Abstract
Background
Gastrointestinal infection is an important risk factor for developing irritable bowel syndrome (IBS). Our aim was to characterise post-infectious IBS (PI-IBS) compared to other IBS patients.
Methods
An internet survey of IBS patients using Rome III diagnostic questionnaire, Hospital Anxiety and Depression Scale (HADS) and Patient Health Questionnaire-12 Somatic Symptom (PHQ12-SS) scale score documenting the mode of onset was conducted.
Results
A total of 7811 participants (63.2% female), of whom 1004 (13.3%) met criteria for PI-IBS, were studied. Seventy per cent of PI-IBS patients described sudden onset, 35% onset while travelling, 49.6% vomiting, 49.9% fever and 20.3% bloody diarrhoea. Compared to other IBS individuals, PI-IBS was significantly associated with living in Northern Europe and North America, having a hysterectomy, not having an appendicectomy, higher PHQ12-SS score and having more than one toilet in the family home. PI-IBS patients had more frequent stools. At one year recovery rate in the PI-IBS and non-PI-IBS group was 19.7% and 22.2%, p = 0.15. Recovery rates were lower for females (20.7%) vs males (38.8%), those with somatisation (23.0%) vs those without (33.2%) and those living in North America or Northern Europe (21.1%) vs living elsewhere (33.9%) p ≤ 0.001.
Conclusion
PI-IBS accounts for around 13% of all IBS in this internet sample, with some distinctive features but a similar prognosis to the remainder.
Keywords: Irritable, infection, prospective, survey, internet
Key summary
Established knowledge on this subject
Bacterial gastroenteritis quadruples the risk of developing irritable bowel syndrome (IBS) but the proportion of all IBS that is post-infectious (PI) is unclear.
Risk factors include severity of initial illness, female gender and adverse psychological factors.
What determines prognosis is uncertain.
What are the significant and/or new findings of this study?
Thirteen per cent of 7811 IBS patients met criteria for PI-IBS.
PI-IBS was associated with childhood hygiene, somatisation and living in Northern Europe/America.
Prognosis in PI-IBS was not different from non-PI-IBS.
High somatisation, female gender and living in North America and Northern Europe were associated with lower recovery rates.
Introduction
Irritable bowel syndrome (IBS) developing de novo after an acute infection in an individual with normal bowel function gives an ideal opportunity to study the underlying mechanisms causing IBS. Earlier studies suggested that patients with post-infectious IBS (PI-IBS) would have a better prognosis and a lower frequency of psychological disorders compared to other IBS patients.1 However, prospective studies found neuroticism, hypochondriasis,2 depression3 and somatisation4 all increase the risk. A recent meta-analysis has summarised the extensive literature finding that the incidence of new IBS 12 months after infection was 10.1% (95% confidence interval (CI) 7.2–14.1). The incidence appears higher after parasitic or protozoan infections at 49% compared to 13.8% after bacterial gastroenteritis. Most studies agree that females and those with anxiety, depression or somatisation are at increased risk.5 Underlying possible mechanisms include ongoing increased permeability,6 abnormal serotonin metabolism,3 and ongoing chronic immune activation together with altered microbiota.7
We hypothesised based on previous studies (for review see Spiller and Garsed8) that PI-IBS would be most likely in those with severe gut inflammation. Many infections such as Campylobacter are less severe in childhood and lead to adaptive immunity.9,10 We hypothesised that a rural upbringing and reduced hygiene in the family home during childhood would reduce the risk of PI-IBS. We also wanted to test the previous findings that psychological vulnerability was associated with PI-IBS and how this might affect recovery rates.
Methods
This was an internet survey which allowed recruitment throughout the world and was funded by the United European Gastroenterology Federation, Gastro 2009. We created two identical websites (http://www.pi-ibs.eu and http://www.postinfectious-ibs.eu) which provided bowel symptom questionnaires in eight languages, namely English, Dutch, German, Belgian, Spanish (two versions: for Spain, for Mexico), Italian, Polish and Romanian.
Ethics approval was sought and received from the University of Nottingham Medical School Ethics Committee, Division of Therapeutics and Molecular Medicine, Nottingham, UK) (P/9/2008, as of 26 September 2008) conforming to the ethical guidelines of the 1975 Declaration of Helsinki. Where legally required, national ethics board permissions were requested and received. Recruitment took place from 5 December 2008 with the main recruitment taking place within the first three years though more continued to accrue until the website was closed in January 2015.
Inclusions and exclusions
Consecutive patients attending clinic were all invited to complete the survey online. Access to one of the websites (www.pi-ibs.eu) was granted for each patient by using a unique password provided by the investigators, who all were specialists in functional gastrointestinal (GI) disease; individuals without a password who found the website themselves without being directed there entered the web at www.postinfectious-ibs.eu. This allowed us to differentiate between patients with an investigator-confirmed diagnosis of IBS using Rome III criteria and the rest who responded yes to the question “Have you been diagnosed with IBS by a doctor?” The introductory information asked those with a history of major GI surgery, inflammatory bowel disease, colon cancer and taking drugs known to alter bowel function, especially opiates and anticholinergics, not to take part. Completing the questionnaire was accepted as giving informed consent.
Questionnaire details
Participants were asked to answer questions of demographics including age, gender, country (of birth and also of current residence), schooling, current occupation, previous surgery and psychiatric treatment. We enquired about childhood living conditions including whether they shared a bed with their siblings or whether they had more than one toilet or running hot water in the home. GI symptoms at the present time were documented with the Rome III IBS modular questionnaire (http://www.romecriteria.org/pdfs/AdultFunctGIQ.pdf), together with bowel frequency, days per week of urgency, bloating and their commonest stool form using the Bristol Stool Form Scale.11
Details of diagnosis of PI-IBS
Participants were asked to describe whether their IBS symptoms began gradually or suddenly and if suddenly whether this was an infectious illness. Evidence to support a diagnosis of PI-IBS included sudden onset of IBS after an infective episode diagnosed either by a positive stool culture showing a named organism or acute onset of new bowel symptoms associated with two or more of the following: fever, vomiting, diarrhoea, rectal bleeding or onset during foreign travel. Participants were then asked to record their bowel habit before the acute episode using the Rome IBS module to determine whether they had IBS before the presumed infectious illness.
Psychological assessments
Participants completed the Hospital Anxiety and Depression Scale (HADS)12 and the Patient Health Questionnaire-12 Somatic Symptom (PHQ12-SS) scale score.13 Values of HADS Anxiety or Depression >7 and PHQ12-SS > 6 were considered abnormal. They were asked to provide a contact email address to which a second questionnaire would be sent out one year later. Provision of the email was taken as permission to do so. Data are reported showing the total population and then those with PI-IBS compared with those with non-PI-IBS.
Analysis
Duplicate entries were removed and country of origin recoded as either Northern Europe (defined as North of Alps or Pyrenees) and North America OR the rest of the world.
We present summary statistics as proportions for categorical variables which we have compared using chi squared tests, and as means and standard deviations or medians and interquartile ranges (IQRs) for normally and non-normally distributed continuous variables which were tested using t tests or Wilcoxon’s rank sum, respectively. We went on to carry out multivariate analyses using logistic regression to examine potential confounding of the relationships between PI-IBS and prognosis.
Results
There were a total of 7836 lines of data in the dataset, and after exclusion of 75 duplicate email addresses and 209 lines with duplicate internet protocol address, age and gender, this provided a total of 7552 participants. The majority of cases came from the investigators’ countries with the largest numbers from Italy (46%), Netherlands (14%), Germany (8%) and Spain (6%) but there was at least one return from 107 countries. Sixty-three per cent met strict Rome III criteria but 37% did not despite having had a diagnosis of IBS by their doctor.
Similarity of participants with and without a password
The 2622 (34.72%) individuals who had a password to access the questionnaire did not differ from those without a password in the proportion meeting Rome III criteria, which was 1648 of 2622 (62.9%) and 3097 of 4930 (62.8%), respectively. There were minor differences between these two groups: Those without a password were more likely to be female (66.6% vs 56.1%, p < 0.001) and were slightly older (mean 40.8 years vs 39.9 years p = 0.0058). They were also less likely to score abnormally high on the HADS Anxiety scale (84.7% vs 87.6%, p = 0.003) though more likely to score abnormally high on the PHQ12-SS (61.4% vs 57.1%, p = 0.002).
Diagnosis of PI-IBS
A total of 1080 individuals met our definition of PI-IBS and, after excluding 76 with prior IBS, the proportion of IBS that was PI-IBS was 1004/7552 (13.3%).Those with PI-IBS were slightly more likely to meet the Rome III criteria than those without (68.5% vs 62.0%, respectively, p < 0.001); they had, however, a similar gender distribution (63% female for both PI-IBS and non-PI-IBS) and age (median(IQR) 38(29-49) for PI-IBS and median(IQR) 38(30-49) for non-PI-IBS). Table 1 shows the frequency of features used to diagnose PI-IBS. The commonest feature was sudden onset, which was seen in 72% followed by fever, vomiting and onset during travel. Only 24% had a positive stool culture. Most of those meeting our criteria met two (62%), with 27% meeting three criteria and 11% four or more. When asked “Do you remember how your doctor treated your infection?” only 713 recorded a response: A total of 293 replied that they received no specific treatment, 275 received antibiotics, 35 probiotics and 45 loperamide with 65 reporting other drugs. Those taking antibiotics were slightly more anxious than those taking no treatment with HADS scores of 10.9 ± 2.7 and 10.6 ± 2.7, respectively, p = 0.036. Antibiotic usage was significantly increased in those with bloody diarrhoea, 12.2% of whom took antibiotics while just 3.2% with no blood in their stools took antibiotics, Fisher’s exact test, p < 0.001.
Table 1.
Frequency of PI-IBS criteria (%).
| PI-IBS | Non-PI-IBS | |
|---|---|---|
| Sudden onset | 72.4 | 16.8 |
| Onset during travel | 34.5 | 2.8 |
| Vomiting | 50.1 | 4.4 |
| Fever | 50.6 | 2.4 |
| Bloody diarrhoea | 19.4 | 2.4 |
| Stool culture positive | 24.0 | 1.8 |
PI-IBS: post-infectious irritable bowel syndrome.
Differences between PI-IBS and non-PI-IBS
Current GI symptoms
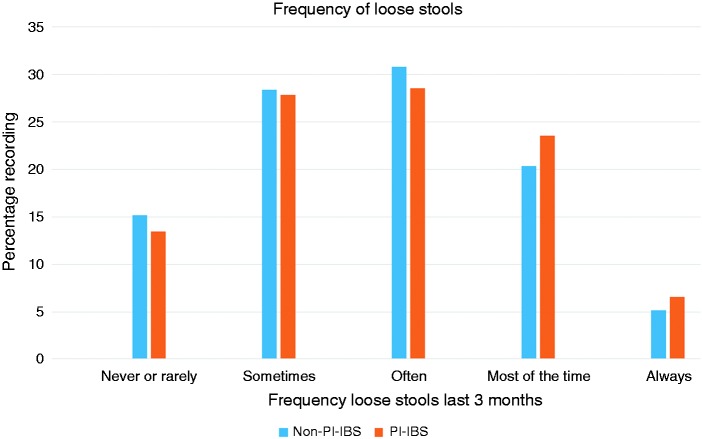
PI-IBS patients tended to have more stools per day, median (IQR) being two (1–3) compared to 1.5 (1–3) (p = 0.0002, Mann-Whitney U test). Comparing both the hardest and softest forms recorded as typical between the groups, distributions were similar for PI-IBS and non-PI-IBS (both p > 0.05). Participants reported the frequency of loose and hard stools on a five-point scale ranging from never or rarely to always. PI-IBS reported significantly more frequent the passage of loose stools (Figure 1, p = 0.03) but not hard ones (p = 0.716).
Figure 1.
Frequency of loose stools as rated by participants on a five-point scale was increased in post-infectious irritable bowel syndrome patients (PI-IBS), chi squared test, p = 0.03.
Psychometrics
The total HADS scores and Anxiety or Depression sub-scores were similar between patients with and without PI-IBS (see Table 2). A high proportion of patients were being treated for anxiety in both the PI-IBS (77.4%) and non-PI-IBS (74.5%) groups (difference not significant p = 0.051). Treatment for depression was also common but in this case those with PI-IBS were slightly less likely to be treated 79.3% vs 82.0% (p = 0.04). Respecting somatisation, a significantly greater proportion of PI-IBS patients scored above the upper limit of normal for the PHQ12-SS (6), 64.3% vs 59.0% (p = 0.002).
Table 2.
Univariate associations of PI-IBS at baseline questionnaire.
| PI-IBS | Non-PI-IBS | p | |
|---|---|---|---|
| Number | 1004 | 6548 | |
| Age (median (IQR)) | 38 (29–49) | 38 (30–49) | 0.33 |
| HADS Anxiety score (median (IQR)) | 11 (9–13) | 11 (9v13) | 0.40 |
| HADS Depression score (median (IQR)) | 10 (8–11) | 10 (8–11) | 0.06 |
| PHQ12-SS (median (IQR)) | 8 (5–11) | 7 (5–11) | 0.003 |
| History of cholecystectomy | 6.0% | 4.7% | 0.075 |
| History of appendicectomy | 14.1% | 17.2% | 0.016 |
| History of hysterectomy (in women) | 8.2% | 5.2% | 0.002 |
| Characteristics of childhood home | |||
| Contact with animals | 8.37% | 8.15% | 0.813 |
| More than one toilet | 50.8% | 45.9% | 0.004 |
| Running hot water | 89.5% | 90.6% | 0.292 |
| Shared a bed | 13.7% | 12.3% | 0.225 |
| Urban | 67.1% | 63.0% | 0.011 |
| Geographic origin | |||
| Northern Europe and United States | 59.1% | 37.3% | <0.001 |
HADS: Hospital Anxiety and Depression Scale; IQR: interquartile range; PHQ12-SS: Patient Health Questionnaire-12 Somatic Symptom scale score; PI-IBS: post-infectious irritable bowel syndrome.
Childhood environment, surgical history and PI-IBS
Childhood living conditions as assessed by having running hot water in the home, having to share a bed as a child or having contact with animals were not associated significantly with PI-IBS (Table 2), but having more than one toilet was commoner in PI-IBS, being reported in 50.1% vs 45.9% in non-PI-IBS (p = 0.004). PI-IBS patients were more often from an urban childhood environment (67.1% vs 63%, p = 0.011). As can be seen in Table 2, 59% of PI-IBS patients came from Northern Europe or North America, significantly more than the 37% of non-PI-IBS. PI-IBS was also significantly associated with a history of hysterectomy among women and was negatively associated with a history of appendicectomy.
Follow-up data
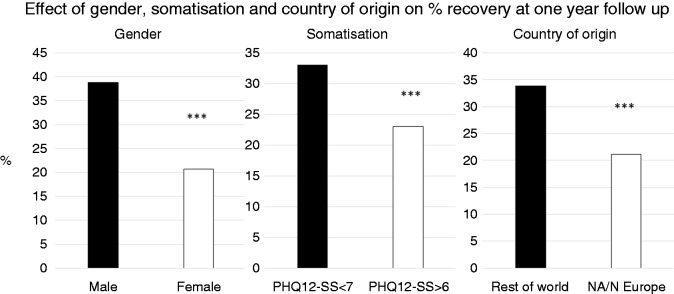
Of the initial 7552 participants, 3256 provided an email address indicating they were prepared to repeat a symptom survey at one year. We obtained follow-up data after one year on 1056 (32.43%) of these, of whom 200 (18.9%) met our definition of PI-IBS. Of the 846 who met Rome III criteria initially, 618 still met the criteria at one year so the rate of spontaneous remission was 27% in the year. The proportion who improved (as judged by ceasing to meet these criteria) varied across the age groups from 23% in those aged 21–30 to 37.5% in those aged over 70; these differences were, however, not significant (p = 0.18). Significant effects on recovery rates were seen for gender, somatisation and country of origin (Figure 2). Significantly more males (38.8%) ceased to meet Rome III than females (20.7%) (p < 0.001). Those with somatisation, indicated by an abnormally high PHQ12-SS, were significantly less likely to recover than those without somatisation, remission rates being 23.0% vs 33.2%, respectively, p < 0.001 The remission rate in PI-IBS at 22.8% was not significantly different from that in the non-PI-IBS group at 27.9% (p = 0.19).
Figure 2.
Remission rates of IBS symptoms were worse for females, those with abnormal Patient Health Questionnaire-12 Somatic Symptom (PHQ12-SS) scale score and those residing in North America and Northern Europe (NA/N Europe). ***p = 0.001.
Remission of symptoms was significantly less in those from North America/Europe at 21.1% compared to those from elsewhere 33.9% (Table 3). A multivariable logistic regression adjusting the effect of PI-IBS upon improvement for the effects of the other variables showed only slight confounding by them: Odds ratio for still being Rome III for PI-IBS vs non-PI-IBS was 1.31 (0.87–1.95) in univariate analysis, and 1.14 (0.75–1.75) in multivariate regression. If we altered the definition of improvement to be a reduction in abdominal pain over the previous three months, or reductions in the number of hard or of loose stools, we similarly found that some individuals improved while others got worse but there was no significant association with PI-IBS according to our definition. Finally, we repeated analyses defining PI-IBS either as only those with a positive stool culture at onset, or as patients who self-identified as PI-IBS. Again, no significant difference in prognosis was noted (data not shown).
Table 3.
Univariate associations of recovery of IBS as measured by ceasing to be ROME III positive among those with follow-up data who were Rome III positive at the outset.
| Rome III negative at follow-up | % | p | |
|---|---|---|---|
| Total | 228 | 27 | |
| Male | 113 | 38.8 | <0.001 |
| Female | 115 | 20.7 | |
| Age bands | |||
| 0–20 | 1 | 33.3 | 0.29 |
| 21–30 | 39 | 23.4 | |
| 31–40 | 62 | 26.2 | |
| 41–50 | 51 | 23.6 | |
| 51–60 | 45 | 32.4 | |
| 61–70 | 20 | 33.9 | |
| >70 | 9 | 37.5 | |
| Country of residence | |||
| Northern Europe/America | 96 | 21.1 | <0.001 |
| Rest of the world | 132 | 33.9 | |
| HADS anxiety | |||
| Normal | 27 | 24.55 | 0.54 |
| Abnormal | 201 | 27.31 | |
| HADS depression | |||
| Normal | 33 | 33.67 | 0.11 |
| Abnormal | 195 | 26.07 | |
| PHQ12-SS | |||
| Normal | 108 | 33.2 | 0.001 |
| Abnormal | 120 | 23.0 | |
| Subtype of IBS | |||
| PI-IBS | 37 | 22.8 | 0.19 |
| Non PI-IBS | 191 | 27.9 |
HADS: Hospital Anxiety and Depression Scale; IBS: irritable bowel syndrome; IQR: interquartile range; PHQ12-SS: Patient Health Questionnaire-12 Somatic Symptom Scale score; PI-IBS: post-infectious irritable bowel syndrome.
Longer duration of IBS symptoms reduced recovery rates. A total of 272 had IBS onset 0–3 years before the survey, of whom 38% recovered at the one-year follow-up while 819 had onset >3 years before, and of these just 25% recovered, chi squared test = 3.3, p = 0.07.
In view of the response rate of only 32.43% at follow-up, we compared baseline data for those with and without follow-up. Amongst those providing email addresses, those providing follow-up were slightly older (mean age 43.2 vs 39.9, p < 0.001), more likely to satisfy Rome III criteria (80.1% vs 72.6%, p < 0.001), more likely to be from Northern Europe or the United States (52.1% vs 31.7%, p < 0.001) but less likely to have an abnormal PHQ12-SS score (59.7% vs 64.6%, p = 0.007, gender and HAD sub-scores (or overall) were not significantly different.
Discussion
This large, pragmatic study of IBS, PI-IBS and its prognosis found that about 13% of included cases satisfied criteria suggestive of PI-IBS. The commonest diagnostic feature was sudden onset followed by vomiting and fever but only 24% reported having a positive stool culture. Compared to non-PI-IBS cases, PI-IBS patients had greater stool frequency and looser stools, which is in keeping with earlier reports that the commonest IBS subtype in PI-IBS was IBS with diarrhoea (IBS-D).3,14 Whether this is related to the common axis of dysbiosis in which PI-IBS and IBS-D overlap remains to be determined.7 Those with PI-IBS had a slightly greater tendency to somatise, a lower rate of previous appendicectomy and a higher rate of hysterectomy. They were also more likely to have lived in an urban environment and in a house with multiple toilets during childhood, and more likely to be from Northern Europe or North America. Contrary to our hypothesis PI-IBS was not associated with a significantly improved prognosis. We were however able to show that prognosis is better in those without somatisation, in males, and in those not from Northern Europe and North America.
Individuals who used a password to access the questionnaire were known to be from participating clinics diagnosed by the authors and their colleagues (all experts in functional GI diseases), and their diagnoses were therefore of known validity. We found no difference between those with and without a password as regards to meeting our gold standard for IBS diagnosis (namely the Rome III criteria). This encourages us to believe that those who took part without a password were also likely truly to suffer from IBS, i.e. to believe that they were valid cases to study. This lack of difference also reassured us that participants from investigators’ clinics were not grossly atypical (a risk when examining the practice of those with a special interest in the area). Where we have limited analysis to those using passwords, the results should be generalisable to patients with IBS seen in secondary care. An inevitable limitation to our study is that we have recruited exclusively patients prepared to complete a quite lengthy survey, which may not be typical of all patients.
There was a risk that our survey would selectively recruit those with a PI origin; however, we do not believe this is the case for a number of reasons. Firstly, the proportion that were PI-IBS (14%) is within the range of 6%–17% reported by others using a number of different recruitment techniques.15 Secondly, others have modelled the proportion of IBS that is PI-IBS and using the most conservative model concluded that 9% of IBS was PI.16 Finally, although the website was labelled “piibs”, we don’t think the general public would necessarily recognise these initials, and questions about infection appear only in the middle of a quite extensive set of questions about other factors.
Though only 28% of our PI-IBS cases reported a positive stool culture, the absence of this does not exclude the diagnosis of PI-IBS since it is common practice to request stool culture only in cases of acute gastroenteritis which are not settling. One prospective survey in the United Kingdom (UK) found that stool samples were requested in only 27% of gastroenteritis cases presenting to primary care17 so the rate of culturing for all cases must be much lower given that many do not seek health care. To examine the possibility of bias, we varied our definition of PI-IBS to include only those with positive stool cultures but this did not appreciably alter our results. We therefore think it unlikely that our definition of PI-IBS has greatly biased the results, and in addition as the criteria we used mirror those we would commonly use in clinical practice, we feel our results are likely both to be valid and to be informative to clinicians.
We found PI-IBS cases to have more frequent bowel movements but to be remarkably similar to non-PI-IBS patients with respect to age, gender and anxiety though they were less likely to have been treated for depression. Contrary to our original hypothesis, we found no evidence that remission at one year differed from non-PI-IBS, being 22.8% and 27.9%, respectively.
Childhood living conditions were generally good with, for example, 86% having hot running water in their homes, but we did find that having more than one toilet in the home was slightly commoner in PI-IBS than non-PI-IBS, partially supporting the idea that childhood affluence might increase one’s risk of developing PI-IBS.
This might explain why the proportion of PI-IBS patients who were from Northern Europe/North America (59%) was significantly higher than the 37.4% of non-PI-IBS (p < 0.001). An alternative explanation is that one-third of PI-IBS develops during foreign travel, which might occur more frequently in those from the more affluent Northern Europe/North America. Campylobacter is the commonest cause of PI-IBS in adults in the UK at least.18 However, the majority of Campylobacter infections in tropical countries occur in childhood19 when they are often mild with a few days of watery diarrhoea. A similar mild character was reported in adult patients infected with Campylobacter jejuni from southern Spain with 96% having only watery diarrhoea.20 By contrast adults with Camplyobacter jejuni infection in the United States experience a severe illness with mucosal ulceration,21 and one-third of adult cases in the UK have bloody diarrhoea and weight loss,18 indicating severe mucosal inflammation which might be more likely to result in prolonged bowel dysfunction. Whether these differences represent differences in bacterial or host characteristics remains to be determined.
The prognosis in PI-IBS did not differ from non-PI-IBS with recovery in approximately one in four in the first year. However, males were almost twice as likely as females to recover in the first year, a striking as yet unexplained difference which should be further explored. However, the response rate for the follow-up study was low at 14% (32% of those originally indicating a willingness to consider further contact), which does introduce a risk of bias. Our analysis of the difference between responders and non-responders shows that of the factors we have found to be associated with prognosis, gender is not associated with response, and the excess of Europeans and North Americans might bias towards a worse prognosis whereas the deficit of somatisers would bias in the other direction. Few other surveys have attempted such follow-up so there are limited data with which to compare our recovery rate but 27% at one year is within the range reported in meta-analyses of PI-IBS.22
Although we found few major differences in clinical features between PI-IBS and non-PI-IBS, it is still important to recognise the condition both to reassure the patient that this is not unusual and also because there is a hint that response to treatment might be different. A recent trial of the anti-inflammatory agent mesalazine showed overall no benefit in IBS with diarrhoea but a post hoc subgroup analysis did suggest that PI-IBS patients did respond.23 This needs repeating but makes sense if the underlying pathophysiology of PI-IBS includes ongoing low-grade inflammation.
Acknowledgements
Author contributions are as follows: PE and RS co-chaired the UEG Working Party; PE established and managed websites www.pi-ibs.eu and www.postinfectiuous-ibs.eu from which the survey was coordinated; RS, PE, GEEB, JS, FA, JM, GB, FM and QA developed the protocol; TC analysed data; and TC and RS produced the first draft. All authors reviewed and approved the final manuscript.
The technical support of Hendrik Buschkamp of Buschkamp-dv, Grevenbroich, Germany, for hosting the websites and providing the data readouts is gratefully acknowledged.
Declaration of conflicting interests
PE has received research funding from Shire-Movetis and SymbioPharm; advisory boards from Allergan Pharma, Almirall, Boehringer-Ingelheim, Heel Pharma, and AstraZeneca, and speaker fees from Almirall, Bayer Healthcare, Falk Pharma, Heel Pharma and SymbioPharm.
RS has received research funding from Lesaffre and Ironwood; advisory boards for Almirall, Yuhan Corporation, Ibsen and Danone; and speaker fees from Menarini.
JKM has served on advisory boards and/or provided consulting for AbbVie, Boehringer-Ingelheim, Celgene, Celltrion, Ferring, Hospira, Janssen, Merck, Pfizer, Pharmascience, Procter & Gamble, Shire, and Takeda; and received speaker fees from AbbVie, Allergan, Ferring, Janssen, Procter & Gamble, Shire, and Takeda.
The other authors have nothing to declare.
Funding
This work was supported by a grant from the United European Gastroenterology Association (UEG) GASTRO 2009.
Ethics approval
Ethics approval for this study was sought and received from the University of Nottingham Medical School Ethics Committee, Division of Therapeutics and Molecular Medicine, Nottingham, UK (P/9/2008, as of 26 September 2008), conforming to the ethical guidelines of the 1975 Declaration of Helsinki. Where legally required, national ethics board permissions were requested and received.
Informed consent
Completing the questionnaire at the outset of the study by participants was accepted as giving informed consent.
References
- 1.Chaudhary NA, Truelove SC. The irritable colon syndrome. Quart J Med 1962; 123: 307–322. [PubMed] [Google Scholar]
- 2.Gwee KA, Leong YL, Graham C, et al. The role of psychological and biological factors in postinfective gut dysfunction. Gut 1999; 44: 400–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dunlop SP, Jenkins D, Neal KR, et al. Relative importance of enterochromaffin cell hyperplasia, anxiety, and depression in postinfectious IBS. Gastroenterology 2003; 125: 1651–1659. [DOI] [PubMed] [Google Scholar]
- 4.Schwille-Kiuntke J, Enck P, Zendler C, et al. Postinfectious irritable bowel syndrome: Follow-up of a patient cohort of confirmed cases of bacterial infection with Salmonella or Campylobacter. Neurogastroenterol Motil 2011; 23: e479–e488. [DOI] [PubMed] [Google Scholar]
- 5.Klem F, Wadhwa A, Prokop LJ, et al. Prevalence, risk factors, and outcomes of irritable bowel syndrome after infectious enteritis: A systematic review and meta-analysis. Gastroenterology 2017; 152: 1042–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dunlop SP, Hebden J, Campbell E, et al. Abnormal intestinal permeability in subgroups of diarrhea-predominant irritable bowel syndromes. Am J Gastroenterol 2006; 101: 1288–1294. [DOI] [PubMed] [Google Scholar]
- 7.Jalanka-Tuovinen J, Salojärvi J, Salonen A, et al. Faecal microbiota composition and host-microbe cross-talk following gastroenteritis and in postinfectious irritable bowel syndrome. Gut 2014; 63: 1737–1745. [DOI] [PubMed] [Google Scholar]
- 8.Spiller R, Garsed K. Postinfectious irritable bowel syndrome. Gastroenterology 2009; 136: 1979–1988. [DOI] [PubMed] [Google Scholar]
- 9.Calva JJ, Ruiz-Palacios GM, Lopez-Vidal AB, et al. Cohort study of intestinal infection with Campylobacter in Mexican children. Lancet 1988; 1: 503–506. [DOI] [PubMed] [Google Scholar]
- 10.Martin PM, Mathiot J, Ipero J, et al. Immune response to Campylobacter jejuni and Campylobacter coli in a cohort of children from birth to 2 years of age. Infect Immun 1989; 57: 2542–2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heaton KW, O’Donnell LJ. An office guide to whole-gut transit time. Patients’ recollection of their stool form. J Clin Gastroenterol 1994; 19: 28–30. [DOI] [PubMed] [Google Scholar]
- 12.Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand 1983; 67: 361–370. [DOI] [PubMed] [Google Scholar]
- 13.Spiller RC, Humes DJ, Campbell E, et al. The Patient Health Questionnaire 12 Somatic Symptom scale as a predictor of symptom severity and consulting behaviour in patients with irritable bowel syndrome and symptomatic diverticular disease. Aliment Pharmacol Ther 2010; 32: 811–820. [DOI] [PubMed] [Google Scholar]
- 14.Wouters MM, Van Wanrooy S, Nguyen A, et al. Psychological comorbidity increases the risk for postinfectious IBS partly by enhanced susceptibility to develop infectious gastroenteritis. Gut 2016; 65: 1279–1288. [DOI] [PubMed] [Google Scholar]
- 15.Longstreth GF, Hawkey CJ, Mayer EA, et al. Characteristics of patients with irritable bowel syndrome recruited from three sources: Implications for clinical trials. Aliment Pharmacol Ther 2001; 15: 959–964. [DOI] [PubMed] [Google Scholar]
- 16.Shah ED, Riddle MS, Chang C, et al. Estimating the contribution of acute gastroenteritis to the overall prevalence of irritable bowel syndrome. J Neurogastroenterol Motil 2012; 18: 200–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wheeler JG, Sethi D, Cowden JM, et al. Study of infectious intestinal disease in England: Rates in the community, presenting to general practice, and reported to national surveillance. The Infectious Intestinal Disease Study Executive. Br Med J 1999; 318: 1046–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neal KR, Hebden J, Spiller R. Prevalence of gastrointestinal symptoms six months after bacterial gastroenteritis and risk factors for development of the irritable bowel syndrome: Postal survey of patients. BMJ 1997; 314: 779–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Georges-Courbot MC, Cassel-Beraud AM, Gouandjika I, et al. A cohort study of enteric Campylobacter infection in children from birth to two years in Bangui (Central African Republic). Trans R Soc Trop Med Hyg 1990; 84: 122–125. [DOI] [PubMed] [Google Scholar]
- 20.Gallardo F, Gascón J, Ruiz J, et al. Campylobacter jejuni as a cause of traveler’s diarrhea: Clinical features and antimicrobial susceptibility. J Travel Med 1998; 5: 23–26. [DOI] [PubMed] [Google Scholar]
- 21.Siegal D, Syed F, Hamid N, et al. Campylobacter jejuni pancolitis mimicking idiopathic ulcerative colitis. Heart Lung 2005; 34: 288–290. [DOI] [PubMed] [Google Scholar]
- 22.Thabane M, Kottachchi DT, Marshall JK. Systematic review and meta-analysis: The incidence and prognosis of post-infectious irritable bowel syndrome. Aliment Pharmacol Ther 2007; 26: 535–544. [DOI] [PubMed] [Google Scholar]
- 23.Lam C, Tan W, Leighton M, et al. A mechanistic multicentre, parallel group, randomised placebo-controlled trial of mesalazine for the treatment of IBS with diarrhoea (IBS-D). Gut 2016; 65: 91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]