ABSTRACT
Human neural angiostrongyliasis is an emerging infectious disease caused by nematode Angiostrongylus cantonensis. The present study investigated the presence of Angiostrongylus spp. in terrestrial molluscs collected from the following areas in the Metropolitan Region of Aracaju, Sergipe State, Brazil: Barra dos Coqueiros, Nossa Senhora do Socorro, Sao Cristovao and Aracaju. In total, 703 specimens representing 13 mollusc species were screened for Angiostrongylus spp. Larvae of Angiostrongylus spp. were found in three species. Larvae recovered from Achatina fulica were used for experimental infection in Wistar rats (Rattus norvegicus). For specific identification of nematodes, the mitochondrial cytochrome c oxidase subunit I (COI) was sequenced from both larvae and adults recovered from molluscs and rats, respectively. Infection with A. cantonensis was detected in all municipalities and in the following three host species: Bulimulus tenuissimus, Cyclodontina fasciata (Barra dos Coqueiros), and A. fulica (Aracaju, Nossa Senhora do Socorro and Sao Cristovao). Co-infections were also found with Caenorhabditis sp. and Strongyluris sp. larvae. This is the first study of the helminth fauna associated with the terrestrial malacofauna in Sergipe State, and confirms that these three snail species are involved in the transmission of A. cantonensis in the state. In addition, B. tenuissimus and C. fasciata are newly reported natural hosts of the parasite.
KEYWORDS: Eosinophilic meningitis, Angiostrongylus, Achatina fulica, Bulimulus tenuissimus, Cyclodontina fasciata, Land snails
INTRODUCTION
Achatina fulica Bowdich, 1822 is listed among 100 of the worst invasive species in the world and is present in almost all Brazilian states 1 , 2 . This species' rapid proliferation is the main reason it is considered an agricultural pest. It can also act as an intermediate host of Angiostrongylus cantonensis (Chen, 1935) and Angiostrongylus costaricensis Morera and Céspedes, 1971. These two species are the etiological agents of human neural angiostrongyliasis and human abdominal angiostrongyliasis, respectively 2 - 4 .
Eosinophilic meningitis is the principal clinical manifestation of human neural angiostrongyliasis, an emerging infectious disease that causes severe impairment of the central nervous system and can lead to death 5 - 7 . This nematode has low specificity for its intermediate hosts, which means that many mollusc species have already been found to be naturally infected globally 8 , 9 . However, A. fulica is an important intermediate host for A. cantonensis 4 , 8 , 10 - 12 .
Human neural angiostrongyliasis occurs primarily by ingestion of snails that are infected with L3 larvae 13 . These larvae reach the brain where they mature into L4 and L5 but are unable to migrate from the brain to the heart (as they do in the definitive rat hosts) but move around in the brain causing neurological damage and, especially when they die, cause intense inflammatory reactions that result in additional neurological damage and symptoms 5 , 7 , 14 .
More than 2,800 human cases of eosinophilic meningitis caused by A. cantonensis have been reported in more than 30 countries 11 since it was first reported in Taiwan in 1945 15 . In Brazil, out of 84 suspected cases, there have been 34 confirmed cases of A. cantonensis infections in humans as well as the confirmation of definitive hosts and infected intermediate hosts in different regions of the country 10 .
In Sergipe State, 19 municipalities have reported infestation of A. fulica (Comissao de Combate ao Caramujo Africano, personal communication) in addition to Barra dos Coqueiros municipality 8 . The objective of this study was to verify the infection rate by nematode larvae in A. fulica and other terrestrial molluscs collected in the Aracaju Metropolitan Region, Sergipe State, Northeastern Brazil.
MATERIALS AND METHODS
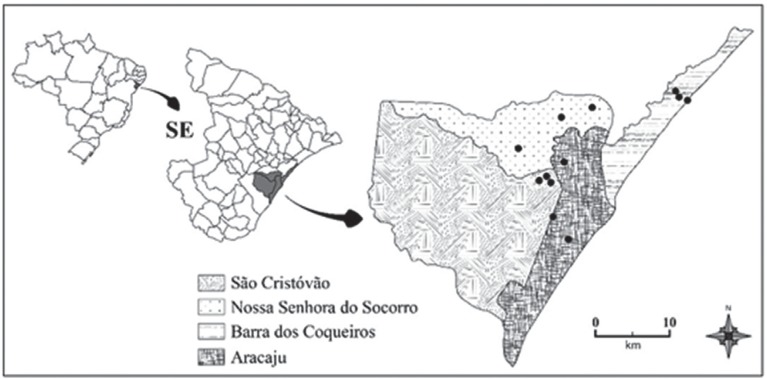
This study was undertaken in urban environments of four municipalities that constitute the Metropolitan Region of Aracaju, Sergipe State (Figure 1): Aracaju, Barra dos Coqueiros, Nossa Senhora do Socorro and Sao Cristovao (Table 1).
Figure 1. Study area: Aracaju Metropolitan Region, including the capital of Sergipe State, the municipalities of Barra dos Coqueiros, Nossa Senhora do Socorro and Sao Cristovao. The black spots represent the collection localities.
Table 1. Geographical coordinates by location of collection of terrestrial molluscs.
| Municipalities | Description of locality | Geographical coordinates |
|---|---|---|
| Square / open area | 10°49'30.1"S 36°56'46.7"W | |
| Barra dos Coqueiros | Outer clothing area | 10°49'42.6"S 36°56'08.2"W |
| School backyard / Rubble | 10°49'12.2"S 36°56'58.9"W | |
| Street / close to houses | 10°55'57.8"S 37°06'26.6"W | |
| Sao Cristovao | Street / close to houses | 10°55'27.7"S 37°06'43.2"W |
| Street and house backyard | 10°55'43.1"S 37°07'16.2"W | |
| Street / close to houses | 10°58'29.7"S 37°06'16.7"W | |
| Aracaju | Ground / adjacent to a commercial establishment | 11°00'11.9"S 37°05'03.2"W |
| Close to sewer-line | 10°54'24.1"S 37°05'23.3"W | |
| Garden and houses backyard | 10°50'19.0"S 37°03'15.0"W | |
| Nossa Senhora do Socorro | Houses backyard | 10°51'02.2"S 37°05'38.1"W |
| Street and wasteland | 10°53'23.0"S 37°08'50.9"W |
Each point of collection was georeferenced, followed by manual random sampling of terrestrial molluscs using forceps and gloves. The collections were carried out in the following three seasons: April (autumn), July (winter) and October (spring) of 2016, at three locations in each municipality. The samples were sent to the National Reference Laboratory for Schistosomiasis - Malacology (LRNEM) of the Instituto Oswaldo Cruz, Fundação Oswaldo Cruz (IOC/Fiocruz, Rio de Janeiro), where they were analyzed. The identification of the molluscs species was done based on conchological characteristics compared to photos and descriptions from catalogues 16 - 19 . We also compared our material with material deposited in the Instituto Oswaldo Cruz. Samples of each species and from each collection locality were deposited in the Mollusca Collection of the Instituto Oswaldo Cruz (CMIOC 10103 - 10134, 11206).
To collect the nematode larvae, the molluscs were artificially digested by a modified Wallace and Rosen technique 20 . The recovered material was observed under a stereoscopic microscope following the LRNEM Identification Guide and based on the literature 4 , 21 . The Angiostrongylus sp. (Nematoda: Metastrongylidae) larvae were identified under a compound microscope according to Thiengo et al. 4 . Ten Angiostrongylus sp. larvae from each sample were collected and cryopreserved at −20 °C until DNA extraction for subsequent molecular analysis. Some rhabditiform larvae were observed and prepared for DNA extraction. In addition, Strongyluris sp. was morphologically identified, based on Thiengo 21 .
To assess the viability of the parasites, Angiostrongylus sp. larvae recovered from A. fulica from the municipality of Aracaju were used to infect two specimens of Rattus norvegicus through an orogastric tube at a concentration of 50 L3 larvae per rodent. Fifty days after infection, the animals were killed and necropsied to confirm the presence of adult A. cantonensis 22 . This procedure was carried out following the Ethics Commission on Animal Use of the Oswaldo Cruz Foundation (LW-47/14).
Molecular diagnosis of nematode larvae
DNA was extracted from 10 Angiostrongylus sp. larvae obtained from each mollusc in which larvae had been found and resuspended in 30 μL of PCR Buffer solution (Thermo Fisher Scientific, Massachusetts, USA). The polymerase chain reaction (PCR) mixtures were prepared in a volume of 20 μL containing 8 μL of ultrapure water, 5 μL of 10% trehalose, 2.5 μL of 10x PCR Reaction Buffer, 2 μL of 2.5 mM dNTPs, 1.25 μL 50 mM MgCl2, 0.5 μL each of 5 μM forward and reverse primer (Nem 3 from Prosser et al. 23 ), and 0.25 μL of recombinant Taq DNA polymerase (Thermo Fisher Scientific). A total of 5 μL of the DNA sample was added to the mixture, producing a final volume of 25 μL for each reaction. For all the reactions, ultrapure water was used as a negative control template, and the positive control was performed with genomic DNA of A. cantonensis as template. The 700 bp fragment of the Angiostrongylus sp. larvae mitochondrial cytochrome c oxidase subunit I (COI) gene was amplified using the following PCR conditions: initial denaturation at 94 °C for 1 min, five cycles at 94 °C for 40 s, 45 °C for 40 s, and 72 °C for 1 min, followed by 30 cycles at 94 °C for 40 s, 51 °C for 40 s, and 72 °C for 1 min and a final extension at 72 °C for 1 min.
For rhabditiform larvae, individual DNA from some isolates was used to amplify the 480 bp ribosomal 18S region 24 .
The PCR products were purified using the Illustra GFX PCR DNA and Gel Band Purification Kit (GE Healthcare, Little Chalfont, UK) following the manufacturer's protocol. Purified products after amplification were bidirectionally sequenced using BigDye Terminator v3.1 Cycle Sequencing kit (Applied Biosystems, California, USA) according to the manufacturer's instructions. Chromatograms of the sequences obtained were analyzed and edited using Geneious version R9 software (http://www.geneious.com), resulting in a consensus sequence (contig). Then, a comparative similarity search was performed on GenBank (www.ncbi.nlm.nih.gov/genbank/) 25 using BLAST (Basic Local Alignment Search Tool) to identify the closest match. The obtained sequences are available in GenBank under the access N° MH511539 - MH511542, and MH547424.
RESULTS
In total, 703 terrestrial gastropods were analyzed (190 in Aracaju, 250 in Barra dos Coqueiros, 159 in Nossa Senhora do Socorro, and 104 in São Cristovão) and identified as the following 13 species: Achatina fulica (Bowdich, 1822), Allopeas gracile (Hutton, 1834), Subulina octona (Bruguière, 1789), Leptinaria unilamellata (d'Orbigny, 1835), Bulimulus tenuissimus (d'Orbigny, 1835), Cyclodontina fasciata (Potiez & Michaud, 1838), Latipes erinaceus (Colosi, 1921), Sarasinula linguaeformis (Semper, 1885), Streptartemon cookeanus (Baker, 1914), Streptartemon quixadensis (Baker, 1914), Tamayoa banghaasi (Thiele, 1927), Helicina sp. and Omalonyx sp.
Angiostrongylus sp. larvae were found in specimens of A. fulica, B. tenuissimus and C. fasciata and were later confirmed by molecular analysis as A. cantonensis (Table 2).
Table 2. Prevalence of nematodes in terrestrial molluscs from the Metropolitan Region of Aracaju, Sergipe State, Northeast Brazil, from April to October 2016.
| Locality | Species | N° specimens (n) | Positive molluscs (n) | ||
|---|---|---|---|---|---|
| A. cantonensis | Caenorhabditis sp. | Strongyluris sp. | |||
| A. fulica | 110 | - | 48 | 22 | |
| B. tenuissimus | 23 | 1 | 3 | - | |
| C. fasciata | 112 | 2 | 48 | 23 | |
| Helicina sp. | - | - | - | - | |
| Barra dos Coqueiros | S. cookeanus | 1 | - | - | - |
| S. linguaeformis | 2 | - | - | 1 | |
| S. octona | 2 | - | - | - | |
| S. quixadensis | - | - | - | - | |
| T. banghaasi | - | - | - | - | |
| A. fulica | 63 | 22 | 25 | 1 | |
| Sao Cristovao | B. tenuissimus | 17 | - | 1 | - |
| S. linguaeformis | 10 | - | 1 | - | |
| S. octona | 14 | - | 1 | - | |
| A. fulica | 97 | 36 | 46 | 12 | |
| A. gracille | - | - | - | - | |
| B. tenuissimus | 19 | - | 2 | - | |
| Aracaju | C. fasciata | 43 | - | 4 | 1 |
| S. cookeanus | 2 | - | - | - | |
| S. linguaeformis | 5 | - | - | - | |
| S. octona | 24 | - | 2 | - | |
| T. banghaasi | - | - | - | - | |
| A. fulica | 85 | 5 | 34 | - | |
| B. tenuissimus | 13 | - | 3 | - | |
| L. erinaceus | - | - | - | - | |
| Nossa Senhora do Socorro | L. unilamellata | 47 | - | 5 | - |
| Omalonyx sp. | 2 | - | 1 | - | |
| S. linguaeformis | 2 | - | - | - | |
| S. octona | 10 | - | 4 | - | |
| T. banghaasi | - | - | - | - | |
Achatina fulica was found in all four municipalities, with snails harboring infection by A. cantonensis in three of them (Aracaju, Sao Cristovao and Nossa Senhora do Socorro). Bulimulus tenuissimus occurred in all municipalities, while specimens of C. fasciata were found only in Aracaju and Barra dos Coqueiros. Both B. tenuissimus and C. fasciata were infected by A. cantonensis in the municipality of Barra dos Coqueiros, near the port area of Sergipe State.
Co-infections were observed between A. cantonensis and other nematodes, such as Strongyluris sp. and rhabditiform larvae (Table 3). The rhabditiform larvae were analyzed molecularly and were found to be 99% similar to a sequence from GenBank identified as Caenorhabditis sp.
Table 3. Prevalence of co-infection between Angiostrongylus cantonensis, Caenorhabditis sp. and Strongyluris sp. in land molluscs from the Aracaju Metropolitan Region, Sergipe State, Northeast Brazil, from April to October 2016.
| Locality | Species | Specimens analyzed (n) | Positive specimens for A. cantonensis | Co-infection (n) | |||
|---|---|---|---|---|---|---|---|
| (n) | (%) | A. cantonensis + Strongyluris sp. | A. cantonensis + Caenorhabditis sp. | A. cantonensis + Caenorhabditis sp. + Strongyluris sp. | |||
| Barra dos | B. tenuissimus | 23 | 1 | 4.3 | - | 1 | - |
| Coqueiros | C. fasciata | 109 | 2 | 1.8 | - | - | - |
| São Cristovao | A. fulica | 63 | 22 | 34.9 | - | 6 | - |
| Aracaju | A. fulica | 99 | 36 | 36.4 | 3 | 10 | 7 |
| Nossa Senhora do Socorro | A. fulica | 85 | 5 | 5.9 | - | - | - |
Infections of Caenorhabditis sp. were observed in A. fulica, B. tenuissimus, C. fasciata, S. octona, L. unilamellata, S. linguaeformis and Omalonyx sp. Infections by Strongyluris sp. were observed in A. fulica, C. fasciata and S. linguaeformis.
Two rats that were experimentally infected with Angiostrongylus sp. isolates displayed symptoms 50 days after infection. Forty A. cantonensis adult worms were recovered in the first rat (37.5% males and 62.5% females), and 42 adult worms were recovered in the second (40.4% males and 59.5% females).
DISCUSSION
The snail A. fulica has already been reported as being parasitized with A. cantonensis by several authors 23 , 4 , 8 , 10 , 19 , 26 . In Brazil, the species is considered one of the main potential transmitters of human neural angiostrongyliasis, considering their widespread distribution, high population densities and proximity to humans 10 , 26 .
Despite knowledge of the presence of the giant African snail in Sergipe State since 2006 27 , 28 , only one previous investigative study has been carried out in this region 8 . Ten years after the first detection of A. cantonensis in Brazil, this study records its presence in Sergipe State for the first time; it is also the first report of C. fasciata and B. tenuissimus as natural intermediate hosts of the parasite. In addition, we observed co-infection with other nematodes, which is consistent with other studies 12 , 26 , 28 .
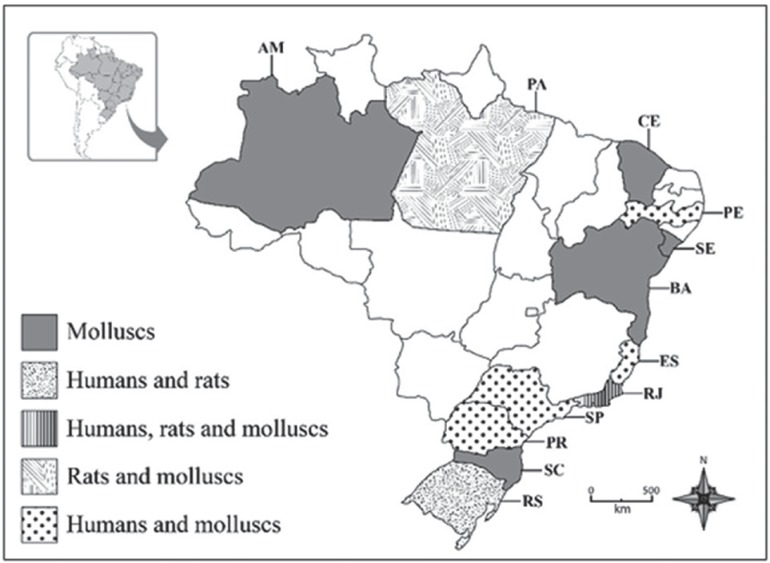
Since 2007, when the first Brazilian case of eosinophilic meningitis caused by A. cantonensis was recorded, in Espirito Santo State 29 , reports of the disease have increased. Currently, the parasite has been found in 12 states (Figure 2) 2 , 7 , 10 , 29 - 31 .
Figure 2. Distribution in Brazil of Angiostrongylus cantonensis in natural vertebrate and invertebrate hosts, and in humans. Adapted from: Thiengo et al. 5 , Carvalho et al. 8 , Morassutti et al. 10 , Espirito Santo et al. 30 , Moreira et al. 31 . AM: Amazonas; PA: Para; CE: Ceara; PE: Pernambuco; SE: Sergipe; BA: Bahia; ES: Espirito Santo; RJ: Rio de Janeiro; SP: Sao Paulo; PR: Parana; SC: Santa Catarina; RS: Rio Grande do Sul.
The first known occurrence of A. fulica naturally infected by A. cantonensis in the northeast region of Brazil was reported in 2008, with human cases of eosinophilic meningitis and naturally infected intermediate hosts in the municipalities of Escada and Olinda, Pernambuco State 4 . A study carried out in Brazilian port areas, including the port of Sergipe State, reported the presence of terrestrial and aquatic molluscs that, although previously negative for A. cantonensis, tested positive in the bordering Bahia State 8 and in Ceara State 10 .
Several other terrestrial molluscs have also been found to be naturally infected with this nematode in Brazil 2 , 3 , 8 , 19 and in other countries 9 , 13 , 14 . Among these species are S. linguaeformis and S. octona 2 , 3 , 8 , 9 , which, however, were not found to be infected in the present study. A possible reason for this is the lower abundance of these species in the sampled localities. The three species found infected by A. cantonensis were also those that were more abundant in the environment.
The present study identifies, for the first time, the following two species as natural hosts of the parasite: C. fasciata, which had not yet been described in the literature as an intermediate host for A. cantonensis or for other nematodes of human and veterinary interest, and B. tenuissimus, described as a host of metacercariae of the genus Postharmostomum 32 . This interaction between exotic and native species has become a new concern in the ecology of the disease and may result in increased parasitic spread as well as pose a threat to native species 9 .
Studies indicate the need to implement control measures against molluscs associated with public health, particularly the exotic species A. fulica. However, passive geographic dispersal is the main form of spread of the giant African snail, which hinders its control 33 .
The present study reinforces the need to investigate the current situation of A. cantonensis dispersal in the Northeastern region of Brazil, since there are reports of its presence in four of its nine states, i.e., Bahia, Ceara, Pernambuco and Sergipe 7 , 8 , as well as the evident dispersal of A. fulica in several municipalities in the Sergipe State.
ACKNOWLEDGMENTS
The authors are grateful to the Genomic Platform DNA Sequencing - RPT01A (Rede de Plataformas Tecnologicas FIOCRUZ) and for the technical support of the LRNEM/ IOC/Fiocruz team, Eduardo Cinilha for help with map preparation, and the CAPES for the scholarship to Jucicleide R. Souza.
REFERENCES
- 1.Lowe S, Browne M, Boudjelas S, De Poorter M. 100 of the world's worst invasive alien species: a selection from the Global Invasive Species Database. Auckland: Invasive Species Specialist Group; 2000. [Google Scholar]
- 2.Thiengo SC, Fernandez MA. Gastrópodes neotropicais continentais de importância médica. In: Coura JR, editor. Dinâmica das doenças infecciosas e parasitárias. 2a ed. Rio de Janeiro: Guanabara Koogan; 2018. pp. 131–153. [Google Scholar]
- 3.Laitano AC, Genro JP, Fontoura R, Branco SS, Maurer RL, Graeff-Teixeira C, et al. Report on the occurrence of Angiostrongylus costaricensis in southern Brazil, in a new intermediate host from the genus Sarasinula (Veronicellidae, Gastropoda) Rev Soc Bras Med Trop. 2001;34:95–97. doi: 10.1590/s0037-86822001000100015. [DOI] [PubMed] [Google Scholar]
- 4.Thiengo SC, Maldonado A, Júnior, Mota EM, Torres EJ, Caldeira R, Carvalho OS, et al. The giant African snail Achatina fulica as natural intermediate host of Angiostrongylus cantonensis in Pernambuco, northeast Brazil. Acta Trop. 2010;115:194–199. doi: 10.1016/j.actatropica.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 5.Thiengo SC, Simões RO, Fernandez M, Maldonado A., Júnior Angiostrongylus cantonensis and rat lung worm disease in Brazil. Hawaii J Med Public Health. 2013;72(Suppl 2):18–22. [PMC free article] [PubMed] [Google Scholar]
- 6.Graeff-Teixeira C, Silva AC, Yoshimura K. Update on eosinophilic meningoencephalitis and its clinical relevance. Clin Microbiol Rev. 2009;22:322–348. doi: 10.1128/CMR.00044-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cowie RH. Biology, systematics, life cycle, and distribution of angiostrongylus cantonensis, the cause of rat lungworm disease. Hawaii J Med Public Health. 2013;72(Suppl 2):6–9. [PMC free article] [PubMed] [Google Scholar]
- 8.Carvalho OS, Scholte RG, Mendonça CL, Passos LK, Caldeira RL. Angiostrongylus cantonensis (Nematode: Metastrongyloidea) in molluscs from harbour areas in Brazil. Mem Inst Oswaldo Cruz. 2012;107:740–746. doi: 10.1590/s0074-02762012000600006. [DOI] [PubMed] [Google Scholar]
- 9.Kim JR, Hayes KA, Yeung NW, Cowie RH. Diverse gastropod hosts of angiostrongylus cantonensis, the rat lungworm, globally and with a focus on the Hawaiian Islands. PloS One. 2014;9:e94969–e94969. doi: 10.1371/journal.pone.0094969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morassutti AL, Thiengo SC, Fernandez M, Sawanyawisuth K, Graeff-Teixeira C. Eosinophilic meningitis caused by Angiostrongylus cantonensis: an emergent disease in Brazil. Mem Inst Oswaldo Cruz. 2014;109:399–407. doi: 10.1590/0074-0276140023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang QP, Lai DH, Zhu XQ, Chen XG, Lun ZR. Human angiostrongyliasis. Lancet Infect Dis. 2008;8:621–630. doi: 10.1016/S1473-3099(08)70229-9. [DOI] [PubMed] [Google Scholar]
- 12.Maldonado A, Júnior, Simões RO, Oliveira AP, Motta EM, Fernandez MA, Pereira ZM, et al. First report of Angiostrongylus cantonensis (Nematoda: Metastrongylidae) in Achatina fulica (Mollusca: Gastropoda) from Southeast and South Brazil. Mem Inst Oswaldo Cruz. 2010;105:938–941. doi: 10.1590/s0074-02762010000700019. [DOI] [PubMed] [Google Scholar]
- 13.Vitta A, Polsut W, Fukruksa C, Yimthin T, Thanwisai A, Dekumyoy P. Levels of infection with the lungworm Angiostrongylus cantonensis in terrestrial snails from Thailand, with Cryptozona siamensis as a new intermediate host. J Helminthol. 2016;90:737–741. doi: 10.1017/S0022149X15001042. [DOI] [PubMed] [Google Scholar]
- 14.Maldonado A, Júnior, Simões R, Thiengo SC. Angiostrongyliasis in the Americas. In: Morales-Lorenzo J, editor. Zoonosis. London: IntechOpen; 2012. pp. 303–320. [Google Scholar]
- 15.Beaver PC, Rosen L. Memorandum on the first report of Angiostrongylus in Man, by Nomura and Lin, 1945. Am J Trop Med Hyg. 1964;13:589–590. doi: 10.4269/ajtmh.1964.13.589. [DOI] [PubMed] [Google Scholar]
- 16.Thomé JW. Estado atual da sistemática dos Veronicellidae (Mollusca, Gastropoda) americanos, com comentários sobre sua importância econômica, ambiental e na saúde. Biociências. 1993;1:61–75. [Google Scholar]
- 17.Salgado NC, Coelho AC. Moluscos terrestres do Brasil (Gastrópodes operculados ou não, exclusive Veronicellidae, Milacidae e Limacidae) Rev Biol Trop. 2003;5(Suppl 3):149–189. [Google Scholar]
- 18.Simone LR. Land and freshwater molluscs of Brazil: an illustrated inventory on the Brazilian malacofauna, including neighbor regions of the South America, respect to the terrestrial and freshwater ecosystems. São Paulo: EGB; 2006. [Google Scholar]
- 19.Ohlweiler FP, Takahashi FY, Guimarães MC, Gomes SR, Kawano T. Manual de gastrópodes límnicos e terrestres do Estado de São Paulo associados às helmintoses. Porto Alegre: Redes; 2010. [Google Scholar]
- 20.Wallace GD, Rosen L. Techniques for recovering and identifying larvae of Angiostrongylus cantonensis from molluscs. Malacologia. 1969;7:427–438. [Google Scholar]
- 21.Thiengo SC. Presence of Strongyluris-like larvae (Nematoda) in some terrestrial molluscs in Brazil. Mem Inst Oswaldo Cruz. 1995;90:619–620. doi: 10.1590/s0074-02761995000500014. [DOI] [PubMed] [Google Scholar]
- 22.Garcia JS, Lúcio CS, Bonfim TC, Maldonado A, Júnior, Tunholi VM, Tunholi-Alves VM, et al. Metabolic and histopathological profile of Rattus norvegicus (Wistar) experimentally infected by Angiostrongylus cantonensis (Chen, 1935) Exp Parasitol. 2014;137:35–40. doi: 10.1016/j.exppara.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 23.Prosser SW, Velarde-Aguilar MG, León-Règagnon V, Hebert PDN. Advancing nematode barcoding: a primer cocktail for the cytochrome c oxidase subunit I gene from vertebrate parasitic nematodes. Mol Ecol Resour. 2013;13:1108–1115. doi: 10.1111/1755-0998.12082. [DOI] [PubMed] [Google Scholar]
- 24.Blaxter ML, De Ley P, Garey JR, Liu LX, Scheldeman P, Vierstraete A, et al. A molecular evolutionary framework for the phylum Nematoda. Nature. 1998;392:71–75. doi: 10.1038/32160. [DOI] [PubMed] [Google Scholar]
- 25.Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, et al. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28:1647–1649. doi: 10.1093/bioinformatics/bts199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oliveira AP, Gentile R, Maldonado A, Júnior, Torres EJ, Thiengo SC. Angiostrongylus cantonensis infection in molluscs in the municipality of São Gonçalo, a metropolitan area of Rio de Janeiro, Brazil: role of the invasive species Achatina fulica in parasite transmission dynamics. Mem Inst Oswaldo Cruz. 2015;110:739–744. doi: 10.1590/0074-02760150106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramos-de-Souza J, Thiengo SC, Gomes SR, Fernandez MA, Clímaco MC, Jain S, et al. Native and alien terrestrial snails in urban areas of Sergipe, Northeast Brasil. Tentacle. 2017;25:9–13. [Google Scholar]
- 28.Thiengo SC. Helmintoses de interesse médico-veterinário transmitidas por moluscos no Brasil. In: Santos SB, Pimenta AD, Thiengo SC, Fernandez MA, Absalão RS, editors. Tópicos em malacologia: ecos do XVIII EBRAM. Rio de Janeiro: Sociedade Brasileira de Malacologia; 2007. pp. 287–294. [Google Scholar]
- 29.Caldeira RL, Mendonça CL, Goveia CO, Lenzi HL, Graeff-Teixeira C, Lima WS, et al. First record of molluscs naturally infected with Angiostrongylus cantonensis (Chen, 1935) (Nematoda: Metastrongylidae) in Brazil. Mem Inst Oswaldo Cruz. 2007;102:887–889. doi: 10.1590/s0074-02762007000700018. [DOI] [PubMed] [Google Scholar]
- 30.Espírito-Santo MC, Pinto PL, Mota DJ, Gryschek RC. The first case of Angiostrongylus cantonensis eosinophilic meningitis diagnosed in the city of São Paulo, Brazil. Rev Inst Med Trop Sao Paulo. 2013;55:129–132. doi: 10.1590/s0036-46652013000200012. [DOI] [PubMed] [Google Scholar]
- 31.Moreira VL, Giese EG, Melo FT, Simões RO, Thiengo SC, Maldonado A, Júnior, et al. Endemic angiostrongyliasis in the Brazilian Amazon: natural parasitism of Angiostrongylus cantonensis in Rattus rattus and R. norvegicus, and sympatric giant African land snails, Achatina fulica. Acta Trop. 2013;125:90–97. doi: 10.1016/j.actatropica.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 32.Thiengo SC, Amato SB. Phyllocaulis variegatus (Mollusca: Veronicellidae), a new intermediate host for Brachylaima sp. (Digenea: Brachylaimatidae) Mem Inst Oswaldo Cruz. 1995;90:621–622. [Google Scholar]
- 33.Colley E, Fisher ML. Avaliação dos problemas enfrentados no manejo do caramujo gigante africano Achatina fulica (Gastropoda: Pulmonata) no Brasil. Zoologia. 2009;26:674–683. [Google Scholar]