Abstract
Objective
Cognitive deficits, especially those of information processing speed (IPS), are common in multiple sclerosis (MS), however, the underlying neurobiological mechanisms remain poorly understood. In this study, we examined structural and functional brain changes separately, but also in an integrative manner, in relation to IPS performance.
Methods
IPS was measured using the symbol digit modalities test (SDMT) in 330 MS patients and 96 controls. Patients with IPS impairment (IPS-I, z-score < −1.5) were compared to patients with preserved IPS performance (IPS-P) on volumetric measures, white matter integrity loss (using diffusion tensor imaging) and the severity of functional connectivity changes (using resting-state fMRI). Significant predictors of IPS performance were used to create groups of mild or severe structural and/or functional damage to determine the relative effect of structural and/or functional changes on IPS.
Results
IPS-I patients, compared to IPS-P patients, showed lower deep gray matter volume and less WM integrity, but stronger increases in functional connectivity. Patients with predominantly structural damage had worse IPS (z-score = −1.49) than patients with predominantly functional changes (z-score = −0.84), although both structural and functional measures remained significant in a regression model. Patients with severe structural and functional changes had worst IPS (z-score = −1.95).
Conclusion
The level of structural damage explains IPS performance better than functional changes. After integrating functional and structural changes, however, we were able to detect more subtle and stepwise decline in IPS. In subgroups with a similar degree of structural damage, more severe functional changes resulted in worse IPS scores than those with only mild functional changes.
Keywords: Multiple sclerosis, Cognition/IPS, fMRI, DTI, Volumetric MRI
Highlights
-
•
Impaired information processing in MS relates to structural and functional changes.
-
•
There is no one-to-one relation between structural and functional damage.
-
•
MS patients with severe structural and functional changes have the lowest IPS.
-
•
Structural changes affect information processing more than functional changes.
-
•
Functional changes seem to mediate the effect of structural damage on IPS.
1. Introduction
Multiple sclerosis (MS) is a progressive inflammatory and neurodegenerative disease of the central nervous system characterized by demyelination and neuronal loss (Stys et al., 2012). In addition to physical disabilities, cognitive deficits are common, affecting approximately 40–70% of the MS patients (Amato et al., 2006; Chiaravalloti and DeLuca, 2008). Among cognitive deficits, problems with information processing speed (IPS) are frequently seen and already present early in the disease (Archibald and Fisk, 2000; Deloire, 2005; Chiaravalloti and DeLuca, 2008; Khalil et al., 2011).
In MS, previous imaging studies have shown that IPS deficits are related to structural or functional brain abnormalities (Benedict et al., 2005; Dineen et al., 2009; Khalil et al., 2011; Leavitt et al., 2012; Schoonheim et al., 2013, Schoonheim et al., 2014; Bergsland et al., 2016; Moroso et al., 2017), including deep gray matter (DGM) atrophy (Batista et al., 2012) and white matter (WM) integrity loss (Dineen et al., 2009), as well as changes in functional connectivity (Leavitt et al., 2012; Schoonheim et al., 2013). Unfortunately, studies that have integrated structural and functional measures to explain IPS deficits are currently lacking. Although structural and functional brain characteristics are intertwined to a certain extent, there is no simple one-to-one relation between these two (Hillary and Grafman, 2017). Therefore, it might be that structural damage may occur in the presence of minor functional changes, but may also involve severe functional changes. As complex cognitive functions like IPS arise from an efficient interplay between the brains' functional and structural architecture, varying levels of structural and/or functional damage may also result in different levels of IPS impairment in MS (Park and Friston, 2013). This emphasizes the need to consider both structural and functional measures simultaneously to be able to better understand IPS deficits.
We hypothesize that to increase our understanding of the underlying neurobiology of IPS deficits an integrated measure of functional and structural brain changes is essential, instead of studying either one or the other (Chard and Trip, 2017). Therefore, we integrated advanced functional and structural MRI measures to examine the relative and joint impact of functional and structural brain changes in explaining IPS performance.
2. Methods
2.1. Participants
All participants with complete functional and structural imaging protocols (see below) of the Amsterdam MS Cohort (Daams et al., 2015; Schoonheim et al., 2015) were included, resulting in 330 MS patients (age 48.14 ± 10.06 years) and 96 healthy controls (HC; age 45.9 ± 10.5 years). All patients were without clinical relapses and steroid treatment for at least two months. The local institutional ethics review board approved the study and written informed consent was obtained from all participants.
2.2. Neuropsychological testing
All subjects underwent neuropsychological evaluation using an expanded Brief Repeatable Battery of Neuropsychological tests, as previously described (Meijer et al., 2017). Of these assessments, we formed groups based on IPS only, as measured with the symbol digit modalities test (SDMT) (Benedict et al., 2017), which was corrected for effects of sex, age and education (Amato et al., 2006). These scores were subsequently converted to z-scores based on the mean and standard deviation (SD) of the HC and used to categorize MS patients into either IPS impaired (IPS-I, z-score ≤ −1.5 on SDMT) or IPS preserved (IPS-P, z-score > −1.5 on SDMT). Differentiation into this subgroups was performed to increase the sensitivity for detecting neural correlates of clinically relevant differences in IPS performance. For descriptive purposes, similar cut-off scores were applied to the remaining cognitive domains.
2.3. MR imaging
MR imaging was performed on a 3 T scanner (GE Signa HDxt, Milwaukee, WI, USA) using an eight-channel head-coil. The structural imaging protocol included a 3D T1-weighted inversion-prepared fast spoiled gradient recall sequence (FSPGR, TR 7.8 ms, TE 3 ms, TI 450 ms, FA 12°, sagittal 1.0-mm sections, 0.94 × 0.94mm2 in-plane resolution) for volumetric measurements, a 3D fluid-attenuated inversion-recovery sequence (FLAIR, TR 8000 ms, TE 125 ms, TI 2350 ms, sagittal 1.2 mm slices, 0.98 × 0.98mm2 in-plane resolution) for lesion detection and a diffusion tensor imaging (DTI) sequence covering the entire brain using five volumes without directional weighting (i.e. b0) and 30 volumes with non-collinear diffusion gradients (EPI, b = 1000s/mm2, TR 13000 ms, TE 91 ms, FA 90°, 2.4 mm contiguous axial slices, 2x2mm2 in-plane resolution). Brain function was assessed using resting-state functional MRI with whole-brain coverage using 202 volumes, of which the first two were discarded (EPI, TR 2200 ms, TE 35 ms, FA 20°, 3 mm contiguous axial slices, 3.3 × 3.3mm2 in-plane resolution).
2.4. Volumetric measures
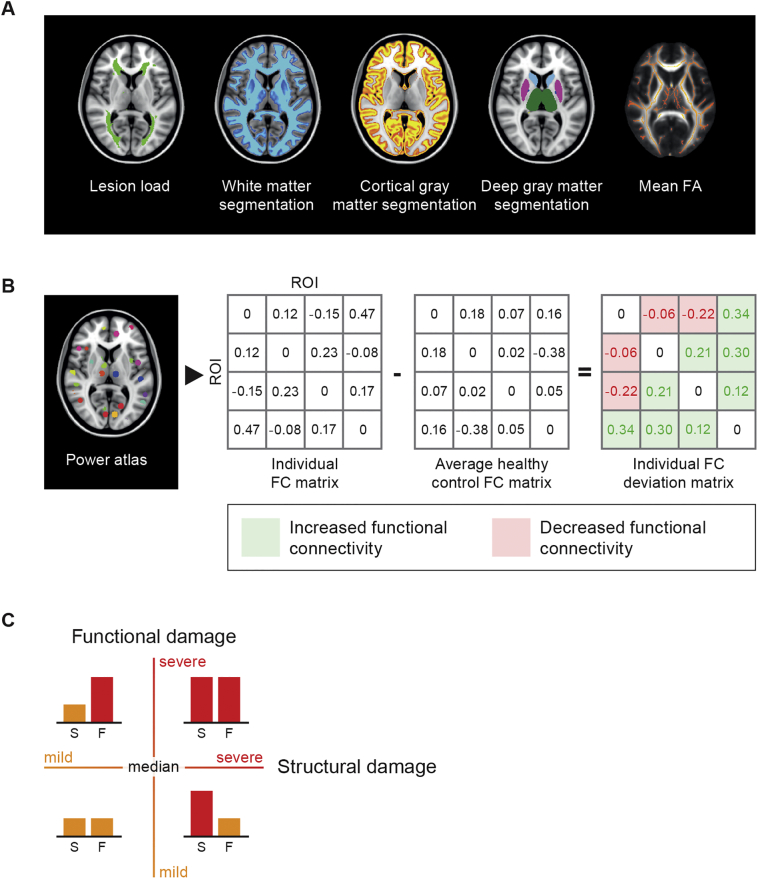
Hyperintense lesions were automatically segmented on the FLAIR images (Steenwijk et al., 2013) and filled on the T1 using LEAP (Chard et al., 2010) to minimize the impact of lesions on volumetric measures and registration algorithms. Normalized gray matter (NGMV) and WM (NWMV) volumes were calculated with SIENAX (part of FSL 5). FIRST was used to segment deep gray matter (DGM) structures and the volume of these structures were computed, summed and normalized for head size, resulting in normalized DGM volume (NDGMV). Normalized cortical volumes (NCGMV) were computed by subtracting FIRST segmentations from the SIENAX-based GM segmentation (Fig. 1A). To examine regional differences, voxelwise gray matter density was compared between IPS-I and IPS-P patients using the standard voxel-based morphometry pipeline (part of FSL) using a permutation algorithm (‘randomise’ from FSL) with 5000 permutations and threshold-free cluster enhancement to correct for multiple comparisons.
Fig. 1.
Data analysis flow chart. To define the structural brain status, the most commonly addressed global structural measures were determined, including brain volumes, lesion load and whole-brain white matter integrity (A) We subsequently aimed to design a whole-brain functional network measure representing the severity of functional connectivity changes in each individual. To compute a measure that could reflect the whole-brain functional brain status, an average healthy control matrix was computed. This matrix was subsequently subtracted from the individual functional connectivity matrices resulting in an individual deviation matrix. An example matrix consisting of the connectivity values between four regions is shown (B). Patients were assigned to one of the four groups based on their level of structural and functional changes (C).
2.5. Severity of fractional anisotropy-based damage
DTI data were pre-processed using FSL5, including motion- and eddy current correction on images and gradient vectors, followed by diffusion tensor fitting. Since fractional anisotropy (FA) is the most commonly examined diffusion measure in MS, we focused on whole-brain FA as a measure of WM integrity, also to limit the number of dependent variables. To obtain skeletonised FA maps, the default tract-based spatial statistics (TBSS) pipeline was used (Smith et al., 2006). Mean FA scores of the WM skeleton were extracted for each subject as a measure of whole-brain WM integrity. To examine regional differences, voxel-wise FA values were compared between IPS-I and IPS-P patients using a permutation algorithm (‘randomise’ from FSL) with 5000 permutations and threshold-free cluster enhancement to correct for multiple comparisons.
2.6. Processing of functional images
Pre-processing of fMRI data was carried out using the default pipeline of MELODIC, consisting of motion correction, removal of non-brain tissue, spatial smoothing using a 5 mm full-width-at-half-maximum Gaussian kernel and high-pass temporal filtering to cut off frequencies below 0.01 Hz. All resting-state fMRI scans were checked for artefacts, excessive motion and registration errors. The individual level of motion was calculated based on the average frame-to-frame motion. The amount of motion was not different between HC and MS (p = .34) and no subject moved >0.3 mm. To remove signal originating from residual non-brain tissue as well as from voxels sensitive to EPI-distortions, voxels with a signal intensity in the lowest quartile of the robust range were excluded (Eijlers et al., 2017; Meijer et al., 2017). For this study we used the power atlas (Power et al., 2011), which was specifically designed to study brain networks and consists of 264 GM regions. This atlas was registered to 3DT1 space with inverted non-linear registration parameters, using nearest neighbour interpolation. The atlas was then multiplied with the SIENAX and FIRST segmentations to include GM only. Subsequently, inverted boundary-based registration matrices were used to register the atlas to each individual fMRI scan using nearest neighbour interpolation. Regions of interest (ROIs) were excluded if these contained missing values in >10% of the subjects after the final registration to fMRI, leaving 238 GM regions in the final atlas for which mean time series were calculated. Functional connectivity matrices were formed by calculating Pearson correlations between the time series of all these pairs of nodes. Since the controversial nature of negative connectivity values, only positive correlation coefficients were considered (Fox et al., 2005; Chai et al., 2012). Since the average level of functional connectivity is known to be highly variable which could hamper between-group comparisons, (Finn et al., 2015) these raw correlation coefficients were converted to relative connectivity z-scores by subtracting each individual's mean connectivity and dividing it by the SD of each participants' functional connectivity matrix (Meijer et al., 2017).
2.7. Severity of functional network changes
While global structural measures (e.g. NGMV, NWMV and whole-brain FA) are commonly computed, there is no such equivalent with regard to functional measures. Studies usually address regional changes in functional connectivity. After computing global structural measures, however, we subsequently aimed to design a whole-brain functional network measure representing the severity of functional connectivity changes in each individual. In other words, we needed an individual quantification of the amount of deviation from normal in functional connectivity levels. To determine “normal” connectivity levels for each link we constructed an average normalized HC matrix (based on all 96 HC). Subsequently, we subtracted each individual normalized connectivity matrix from aforementioned average HC matrix, resulting in a deviation score per element of the connectivity matrix. The values these deviation matrices could thus either be positive, reflecting an increase in functional connectivity, or negative, reflecting a decrease in functional connectivity (Fig. 1B). For each individual matrix, the average connectivity level of increased and decreased links were used as two measures of the severity of functional network changes for subsequent analyses.
2.8. Function versus structure
Together, this pipeline resulted in four measures of structural damage (i.e. NDGMV, NCGMV, lesion volume and severity of WM integrity loss) and two measures of functional damage (i.e. the severity of increased and decreased functional connectivity changes). First we compared these measures between IPS-I and IPS-P patients. Subsequently, in the entire MS group, a backward regression model was conducted to determine which of these significant functional and/or structural variables were the main independent predictors of IPS performance. Finally, to be able to integrate structural and functional measures, structural predictors were paired with functional predictors to determine the combined amount of damage for each patient individually. Four groups of patients, classified based on median splits of functional and structural damage, were examined in relation to IPS performance, namely
-
1.
Patients with mild functional damage and mild structural damage;
-
2.
Patients with severe functional damage but only mild structural damage, from here on referred to as “predominantly functional damage”;
-
3.
Patients with mild functional damage but severe structural damage, from here on referred to as “predominantly structural damage”;
-
4.
Patients with both severe functional and severe structural damage (Fig. 1C).
Mild damage was defined as scores lower than the median score (based on the MS group), whereas severe damage was defined as scores higher than the median score. In all groups the relation between the severity of structural and functional damage with IPS performance was investigated. To limit the number of comparisons, only consecutive groups were compared on IPS performance.
2.9. Statistical analysis
Statistical analyses were conducted in SPSS version 22.0 (Chicago, IL, USA). All variables were checked for normal distributions by histogram inspection and normality tests. Before conducting statistical analyses, it was checked whether the required assumptions were met. General linear models were used to compare measures of interest between groups including age, sex and education as covariates. Non-parametric tests were used to compare not normally distributed measures of interest. The linear regression model used to determine independent predictors of IPS followed a backward selection procedure. To examine how functional and structural measures were interrelated Pearson correlations were calculated for normally distributed variables, or Spearman's Rank-Order correlations for non-normally distributed variables. Test statistics were considered significant with p-values <.05 (Bonferroni corrected). The threshold for Bonferroni correction was based on the number of statistical tests conducted for each modality. This means that the statistical analyses regarding structural measures were corrected for six tests (i.e. NGMV, NWMV, NDGMV, NCGMV, lesion load, FA), whereas statistical analyses regarding functional measures were corrected for two tests (i.e. increased and decreased whole-brain functional connectivity). Post hoc tests for testing differences between the four groups of patients, classified based on median splits of functional and structural damage, were corrected for the total number of comparisons.
3. Results
3.1. Demographics and cognitive profiles: IPS-I versus IPS-P
Of all MS patients, 130 (39%) were defined as IPS-I, and 200 (61%) as IPS-P (see Table 1). No difference in sex was found for the two groups. However, IPS-I patients were older and had a lower educational level compared to IPS-P patients. The IPS-I group consisted of a higher percentage of progressive patients (36% versus 20%; χ2 = 0.59; p = .001) and had higher EDSS scores (EDSS = 3.5 versus EDSS = 3.0; H = 28.318; p < .001) compared to IPS-P patients. The symptom duration was longer in IPS-I patients compared to IPS-P patients (H = 7.09; p = .008). Of the IPS-I patients, 33% showed impairment on one additional cognitive domain, 15% on two cognitive domains, 14% on three cognitive domains and 14% on more than three cognitive domains. Of the IPS-P patients, 24% showed impairment on one cognitive domain, 14% on two cognitive domains, 6% on three cognitive domains and 6% on more than three cognitive domains.
Table 1.
Demographics IPS impaired and preserved patients and HC.
| IPS impaired |
IPS preserved |
HC |
Test statistic |
P-value |
|
|---|---|---|---|---|---|
| N | 130 | 200 | 96 | ||
| Age, years | 50.01 (11.33) | 46.93 (10.74) | 45.87 (10.45) | F = 4.80 | 0.01a, c |
| Women/men | 85/45 | 140/60 | 56/40 | χ2 = 3.96 | 0.14 |
| Educational level, years* | 4.00 (3.00–6.00) | 5.00 (4.00–6.00) | 6.00 (4.00–7.00) | H = 12.65 | 0.002a, b |
| RRMS/SPMS/PPMS | 83/31/16 | 160/20/20 | – | χ2 = 0.59 | 0.002 |
| Symptom duration, years* | 15.82 (7.8–21.59) | 9.80 (6.63–20.32) | – | H = 7.09 | 0.001 |
| EDSS* | 4.0 (3.0–6.0)) | 3.0 (2.00–4.00) | – | H = 28.318 | 0.04 |
| SDMT | 37 (8.6) | 58 (8.9) | 61 (9.81) | F = 255.73 | 0.001a, b, c |
IPS: information processing speed; RRMS: relapsing remitting multiple sclerosis; SPMS: secondary progressive multiple sclerosis; PPMS: primary progressive multiple sclerosis; EDSS: expanded disability status scale. * not normally distributed data for which median (interquartile range) are provided.. Test statistics were provided for the statistical analyses that was used to compare the three groups.
Significant difference between IPS impaired and HC.
Significant difference between IPS preserved and HC.
Significant difference between IPS impaired and IPS preserved.
3.2. Structural and functional damage: IPS-P versus IPS-I
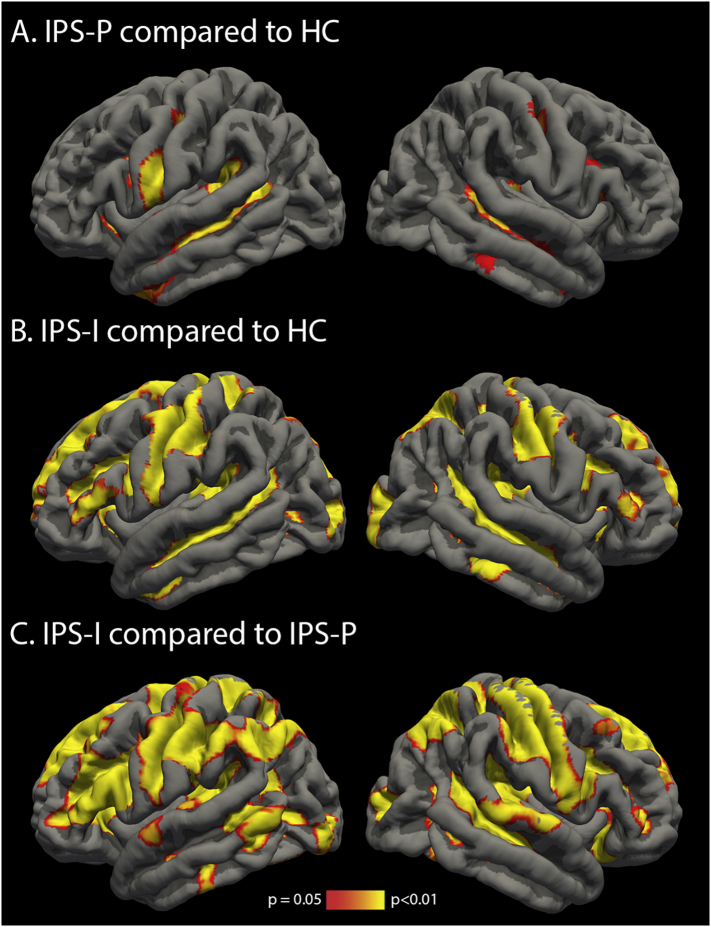
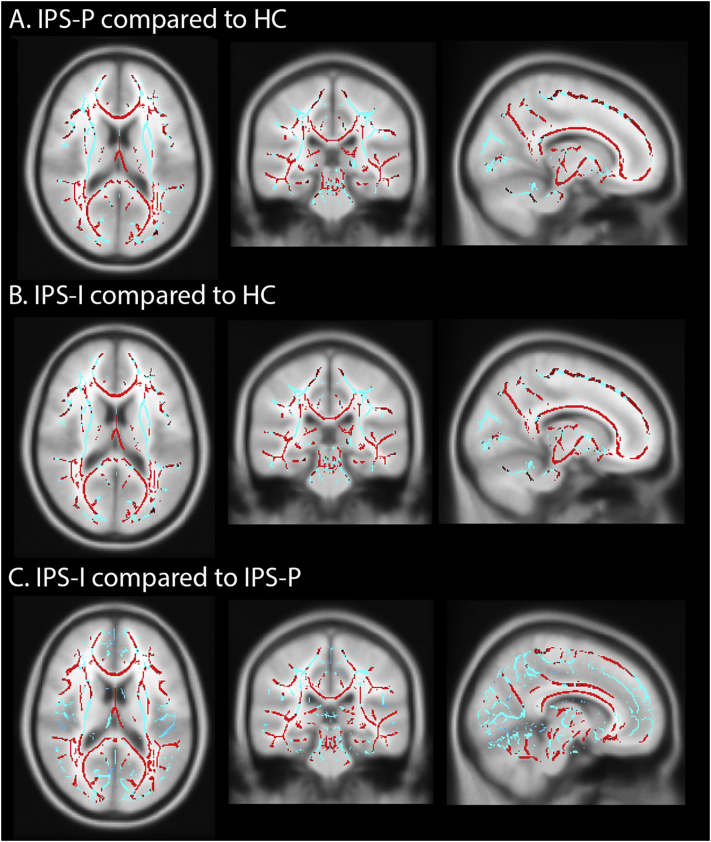
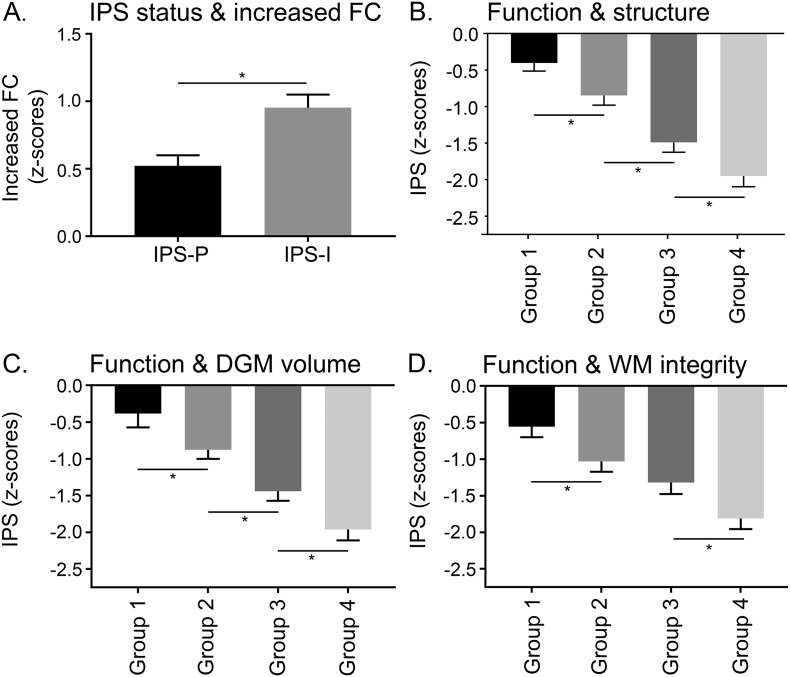
Both patient groups showed lower brain volumes and loss of WM integrity, as well as increased and decreased functional connectivity compared to HC (all p < .001, see Table 2). Compared to IPS-P, IPS-I patients showed the lowest brain volumes, lowest WM integrity and highest lesion volumes (all p < .001). The VBM analysis showed regions with lower volume throughout the cortical gray matter in the IPS-I patients compared to the IPS-P patients (Supplementary Fig. 1C). The TBSS analysis demonstrated more severe and widespread WM integrity loss in IPS-I patients compared to IPS-P patients (Supplementary Fig. 2C). Additionally, IPS-I patients showed increased functional connectivity compared to IPS-P patients (F = 8.17; pcor = 0.01; Fig. 2A). In the entire MS group, increased functional connectivity was associated with lower NCGMV and NDGMV (r = −0.218 and r = −0.179, pcor ≤ 0.001), as well as higher lesion load and loss of WM integrity (rho = 0.180 and r = −0.231; pcor ≤ 0.001). Similar findings were obtained when comparing IPS-I and IPS-P groups after excluding progressive MS patients.
Table 2.
Structural and functional MRI characteristics of IPS-I patients, IPS-P patients and HC.
| IPS impaired |
IPS preserved |
HC |
Test statistic |
P-value |
|
|---|---|---|---|---|---|
| N | 130 | 200 | 96 | ||
| NGMV (ml) | 758.81 (60.42) | 799.98 (59.04) | 818.54 (53.13) | F = 38.51 | <0.001a, b, c |
| NWMV (ml) | 657.55 (35.95) | 675.98 (32.87) | 697.09 (31.29) | F = 37.01 | <0.001a, b, c |
| NDGMV (ml) | 52.76 (7.49) | 58.39 (5.28) | 62.91 (37.35) | F = 85.25 | <0.001a, b, c |
| NCGMV (ml) | 726.33 (56.77) | 763.64 (47.06) | 779.41 (52.27) | F = 32.76 | <0.001a, b, c |
| Normalized TLL (ml)* | 21.67 (10.71–35.59) | 10.51 (5.62–17.83) | – | H = 40.95 | <0.001 |
| FA whole-brain | 0.39 (0.03) | 0.40 (0.02) | 0.42 (0.02) | F = 55.45 | <0.001a, b, c |
| Decreased FC | −0.70 (0.95) | −0.42 (0.97) | 0 (1.0) | F = 12.61 | <0.001a, b |
| Increased FC | 0.95 (1.13) | 0.52 (1.12) | 0 (1.0) | F = 15.85 | <0.001a, b, c |
IPS: information processing speed; NGMV: normalized gray matter volume; NWMV: normalized white matter volume; NDGMV: normalized deep gray matter volume; NCGMV: normalized cortical gray matter volume; TLL: total lesion load; FA: fractional anisotropy; FC: functional connectivity. * not normally distributed data for which median (interquartile range) are provided. Test statistics were provided for the statistical analyses that was used to compare the three groups. For descriptive purposes, functional connectivity values were converted to z-scores based on the mean and SD of the HC.
Significant difference between IPS impaired and HC.
Significant difference between IPS preserved and HC.
Significant difference between IPS impaired and IPS preserved.
Supplementary Fig. 1.
Reduced regional cortical grey matter volume in (A) IPS-P patients compared to HC, (B) IPS-I patients compared to HC and (C) IPS-I patients compared to IPS-P patients.
Supplementary Fig. 2.
Regionally loss of white matter integrity in (A) IPS-P patients compared to HC, (B) IPS-I patients compared to HC and (C) IPS-I patients compared to IPS-P patients.
Fig. 2.
IPS status is reflected by different severities of functional and structural changes. IPS impaired (IPS-I) patients showed more severe increases in functional connectivity than IPS preserved (IPS-P) patients (A). A similar stepwise deterioration in IPS performance was observed for functional changes combined with a composite score of structural damage (B), functional changes combined with DGM volume loss (C) and functional changes combined with WM integrity loss (D). In general, mild functional and mild structural changes were associated with the best IPS performance. Severe functional changes were associated with better IPS than severe structural changes, whereas severe functional changes combined with severe structural changes were associated with the worst IPS. * indicates a significant difference between groups after Bonferroni correction.
3.3. Which measure is the best predictor of IPS deficits in MS?
The final model (R2 = 0.454; p < .001) contained the following predictors for worse IPS performance: lower NDGMV (standardized β = 0.374; p < .001), older age (β = −0.206; p < .001), lower education (β = 0.180; p < .001), loss of WM integrity (β = 0.152; p = .012), male sex (β = −0.116; p = .010) and increased functional connectivity (β = −0.102; p = .021). Based on this regression model, NDGMV, loss of WM integrity and increased functional connectivity were selected as structural and functional measures of interest. To obtain the individual effect sizes of these measures, separate models were conducted containing demographic variables and the measure of interest. As a result, NDGMV volume explained 42% of the variance in IPS (p < .001), WM integrity 37% (p < .001) and increased functional connectivity 24% (p < .001).
3.4. Impact of different severities of functional and structural damage
After identifying NDGMV, loss of WM integrity and functional connectivity as independent predictors of IPS, four groups were created based on the severity of functional and structural damage (Supplementary Table. 1): mild structural and functional damage (group 1), predominantly functional damage (group 2), predominantly structural damage (group 3) and functional as well as structural damage (group 4). Since two structural measures appeared to be predictors of IPS, NDGMV and WM integrity were combined in one composite score by first creating z-scores relative to the healthy controls for both measures separately and then add these z-scores. In this way, NDGMV and loss of WM integrity were averaged into one structural damage composite score (Fig. 2D). The four different groups with different severities of structural and functional damage did differ on IPS performance (F = 18.69; p ≤ .001). As expected, group 4 had worst IPS performance compared to all other groups (z-score = −1.95(1.41); group 4 vs 3; pcor = 0.02). Group 1 had best IPS performance (z-score = −0.40(1.10); group 1 vs 2; pcor = 0.02). Additionally, group 3 had worse IPS performance (z-score = −1.49(1.12)) than group 2 (z-score = −0.84(1.10); pcor = 0.002). Groups 1 vs 3 and 2 vs 4 did not differ on the severity of functional connectivity, while groups 1 vs 2 and 3 vs 4 did not differ on the level of structural damage.
3.5. Impact of DGM atrophy and loss of WM integrity separately
The results of creating patient groups based on the two structural imaging measures separately are highly similar to abovementioned results, and can be found in Fig. 2B and 2C. A similar stepwise decline in IPS performance was observed for NDGMV loss together with increased functional connectivity (F = 34.83; pcor ≤ 0.001; Fig. 2C). For WM integrity also a significant group effect was seen (F = 25.16; pcor ≤ 0.001), but no difference in IPS performance was observed between patients with predominantly WM integrity loss and those with predominantly functional damage (pcor ≥ 0.05; Fig. 2B).
4. Discussion
Using innovative and integrated measures for functional and structural damage, we were able to demonstrate that different severities of functional and structural damage reflect stepwise worsening of IPS. MS patients with mild functional and mild structural damage had the best, although lower than HC, IPS. A further decline in IPS was found in MS patients with predominantly functional damage. MS patients with predominantly structural damage had worse IPS than the previous two groups, whereas MS patients with both severe functional and severe structural damage were worst off. The severity of functional network changes seemed to have an additive effect on IPS performance, as a similar degree of structural damage can be accompanied with either mild or severe functional network changes, resulting in different levels of IPS.
Until now functional connectivity studies mostly focused on a few selected regions (Hulst et al., 2012; Schoonheim et al., 2015; Rocca et al., 2017b). In this study, we defined an innovative whole-brain functional connectivity measure to assess the severity of connectivity changes in one single measure, which allows to map widespread functional connectivity changes. Both increased and decreased functional connectivity were observed in MS patients compared to HC, but only increased functional connectivity changes discriminated IPS-P from IPS-I patients. Several studies have reported increased levels of functional connectivity during rest as a correlate of cognitive deficits (Hawellek et al., 2011; Leavitt et al., 2014; Schoonheim et al., 2015; Meijer et al., 2017). This increase in functional connectivity could indicate altered functional network activities, including increased levels of network synchrony and abnormal oscillatory rhythm (Denève and Machens, 2016). In the current study, changes in whole-brain measures were related to IPS performance. The rationale for investigating IPS was based on its high frequency and early presence in the disease. In addition, this domain is likely to depend on the interaction across many distant brain regions and cannot be assigned to one single brain region, and therefore differences in performance might reflect changes in whole-brain structural and functional measures. Our regional analyses (VBM and TBSS) support this hypothesis that worse IPS performance cannot be assigned to one single brain region, but involves changes across the brain. However, since IPS scores might influence and might be influenced by deficits in other cognitive domains, it is unfortunately not feasible to examine IPS deficits in isolation (DeLuca et al., 2004; Forn et al., 2008). This is also shown by our own data since more cognitive deficits were detected in the IPS-I group.
Since clinically relevant thresholds of WM integrity loss, GM atrophy and functional connectivity changes are currently lacking, we have used a median split in the patient group as cut-off to categorize patients according to the severity of functional and structural damage. After integrating structural and functional measures, our findings show that in the presence of predominantly structural damage worse IPS performance was observed (z-score = −1.49) when compared to predominantly functional damage (z-score = −0.84). Larger cognitive consequences of structural damage were also shown by the regression models, i.e. structural measures possessed a stronger predictive value. The strong influence of structural measures on IPS performance was previously reported as well. Not only loss of WM integrity (Dineen et al., 2009; Schoonheim et al., 2014), but also loss of DGM volume (Batista et al., 2012; Bergsland et al., 2016) was associated with worse IPS. This might be explained by the relatively rigid nature of the brains' structural architecture, whereas the functional network might be better able to circumvent damage as a result of its dynamic and more flexible properties (Park and Friston, 2013). In addition, in our cohort the structural measures were more abnormal than the observed functional changes, which could also explain the larger predictive value of structural measures for IPS performance.
Among the structural measures, it seemed that DGM volume had a stronger effect than WM integrity loss. Contrary to what we observed for DGM volume, there was no significant difference in IPS performance between patients with predominantly WM integrity loss and those with predominantly functional damage. Like fMRI changes, WM integrity loss is likely to reflect more subtle damage, especially in the so-called normal appearing WM (Miller et al., 2003; Moll et al., 2011), while a measure like DGM atrophy is an MRI marker for (substantial) neurodegeneration (Popescu et al., 2015; Rocca et al., 2017a), possibly explaining the larger impact of the latter on IPS. It is important, however, to note that we focused on global brain measures for this analysis, while certain focal structural and functional changes might influence the results differently.
Although structural damage was a strong predictor for IPS performance, investigating the joint impact of structural and functional measures showed that some subgroups with a similar degree of structural damage (i.e. both group 1 and group 2 as well as group 3 and group 4) had different degrees (i.e. mild or severe) of functional changes. For example, in some patients, severe structural damage occurred simultaneously with severe functional changes (group 4), associated with worse IPS scores than when accompanied with mild functional changes (group 3). One could hypothesize that in this subgroup the functional network “suffers” from the structural damage. The absence of a strict one-to-one relation between the level of structural and functional damage, emphasizes the value of integrating both measures. Our findings showed that adding information about the severity of functional changes is needed to distinguish between different levels of IPS performance in patients with similar degrees of structural damage. This might indicate that the functional network acts as mediating factor between the level of structural damage and IPS performance. Additionally, the resilience of the functional brain network might also limit cognitive consequences, as observed in patients with severe structural damage and only mild functional damage. More resilient networks might theoretically be able to cope with a larger amount of structural damage (Albert et al., 2000; Aerts et al., 2016).
In summary, our findings suggest that damage to the structural brain architecture has larger consequences for IPS than functional brain changes. After integrating functional and structural changes we were able to detect subtle stepwise changes in IPS performance. Insight into functional network changes is especially relevant to distinguish between patients with similar levels of structural damage, but different levels of IPS performance. This emphasizes the added value for an integrated measure to be able to explain IPS performance more accurately in patients with MS.
The following are the supplementary data related to this article.
Demographics and MRI characteristics of the groups with mild and severe structural and/or functional changes.
Acknowledgments
Acknowledgements
This study was supported by the Dutch MS Research Foundation, grant numbers 08-650, 13-820 and 14-358e.
Declarations of interest
None.
References
- Aerts H., Fias W., Caeyenberghs K., Marinazzo D. Brain networks under attack: Robustness properties and the impact of lesions. Brain. 2016;139:3063–3083. doi: 10.1093/brain/aww194. [DOI] [PubMed] [Google Scholar]
- Albert R, Jeong H, Barabási A-L (2000) Error and attack tolerance of complex networks. Nature, 406:378–382. 10.1038/35019019 [DOI] [PubMed]
- Amato M.P., Portaccio E., Goretti B., Zipoli V., Ricchiuti L., De Caro M.F., Patti F., Vecchio R., Sorbi S., Trojano M. The Rao ’ s Brief Repeatable Battery and Stroop test : normative values with age, education and gender corrections in an Italian population. Mult. Scler. J. 2006;12:787–793. doi: 10.1177/1352458506070933. [DOI] [PubMed] [Google Scholar]
- Archibald C.J., Fisk J.D. Information processing efficiency in patients with multiple sclerosis. J. Clin. Exp. Neuropsychol. 2000;22:686–701. doi: 10.1076/1380-3395(200010)22:5;1-9;FT686. [DOI] [PubMed] [Google Scholar]
- Batista S., Zivadinov R., Hoogs M., Bergsland N., Heininen-Brown M., Dwyer M.G., Weinstock-Guttman B., Benedict R.H.B. Basal ganglia, thalamus and neocortical atrophy predicting slowed cognitive processing in multiple sclerosis. J. Neurol. 2012;259:139–146. doi: 10.1007/s00415-011-6147-1. [DOI] [PubMed] [Google Scholar]
- Benedict R.H.B., Zivadinov R., Carone D.A., Weinstock-Guttman B., Gaines J., Maggiore C., Sharma J., Tomassi M.A., Bakshi R. Regional lobar atrophy predicts memory impairment in multiple sclerosis. Am. J. Neuroradiol. 2005;26:1824–1831. 26/7/1824 [PMC free article] [PubMed] [Google Scholar]
- Benedict R.H., DeLuca J., Phillips G., Larocca N., Hudson L.D., Rudick R., Multiple Sclerosis Outcome Assessments Consortium Validity of the Symbol Digit Modalities Test as a cognition performance outcome measure for multiple sclerosis. Mult. Scler. 2017;23:721–733. doi: 10.1177/1352458517690821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergsland N., Zivadinov R., Dwyer M.G., Weinstock-Guttman B., Benedict R.H. Localized atrophy of the thalamus and slowed cognitive processing speed in MS patients. Mult. Scler. J. 2016;22:1327–1336. doi: 10.1177/1352458515616204. [DOI] [PubMed] [Google Scholar]
- Chai X.J., Castañán A.N., Öngür D., Whitfield-Gabrieli S. Anticorrelations in resting state networks without global signal regression. NeuroImage. 2012;59:1420–1428. doi: 10.1016/j.neuroimage.2011.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chard D., Trip S. Resolving the clinico-radiological paradox in multiple sclerosis. F1000Res. 2017;6:1828–1837. doi: 10.12688/f1000research.11932.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chard D.T., Jackson J.S., Miller D.H., Wheeler-Kingshott C.A.M. Reducing the impact of white matter lesions on automated measures of brain gray and white matter volumes. J. Magn. Reson. Imaging. 2010;32:223–228. doi: 10.1002/jmri.22214. [DOI] [PubMed] [Google Scholar]
- Chiaravalloti N.D., Deluca J. Cognitive impairment in multiple sclerosis. Lancet. 2008;7:1139–1151. doi: 10.1016/S1474-4422(08)70259-X. [DOI] [PubMed] [Google Scholar]
- Daams M., Steenwijk M.D., Wattjes M.P., Geurts J.J.G., Uitdehaag B.M.J., Tewarie P.K., Balk L.J., Pouwels P.J.W., Killestein J., Barkhof F. Unraveling the neuroimaging predictors for motor dysfunction in long-standing multiple sclerosis. Neurology. 2015;85:248–255. doi: 10.1212/WNL.0000000000001756. [DOI] [PubMed] [Google Scholar]
- Deloire M.S.A. Cognitive impairment as marker of diffuse brain abnormalities in early relapsing remitting multiple sclerosis. J. Neurol. Neurosurg. Psychiatry. 2005;76:519–526. doi: 10.1136/jnnp.2004.045872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deluca J., Chelune G.J., Tulsky D.S., Lengenfelder J., Chiaravalloti N.D. Is speed of Processing or Working memory the primary Information Processing Deficit in Multiple Sclerosis? J. Clin. Exp. Neuropsychol. 2004;26:550–562. doi: 10.1080/13803390490496641. [DOI] [PubMed] [Google Scholar]
- Denève S., Machens C.K. Efficient codes and balanced networks. Nat. Neurosci. 2016;19:375–382. doi: 10.1038/nn.4243. [DOI] [PubMed] [Google Scholar]
- Dineen R.A., Vilisaar J., Hlinka J., Bradshaw C.M., Morgan P.S., Constantinescu C.S., Auer D.P. Disconnection as a mechanism for cognitive dysfunction in multiple sclerosis. Brain a J Neurol. 2009;132:239–249. doi: 10.1093/brain/awn275. [DOI] [PubMed] [Google Scholar]
- Eijlers A.J.C., Meijer K.A., Wassenaar T.M., Steenwijk M.D., Uitdehaag B.M.J., Barkhof F., Wink A.M., Geurts J.J.G., Schoonheim M. Increased default-mode network centrality in cognitively impaired multiple sclerosis patients. Neurology. 2017;88:952–960. doi: 10.1212/WNL.0000000000003689. [DOI] [PubMed] [Google Scholar]
- Finn E.S., Shen X., Scheinost D., Rosenberg M.D., Huang J., Chun M.M., Papademetris X., Todd Constable R. Functional connectome fingerprinting: identifying individuals using patterns of brain connectivity. Nat. Neurosci. 2015;18:1–11. doi: 10.1038/nn.4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forn C., Belenguer A., Parcet-Ibars M.A., Ávila C. Information-processing speed is the primary deficit underlying the poor performance of multiple sclerosis patients in the Paced Auditory serial Addition Test (PASAT) J. Clin. Exp. Neuropsychol. 2008;30:789–796. doi: 10.1080/13803390701779560. [DOI] [PubMed] [Google Scholar]
- Fox M.D., Snyder A.Z., Vincent J.L., Corbetta M., Van Essen D.C., Raichle M.E. The human brain is intrinsically organized into dynamic. anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawellek D.J., Hipp J.F., Lewis C.M., Corbetta M., Engel A.K. Increased functional connectivity indicates the severity of cognitive impairment in multiple sclerosis. Proc. Natl. Acad. Sci. U. S. A. 2011;108:19066–19071. doi: 10.1073/pnas.1110024108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillary F.G., Grafman J.H. Injured Brains and Adaptive Networks: the Benefits and costs of Hyperconnectivity. Trends Cogn. Sci. 2017;21:385–401. doi: 10.1016/j.tics.2017.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulst H.E., Schoonheim M.M., Roosendaal S.D., Popescu V., Schweren L.J.S., van der Werf Y.D., Visser L.H., Polman C.H., Barkhof F., Geurts J.J.G. Functional adaptive changes within the hippocampal memory system of patients with multiple sclerosis. Hum. Brain Mapp. 2012;33:2268–2280. doi: 10.1002/hbm.21359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil M., Enzinger C., Langkammer C., Petrovic K., Loitfelder M., Tscherner M., Jehna M., Bachmaier G., Wallner-Blazek M., Ropele S., Schmidt R., Fuchs S., Fazekas F. Cognitive impairment in relation to MRI metrics in patients with clinically isolated syndrome. Mult. Scler. J. 2011;17:173–180. doi: 10.1177/1352458510384009. [DOI] [PubMed] [Google Scholar]
- Leavitt V.M., Wylie G., Genova H.M., Chiaravalloti N.D., Deluca J. Altered effective connectivity during performance of an information processing speed task in multiple sclerosis. Mult. Scler. 2012;18:409–417. doi: 10.1177/1352458511423651. [DOI] [PubMed] [Google Scholar]
- Leavitt V.M., Wylie G.R., Girgis P.A., Deluca J., Chiaravalloti N.D. Increased functional connectivity within memory networks following memory rehabilitation in multiple sclerosis. Brain Imaging Behav. 2014;8:394–402. doi: 10.1007/s11682-012-9183-2. [DOI] [PubMed] [Google Scholar]
- Meijer K.A., Eijlers A.J.C., Douw L., Uitdehaag B.M., Barkhof F., Geurts J.J.G., Schoonheim M.M. Increased connectivity of hub networks and cognitive impairment in multiple sclerosis. Neurology. 2017;88:2107–2114. doi: 10.1212/WNL.0000000000003982. [DOI] [PubMed] [Google Scholar]
- Miller D.H., Thompson A.J., Filippi M. Magnetic resonance studies of abnormalities in the normal appearing white matter and grey matter in multiple sclerosis. J. Neurol. 2003;250:1407–1419. doi: 10.1007/s00415-003-0243-9. [DOI] [PubMed] [Google Scholar]
- Moll N.M., Rietsch A.M., Thomas S., Ransohoff A.J., Lee J.-C., Fox R., Chang A., Ransohoff R.M., Fisher E. Multiple sclerosis normal-appearing white matter: Pathology-imaging correlations. Ann. Neurol. 2011;70:764–773. doi: 10.1002/ana.22521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroso A., Ruet A., Lamargue-Hamel D., Munsch F., Deloire M., Coupé P., Ouallet J.-C., Planche V., Moscufo N., Meier D.S., Tourdias T., Guttmann C.R.G., Dousset V., Brochet B. Posterior lobules of the cerebellum and information processing speed at various stages of multiple sclerosis. J. Neurol. Neurosurg. Psychiatry. 2017;88:146–151. doi: 10.1136/jnnp-2016-313867. [DOI] [PubMed] [Google Scholar]
- Park H.-J., Friston K. Structural and Functional Brain Networks: from Connections to Cognition. Science. 2013;342:579–587. doi: 10.1126/science.1238411. [DOI] [PubMed] [Google Scholar]
- Popescu V., Klaver R., Voorn P., Galis-De Graaf Y., Knol D., Twisk J., Versteeg A., Schenk G., Van der Valk P., Barkhof F., De Vries H., Vrenken H., Geurts J. What drives MRI-measured cortical atrophy in multiple sclerosis? Mult. Scler. 2015:1–11. doi: 10.1177/1352458514562440. [DOI] [PubMed] [Google Scholar]
- Power J.D., Cohen A.L., Nelson S.M., Wig G.S., Barnes K.A., J A Church, Vogel A.C., Laumann TO, Miezin F.M., Schlaggar B.L., Petersen S.E. Functional network organization of the human brain. Neuron. 2011;72:665–678. doi: 10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocca M.A., Battaglini M., Benedict R.H.B., De Stefano N., Geurts J.J.G., Henry R.G., Horsfield M.A., Jenkinson M., Pagani E., Filippi M. Brain MRI atrophy quantification in MS. Neurology. 2017;88:403–413. doi: 10.1212/WNL.0000000000003542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocca M.A., Valsasina P., Leavitt V.M., Rodegher M., Radaelli M., Riccitelli G.C., Martinelli V., Martinelli-Boneschi F., Falini A., Comi G., Filippi M. Functional network connectivity abnormalities in multiple sclerosis: Correlations with disability and cognitive impairment. Mult. Scler. 2017;24:459–471. doi: 10.1177/1352458517699875. [DOI] [PubMed] [Google Scholar]
- Schoonheim M.M., Geurts J.J.G., Landi D., Douw L., van der Meer M.L., Vrenken H., Polman C.H., Barkhof F., Stam C.J. Functional connectivity changes in multiple sclerosis patients: a graph analytical study of MEG resting state data. Hum. Brain Mapp. 2013;34:52–61. doi: 10.1002/hbm.21424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoonheim M.M., Vigeveno R.M., Rueda Lopes F.C., Pouwels P.J.W., Polman C.H., Barkhof F., Geurts J.J.G. Sex-specific extent and severity of white matter damage in multiple sclerosis: implications for cognitive decline. Hum. Brain Mapp. 2014;35:2348–2358. doi: 10.1002/hbm.22332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoonheim M.M., Hulst H.E., Brandt R., Strik M., Wink A M., Uitehaag B., Barkhof F., Geurts J.J. Thalamus structure and function determines severity of cognitive impairment in multiple sclerosis. Neurology. 2015;84:776–783. doi: 10.1212/WNL.0000000000001285. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Johansen-Berg H., Rueckert D., Nichols T.E., Mackay C.E., Watkins K.E., Ciccarelli O., Cader M.Z., Matthews P.M., Behrens T.E.J. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. NeuroImage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Steenwijk M.D., Pouwels P.J.W., Daams M., Van Dalen J.W., Caan M.W.A., Richard E., Barkhof F., Vrenken H. Accurate white matter lesion segmentation by k nearest neighbor classification with tissue type priors (kNN-TTPs) NeuroImage Clin. 2013;3:462–469. doi: 10.1016/j.nicl.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stys P.K., Zamponi G.W., van Minnen J., Geurts J.J.G. Will the real multiple sclerosis please stand up? Nat. Rev. Neurosci. 2012;13:507–514. doi: 10.1038/nrn3275. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Demographics and MRI characteristics of the groups with mild and severe structural and/or functional changes.