinical relapse. (97%)
– FC measurements can be performed according to the risk profile of the patient every three to six months. (94%)
– In case of elevated FC: (97%)
– Exclude confounders (C. difficile infections, use of NSAIDs).
– Confirm disease activity by imaging (magnetic resonance imaging, endoscopy).
Figure 3.
Interpretation of the different cut-off levels of FC in asymptomatic IBD patients.
aRegular testing, e.g. at diagnosis, for monitoring, or at time of major therapeutic changes will allow for comparison within an individual patient.
CD: Crohn’s disease; FC: faecal calprotectin; IBD: inflammatory bowel disease; MRI: magnetic resonance imaging; UC: ulcerative colitis.
Faecal biomarkers have been identified as surrogate makers of endoscopic and histological healing in IBD patients. An FC cut-off value of less than 250 µg/g is associated with the absence of large ulcers in CD, according to the Simple Endoscopic Score for Crohn’s Disease, with a specificity and a sensitivity of 61% and 80%, respectively, and can discriminate a Mayo endoscopic sub-score of zero vs ≥1 in UC with a sensitivity and a specificity of 71% and 100%, respectively.19–21,30 Prospective studies have demonstrated that FC can discriminate patients with a higher risk of future relapse even if they are in clinical remission.31 In CD with either ileal or colonic involvement, the patients having an FC value ≤200 µg/g had a four-fold lower risk of clinical relapse within the following year compared to patients with an FC > 200 µg/g. In UC and colonic CD, a lower cut-off was able to identify the patients with a low risk of clinical relapse: FC ≤120 µg/g was associated with a six-fold lower risk of clinical relapse within the following year.32 In UC it was demonstrated that patients in deep remission on infliximab experienced an increase of FC three to four months before clinical relapse.27 In CD, FC progressively increased two to four months before clinical relapse in patients whose anti-tumour necrosis factor was withdrawn while in clinical remission. FC > 200 to 300 µg/g predicted the relapse with 83% specificity and 50% sensitivity.33,34 The implication in clinical practice is considerable because the patient might be offered an escalated treatment strategy at earlier stages of inflammation.
FC in the postoperative setting in IBD
Clinical use of FC in postoperative IBD patients
– Endoscopy six to 12 months after surgery remains the gold standard to assess postoperative recurrence in CD. (100%)
– FC <100 µg/g six to 12 months after surgery has a 90% negative predictive value for endoscopic recurrence (i2 or more) and is well correlated to the Rutgeerts score. (81%)
– FC cannot replace an endoscopy six months after surgery. FC measurements three months after surgery can identify patients with early endoscopic recurrence and can select the patients who need an early postoperative endoscopy. (85%)
– In UC pouchoscopy remains the gold standard to assess inflammatory activity after pouch surgery, but FC can be a useful marker to diagnose pouchitis noninvasively. (81%)
Despite an expanded therapeutic armamentarium, about one-half of patients with CD still require surgery in the first 10 years of the disease course.35 Post-surgical recurrence is inevitable in a vast majority of patients although aggressive postoperative approaches have been introduced, mainly in patients with a high-risk profile.36 Endoscopic evaluation six to 12 months after surgery is still the gold standard to detect the recurrence of early endoscopic lesions and to introduce an effective treatment strategy before the occurrence of severe lesions and tissue damage.37 A single FC measurement six to 12 months after surgery is predictive for clinical recurrence over time.38 A cut-off of 100 µg/g has a negative predictive value of 90% to identify patients without endoscopic recurrence and is well correlated to the Rutgeerts score.22,38,39 In a post-hoc analysis of the Post-Operative Crohn’s Disease Endoscopic Recurrence trial, the combined FC levels at six and 18 months postoperatively correlated with endoscopic recurrence by using a cut-off of 100 µg/g.39 Another approach is the serial measurement of FC in the postoperative setting to guide the clinician for the best timing to perform the endoscopy. Continued low FC levels indicate continuous remission.40
In patients with UC who underwent a proctocolectomy with ileo-anal pouch, increased FC has been correlated with pouchitis (using a cut-off of 56 µg/g) and increases two months before the occurrence of clinical symptoms and of endoscopic inflammation of the pouch.41
Conclusion
FC is a sensitive biomarker to detect histological inflammation in the gastrointestinal tract. Although nonspecific, the presence of high titres of FC in chronic symptomatic patients is indicative for (active) IBD and urges further examination including endoscopy with biopsies.
In known IBD patients, FC is increasingly used to predict response to therapy, and for monitoring disease activity and postoperative recurrence, thereby avoiding repeated endoscopic investigations. However, FC should be used not only as a marker of treatment efficacy, but it could also play an important role in the assessment of disease activity to alert the clinician of the need for further endoscopic or radiographic evaluation.
Acknowledgements
We acknowledge all BIRD members who kindly answered the survey: Dr T Billiet (UZ Leuven), Dr JL. Coenegrachts (Hasselt), Dr A. Colard (Cliniques Saint Joseph Liège), Dr Delen (Ziekenhuis Maas en Kempen, Maaseik), Dr N. de Suray (G.H.d.C. Gilly), Dr P. Dewint (AZ Maria Middelares), Dr O. Dewit (Cliniques Universitaires Saint-Luc), Dr Dutre (XX), Dr M. Etienne (CHC Hermalle), Dr M. Ferrante (UZ Leuven), Dr G. Lambrechts (AZ Damiaan Oostende), Dr C. Liefferinckx (ULB Erasme), Dr E. Louis (CHU Liège), Dr E. Macken (UZ Antwerpen), Dr F. Mana (UZ Brussels), Dr F. Mokkadem (Clinique Libramont), Dr T. Moreels (Cliniques Universitaires Saint-Luc), Dr Ouziel (CHU de Charleroi), Dr H. Peeters (AZ Sint Lucas Gent), Dr Ph. Potvin (Sint-Jozefkliniek Bornem), Dr Ph. Van Hootegem (AZ Sint Lucas Brugge), Dr Van De Mierop (GZA Ziekenhuizen, Wilrijk), Dr H. Vafa (ULB Erasme), Dr C. Vankemseke (CHU Liège), Dr S. Vermeire (UZ Leuven), Dr B. Verstockt (UZ Leuven) and Dr B. Willandt (AZ Sint-Jan Brugge).
The authors thank Saskia Appelmans, chief operating officer of the BIRD, for her excellent support in driving this project.
Declaration of conflicting interests
Catherine Reenaers has received speakers and consultancy fees from Abbvie, Cellgen, Ferring, Janssens, MSD, Mundipharma, Pfizer, and Takeda. Peter Bossuyt is part of the advisory board for Mundipharma, Dr Falk Benelux, MSD, Hospira, Takeda, Pfizer, Janssens, and Roche; has received lecture fees from Abbvie, Takeda, and Vifor Pharma; and has received an educational grant from Abbvie. Pieter Hindryckx has received consulting fees from Abbvie and Takeda; and speakers fees from Ferring, Falk Pharma, Vifor Pharma, Tillotts Pharma, Chiesi, Takeda and Abbvie. Hilde Vanpoucke has nothing to declare. Anneline Cremer has received a grant from the Fonds Erasme for medical research. Filip Baert’s department has received research grants from Abbvie, Chiesi, Ipsen, MSD, and Roche. He has received speakers and consultancy fees from Abbvie, Falk, Ferring, Janssen, Mundipharma, MSD, Pfizer, Takeda, and Vifor.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. The study was performed according the the recommendations of the ethical committee.
References
- 1.Magro F, Gionchetti P, Eliakim R, et al. Third European Evidence-Based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 1: Definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and ileo-anal pouch disorders. J Crohns Colitis 2017; 11: 649–670. [DOI] [PubMed] [Google Scholar]
- 2.Gomollón F, Dignass A, Annese V, et al. 3rd European Evidence-Based Consensus on the Diagnosis and Management of Crohn’s Disease 2016: Part 1: Diagnosis and medical management. J Crohns Colitis 2017; 11: 3–25. [DOI] [PubMed] [Google Scholar]
- 3.Birko S, Dove ES, Özdemir V. Evaluation of nine consensus indices in Delphi foresight research and their dependency on Delphi survey characteristics: A simulation study and debate on Delphi design and interpretation. PLoS One 2015; 10: e0135162–e0135162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lasson A, Stotzer PO, Öhman L, et al. The intra-individual variability of faecal calprotectin: A prospective study in patients with active ulcerative colitis. J Crohns Colitis 2015; 9: 26–32. [DOI] [PubMed] [Google Scholar]
- 5.Røseth AG, Fagerhol MK, Aadland E, et al. Assessment of the neutrophil dominating protein calprotectin in feces. A methodologic study. Scand J Gastroenterol 1992; 27: 793–798. [DOI] [PubMed] [Google Scholar]
- 6.Coorevits L, Baert FJ, Vanpoucke HJ. Faecal calprotectin: Comparative study of the Quantum Blue rapid test and an established ELISA method. Clin Chem Lab Med 2013; 51: 825–831. [DOI] [PubMed] [Google Scholar]
- 7.Elkjaer M, Burisch J, Voxen Hansen V, et al. A new rapid home test for faecal calprotectin in ulcerative colitis. Aliment Pharmacol Ther 2010; 31: 323–330. [DOI] [PubMed] [Google Scholar]
- 8.De Sloovere MMW, De Smet D, Baert FJ, et al. Analytical and diagnostic performance of two automated fecal calprotectin immunoassays for detection of inflammatory bowel disease. Clin Chem Lab Med 2017; 55: 1435–1446. [DOI] [PubMed] [Google Scholar]
- 9.Heida A, Knol M, Kobold AM, et al. Agreement between home-based measurement of stool calprotectin and ELISA results for monitoring inflammatory bowel disease activity. Clin Gastroenterol Hepatol 2017; 15: 1742–1749. [DOI] [PubMed] [Google Scholar]
- 10.Amcoff K, Stridsberg M, Lampinen M, et al. Clinical implications of assay specific differences in f-calprotectin when monitoring inflammatory bowel disease activity over time. Scand J Gastroenterol 2017; 52: 344–350. [DOI] [PubMed] [Google Scholar]
- 11.Menees SB, Powell C, Kurlander J, et al. A meta-analysis of the utility of C-reactive protein, erythrocyte sedimentation rate, fecal calprotectin, and fecal lactoferrin to exclude inflammatory bowel disease in adults with IBS. Am J Gastroenterol 2015; 110: 444–454. [DOI] [PubMed] [Google Scholar]
- 12.van Rheenen PF, Van de Vijver E, Fidler V. Faecal calprotectin for screening of patients with suspected inflammatory bowel disease: Diagnostic meta-analysis. BMJ 2010; 341: c3369–c3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Degraeuwe PL, Beld MP, Ashorn M, et al. Faecal calprotectin in suspected paediatric inflammatory bowel disease. J Pediatr Gastroenterol Nutr 2015; 60: 339–346. [DOI] [PubMed] [Google Scholar]
- 14.D’Haens G, Ferrante M, Vermeire S, et al. Fecal calprotectin is a surrogate marker for endoscopic lesions in inflammatory bowel disease. Inflamm Bowel Dis 2012; 18: 2218–2224. [DOI] [PubMed] [Google Scholar]
- 15.Peyrin-Biroulet L, Sandborn W, Sands BE, et al. Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE): Determining therapeutic goals for treat-to-target. Am J Gastroenterol 2015; 110: 1324–1338. [DOI] [PubMed] [Google Scholar]
- 16.Sipponen T, Savilahti E, Kolho KL, et al. Crohn’s disease activity assessed by fecal calprotectin and lactoferrin: Correlation with Crohn’s disease activity index and endoscopic findings. Inflamm Bowel Dis 2008; 14: 40–46. [DOI] [PubMed] [Google Scholar]
- 17.Mosli MH, Zou G, Garg SK, et al. C-reactive protein, fecal calprotectin, and stool lactoferrin for detection of endoscopic activity in symptomatic inflammatory bowel disease patients: A systematic review and meta-analysis. Am J Gastroenterol 2015; 110: 802–819; quiz 820. [DOI] [PubMed] [Google Scholar]
- 18.Hoekman DR, Diederen K, Koot BG, et al. Relationship of clinical symptoms with biomarkers of inflammation in pediatric inflammatory bowel disease. Eur J Pediatr 2016; 175: 1335–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schoepfer AM, Beglinger C, Straumann A, et al. Fecal calprotectin correlates more closely with the Simple Endoscopic Score for Crohn’s disease (SES-CD) than CRP, blood leukocytes, and the CDAI. Am J Gastroenterol 2010; 105: 162–169. [DOI] [PubMed] [Google Scholar]
- 20.Lewis JD. The utility of biomarkers in the diagnosis and therapy of inflammatory bowel disease. Gastroenterology 2011; 140: 1817–1826.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naismith GD, Smith LA, Barry SJ, et al. A prospective single-centre evaluation of the intra-individual variability of faecal calprotectin in quiescent Crohn’s disease. Aliment Pharmacol Ther 2013; 37: 613–621. [DOI] [PubMed] [Google Scholar]
- 22.Mooiweer E, Severs M, Schipper ME, et al. Low fecal calprotectin predicts sustained clinical remission in inflammatory bowel disease patients: A plea for deep remission. J Crohns Colitis 2015; 9: 50–55. [DOI] [PubMed] [Google Scholar]
- 23.Sipponen T, Björkesten CG, Färkkilä M, et al. Faecal calprotectin and lactoferrin are reliable surrogate markers of endoscopic response during Crohn’s disease treatment. Scand J Gastroenterol 2010; 45: 325–331. [DOI] [PubMed] [Google Scholar]
- 24.Theede K, Kiszka-Kanowitz M, Nielsen AM, et al. The correlation between fecal calprotectin, simple clinical colitis activity index and biochemical markers in ulcerative colitis during high-dose steroid treatment. Scand J Gastroenterol 2014; 49: 418–423. [DOI] [PubMed] [Google Scholar]
- 25.De Vos M, Dewit O, D’Haens G, et al. Fast and sharp decrease in calprotectin predicts remission by infliximab in anti-TNF naïve patients with ulcerative colitis. J Crohns Colitis 2012; 6: 557–562. [DOI] [PubMed] [Google Scholar]
- 26.Colombel JF, Panaccione R, Bossuyt P, et al. Effect of tight control management on Crohn’s disease (CALM): A multicentre, randomised, controlled phase 3 trial. Lancet 2018; 390: 2779–2789. [DOI] [PubMed] [Google Scholar]
- 27.Sandborn WJ, Feagan BG, Rutgeerts P, et al. Vedolizumab as induction and maintenance therapy for Crohn’s disease. N Engl J Med 2013; 369: 711–721. [DOI] [PubMed] [Google Scholar]
- 28.Molander P, af Björkesten CG, Mustonen H, et al. Fecal calprotectin concentration predicts outcome in inflammatory bowel disease after induction therapy with TNFα blocking agents. Inflamm Bowel Dis 2012; 18: 2011–2017. [DOI] [PubMed] [Google Scholar]
- 29.Turner D, Leach ST, Mack D, et al. Faecal calprotectin, lactoferrin, M2-pyruvate kinase and S100A12 in severe ulcerative colitis: A prospective multicentre comparison of predicting outcomes and monitoring response. Gut 2010; 59: 1207–1212. [DOI] [PubMed] [Google Scholar]
- 30.Sipponen T, Kärkkäinen P, Savilahti E, et al. Correlation of faecal calprotectin and lactoferrin with an endoscopic score for Crohn’s disease and histological findings. Aliment Pharmacol Ther 2008; 28: 1221–1229. [DOI] [PubMed] [Google Scholar]
- 31.Mao R, Xiao YL, Gao X, et al. Fecal calprotectin in predicting relapse of inflammatory bowel diseases: A meta-analysis of prospective studies. Inflamm Bowel Dis 2012; 18: 1894–1899. [DOI] [PubMed] [Google Scholar]
- 32.García-Sánchez V, Iglesias-Flores E, González R, et al. Does fecal calprotectin predict relapse in patients with Crohn’s disease and ulcerative colitis? J Crohns Colitis 2010; 4: 144–152. [DOI] [PubMed] [Google Scholar]
- 33.Louis E, Mary JY, Vernier-Massouille G, et al. Maintenance of remission among patients with Crohn’s disease on antimetabolite therapy after infliximab therapy is stopped. Gastroenterology 2012; 142: 63–70.e5. quiz 70.e31. [DOI] [PubMed] [Google Scholar]
- 34.Molander P, Färkkilä M, Ristimäki A, et al. Does fecal calprotectin predict short-term relapse after stopping TNFα-blocking agents in inflammatory bowel disease patients in deep remission? J Crohns Colitis 2015; 9: 33–40. [DOI] [PubMed] [Google Scholar]
- 35.Cosnes J, Gower-Rousseau C, Seksik P, et al. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology 2011; 140: 1785–1794. [DOI] [PubMed] [Google Scholar]
- 36.Regueiro M, Strong SA, Ferrari L, et al. Postoperative medical management of Crohn’s disease: Prevention and surveillance strategies. J Gastrointest Surg 2016; 20: 1415–1420. [DOI] [PubMed] [Google Scholar]
- 37.Rutgeerts P, Geboes K, Vantrappen G, et al. Predictability of the postoperative course of Crohn’s disease. Gastroenterology 1990; 99: 956–963. [DOI] [PubMed] [Google Scholar]
- 38.Yamamoto T, Shiraki M, Bamba T, et al. Faecal calprotectin and lactoferrin as markers for monitoring disease activity and predicting clinical recurrence in patients with Crohn’s disease after ileocolonic resection: A prospective pilot study. United European Gastroenterol J 2013; 1: 368–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wright EK, Kamm MA, De Cruz P, et al. Measurement of fecal calprotectin improves monitoring and detection of recurrence of Crohn’s disease after surgery. Gastroenterology 2015; 148: 938–947.e1. [DOI] [PubMed] [Google Scholar]
- 40.Yamamoto T, Shimoyama T, Umegae S, et al. Serial monitoring of faecal calprotectin for the assessment of endoscopic recurrence in asymptomatic patients after ileocolonic resection for Crohn’s disease: A long-term prospective study. Therap Adv Gastroenterol 2016; 9: 664–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yamamoto T, Shimoyama T, Bamba T, et al. Consecutive monitoring of fecal calprotectin and lactoferrin for the early diagnosis and prediction of pouchitis after restorative proctocolectomy for ulcerative colitis. Am J Gastroenterol 2015; 110: 881–887. [DOI] [PubMed] [Google Scholar]