Abstract
Background
Whether the etiology of potential small-bowel bleeding depends on the age and gender of the patient is not yet fully understood.
Methods
A total of 1953 patients who underwent video capsule endoscopy (VCE) to evaluate potential small-bowel bleeding and were registered in the Capsule Endoscopy Nationwide Database Registry from 2003 to 2014 were eligible for this study. VCE findings and the etiology of small-bowel bleeding were analyzed by age and gender.
Results
The diagnostic yield of VCE was 48.4% (95% CI: 46.2%–50.6%) and the diagnosis rate of etiology of potential small-bowel bleeding was 61.4% (95% CI: 59.2%–63.6%). The etiology of potential small-bowel bleeding depends on the age and gender of the patient. Crohn's disease and small-bowel diverticular diseases were more prevalent etiology of potential small-bowel bleeding in the young adults group (< 40 years) whereas angiodysplasia was revealed to be a most common etiology in elderly group (≥ 60 years), reaching statistical significance (p<0.00152) by Bonferroni correction.
Conclusions
The etiology of potential small-bowel bleeding depends on the age of the patient. Thus, an individualized lesion-specific diagnostic approach based on age might be needed for patients with potential small-bowel bleeding.
Keywords: Potential small-bowel bleeding, obscure gastrointestinal bleeding, video capsule endoscopy, age, gender
Introduction
It is known that management and outcomes of patients with gastrointestinal (GI) bleeding depend on disease etiology.1 The underlying etiology might not be evident after an initial evaluation using esophagogastroduodenoscopy (EGD) and colonoscopy in 5%–10% of patients with GI bleeding.2 Such patients are considered to have potential small-bowel bleeding.3–6 The etiology of potential small-bowel bleeding is thought to depend on the age of the patient.7 Patients <40 years are more likely to have inflammatory bowel disease or Meckel’s diverticulum whereas patients ≥40 years are more likely to have angiodysplasia, other vascular lesions, or ulcers secondary to anti-inflammatory agents as the cause of GI bleeding.7 Because of the difficulty in identifying the cause of potential small-bowel bleeding, however, no population-based or large-scale multicenter studies have been published on this subject.
Video capsule endoscopy (VCE) allows for noninvasive evaluation of the entire small bowel in 79–90% of patients, with a diagnostic yield of 38%–83% in patients with suspected small-bowel bleeding.7 Current guidelines recommend VCE as a first-line diagnostic modality for potential small-bowel bleeding. Analyzing data from patients who underwent VCE after negative EGD and colonoscopy can help elucidate the etiology of potential small-bowel bleeding.
The Korean Gut Image Study Group of the Korean Society of Gastrointestinal Endoscopy constructed a prospective web-based CAPsule Endoscopy Nationwide database regisTRY (CAPENTRY) in 2003.8 The objective of this study was to analyze etiologies of potential small-bowel bleeding according to patients’ age and gender.
Patients and methods
Patients
CAPENTRY prospectively registered 3298 sets of VCE data from March 2003 to December 2014. A total of 14 referral centers in Korea registered deidentified data using a web-based case report form, including each patient’s age, gender, indication, VCE manufacturer, bowel preparation quality, complete small-bowel visualization, incidence of VCE retention, VCE findings, and etiologic diagnoses of small bowel pathology. This study conformed to the ethical guidelines of the 1975 Declaration of Helsinki. On June 25, 2014, the institutional review board of each CAPENTRY-enrolled hospital approved this study.
The inclusion criteria were as follows: (1) the indication for VCE was potential small-bowel bleeding, and (2) patients who received EGD and colonoscopy with negative results prior to VCE. Exclusion criteria were as follows: (1) age <15 years, (2) incomplete small bowel visualization, (3) inadequate bowel preparation, (4) capsule retention, (5) capsule stasis in the stomach, (6) technical failure of VCE, or (7) incomplete data. When patients underwent repeated VCE, the first VCE finding was included in the analysis.
VCE procedure
All patients were examined using a PilCam SB® (SB1 and SB2, Given Imaging, Yogneam, Israel) or a MiroCam® (Intromedic, Seoul, Korea). Written informed consent was obtained from each patient before VCE. Board-certificated gastroenterologists reviewed and analyzed VCE findings.9 Bowel preparation status of the VCE was rated according to the following five categories: excellent (no liquid or bubbles), good (some clear liquid without limiting the examination), fair (some dark liquid and bubbles that limited the reliability of the examination), poor (significant fluid or debris present such that the examination was unreliable) and inadequate (incomplete small-bowel visualization requiring an additional examination).
VCE findings
Lesions of interest found by VCE were classified as bleeding or having a potential for bleeding. Lesions with potential for bleeding were classified using the following three categories: P2 (active bleeding or high bleeding potential such as angiodysplasia, Dieulafoy’s lesions, ulcers, tumors, or varices), P1 (uncertain bleeding potential such as red spots or erosions) and P0 (no bleeding potential such as nodules, visible submucosal veins, or diverticula without the presence of blood).13 Angiodysplasia (arteriovenous malformations, angioectasia, vascular lesions, vascular abnormalities and vascular malformations) was defined as the presence of abnormal, dilated, tortuous and usually small (<10 mm) blood vessels visualized within the small-bowel mucosa.11,12 Dieulafoy’s lesion was described as a punctulate lesion with pulsatile bleeding or a pulsatile red protrusion without surrounding venous dilation.12
A positive VCE finding was defined when P2 lesions detected by VCE could explain the potential small-bowel bleeding. Diagnostic yield of VCE was defined as the percentage of positive findings detected by VCE over the total number of VCEs performed for potential small-bowel bleeding.
Repeat-EGD and –colonoscopy
Repeat-EGD and -colonoscopy were performed, if necessary, according to previously established practice guidelines.2,3,7 Repeat-EGD and -colonoscopy were performed in cases of strongly suspicious upper or lower GI bleeding, inadequate quality of previous endoscopy, or possible bleeding focus detected by VCE or other subsequent small-bowel workups.
Etiologies of potential small-bowel bleeding
The diagnosis and management of patients with potential small-bowel bleeding followed practical guidelines.2–7 VCE is a noninvasive diagnostic modality that scans the entire bowel. It should be followed by an algorithmic approach to identify the etiology of potential small-bowel bleeding using balloon-assisted enteroscopy, computed tomography (CT) enterography, angiography, or exploratory surgery.6,7 After a workup for potential small-bowel bleeding, the final diagnosis of its etiology was categorized as angiodysplasia, other vascular diseases (including Dieulafoy’s lesion, hemangioma, varices, portal hypertensive enteropathy, ischemic enteritis, vasculitis, lymphangiectasia and pseudoaneurysm), Crohn’s disease, nonsteroidal anti-inflammatory drug (NSAID)-induced enteropathy, small bowel ulcers, other inflammatory diseases (including erosive enteropathy, erythematous/hemorrhagic enteropathy, eosinophilic enteritis, infectious enteritis, intestinal tuberculosis, radiation enteritis, intestinal Behçet’s disease and cryptogenic multifocal ulcerous stenosing enteritis), neoplastic diseases (including adenocarcinoma, metastatic cancer, gastrointestinal stromal tumor, lymphoma, submucosal tumor, small-bowel benign tumor, Peutz-Jegher syndrome and polyps), diverticular diseases (Meckel’s diverticulum and unspecified diverticulum), diseases outside the small bowel (esophagus, stomach, hepatobiliary tract and colorectum and anus), other uncommon diseases (including amyloidosis, congestive enteropathy, intussusception, parasite infestation, foreign body, postsurgical anastomosis stricture, malabsorption syndrome and protein-losing enteropathy) and obscure gastrointestinal bleeding (OGIB, patients not found to have a source of bleeding after the performance of EGD and colonoscopy, VCE, device-assisted enteroscopy, and radiographic testing).7 The diagnostic rate of potential small-bowel bleeding was defined as the percentage of patients having a final etiologic diagnosis of potential small-bowel bleeding over the total number of enrolled patients.
Statistical analysis
Differences between continuous variables were analyzed using unpaired Student t test while differences between categorical variables were analyzed using χ2 test and Fisher exact test as appropriate. A p value < 0.05 was considered statistically significant. Included patients were divided into three age groups using a decision tree analysis model with chi-square automatic interaction detector algorithm: elderly (≥60 years), middle-aged (40–59 years) and young adults (<40 years). Bonferroni correction was used for multiple comparisons.13–15 The relationship between age by decade and VCE findings and etiology of potential small-bowel bleeding was analyzed using linear-by-linear association. Logistic regression analysis was performed to identify independent risk factors for specific etiologies of potential small-bowel bleeding after adjusting for age group, gender, when VCE was performed (March 2003–August 2008 vs September 2008–December 2014) and bowel preparation (excellent/good vs fair/poor). For each variable, odds ratio (OR) and 95% confidence interval (CI) are reported. All statistical analyses were performed using SPSS software version 24.0 for Windows (SPSS Inc, Chicago, IL, USA).
Results
Baseline characteristics and VCE findings of enrolled patients
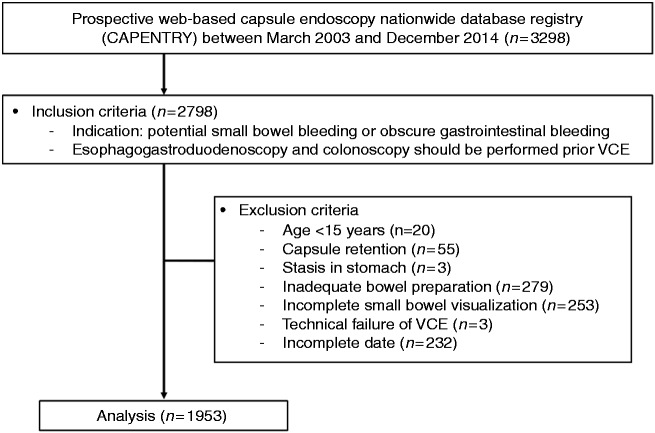
Among 3298 sets of VCE data registered in CAPENTRY between 2003 and 2014, 2798 patients underwent VCE after initial EGD and colonoscopy that failed to find the source of GI bleeding. A total of 845 patients were excluded. Ultimately, 1953 patients were eligible for this study (Figure 1).
Figure 1.
Flow diagram of this study.
VCE: video capsule endoscopy.
Clinical characteristics of enrolled patients are shown in Table 1. The mean age of enrolled patients was 56.1 ± 17.7 years. There were 1179 (60.4%) male patients and 774 (39.6%) female patients. Male patients were more common among the young adults than among the elderly (p < 0.001). There were 1501 (76.9%) patients with overt bleeding that was more frequent in the elderly and male (p < 0.001 for both). There were no significant differences in bowel preparation status, VCE manufacturer, or when VCE was preformed between different age groups.
Table 1.
Clinical characteristics of enrolled patients.
| Variables | Total (n = 1953), n (%) | Age group |
Gender |
||||||
|---|---|---|---|---|---|---|---|---|---|
| <40 years (n = 378), n (%) | 40–59 years (n = 659), n (%) | ≥60 years (n = 916), n (%) | P | Male (n = 1179), n (%) | Female (n = 774), n (%) | p | |||
| Age | <30 years | 183 (9.4) | 183 (48.4) | – | – | 123 (10.4) | 60 (7.8) | <0.001 | |
| 30–39 years | 195 (10.0) | 195 (51.6) | – | – | 134 (11.4) | 60 (7.8) | |||
| 40–49 years | 288 (14.7) | – | 288 (43.7) | – | 192 (16.3) | 97 (12.5) | |||
| 50–59 years | 371 (19.0) | – | 371 (56.3) | – | 229 (19.4) | 142 (18.3) | |||
| 60–69 years | 406 (20.8) | – | – | 406 (44.3) | 247 (20.9) | 159 (20.5) | |||
| 70–79 years | 361 (18.5) | – | – | 361 (39.4) | 188 (15.9) | 173 (22.4) | |||
| ≥80 years | 149 (7.6) | – | – | 149 (16.3) | 66 (5.6) | 83 (10.7) | |||
| Gender | Male | 1179 (60.4) | 257 (68.0) | 421 (63.9) | 501 (54.7) | <0.001 | – | – | – |
| Female | 774 (39.6) | 121 (32.0) | 238 (36.1) | 415 (45.3) | – | – | |||
| Clinical presentation | Overt bleeding | 1501 (76.9) | 260 (68.8) | 510 (77.4) | 731 (79.8) | <0.001 | 941 (79.8) | 560 (72.4) | <0.001 |
| Occult bleeding | 452 (23.1) | 118 (31.2) | 149 (22.6) | 185 (20.2) | 238 (20.2) | 214 (27.6) | |||
| Bowel preparation status | Excellent, good | 1223 (62.6) | 253 (66.9) | 420 (63.7) | 550 (60.0) | 0.054 | 730 (61.9) | 493 (63.7) | 0.444 |
| Fair, poor | 730 (37.4) | 125 (33.1) | 239 (36.3) | 366 (40.0) | 449 (38.1) | 281 (36.3) | |||
| When VCE was performed | Mar 2003–Aug 2008 | 924 (47.3) | 176 (46.6) | 302 (45.8) | 446 (48.7) | 0.497 | 556 (47.2) | 368 (47.5) | 0.889 |
| Sep 2008–Dec 2014 | 1029 (52.7) | 202 (53.4) | 357 (54.2) | 470 (51.3) | 623 (52.8) | 406 (52.5) | |||
| VCE Manufacturer | PilCam | 1583 (81.1) | 302 (79.9) | 535 (81.2) | 746 (81.4) | 0.856 | 958 (81.3) | 625 (80.7) | 0.813 |
| MiroCam | 370 (18.9) | 76 (20.1) | 124 (18.8) | 170 (18.6) | 221 (18.7) | 149 (19.3) | |||
VCE: video capsule endoscopy.
VCE finding for potential small-bowel bleeding according to age by decade
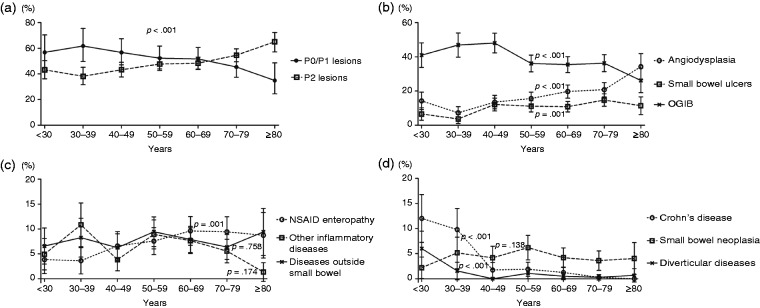
VCE was able to detect P2 lesions in 945 patients, rendering a diagnostic yield of 48.4% (95% CI: 46.2%–50.6%). The diagnostic yield of VCE in patients with potential small-bowel bleeding differed according to age by decade (Table 2). The detection rate of P2 lesions was increased significantly with age (p < 0.001, Figure 2(a)). Among P2 lesions, small-bowel ulcers were the most frequent etiology (n = 402, 42.5%), followed by angiodysplasia (n = 345, 36.5%) and active bleeding without identifiable etiology (n = 123, 13.0%) (Supplementary material 1). As age increased, the detection of angiodysplasia also increased (p = 0.033). However, the detection of ulcers decreased (p = 0.005) with increasing age (Supplementary material 2).
Table 2.
VCE findings and final etiologic diagnosis of potential small bowel bleeding according to age by decade.
| Total (n = 1953) | <30 years | 30–39 years | 40–49 years | 50–59 years | 60–69 years | 70–79 years | ≥80 years | ptrend | |
|---|---|---|---|---|---|---|---|---|---|
| (n = 183) | (n = 195) | (n = 288) | (n = 371) | (n = 406) | (n = 361) | (n = 149) | |||
| VCE findings | |||||||||
| P0/P1 lesions, n (%) | 1008 (51.6) | 104 (56.8) | 121 (62.1) | 163 (56.6) | 194 (52.3) | 210 (51.7) | 164 (45.4) | 52 (34.9) | <0.001 |
| P2 lesions, n (%) | 945 (48.4) | 79 (43.2) | 74 (37.9) | 125 (43.4) | 177 (47.7) | 196 (48.3) | 197 (54.6) | 97 (65.1) | |
| Final etiologic diagnosis | |||||||||
| Angiodysplasia, n (%) | 343 (17.6) | 26 (14.2) | 14 (7.2) | 39 (13.5) | 58 (15.6) | 80 (19.7) | 75 (20.8) | 51 (34.2) | <0.001 |
| Other vascular disease, n (%) | 42 (2.2) | 4 (2.2) | 5 (2.6) | 10 (3.5) | 5 (1.3) | 6 (1.5) | 8 (2.2) | 4 (2.7) | 0.621 |
| Crohn’s disease, n (%) | 59 (3.0) | 22 (12.0) | 19 (9.7) | 5 (1.7) | 7 (1.9) | 5 (1.2) | 1 (0.3) | 0 (0.0) | <0.001 |
| NSAID enteropathy, n (%) | 147 (7.5) | 7 (3.8) | 7 (3.6) | 19 (6.6) | 28 (7.5) | 39 (9.6) | 34 (9.4) | 13 (8.7) | 0.001 |
| Small bowel ulcers, n (%) | 209 (10.7) | 12 (6.6) | 7 (3.6) | 35 (12.2) | 41 (11.1) | 44 (10.8) | 53 (14.7) | 17 (11.4) | 0.001 |
| Other inflammatory disease, n (%) | 127 (6.5) | 9 (4.9) | 21 (10.8) | 11 (3.8) | 33 (8.9) | 31 (7.6) | 20 (5.5) | 2 (1.3) | 0.174 |
| Small bowel neoplasia, n (%) | 85 (4.4) | 4 (2.2) | 10 (5.1) | 12 (4.2) | 23 (6.2) | 17 (4.2) | 13 (3.6) | 6 (4.0) | 0.138 |
| Diverticular disease, n (%) | 22 (1.1) | 11 (6.0) | 3 (1.5) | 0 (0.0) | 4 (1.1) | 2 (0.5) | 1 (0.3) | 1 (0.7) | <0.001 |
| Disease outside small bowel, n (%) | 150 (7.7) | 12 (6.6) | 17 (8.7) | 17 (5.9) | 35 (9.4) | 32 (7.9) | 23 (6.4) | 14 (9.4) | 0.758 |
| Other disease, n (%) | 16 (0.8) | 1 (0.5) | 1 (0.5) | 1 (0.3) | 3 (0.8) | 6 (1.5) | 2 (0.6) | 2 (1.3) | 0.300 |
| Obscure gastrointestinal bleeding, n (%) | 753 (38.6) | 75 (41.0) | 91 (46.7) | 139 (48.3) | 134 (36.1) | 144 (35.5) | 131 (36.3) | 39 (26.2) | <0.001 |
NSAID: nonsteroidal anti-inflammatory drug; P2 lesion: VCE finding having active bleeding or high potential for bleeding; P1 lesion: VCE finding having uncertain bleeding potential; P0 lesion: VCE finding having no potential for bleeding; VCE: video capsule endoscopy.
Figure 2.
VCE findings and etiologic diagnosis in patients with potential small-bowel bleeding according to age by decade. (a) Trend of VCE findings in patients with potential small-bowel bleeding according to age by decade. (b) Trend of etiologic diagnosis with >10% prevalence (angiodysplasia, small bowel ulcers and GI bleeding of unknown origin) of potential small-bowel bleeding according to age by decade. (c) Trend of etiologic diagnosis with 5% to 10% prevalence (NSAID enteropathy, other inflammatory diseases and diseases outside the small bowel) of potential small-bowel bleeding according to age by decade. (d) Trend of etiologic diagnosis with <5% prevalence (Crohn’s disease, small-bowel neoplasia and diverticular diseases) of potential small-bowel bleeding according to age by decade. GI: gastrointestinal; NSAID: nonsteroidal anti-inflammatory drug; OGIB: obscure gastrointestinal bleeding; VCE: video capsule endoscopy.
The etiologic diagnosis of potential small-bowel bleeding according to age by decade
The diagnosis rate of etiology of potential small-bowel bleeding was 61.4% (95% CI: 59.2%–63.6%) after VCE and further diagnostic measures. VCE was able to detect P2 lesions in 945 patients, rendering a diagnostic yield of 48.4% (95% CI: 46.2%–50.6%), and the diagnosis rate of etiology of potential small-bowel bleeding was 61.4% (95% CI: 59.2%–63.6%) after VCE and further diagnostic measures.
Etiologic diagnoses of potential small-bowel bleeding are listed in Table 2 and Supplementary material 3. Among final etiologic diagnoses for potential small-bowel bleeding, angiodysplasia was the most prevalent (n = 343, 17.6%), followed by small-bowel ulcers (n = 209, 10.7%) and diseases outside the small bowel (n = 150, 7.7%). Despite complete small-bowel evaluation being performed using VCE and/or endoscopic and radiographic evaluation, we failed to find the source of bleeding in 753 (38.6%) patients with OGIB. The prevalence of etiologic diagnoses for potential small-bowel bleeding differed according to age by decade (Figure 2(b)–(d)). The prevalence of OGIB, Crohn’s disease and diverticular disease decreased significantly with increasing age (p < 0.001 for all), whereas that of angiodysplasia, small-bowel ulcers and NSAID enteropathy increased significantly with increasing age (p < 0.001, p = 0.001 and p = 0.001, respectively).
VCE findings and etiologic diagnosis of potential small-bowel bleeding by gender
There was no significant difference in VCE findings between male and female patients with potential small-bowel bleeding (Table 3). However, Crohn’s disease and lesions outside the small bowel were more prevalent in male patients (p < 0.002273 by Bonferroni correction).
Table 4.
VCE findings and final etiologic diagnosis of potential small bowel bleeding by age group.
| Young adults (<40 years, n = 378) |
Middle-aged (40–59 years, n = 659) |
Elderly (≥60 years, n = 916) |
||||
|---|---|---|---|---|---|---|
| n (%) | p | n (%) | p | n (%) | p | |
| VCE findings | ||||||
| P2 lesions | 153 (40.6) | <0.001a | 302 (45.8) | 0.106 | 490 (53.5) | <0.001a |
| P0/P1 lesions | 225 (59.4) | 357 (54.2) | 426 (46.5) | |||
| Final etiologic diagnosis | ||||||
| Angiodysplasia | 40 (10.6) | <0.001b | 97 (14.7) | 0.018 | 206 (22.5) | <0.001b |
| Other vascular disease | 9 (2.4) | 0.731 | 15 (2.3) | 0.785 | 18 (2.0) | 0.595 |
| Small bowel ulcers | 19 (5.0) | <0.001b | 76 (11.5) | 0.396 | 114 (12.4) | 0.019 |
| Crohn’s disease | 41 (10.9) | <0.001b | 12 (1.8) | 0.027 | 6 (0.7) | <0.001b |
| NSAID enteropathy | 14 (3.7) | 0.002 | 47 (7.1) | 0.637 | 86 (9.4) | 0.003 |
| Other inflammatory disease | 30 (8.0) | 0.208 | 44 (6.7) | 0.824 | 53 (5.8) | 0.227 |
| Small bowel neoplasia | 14 (3.7) | 0.491 | 35 (5.3) | 0.138 | 36 (3.9) | 0.390 |
| Small bowel diverticular disease | 14 (3.7) | <0.001b | 4 (0.6) | 0.121 | 4 (0.4) | 0.007 |
| Disease outside small bowel | 28 (7.4) | 0.994 | 53 (8.0) | 0.803 | 69 (7.5) | 0.818 |
| Other disease | 2 (0.5) | 0.486 | 4 (0.6) | 0.458 | 10 (1.1) | 0.209 |
| Obscure gastrointestinal bleeding | 166 (44.0) | 0.017 | 273 (41.4) | 0.063 | 314 (34.3) | <0.001b |
NSAID: nonsteroidal anti-inflammatory drug; VCE: video capsule endoscopy.
Bonferroni correction was used for multiple comparisons between age groups and etiologies, with p < 0.00833 being statistically significant.
Bonferroni correction was used for multiple comparisons between age groups and etiologies, with p < 0.00152 being statistically significant.
Table 3.
VCE findings and etiology of potential small bowel bleeding by gender.
| Male (n = 1179) | Female (n = 774) | p | ||
|---|---|---|---|---|
| VCE findings | ||||
| P2 lesion | 577 (48.9) | 368 (47.5) | 0.546 | |
| P1 lesion | 602 (51.1) | 406 (52.5) | ||
| Etiology | ||||
| Angiodysplasia | 202 (17.1) | 141 (18.2) | 0.538 | |
| Other vascular disease | 33 (2.8) | 9 (1.2) | 0.015 | |
| Small bowel ulcers | 120 (10.2) | 89 (11.5) | 0.356 | |
| Crohn’s disease | 48 (4.1) | 11 (1.4) | 0.001b | |
| NSAID enteropathy | 86 (7.3) | 61 (7.9) | 0.631 | |
| Inflammatory disease | 74 (6.3) | 53 (6.8) | 0.617 | |
| Neoplastic disease | 54 (4.6) | 31 (4.0) | 0.542 | |
| Diverticular disease | 18 (1.5) | 4 (0.5) | 0.039 | |
| Disease outside small bowel | 109 (9.2) | 41 (5.3) | 0.001b | |
| Other disease | 11 (0.9) | 5 (0.6) | 0.491 | |
| Obscure gastrointestinal bleeding | 424 (36.0) | 329 (42.5) | 0.004 |
NSAID: nonsteroidal anti-inflammatory drug; P2 lesion: VCE finding having active bleeding or high potential for bleeding; P1 lesion: VCE finding having uncertain bleeding potential; VCE: video capsule endoscopy.
A standardized adjusted residual score level >2 was considered significant.
Bonferroni correction was used for multiple comparisons between gender and etiologies, with p < 0.002273 being statistically significant.
The etiology of potential small-bowel bleeding depending on age group
Furthermore, included patients were divided into groups of elderly (≥ 60 years), middle-aged (40–59 years) and young adults (<40 years) (Table 4). Bonferroni correction was used for multiple comparisons between age groups and etiologies of potential small bowel bleeding. Crohn’s disease and small-bowel diverticular diseases were more prevalent whereas angiodysplasia and small-bowel ulcers were less prevalent in young adults (p < 0.00152 by Bonferroni correction). In the elderly, angiodysplasia was revealed to be a common etiology whereas Crohn’s disease and OGIB were found to be uncommon etiologies (p < 0.00152 by Bonferroni correction).
The decision tree analysis was applied to 1015 patients. Patients classified as having other vascular diseases, other inflammatory diseases, or other uncommon diseases were excluded in the decision tree analysis because they consisted of rare and heterogeneous disease entities (Supplementary material 4). The decision tree analysis revealed that age had a stronger influence (χ2 = 187, p < 0.001) than gender (χ2 = 14, p = 0.030).
Multivariate analysis using age group as a risk factor for various etiologies of potential small-bowel bleeding
Multivariate logistic regression analysis was performed to evaluate risk factors for individual etiologies of potential small-bowel bleeding (Table 5). The elderly group showed increased risk of angiodysplasia, NSAID enteropathy, and small-bowel ulcers (OR: 2.37, 95% CI: 1.64–3.41; OR: 2.54, 95% CI: 1.42–4.55; and OR: 2.65, 95% CI: 1.60–4.41, respectively). However, this group showed a decreased risk of Crohn’s disease, small-bowel diverticular diseases and OGIB (OR: 0.06; 95% CI: 0.02–0.13; OR: 0.11, 95% CI: 0.04–0.35; and OR: 0.68, 95% CI: 0.52–0.87, respectively). The middle-aged group showed increased risk of NSAID enteropathy and small-bowel ulcers (OR: 1.90, 95% CI: 1.03–3.50 and OR: 2.42, 95% CI: 1.44–4.09, respectively) with decreased risk of Crohn’s disease and small-bowel diverticular disease (OR: 0.15; 95% CI: 0.08–0.29 and OR: 0.15, 95% CI: 0.05–0.46, respectively). In addition, male gender had an increased risk of Crohn’s disease and diseases outside the small bowel (OR: 2.29, 95% CI: 1.64–4.62 and OR: 1.79, 95% CI: 1.23–2.60, respectively) but a decreased risk of OGIB (OR: 0.76; 95% CI: 0.63–0.92).
Table 5.
Multivariate analysis of risks of various etiologies of potential small bowel bleeding.
| Small Bowel diverticular disease |
Disease outside small bowel |
Obscure gastrointestinal disease |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n (%) | OR (95% CI)a | p | n (%) | OR (95% CI)a | p | n (%) | OR (95% CI)a | p | ||
| Age group | Young adults (<40 years) | 40 (10.6) | 1 | <0.001 | 41 (10.9) | 1 | <0.001 | 14 (3.7) | 1 | 0.005 |
| Middle-aged (40–59 years) | 97 (14.7) | 1.42 (0.96–2.10) | 0.081 | 12 (1.8) | 0.15 (0.08–0.29) | <0.001 | 47 (7.1) | 1.90 (1.03–3.50) | 0.041 | |
| Elderly (≥60 years) | 206 (22.5) | 2.37 (1.64–3.41) | <0.001 | 6 (0.7) | 0.06 (0.02–0.13) | <0.001 | 86 (9.4) | 2.54 (1.42–4.55) | 0.002 | |
| Gender | Female | 141 (18.2) | 1 | 0.896 | 11 (1.4) | 1 | 0.017 | 61 (7.9) | 1 | 0.729 |
| Male | 202 (17.1) | 0.98 (0.77–1.25) | 48 (4.1) | 2.29 (1.64–4.62) | 86 (7.3) | 0.94 (0.67–1.33) | ||||
| Clinical presentation | Occult bleeding | 62 (13.7) | 1 | 0.048 | 13 (2.9) | 1 | 0.466 | 20 (4.4) | 1 | 0.008 |
| Overt bleeding | 281 (18.7) | 1.36 (1.00–1.83) | 46 (3.1) | 1.27 (0.67–2.44) | 127 (8.5) | 1.93 (1.18–3.14) | ||||
| When VCE was performed | Mar 2003–Aug 2008 | 172 (18.6) | 1 | 0.362 | 33 (3.6) | 1 | 0.180 | 61 (6.6) | 1 | 0.088 |
| Sep 2008–Dec 2014 | 171 (16.7) | 0.90 (0.71–1.14) | 26 (2.5) | 0.69 (0.40–1.19) | 86 (8.4) | 1.35 (0.96–1.90) | ||||
| Bowel preparation status | Excellent, good | 214 (17.5) | 1 | 0.906 | 32 (2.6) | 1 | 0.060 | 92 (7.5) | 1 | 0.844 |
| Fair, poor | 129 (17.7) | 0.99 (0.77–1.26) | 27 (3.7) | 1.68 (0.98–2.89) | p | 55 (7.5) | 0.97 (0.68–1.37) | |||
| Small bowel ulcers |
Other inflammatory disease | Small bowel neoplasia | ||||||||
|
|
|
n (%) |
OR (95% CI)a |
p
|
n (%) |
OR (95% CI)a |
p
|
n (%) |
OR (95% CI)a |
p
|
| Age group | Young adults (<40 years) | 19 (5.0) | 1 | 0.001 | 30 (8.0) | 1 | 0.321 | 14 (3.7) | 1 | 0.342 |
| Middle-aged (40–59 years) | 76 (11.5) | 2.42 (1.44–4.09) | 0.001 | 44 (6.7) | 0.82 (0.50–1.32) | 0.409 | 35 (5.3) | 1.46 (0.77–2.75) | 0.246 | |
| Elderly (≥60 years) | 114 (12.4) | 2.65 (1.60–4.41) | <0.001 | 53 (5.8) | 0.70 (0.43–1.12) | 0.133 | 36 (3.9) | 1.07 (0.57–2.02) | 0.836 | |
| Gender | Female | 89 (11.5) | 1 | 0.544 | 53 (6.8) | 1 | 0.475 | 31 (4.0) | 1 | 0.595 |
| Male | 120 (10.2) | 0.91 (0.68–1.23) | 74 (6.3) | 0.87 (0.60–1.27) | 54 (4.6) | 1.13 (0.72–1.79) | ||||
| Clinical presentation | Occult bleeding | 36 (8.0) | 1 | 0.098 | 26 (5.8) | 1 | 0.380 | 18 (4.0) | 1 | 0.779 |
| Overt bleeding | 173 (11.5) | 1.38 (0.94–2.02) | 101 (6.7) | 1.22 (0.78–1.92) | 67 (4.5) | 1.08 (0.63–1.85) | ||||
| When VCE was performed | Mar 2003–Aug 2008 | 113 (12.2) | 1 | 0.086 | 63 (6.8) | 1 | 0.671 | 46 (5.0) | 1 | 0.203 |
| Sep 2008–Dec 2014 | 96 (9.3) | 0.78 (0.58–1.04) | 64 (6.2) | 0.93 (0.64–1.33) | 39 (3.8) | 0.75 (0.49–1.17) | ||||
| Bowel preparation status | Excellent, good | 155 (12.7) | 1 | <0.001 | 90 (7.4) | 1 | 0.064 | 55 (4.5) | 1 | 0.753 |
| Fair, poor | 54 (7.4) | 0.54 (0.39–0.75) | 37 (5.1) | 0.69 (0.46–1.02) | 30 (4.1) | 0.93 (0.59–1.47) | ||||
| Small bowel diverticular disease |
Disease outside small bowel | Obscure gastrointestinal bleeding | ||||||||
|
|
|
n (%) |
OR (95% CI)a |
p
|
n (%) |
OR (95% CI)a |
p
|
n (%) |
OR (95% CI)a |
p
|
| Age group | Young adults (<40 years) | 14 (3.7) | 1 | <0.001 | 28 (7.4) | 1 | 0.954 | 166 (44.0) | 1 | 0.001 |
| Middle-aged (40–59 years) | 4 (0.6) | 0.15 (0.05–0.46) | 0.001 | 53 (8.0) | 1.08 (0.67–1.74) | 0.759 | 273 (41.4) | 0.93 (0.72–1.21) | 0.584 | |
| Elderly (≥60 years) | 4 (0.4) | 0.11 (0.04–0.35) | <0.001 | 69 (7.5) | 1.06 (0.67–1.68) | 0.814 | 314 (34.3) | 0.68 (0.52–0.87) | 0.002 | |
| Gender | Female | 4 (0.5) | 1 | 0.129 | 41 (5.3) | 1 | 0.002 | 329 (42.5) | 1 | 0.005 |
| Male | 18 (1.5) | 2.35 (0.78–7.06) | 109 (9.2) | 1.79 (1.23–2.60) | 424 (36.0) | 0.76 (0.63–0.92) | ||||
| Clinical presentation | Occult bleeding | 3 (0.7) | 1 | 0.192 | 28 (6.2) | 1 | 0.225 | 236 (52.2) | 1 | <0.001 |
| Overt bleeding | 19 (1.3) | 2.28 (0.66–7.86) | 122 (8.1) | 1.31 (0.85–2.01) | 517 (34.4) | 0.51 (0.41–0.63) | ||||
| When VCE was performed | Mar 2003–Aug 2008 | 9 (1.0) | 1 | 0.489 | 56 (6.1) | 1 | 0.011 | 346 (37.4) | 1 | 0.655 |
| Sep 2008–Dec 2014 | 13 (1.3) | 1.36 (0.57–3.23) | 94 (9.1) | 1.56 (1.11–2.21) | 407 (39.6) | 1.04 (0.87–1.26) | ||||
| Bowel preparation status | Excellent, good | 12 (1.0) | 1 | 0.310 | 88 (7.2) | 1 | 0.389 | 455 (37.2) | 1 | 0.086 |
| Fair, poor | 10 (1.4) | 1.56 (0.66–3.67) | 62 (8.5) | 1.16 (0.83–1.64) | 298 (40.8) | 1.18 (0.98–1.43) | ||||
CI: confidence interval; NSAID: nonsteroidal anti-inflammatory drug; OR: odds ratio; VCE: video capsule endoscopy.
Adjusted for age group, gender, clinical presentation, when VCE was performed, and bowel preparation status.
Discussion
Age has been assumed to be one factor determining the type of small-bowel pathology detected.2,6,7 However, this has not been supported by any large-scale multicenter studies. As a proof of concept, our study confirmed that age was a determinant for the source of potential small-bowel bleeding. The diagnostic yield of VCE and the diagnosis rate of etiology of potential small-bowel bleeding increased with age. Angiodysplasia was the most common etiology of potential small-bowel bleeding. However, young adults were more likely to have Crohn’s disease. These findings might be translated into practice by prompting gastroenterologists to consider the selection of diagnostic modalities in patients with potential small-bowel bleeding.
To evaluate the etiology of potential small-bowel bleeding, a proper evaluation is essential. VCE has several advantages, including the ability to routinely examine the entire small bowel and localize GI bleeding with an excellent safety profile and high patient tolerability.4 In this study, visualization of the entire small bowel was achieved in 88.8% of cases, consistent with those (79%–90% of patients) reported by previous studies.7,16–19 In this study, we excluded patients with incomplete evaluations of the small bowel. The overall diagnostic yield of VCE for potential small-bowel bleeding was 48.4%, consistent with the diagnostic yield of 38%–83% reported in previous studies.7,17–19 The diagnostic yield of VCE could be influenced by multiple factors. Patients with overt bleeding, male gender, the elderly, and inpatient status have a higher likelihood of positive findings.20 In this study, positive VCE findings were associated with age by decade (OR: 1.14, 95% CI: 1.08–1.20) and overt bleeding (OR: 1.93, 95% CI: 1.55–2.41) (Supplementary material 5). In this study capsule retention, which is a potential complication, occurred in 55 (2.0%) patients, similar to previous reports showing an incidence of 1.5% in patients undergoing evaluations for potential small-bowel bleeding.7,21 These results indicate that the quality of VCE data in this study is reliable and comparable to data from previous studies.
The etiology of potential small-bowel bleeding is diverse. In many cases, the source of bleeding is not even within the small bowel. In this study, bleeding was caused by more than 50 diseases occurring throughout the GI tract. Angiodysplasia was the most common etiology of small-bowel bleeding. This has been well established in previous studies.7,16–19 Linear-by-linear association showed that young adults were more likely to have Crohn’s disease or Meckel’s diverticulum. Our decision tree model revealed that age was a strong determinant of etiology of potential small-bowel bleeding. Based on the results of the decision tree analysis, we divided patients into three age groups: the elderly (≥60 years), middle-aged (40–59 years), and young adults (<40 years). In young adults, Crohn’s disease was the most prevalent, followed by angiodysplasia and diseases outside the small bowel. In the middle-aged group, angiodysplasia was the most frequent, followed by small-bowel ulcers and diseases outside the small bowel. However, in female elderly, NSAID enteropathy was the third common etiology of potential small-bowel bleeding instead of disease outside the small bowel.
To the best of our knowledge, this is the largest multicenter study of patients with potential small-bowel bleeding who underwent VCE. As explained above, the diagnostic yield and quality of the VCE were acceptable. Nonetheless, this study has several limitations. First, our study was based on a VCE registry database. It was unclear which further evaluation was needed to make a final etiologic diagnosis. Furthermore, there are several inherent limitations of VCE, including a lack of therapeutic capability, inability to control its movement through the GI tract, and difficulty in localizing a lesion. Patients often require follow-up small-bowel examinations, including angiography, scintigraphy, CT/magnetic resonance (MR) enterography, and device-assisted enteroscopy. Second, patients at risk for obstruction generally undergo CT/MR enterography for the initial small-bowel evaluation because of the risk of capsule retention. Third, CAPENTRY included only deidentified information. Thus, recurrence or prognosis could not be evaluated. Fourth, this entire cohort was composed of ethnic Korean individuals. It is unclear whether results of this study can be applied to other populations with different sociodemographic characteristics.
In conclusion, this study is the largest multicenter study ever published to date that evaluates the etiology of potential small-bowel bleeding by age and gender. The diagnostic yield of VCE and the diagnosis rate of the etiology of potential small-bowel bleeding increased with increasing age. Angiodysplasia was the most prevalent etiology for small-bowel bleeding. However, Crohn’s disease should be considered as a significant etiology in young adults. Classifying patients with potential small-bowel bleeding into young adults, middle-aged, and elderly can be useful to categorize the etiology of potential small-bowel bleeding. This analysis provides insights into individualized lesion-specific diagnostic approaches for patients with potential small-bowel bleeding. Further population-based and longitudinal studies are needed.
Supplemental Material
Supplemental Material for The etiology of potential small-bowel bleeding depending on patient’s age and gender by Joo Hye Song, Sung Noh Hong, Dong Kyung Chang, Seong Ran Jeon, Jin-Oh Kim, Jinsu Kim, Bo-In Lee, Myung-Gyu Choi, Kyeong Ok Kim, Dong-Hoon Yang, Hyun Joo Song, Jae Hyuk Do, Yun Jeong Lim, Ki-Nam Shim, Soo Jung Park, Ji Hyun Kim, Jeong Seop Moon, Hyun Joo Jang and Hoon Jai Chun in United European Gastroenterology Journal
Acknowledgment
We would like to thank all of the participating patients and the Korean Gut Image Study Group.
Informed consent
Written informed consent was obtained from each patient before VCE.
Ethics approval
This study conformed to the ethical guidelines of the 1975 Declaration of Helsinki. On June 25, 2014, the institutional review board of each CAPENTRY-enrolled hospital approved this study.
Declaration of conflicting interests
None declared.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Pasha SF, Hara AK, Leighton JA. Diagnostic evaluation and management of obscure gastrointestinal bleeding: A changing paradigm. Gastroenterol Hepatol (N Y) 2009; 5: 839–850. [PMC free article] [PubMed] [Google Scholar]
- 2.Raju GS, Gerson L, Das A, et al. American Gastroenterological Association (AGA) Institute technical review on obscure gastrointestinal bleeding. Gastroenterology 2007; 133: 1697–1717. [DOI] [PubMed] [Google Scholar]
- 3.Shim KN, Moon JS, Chang DK, et al. Guideline for capsule endoscopy: Obscure gastrointestinal bleeding. Clin Endosc 2013; 1: 45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pennazio M, Spada C, Eliakim R, et al. Small-bowel capsule endoscopy and device-assisted enteroscopy for diagnosis and treatment of small-bowel disorders: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy 2015; 47: 352–76. [DOI] [PubMed] [Google Scholar]
- 5.Raju GS, Gerson L, Das A, et al. American Gastroenterological Association (AGA) Institute medical position statement on obscure gastrointestinal bleeding. Gastroenterology 2007; 133: 1694–1696. [DOI] [PubMed] [Google Scholar]
- 6.ASGE Standards of Practice Committee, Fisher L, Lee Krinsky M, et al. The role of endoscopy in the management of obscure GI bleeding. Gastrointest Endosc 2010; 72: 471–479. [DOI] [PubMed]
- 7.Gerson LB, Fidler JL, Cave DR, et al. ACG Clinical Guideline: Diagnosis and management of small bowel bleeding. Am J Gastroenterol 2015; 110: 1265–1287; quiz 1288. [DOI] [PubMed] [Google Scholar]
- 8.Min YW, Kim JS, Jeon SW, et al. Long-term outcome of capsule endoscopy in obscure gastrointestinal bleeding: A nationwide analysis. Endoscopy 2013; 46: 59–65. [DOI] [PubMed] [Google Scholar]
- 9.Lim YJ, Moon JS, et al. Gut Image Study Group. Korean Society of Gastrointestinal Endoscopy (KSGE) guidelines for credentialing and granting previleges [sic] for capsule endoscopy. Korean J Gastrointest Endosc 2008; 37: 393–402. [Google Scholar]
- 10.Saurin JC, Delvaux M, Gaudin JL, et al. Diagnostic value of endoscopic capsule in patients with obscure digestive bleeding: Blinded comparison with video push-enteroscopy. Endoscopy 2003; 35: 576–584. [DOI] [PubMed] [Google Scholar]
- 11.Sami SS, Al-Araji SA, Ragunath K. Review article: Gastrointestinal angiodysplasia—pathogenesis, diagnosis and management. Aliment Pharmacol Ther 2014; 39: 15–34. [DOI] [PubMed] [Google Scholar]
- 12.Yano T, Yamamoto H, Sunada K, et al. Endoscopic classification of vascular lesions of the small intestine (with videos). Gastrointest Endosc 2008; 67: 169–172. [DOI] [PubMed] [Google Scholar]
- 13.Beasley TM, Schumacker RE. Multiple regression approach to analyzing contingency tables: Post hoc and planned comparison procedures. J Exp Educ 1995; 64: 79–93. [Google Scholar]
- 14.García-Pérez M, Núñez-Antón V. Cellwise residual analysis in two-way contingency tables. Educ Psychol Meas 2003; 63: 825–839. [Google Scholar]
- 15.Monge-Rojas R, Smith-Castro V, Colón-Ramos U, et al. Psychosocial factors influencing the frequency of fast-food consumption among urban and rural Costa Rican adolescents. Nutrition 2013; 29: 1007–1012. [DOI] [PubMed] [Google Scholar]
- 16.Adler DG, Knipschield M, Gostout C. A prospective comparison of capsule endoscopy and push enteroscopy in patients with GI bleeding of obscure origin. Gastrointest Endosc 2004; 59: 492–498. [DOI] [PubMed] [Google Scholar]
- 17.Mylonaki M, Fritscher-Ravens A, Swain P. Wireless capsule endoscopy: A comparison with push enteroscopy in patients with gastroscopy and colonoscopy negative gastrointestinal bleeding. Gut 2003; 52: 1122–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hadithi M, Heine GD, Jacobs MA, et al. A prospective study comparing video capsule endoscopy with double-balloon enteroscopy in patients with obscure gastrointestinal bleeding. Am J Gastroenterol 2006; 101: 52–57. [DOI] [PubMed] [Google Scholar]
- 19.Triester SL, Leighton JA, Leontiadis GI, et al. A meta-analysis of the yield of capsule endoscopy compared to other diagnostic modalities in patients with obscure gastrointestinal bleeding. Am J Gastroenterol 2005; 100: 2407–2418. [DOI] [PubMed] [Google Scholar]
- 20.Lepileur L, Dray X, Antonietti M, et al. Factors associated with diagnosis of obscure gastrointestinal bleeding by video capsule enteroscopy. Clin Gastroenterol Hepatol 2012; 10: 1376–1380. [DOI] [PubMed] [Google Scholar]
- 21.Pennazio M. Capsule endoscopy: Where are we after 6 years of clinical use? Dig Liver Dis 2006; 38: 867–878. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for The etiology of potential small-bowel bleeding depending on patient’s age and gender by Joo Hye Song, Sung Noh Hong, Dong Kyung Chang, Seong Ran Jeon, Jin-Oh Kim, Jinsu Kim, Bo-In Lee, Myung-Gyu Choi, Kyeong Ok Kim, Dong-Hoon Yang, Hyun Joo Song, Jae Hyuk Do, Yun Jeong Lim, Ki-Nam Shim, Soo Jung Park, Ji Hyun Kim, Jeong Seop Moon, Hyun Joo Jang and Hoon Jai Chun in United European Gastroenterology Journal