Abstract
High-risk neuroblastoma continues to carry a poor prognosis. Nearly 50% of these tumors relapse following extensive treatment regimens. Protein phosphatase 2A (PP2A), a tumor suppressor, has been shown to be downregulated in many human cancers via multiple mechanisms including upregulation of its endogenous inhibitors, I2PP2A or CIP2A. We hypothesized that inhibition of the endogenous PP2A inhibitors or activation of PP2A would decrease tumorigenicity in human neuroblastoma cells. Four human neuroblastoma cell lines were utilized. Expression of PP2A and its endogenous inhibitors I2PP2A and CIP2A was confirmed by immunoblotting. PP2A activation was measured via phosphatase activation assay. Multiple parallel methods including siRNA inhibition of the endogenous PP2A inhibitors and pharmacologic activation of PP2A were utilized. Cell viability, proliferation, migration, and invasion assays were performed. In vivo studies were utilized to determine the effects of PP2A activation on neuroblastoma tumor growth. Inhibition of the endogenous inhibitors of PP2A or pharmacologic activation of PP2A with the PP2A activator FTY720 led to decreased neuroblastoma cell viability, proliferation, migration, and invasion. Treatment of mice bearing SK-N-AS or SK-N-BE(2) neuroblastoma tumors with FTY720 resulted in a significant decrease in tumor growth compared to vehicle-treated animals. In conclusion, activation of PP2A may provide a novel therapeutic target for neuroblastoma.
Introduction
Neuroblastoma is the most common primary malignant extracranial nervous system tumor in children and is responsible for over 15% of all pediatric cancer deaths [1]. Little progress has been made in improving the outcome for advanced-stage disease, and the 5-year survival remains less than 50% [2], [3]. The 5-year survival of those with refractory or relapsed disease is even worse at only 5% [2], [4]. These children have limited new therapeutic options available and virtually none that have resulted in long-term survival. Clearly, novel and innovative therapies will be required to address this disease.
Protein phosphatase 2A (PP2A) is a serine/threonine phosphatase that regulates a variety of cellular functions including cell survival, proliferation, and mobility. In cancer, PP2A plays a role in cellular transformation [5], [6] and interacts with oncoproteins such as c-Myc [7], Bcr-Abl [8], and p53 [9] to suppress tumor formation. PP2A functions to maintain cell adhesion and has been shown to reduce invasiveness of lung carcinoma [10] and prostate cancer cells [11]. There are two endogenous PP2A inhibitors, inhibitor of protein phosphatase 2A (I2PP2A, SET) and cancerous inhibitor of protein phosphatase 2A (CIP2A), which form inhibitory protein complexes with PP2A limiting its tumor suppressor function [12].
We hypothesized that augmenting PP2A in neuroblastoma cell lines would result in decreased cell proliferation and motility, and impede tumor growth in vivo. In the current studies, multiple methods were utilized to increase PP2A including inhibition of the endogenous PP2A inhibitors and treatment with the PP2A activators forskolin and fingolimod (FTY720).
Materials and Methods
Cells and Cell Culture
The human neuroblastoma cell lines SK-N-AS (CRL-2137) and SK-N-BE(2) (CRL-2271) were obtained from American Type Culture Collection (ATCC, Manassas, VA). SH-EP and WAC(2) human neuroblastoma cell lines were a kind gift from M. Schwab (Deutsches Krebsforschungszentrum, Heidelberg, Germany) and have been described in detail [13]. All cell lines were maintained under standard conditions at 37°C and 5% CO2. SK-N-AS cells were maintained in Dulbecco's modified Eagle's medium (DMEM, 30-2601, ATCC) containing 10% fetal bovine serum (Hyclone, Suwanee, GA), 4 mM L-glutamine (Thermo Fisher Scientific Inc., Waltham, MA), 1 μM nonessential amino acids and 1 μg/ml penicillin/streptomycin (Gibco, Carlsbad, CA). SK-N-BE(2) cells were maintained in a 1:1 mixture of minimum Eagle's medium and Ham's F-12 medium (30-2004, ATCC) with 10% fetal bovine serum (Hyclone), 2 mM L-glutamine (Thermo Fisher Scientific), 1 μM nonessential amino acids and 1 μg/ml penicillin/streptomycin (Gibco). SH-EP and WAC2 cell lines were maintained in RPMI 1640 medium (30-2001, ATCC) with 10% fetal bovine serum (Hyclone), 2 mM L-glutamine (Thermo Fisher Scientific) and 1 μg/ml penicillin/streptomycin (Gibco). All four cell lines were verified within the last 12 months using short tandem repeat analysis [Heflin Center for Genomic Sciences, University of Alabama, Birmingham (UAB), Birmingham, AL].
Reagents and Antibodies
Forskolin was purchased from Millipore (Millipore Sigma, Burlington, MA), rapamycin from Calbiochem (Millipore Sigma), and FTY720 from Cayman Chemical (10006292, Cayman Chemical, Ann Arbor, MI). Primary antibodies used for Western blotting included the following: anti-I2PP2A (H-120) (sc-25564) from Santa Cruz Biotechnology (Santa Cruz, CA), anti-PP2A (ab32104) and anti-CIP2A (ab99518) from Abcam (Cambridge, MA), anti–total AKT (9272), anti–phospho-AKT (S473; 9271), anti-MYCN (9405), p44/42 MAP Kinase [ERK1/2 (9102)], anti–phospho-p44/42 MAPK [phospho-ERK, T202/T204, (4377)] from Cell Signaling Technology (Danvers, MA), and anti–β-actin from Sigma (A1978, Sigma Aldrich, St. Louis, MO).
siRNA Transfection
Neuroblastoma cells (4 × 105) were transfected for 48 hours with small interfering RNAs (siRNAs) directed to either I2PP2A, CIP2A, both together (dual), or control (siNeg) at 20 nM concentration with Lipofectamine RNAiMax (Thermo Fisher Scientific). Control siRNA (siNeg) (ON-TARGETplus Non-targeting siRNA #1, sequence: UGGUUUACAUGUCGACUAA) was obtained from Dharmacon (GE Dharmacon, Thermo Fisher Scientific). I2PP2A siRNA was from Dharmacon as ON-TARGETplusSMARTpool (SO-2460229G), and CIP2A siRNA was custom designed from Dharmacon (SO-2590255G, sequence: sense; CUGUGGUUGUGUUUGCACUUU, antisense; AGUGCAAACACAACCACAGUU).
Immunoblotting
Briefly, cells were lysed on ice for 30 minutes in a buffer consisting of 50 mM Tris–HCl (pH 7.4), 150 mM NaCl, 1 mM EDTA, 1% Triton x-100, 1% sodium deoxcycholate, 0.1% SDS, phosphatase inhibitor (P5726, Sigma Aldrich), protease inhibitor (P8340, Sigma Aldrich), and phenylmethylsulfonyl fluoride (P7626, Sigma Aldrich). The lysates were then centrifuged at 14,000 rpm for 30 minutes at 4°C. Protein concentrations were determined using a Micro BCA Protein Assay Kit (Thermo Fisher Scientific), separated by electrophoresis on SDS-PAGE gels, and transferred to Immobilon-P polyvinylidene fluoride transfer membrane (EMD Millipore). Precision Plus Protein Kaleidoscope Standards (161-0375, Bio-Rad, Hercules, CA) were used for molecular weight markers to confirm expected size of target proteins. Antibodies were used in accordance with the manufacturers' recommended protocol. Samples were visualized by enhanced chemiluminescence using Luminata Classico and Luminata Crescendo Western horseradish peroxidase substrates (EMD Millipore). Anti–β-actin was used as an internal control to ensure equal protein loading between samples.
Cell Viability and Proliferation Assays
An alamarBlue assay (Thermo Fisher Scientific) was performed to assess cell viability following treatment with siRNA or FTY720. For siRNA, cells were treated with 20 nM siNeg, siI2PP2A, siCIP2A, or dual inhibition (siDual) with siI2PP2A (20 nM) and siCIP2A (20 nM) for 48 hours. Cells were plated (1.5 × 103 cells) onto 96-well plates, and after 24 hours, 10 μl of alamarBlue dye was added to each well. The plates were read using a microplate reader (Epoch Microplate Spectrophotometer, BioTek Instruments, Winooski, VT) to record the absorbance at 570 nm using 600 nm as a reference wavelength. For the FTY720 experiments, cells (1.5 × 103 cells) were plated and treated with increasing concentrations of FTY720 (0, 1, 2, 5, 8, 10, 25 μM). After 24 hours, 10 μl of alamarBlue dye was added to each well, and the plates were read as described above. Experiments were completed in triplicate and viability reported as fold change ± standard error of the mean (SEM).
Proliferation was assessed using the CellTiter 96 Aqueous One Solution Cell Proliferation assay (Promega, Madison, WI). For siRNA, cells were treated with 20 nM siNeg, siI2PP2A, siCIP2A, or dual inhibition (siDual) with siI2PP2A (20 nM) and siCIP2A (20 nM) for 48 hours. Cells were plated (1.5 × 103 cells) onto 96-well plates, and after 24 hours, 10 μl CellTiter 96 dye was added to each well, and the absorbance was measured at 490 nm using a microplate reader (Epoch Microplate Spectrophotometer). Proliferation was also examined following forskolin or FTY720 treatment. Cells (5 × 103 cells) were plated and treated with increasing concentrations of forskolin (0, 10, 20, 40 μM) for 48 hours or FTY720 (0, 1, 2, 5, 8, 10 μM) for 24 hours. CellTiter 96 dye (10 μl) was added to each well, and the absorbance was measured at 490 nm using a microplate reader (Epoch Microplate Spectrophotometer). Experiments were repeated in triplicate and proliferation reported as fold change ± SEM.
MYCN and I2PP2A Vectors and Transfection
The MYCN overexpression vector has been previously described [14]. Empty vector (ev, pcDNA3.1D/V5-His-TOPO) was used as a control for comparison. shEV (empty vector, pLKO.1-puro) and shSET (shI2PP2A) knockdown plasmids were kind gifts from AM Leopoldino and have been previously described [15]. All plasmids were sequenced for verification (Heflin Center for Genomic Sciences, UAB). Transfection was carried out using FuGENE HD Transfection Reagent (Promega, Madison, WI) per the manufacturer's protocol. Briefly, cells were plated on the day prior to transfection. The appropriate plasmid was incubated for 15 minutes at room temperature in OptiMEMTM media (Thermo Fisher Scientific) with FuGENE HD Transfection Reagent in a 3:2 ratio of transfection reagent to DNA, with 7.5 μg DNA per 1 × 106 cells. Cells were transfected 48-72 hours prior to use in experiments, and immunoblotting was utilized to confirm adequate plasmid transfection.
Cell Migration and Invasion Assays
Cell migration and invasion assays were performed using 6.5-mm Transwell inserts with 8 μM pore polycarbonate membrane (Corning Inc., Corning, NY) in 24-well culture plates. The bottoms of the inserts were coated with collagen Type I (10 mg/ml, 50 μl for 4 hours at 37°C). For invasion assays, the inside of the inserts was also coated with Matrigel (1 mg/ml, 50 μl; BD Biosciences) for 4 hours at 37°C. Cells were pretreated with siRNA (20 nM) for 48 hours, and then 1.5 × 105 cells were plated into the top of the insert. The insert was then placed into a well containing 300 μl of media containing 10% fetal bovine serum (FBS) as a chemoattractant. After 24 hours, the cells on top of the inserts were removed and fixed in 3% paraformaldehyde prior to staining with crystal violet. For forskolin and FTY720, cells were pretreated for 24 hours (0-10 μM, forskolin; 0-10 μM FTY720), and then 1.5 × 105 cells were plated into the top of the insert. The insert was then placed into a well containing 300 μl of treated media with forskolin or FTY720 and 10% FBS as a chemoattractant. After 24 hours, the cells on top of the inserts were removed and fixed in 3% paraformaldehyde prior to staining with crystal violet. The imaging software SPOT Basic 5.2 (Diagnostic Instruments Inc., Sterling Heights, MI) was used to take pictures of the inserts at predetermined locations with a microscope at 100×, and then the cells were quantified using ImageJ software (Ver 1.49, available online at http://imagej.nih.gov/ij). Experiments were repeated in triplicate and migration and invasion reported as fold change ± SEM.
PP2A Activity Assay
Cells (1 × 106 cells) were treated with siRNA (20 nM) or FTY720 (5 μM) for 4 hours and then lysed using NP-40 lysis buffer. PP2A activity was measured using a PP2A Immunoprecipitation Phosphatase Assay Kit (17–313, EMD Millipore). Briefly, protein lysates were incubated with PP2A antibody at 4°C with continuous rotation for 2 hours. Following the addition of assay buffers and malachite green solution, the plate was read at an absorbance of 650 nm using a microplate reader (Epoch Microplate Spectrophotometer). Phosphatase activity was determined using a standard curve. Experiments were repeated at least in triplicate, and phosphatase activity was reported as mean fold change ± SEM from the untreated sample for each cell line.
Animal Statement
Animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC-09355) and were conducted within institutional, national, and NIH guidelines.
In Vivo Tumor Growth
For the first animal experiment, SH-EP and WAC2 cells were stably transfected with shEV or shI2PP2A plasmids. Clones were selected under WB confirmed decreased target expression. Cells (2.5 × 106 cells in 25% Matrigel, Corning, Inc.) with shEV were injected into the right flank and cells with shI2PP2A were injected into the left flank of 6-week-old, female, athymic nude mice (n = 5 per cell line) (Envigo, Prattville, AL). Tumors were measured twice weekly, and tumor volumes were calculated with the formula [(width2 × length/2] with width being the smallest measurement. When tumor size reached IACUC parameters, the animals were humanely euthanized. For the FTY720 experiments, SK-N-AS or SK-N-BE(2) cells (2.5 × 106 cells in 25% Matrigel, Corning, Inc.) were injected into the right flank of athymic nude mice. Once tumors were palpable (100 mm3), the mice were randomized to receive either 50 μl suspension vehicle (ORA-Plus, Perrigo, Allegan, MI) or FTY720 10 mg/kg/day suspended in 50 μl ORA-Plus once daily via oral gavage. The FTY720 dosing was based on previous animal studies [16], [17], [18]. The flank tumors were measured twice weekly using calipers, and tumor volumes were calculated. The animals were humanely euthanized when IACUC parameters were met.
Statistical Analyses
Isobolograms were constructed using the methods of Chou-Talalay [19]. Experiments were performed at a minimum of triplicate. Data were reported as the mean ± standard error of the mean. Parametric data between groups were compared using an analysis of variance or Student's t test as appropriate. Nonparametric data were analyzed with Mann-Whitney rank sum test. Statistical significance was defined as P ≤ .05.
Results
Determination of PP2A and Its Endogenous Inhibitors in Human Neuroblastoma Cell Lines
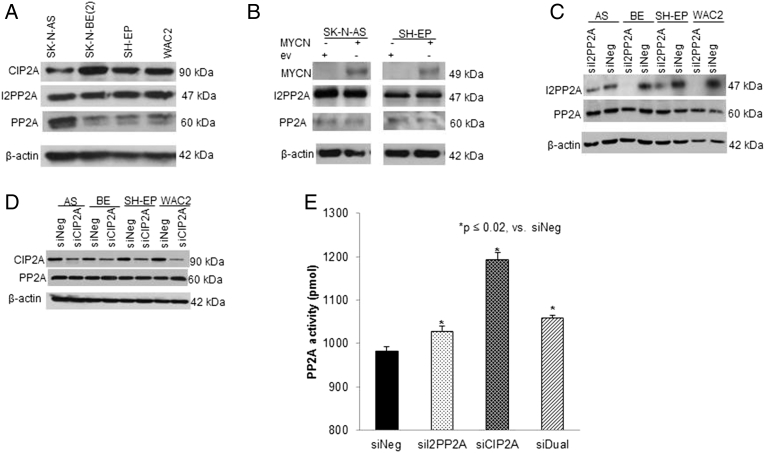
Documentation of PP2A and its inhibitors expression was necessary prior to initiating other investigations. Immunoblotting revealed that PP2A and the endogenous inhibitors I2PP2A (SET) and CIP2A were present in all four cell lines evaluated (Figure 1A). There were inconsistent differences in expression of PP2A between MYCN nonamplified (SK-N-AS) and MYCN amplified [SK-N-BE(2), WAC2] cell lines, with higher expression in the nonamplified MYCN SK-N-AS cell line compared to SK-N-BE(2) but nearly equivalent expression in the SHEP (nonamplified) and WAC2 (amplified) cells (Figure 1A). Therefore, nonamplified cell lines (SK-N-AS and SH-EP) were transfected with an MYCN expression plasmid. There were no differences in PP2A expression between the empty vector controls (ev) and the MYCN expressing cells (Figure 1B). In addition, when the MYCN isogenic cell lines SH-EP (nonamplified) and WAC2 (amplified) cells were compared, there were no differences in expression of PP2A, I2PP2A, or CIP2A (Figure 1A), indicating that these proteins were not likely to be MYCN dependent.
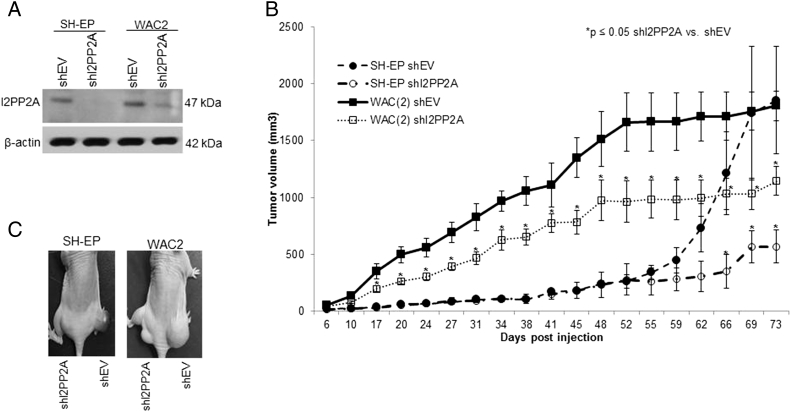
Figure 1.
CIP2A, I2PP2A, and PP2A in neuroblastoma cell lines. (A) Immunoblotting revealed CIP2A, I2PP2A, and PP2A expression in all four neuroblastoma cell lines studied. There were no differences in expression between the MYCN nonamplified SH-EP and the isogenic MYCN amplified WAC2 cells. (B) SK-N-AS and SH-EP neuroblastoma cells (MYCN nonamplified) were transfected with MYCN overexpression vector and cell lysates examined for I2PP2A and PP2A. MYCN was successfully expressed in both cell lines. Expression of I2PP2A and PP2A was not affected by MYCN overexpression. (C). Neuroblastoma cell lines were treated with siRNA knockdown of I2PP2A. Whole cell lysates revealed knockdown of I2PP2A with no change in PP2A expression. (D) Neuroblastoma cell lines were treated with siRNA knockdown of CIP2A. Whole cell lysates revealed knockdown of CIP2A with no change in PP2A expression. (E) PP2A activity was measured in SK-N-AS cells following siRNA inhibition. Inhibition of I2PP2A, CIP2A, or dual inhibition led to significant increases in PP2A activity.
siRNA Knockdown of I2PP2A and CIP2A
We commenced to evaluate the effects of inhibition of the endogenous PP2A inhibitors, using siRNA to target the expression of I2PP2A (SET) and CIP2A. Immunoblotting confirmed target knockdown (Figure 1, C and D). Overall PP2A expression was not affected by I2PP2A or CIP2A knockdown (Figure 1, C and D), and dual knockdown successfully targeted I2PP2A and CIP2A expression in SK-N-BE(2), SH-EP, WAC2, and to a lesser degree SK-N-AS (Supplementary Figure 1A). siRNA knockdown of one inhibitor did not lead to a compensatory increase in the other or change PP2A expression (Supplementary Figure1, B and C). PP2A activation was evaluated after inhibition of I2PP2A and CIP2A in SK-N-AS cells using a PP2A immunoprecipitation phosphatase assay. There was a significant increase in PP2A activation after siRNA knockdown (Figure 1E).
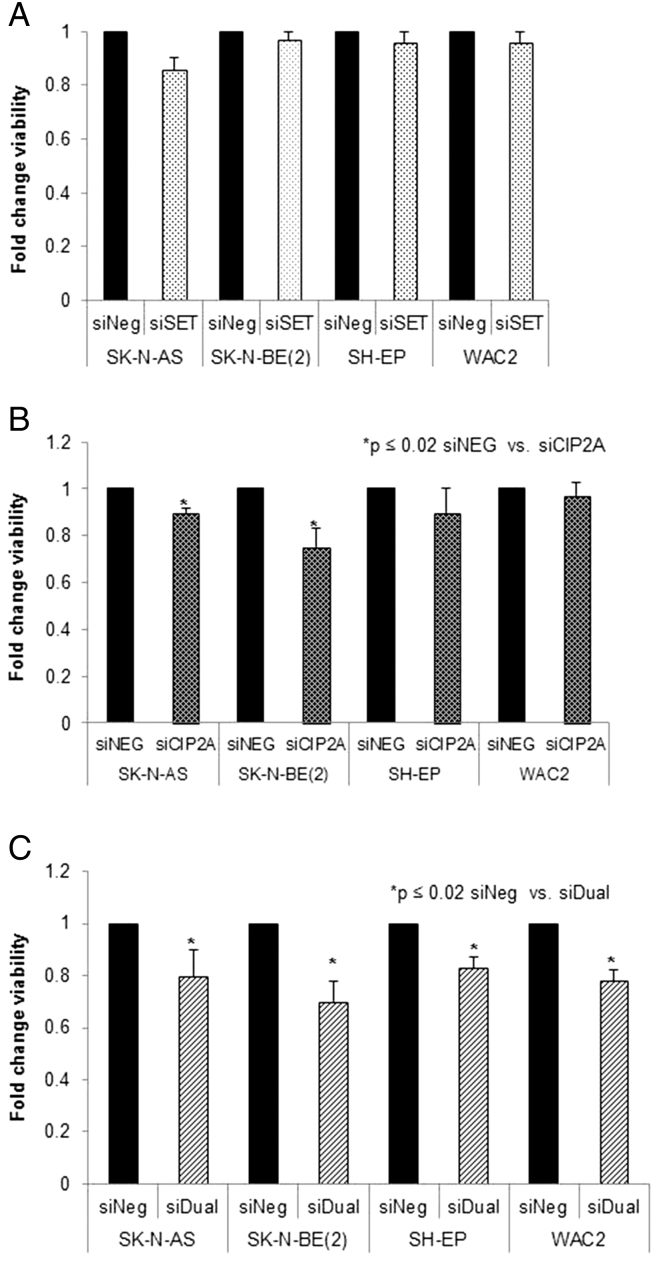
Viability was measured with alamarBlue assays. Inhibition of I2PP2A (SET) did not affect viability in any of the four neuroblastoma cell lines (Figure 2A). Inhibition of CIP2A decreased viability in SK-N-AS and SK-N-BE(2) only (Figure 2B). When cells were treated with dual inhibition, there was a significant decrease in viability in all four cell lines (Figure 2C).
Figure 2.
siRNA knockdown of I2PP2A and CIP2A decreased cell viability. (A) Neuroblastoma cell lines were treated with I2PP2A siRNA (siSET) or control siRNA (siNeg) and cell viability measured with alamarBlue assay. There were no significant differences in viability in any of the four cell lines. (B) Neuroblastoma cell lines were treated with CIP2A siRNA (siCIP2A) or control siRNA (siNeg), and cell viability was measured with alamarBlue assay. Viability was significantly decreased in the SK-N-AS and the SK-N-BE(2) cell lines but not in the SH-EP or WAC2 cells. (C) Neuroblastoma cell lines were treated with both I2PP2A and CIP2A siRNA (siDual) or control siRNA (siNeg), and cell viability was measured with alamarBlue assay. Viability was significantly decreased in all four cell lines with dual inhibition. Experiments were repeated at least in triplicate and reported as mean fold change ± SEM.
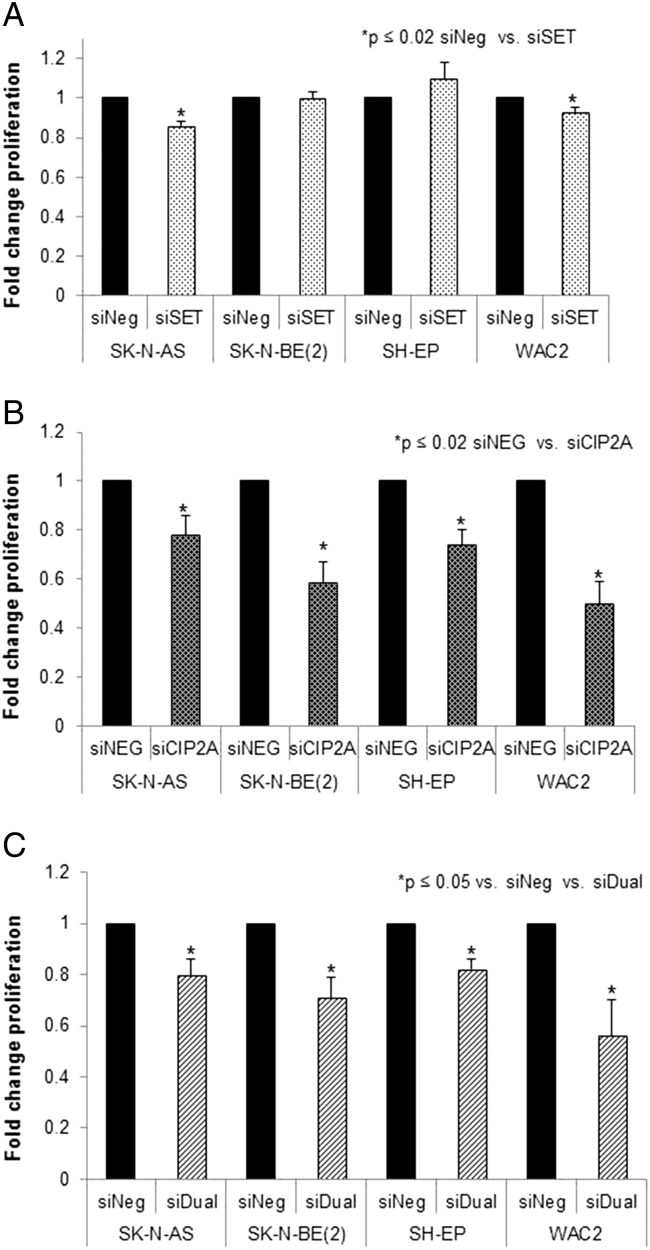
Similar findings were noted with cell proliferation. siRNA inhibition of I2PP2A had less of an effect on proliferation (Figure 3A) than did inhibition of CIP2A (Figure 3B) or dual inhibition (Figure 3C).
Figure 3.
siRNA knockdown of I2PP2A and CIP2A decreased cell proliferation. (A) Neuroblastoma cell lines were treated with I2PP2A siRNA (siSET) or control siRNA (siNeg) for 48 hours, and proliferation was measured with CellTiter 96 assay. Proliferation was significantly decreased in the SK-N-AS and the WAC2 cell lines. (B) Neuroblastoma cell lines were treated with CIP2A siRNA (siCIP2A) or control siRNA (siNeg), and cell proliferation was measured. Proliferation was significantly decreased in all four cell lines after CIP2A knockdown. (C) Neuroblastoma cell lines were treated with both I2PP2A and CIP2A siRNA (siDual) or control siRNA (siNeg), and proliferation was measured. Proliferation was significantly decreased in all four cell lines. Experiments were repeated at least in triplicate and reported as mean fold change ± SEM.
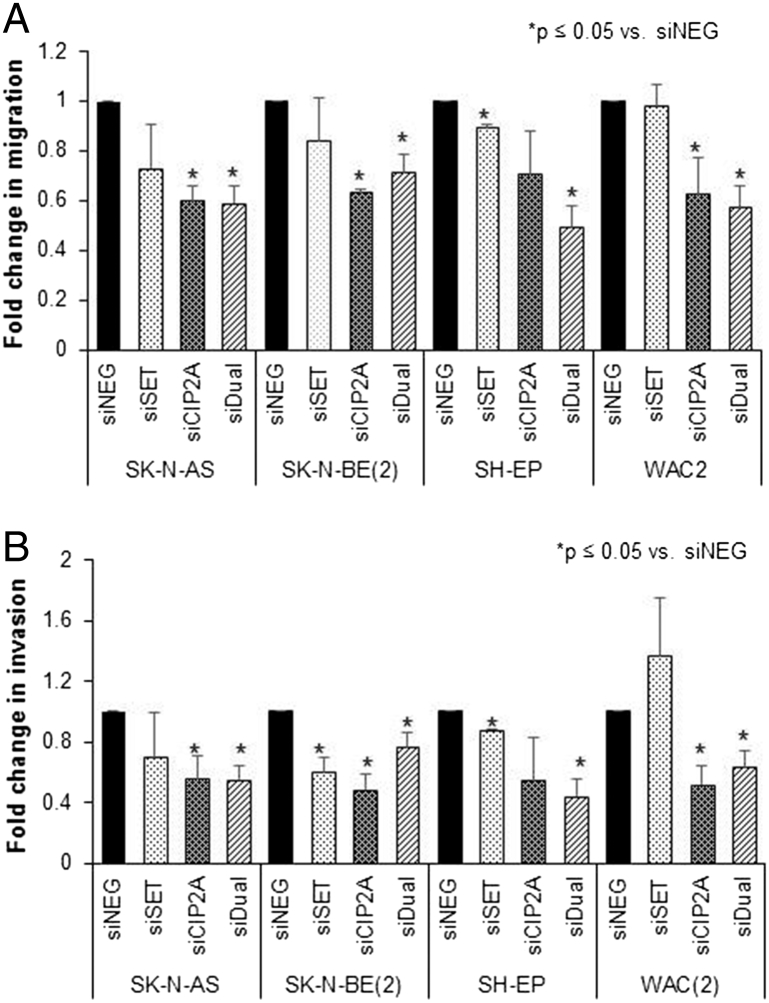
Since I2PP2A and CIP2A are known to affect cell motility, migration and invasion were examined. There was a significant decrease in cell migration in all four neuroblastoma cell lines seen with dual inhibition of I2PP2A and CIP2A when compared to controls (Figure 4A) which was not consistently seen with inhibition of each alone. Similar findings were noted with cell invasion. Dual inhibition more reliably led to significant decreases in invasion than inhibition of either I2PP2A (SET) or CIP2A alone (Figure 4B).
Figure 4.
siRNA knockdown of I2PP2A and CIP2A decreased cell motility. (A) Neuroblastoma cell lines were treated with control siRNA (siNEG) or siRNA to I2PP2A (siSET), CIP2A (siCIP2A) or both (siDual) for 48 hours. (A) Cells were plated and allowed to migrate through a micropore membrane for 24 hours. Cells were fixed and stained, and number of cells were counted. Migration was significantly decreased in all cell lines with dual inhibition. siSET only affected migration in SH-EP cells. Also, SH-EP cell line was the only one not significantly affected by siCIP2A. (B) Similar to migration, treated cells were plated and allowed to invade into a Matrigel layer for 24 hours. Also similar to migration, all four cell lines had a significant decrease in invasion after dual inhibition, but only the SH-EP cell line was unaffected by siCIP2A and significantly affected by siSET. Experiments were repeated at least in triplicate and reported as mean fold change ± SEM.
Inhibition of I2PP2A with shRNA Resulted in Decreased Tumor Growth In Vivo
To further demonstrate that inhibition of I2PP2A was pertinent, SH-EP and WAC2 cells were stably transfected with plasmid containing short hairpin RNA for I2PP2A (shI2PP2A). Empty vector plasmid (shEV) was utilized as a control. Immunoblotting confirmed target knockdown in the cells (Figure 5A). These shI2PP2A or shEV cells were injected into the left and right flank, respectively, of athymic nude mice, and animals were followed for tumor growth. The shI2PP2A cells formed significantly smaller tumors than the empty vector controls (shEV, Figure 5B). Representative photographs of tumors are shown in Figure 5C.
Figure 5.
Inhibition of I2PP2A led to decreased neuroblastoma tumor growth. (A) SH-EP and WAC2 cells were transfected with shI2PP2A or control (shEV) plasmids. Immunoblotting confirmed target knockdown. (B) Cells (2.5 × 106 in 25% Matrigel) were injected into the flanks of immunosuppressed mice, and animals were followed for tumor growth. The shI2PP2A transfected cells grew significantly smaller tumors than the empty vector (shEV) controls in both the SH-EP and WAC2 cell lines. (C) Photo of representative animals demonstrated significantly smaller tumors from the shI2PP2A transfected cells.
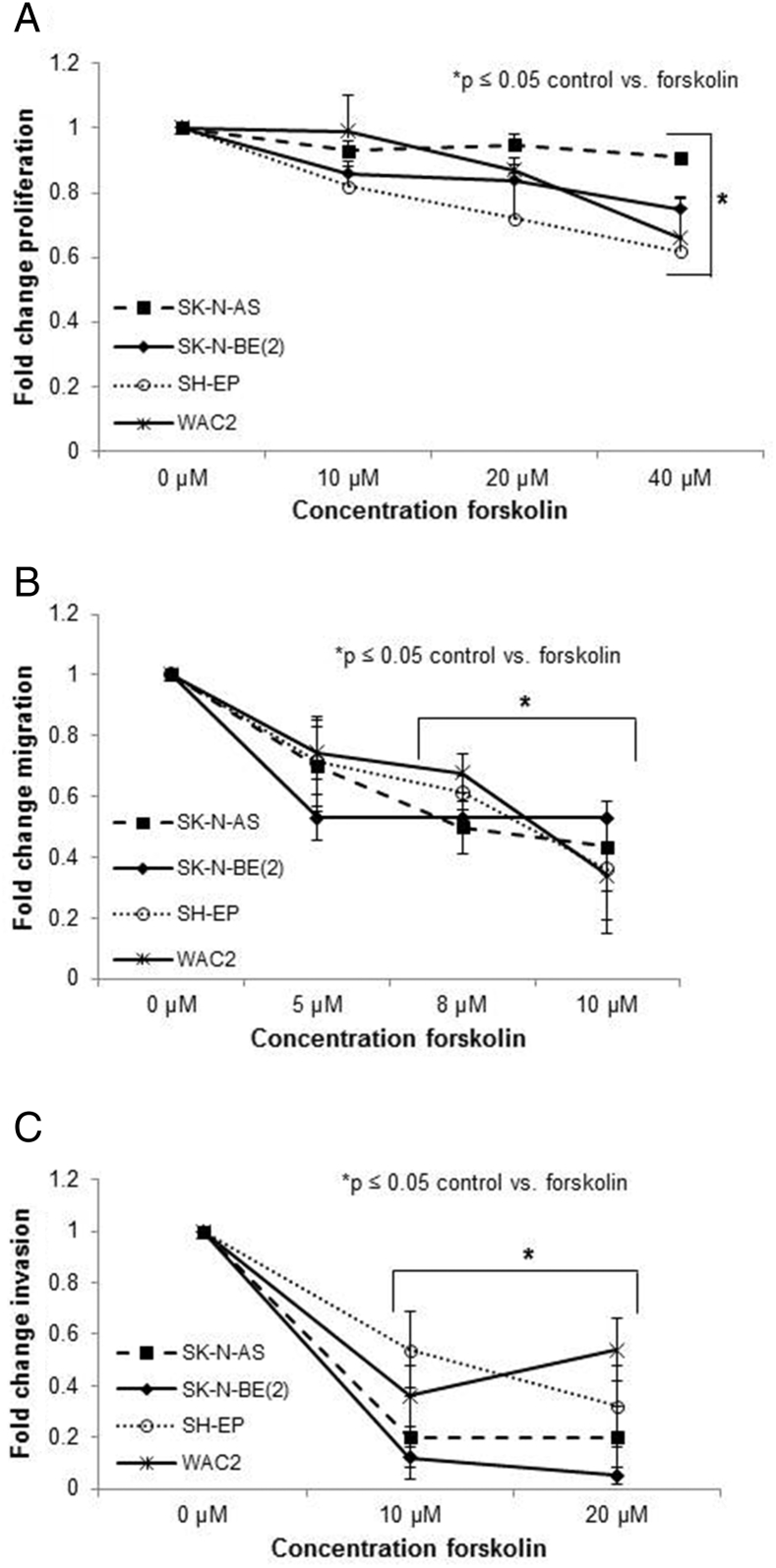
Forskolin Treatment Resulted in Decreased Proliferation and Motility in Neuroblastoma Cells
Forskolin is a known activator of PP2A [20]. Therefore, we chose to evaluate the phenotypic changes in neuroblastoma cells following forskolin treatment. Proliferation was studied with CellTiter 96 assays. Forskolin significantly decreased proliferation in all four neuroblastoma cell lines (Figure 6A). Importantly, the ability of the cells to migrate or invade was significantly diminished at concentrations as low as 8 μM and 10 μM, respectively (Figure 6, B and C), which were well below the inhibitory concentration 50% (IC50) of the cells.
Figure 6.
Forskolin treatment decreased cell proliferation and motility. (A) Neuroblastoma cells were treated with increasing concentrations of forskolin for 48 hours, and proliferation was measured using CellTiter 96 assay. There was a significant decrease in proliferation in all four cell lines. (B) Cells were treated with increasing concentrations of forskolin for 24 hours, plated, and allowed to migrate for 24 hours. There was a significant decrease in migration in all four cell lines beginning at 8-μM concentration. (C) Cells were treated with increasing concentrations of forskolin for 24 hours, plated, and allowed to invade for 24 hours. Invasion was significantly decreased in all four cell lines after forskolin treatment, beginning at 10-μM concentration. Experiments were repeated at least in triplicate and reported as mean fold change ± SEM.
FTY720 Activated PP2A and Led to Decreased Viability, Proliferation, and Motility in Neuroblastoma Cell Lines
Since forskolin, a PP2A activator, had a significant effect upon neuroblastoma cell proliferation and motility, we next wished to investigate a compound that may be useful as a clinical therapeutic. We chose FTY720, as activation of PP2A has been proposed as a mechanism by which its produces its antitumor effects [21], [22]. We examined the ability of FTY720 to activate PP2A with a phosphatase activation kit. Following treatment of SK-N-AS, SK-N-BE(2), SH-EP, and WAC2 cells with FTY720 (5 μM) for 4 hours, the phosphatase activity of PP2A was significantly increased over baseline relative to control in all four lines (Supplementary Figure 2).
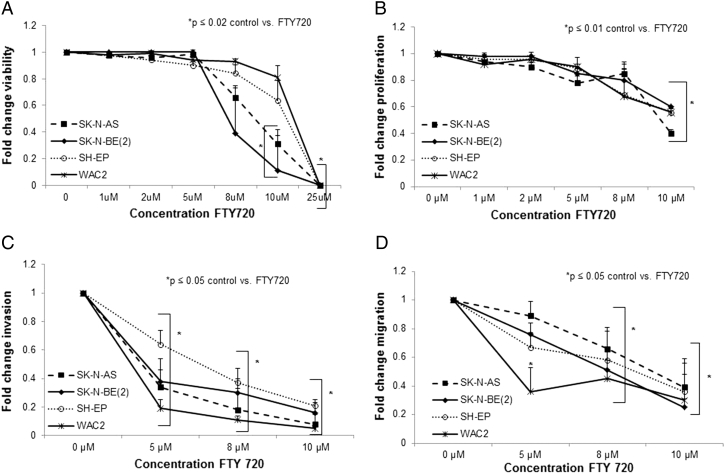
FTY720 has been shown to decrease cancer cell viability [20], [23], [24] and motility [25] in a variety of tumor types, so we examined whether FTY720 would have similar effects on neuroblastoma cells. Cells were treated with increasing concentrations of FTY720 for 48 hours, and viability was measured. FTY720 significantly decreased viability in all four neuroblastoma cell lines (Figure 7A). The lethal dose 50% (LD50) for FTY720 was 5.2 μM in SK-N-AS cells, 4.7 μM in SK-N-BE(2) cells, 5.9 μM in SH-EP cells, and 6.5 μM in WAC2 cells. Cell proliferation was measured following treatment for 24 hours with increasing concentrations of FTY720. There was a significant decrease in proliferation seen in all four cell lines following FTY720 treatment (Figure 7B).
Figure 7.
FTY720 treatment resulted in decreased viability, proliferation, and motility of neuroblastoma cells. (A) Cell viability was measured using alamarBlue assays. Neuroblastoma cells were treated with increasing concentrations of FTY720 for 24 hours. Viability was significantly decreased in the SK-N-AS and SK-N-BE(2) cells beginning at a concentration of 10 μM (*P ≤ .02). SH-EP and WAC2 cells showed significant decreases in viability at concentrations of 25 μM (*P ≤ .02). (B) CellTiter 96 assays were used to measure proliferation. Neuroblastoma cells were treated with increasing concentrations of FTY720 for 24 hours. Proliferation was significantly decreased in all four cell lines at 10 μM (*P ≤ .01). (C) Invasion investigations were completed using Transwell inserts with 8-μM pores, coated on the bottom with Collagen Type I and on the inside with Matrigel. Cells were treated with increasing concentrations of FTY720 for 24 hours, plated, and allowed to invade for 24 hours. Invasion was significantly decreased in all four cell lines at 5-μM concentrations. (D) Migration was determined using Transwell inserts with 8-μM pores, coated on the bottom with Collagen Type I. Cells were treated with FTY720 at increasing concentrations for 24 hours, plated, and allowed to migrate for 24 hours. Migration in all four cell lines was significantly decreased with concentrations of 8 μM. Graphs are means of three experiments with data reported as mean ± SEM. Experiments were repeated at least in triplicate and reported as mean fold change ± SEM.
The ability to migrate and invade is a hallmark behavior of cancer cells. Therefore, we investigated whether FTY720 treatment decreased neuroblastoma cell motility. Following treatment with FTY720 at increasing concentrations for 24 hours, invasion and migration were assessed. FTY720 resulted in a significant decrease in both invasion (Figure 7C) and migration (Figure 7D) in all four neuroblastoma cell lines.
FTY720 Decreased Neuroblastoma Tumor Growth In Vivo
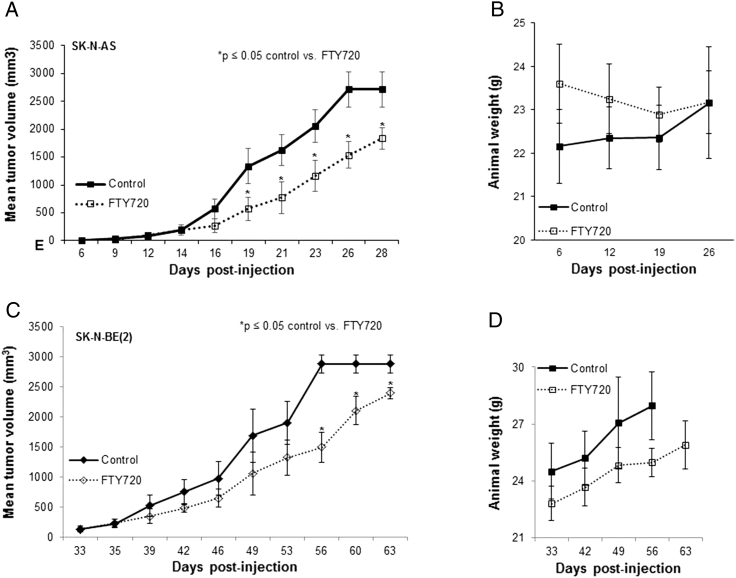
For in vivo testing of FTY720 against neuroblastoma tumor growth, SK-N-AS or SK-N-BE(2) human neuroblastoma cells (2.5 × 106 in Matrigel) were injected into the right flank of athymic nude mice. Once tumors were palpable (100 mm3), animals were randomized in equal numbers to receive daily oral doses of vehicle or FTY720 (10 mg/kg/day). This dosage was chosen based on previous literature reports [25], [26], [27]. The animals bearing SK-N-AS (n = 14) tumors treated with FTY720 showed a significant decrease in tumor volume when compared to vehicle-treated animals (Figure 8A). Similar findings were seen in animals bearing SK-N-BE(2) (n = 10) tumors (Figure 8C). FTY720 did not affect the weight of the animals (Figure 8, B and D).
Figure 8.
FTY720 decreased neuroblastoma tumor growth in vivo. (A) SK-N-AS cells (2.5 × 106 cells in 25% Matrigel) were injected into the right flank of 6-week-old athymic nude mice. When tumors reached an average of 100 mm3, mice were randomized to receive either 50 μl ORA-Plus (suspension vehicle, n = 7) or FTY720 10 mg/kg/day suspended in 50 μl ORA-Plus (n = 7) once daily via oral gavage. Animals treated with FTY720 (open squares) had significantly smaller tumors than those treated with vehicle alone (closed squares). (B) Mice were weighed at the beginning of the experiment and at the time of euthanasia. There was no difference in animal weights between those treated with vehicle and those treated with FTY720. (C) SK-N-BE(2) cells (2.5 × 106 cells in 25% Matrigel) were injected into the right flank of 6-week-old athymic nude mice. When tumors reached an average of 100 mm3, mice were randomized to receive either 50 μl ORA-Plus (suspension vehicle, n = 5) or FTY720 10 mg/kg/day suspended in 50 μl ORA-Plus (n = 5) once daily via oral gavage. Animals treated with FTY720 (open diamonds) had significantly smaller tumors than those treated with vehicle alone (closed diamonds). (D) Mice were weighed at the beginning of the experiment and at the time of euthanasia. There was no difference in weights between those animals treated with vehicle and those treated with FTY720.
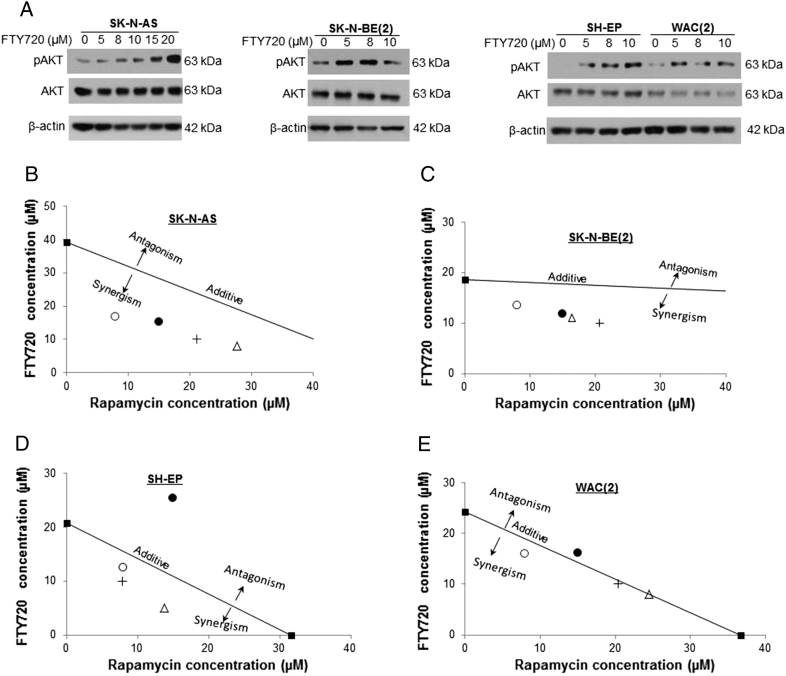
FTY720 Resulted in an Increase in AKT Phosphorylation
Other investigators have postulated that FTY720 may function through downstream targets of PP2A such as changes in ERK or AKT phosphorylation [26], [27]. SH-EP and WAC2 cells were treated with FTY720 for 24 hours at increasing concentrations and whole cell lysates with immunoblotting. There were mild increases in ERK phosphorylation seen, but total ERK expression was also increased, diminishing the importance of the phosphorylation findings (Supplemental Figure 3). When AKT phosphorylation was examined, it was found that AKT phosphorylation increased in all four cell lines with increasing concentrations of FTY720 at 24 hours (Figure 9A). Total AKT expression was not affected. These results prompted an investigation into whether combination therapy with FTY720 and an AKT inhibitor would be relevant. Neuroblastoma cells were treated with FTY720 and the AKT inhibitor rapamycin for 24 hours, and cell viability was measured with alamarBlue. Isobolograms were constructed using the method of Chou-Talalay [19] with a combination index <1 representing a synergistic response, >1 an antagonistic response, and =1 an additive response. In the SK-N-AS (Figure 9B) and SK-N-BE(2) (Figure 9C) cells, there was a marked synergistic response seen with FTY720 treatment combined with rapamycin. In the SH-EP (Figure 9D) and WAC2 (Figure 9E) cell lines, most combinations with FTY720 and rapamycin were synergistic or at least additive (Table 1).
Figure 9.
FTY720 led to increased AKT phosphorylation. (A) Neuroblastoma cell lines were treated with FTY720 at increasing concentrations for 24 hours. Immunoblotting of whole cell lysates revealed increased AKT phosphorylation in all four cell lines after FTY720 treatment. Total AKT expression was unchanged. (B) SK-N-AS cells were treated for 24 hours with varying doses of rapamycin and FTY720. Viability was assessed with alamarBlue. Isobolograms were constructed, and combination indices (CIs) were calculated. Combining the two compounds resulted in a synergistic effect on viability. (C) SK-N-BE(2) cells were treated for 24 hours with varying doses of rapamycin and FTY720. Viability was assessed with alamarBlue. Isobolograms were constructed, and CIs were calculated. Combining the two compounds resulted in a synergistic effect. (D) SH-EP cells were treated for 24 hours with varying doses of rapamycin and FTY720. Viability was assessed with alamarBlue. Isobolograms were constructed, and CIs were calculated. Combining the two compounds resulted in a mostly synergistic effect. (E) WAC2 cells were treated for 24 hours with varying doses of rapamycin and FTY720. Viability was assessed with alamarBlue. Isobolograms were constructed, and CIs were calculated. Combining the two compounds resulted in a mostly additive effect in this cell line.
Table 1.
Combination Indices (CI) for Rapamycin and FTY720
| Rapamycin | CI | FTY720 | CI | |
|---|---|---|---|---|
| SK-N-AS | 8 | 0.58 | 8 | 0.72 |
| 15 | 0.67 | 10 | 0.65 | |
| SK-N-BE(2) | 8 | 0.75 | 8 | 0.67 |
| 15 | 0.68 | 10 | 0.67 | |
| SH-EP | 8 | 0.56 | 8 | 0.68 |
| 15 | 1.6 | 10 | 0.73 | |
| WAC(2) | 8 | 0.87 | 8 | 1.1 |
| 15 | 1.1 | 10 | 0.96 |
Discussion
Although significant advances have been made in the treatment of childhood solid tumors, high-risk neuroblastoma continues to carry a dismal prognosis. It is obvious that the development of novel therapeutic approaches is urgently needed. Recent studies have purported I2PP2A oncogene as a treatment target for multiple cancers [28], [29], [30] and served as an initiating point for the investigations in the current study.
I2PP2A (SET) and CIP2A, the endogenous inhibitors of PP2A, have been shown to be overexpressed in numerous cancer types [27], [31] including chronic myelogenous leukemia [32], cholangiocarcinoma [33], colorectal and squamous cell cancer [34], and neuroblastoma [35]. Amplification of the MYCN oncogene is the most important negative prognostic indicator in neuroblastoma [36]. This oncogene has numerous targets, many of which have not been well characterized. Khanna et al. recently found that CIP2A expression was MYCN independent [35]. We wished to determine whether expression of I2PP2A in neuroblastoma was related to MYCN status. In the cell lines included in this study, it did not appear as though I2PP2A expression was dependent upon MYCN. Similarly, PP2A expression did not appear to be affected by MYCN, as its expression was not different by immunoblotting in both the MYCN isogenic SH-EP and WAC2 cell lines and the SK-N-AS and SH-EP cell lines after MYCN transfection.
Other authors have investigated the utility of targeting these endogenous PP2A inhibitors as a treatment strategy for cancer. Farrell et al. established that shRNA inhibition of CIP2A or I2PP2A resulted in decreased growth of pancreatic tumor cells [29]. Mukhopadhyay and colleagues showed that ceramide inhibition of I2PP2A led to decreased viability in some prostate cancer cell lines [37]. Niclosamide, an anthelmintic drug, was shown to inhibit CIP2A in non–small cell lung cancer cells, which led to decreased cell proliferation and attachment independent growth [30]. Similar to the current findings in neuroblastoma, using short hairpin knockdown of I2PP2A, investigators demonstrated decreased tumor growth in immunocompromised mice in squamous cell carcinoma [15]. The unifying factor in the previous studies was the inhibition of CIP2A or I2PP2A resulted in activation of PP2A leading to the recognized downstream effects [15], [30]. We also utilized siRNA inhibition of I2PP2A (SET) and CIP2A. These studies showed decreased tumor growth and motility. These phenotypic changes related to I2PP2A or CIP2A knockdown were variable between cell lines and were not necessarily additive. Further, they did not appear to be secondary to increased expression of CIP2A or I2PP2A in response to knockdown of the other protein or to PP2A expression (Supplemental Figure 1A). Rather, they may have been due to compensatory changes in other PP2A regulating mechanisms such as JAK2 [38]; but these postulations will be the subject of future study. Additionally, there was not an increase in PP2A expression with I2PP2A or CIP2A knockdown (Supplemental Figure 1, B and C), but PP2A activation was increased, leading to the hypothesis that this activation is the mechanism leading to the observed alterations in tumor cell viability, proliferation, invasion, migration, and in vivo tumor growth.
Because of the inconsistencies seen with siRNA targeting of these endogenous PP2A inhibitors and lack of currently available clinical therapeutic formulations, studies were advanced to targeting PP2A itself. Forskolin is a known activator of PP2A [39]. Forskolin treatment resulted in slightly decreased proliferation but had a more pronounced effect on proliferation and motility in neuroblastoma cells than would have been expected with that seen in proliferation. Similar results were seen by other investigators in acute myeloid leukemia cells [39] and prostate cancer cells [20].
FTY720 (2-amino-2-[2-(4-octylphenyl)]-1,3-propanediol, fingolimod) is a synthetic sphingosine immunosuppressant that was approved by the United States Food and Drug Administration for the treatment of multiple sclerosis [40], [41]. Over the past decade, FTY720 has also been shown to have antitumor properties in several human malignancies [16], [23], [26], [42], [43], [44], including the pediatric tumor medulloblastoma [25], prompting its use in the current investigations. Many mechanisms have been proposed for the effects of FTY720, including activation of PP2A [21], [22], generation of reactive oxygen species [44], [45], [46], and inhibition of sphingosine kinase 1 (SphK1) [47], [48]. Li and others showed that FTY720 inhibited sphingosine kinase 2 in neuroblastoma [49], and Lange et al studied the effects on calcium channel signaling in neuroblastoma [50], but neither group explored whether PP2A activation was involved [49], [50]. In the current study, we found that FTY720 treatment significantly increased PP2A activity in neuroblastoma cells.
In addition to reducing cell viability, FTY720 has been shown to affect tumor cell motility. Zhou demonstrated decreased migration and invasion with FTY720 treatment in the human prostate cell lines DU145 and PC3 [51]. Zhang and others showed that human glioma cells had a marked reduction in migration and invasion following FTY720 treatment [26], and Garner demonstrated similar effects in human medulloblastoma patient-derived xenograft cells [25]. Similarly, in this study, treatment of human neuroblastoma cells with FTY720 in doses well below the LD50 resulted in significant reductions in both migration and invasion.
Previous investigations have postulated that the effects of FTY720-induced PP2A activation may be dependent upon dephosphorylation of AKT. Loss of AKT phosphorylation has been noted in breast and prostate cancer cells and mesothelioma [17], [20], [23]. In this study, FTY720 treatment resulted in an increase in AKT phosphorylation in all four neuroblastoma cell lines tested. Studies conducted in neurologic noncancer models found that FTY720 led to activation of AKT kinase [52], [53], indicating that our findings may have important clinical relevance. To that end, we demonstrated that FTY720 combined with AKT inhibition decreased neuroblastoma cell proliferation in a mostly synergistic, and at least additive, fashion, implying that it may sensitize neuroblastoma cells to conventional rapalogue chemotherapeutic agents. Alterations in ERK phosphorylation have also been proposed as a potential mechanistic target for FTY720. Rincon and colleagues showed decreased activation of ERK in BT-474 and MDA-MB-231 breast cancer cells following treatment with FTY720 [27], and others reported similar results with PC-3 prostate cancer cells [20]. In the current study, SH-EP and WAC2 cells showed increased ERK phosphorylation, but these changes followed changes noted in expression of total ERK (Supplemental Figure 2). These data indicated that the effects of FTY720 on kinases are likely cell line dependent and, along with the findings for AKT, will serve as a basis for further mechanistic studies of FTY720 in neuroblastoma.
Conclusions
The data in the current study provide evidence that inhibition of I2PP2A or CIP2A, or activation of PP2A, decreased neuroblastoma cell proliferation, motility, and in vivo tumor growth. These findings suggest that development of novel therapeutics that antagonize endogenous PP2A inhibitors and also enhance PP2A activity has potential for clinical applications in neuroblastoma. Further, these data suggest that potentially combining PP2A activation with kinase inhibition may be beneficial in neuroblastoma. These findings provide the impetus to further investigate these pathways for the treatment of neuroblastoma.
Additional Information
None of the authors have any competing financial interests.
Data Availability Statement
No data sets were generated in the current studies.
Acknowledgements
This work was partially funded by institutional grants from the National Cancer Institute including T32 CA091078 training grant in surgical oncology (E.F. Garner, A.M. Waters, L.L. Stafman) and T32 CA183926 training grant in translational oncology (A.P. Williams) and P30 AR048311 and P30 AI27667 to the University of Alabama Flow Cytometry Core. Funding was also provided by Hyundai Hope on Wheels (E.A. Beierle). The funding sources had no role in study design, analysis or interpretation of the data, the writing of the manuscript, or the decision for publication submission.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.tranon.2018.09.011.
Appendix A. Supplementary Data
Supplementary figures
References
- 1.Maris JM. The biologic basis for neuroblastoma heterogeneity and risk stratification. Curr Opin Pediatr. 2005;17:7–13. doi: 10.1097/01.mop.0000150631.60571.89. [DOI] [PubMed] [Google Scholar]
- 2.Mossé YP, Deyell RJ, Berthold F, Nagakawara A, Ambros PF, Monclair T, Cohn SL, Pearson AD, London WB, Matthay KK. Neuroblastoma in older children, adolescents and young adults: a report from the International Neuroblastoma Risk Group project. Pediatr Blood Cancer. 2014;61:627–635. doi: 10.1002/pbc.24777. [DOI] [PubMed] [Google Scholar]
- 3.Cotterill SJ, Parker L, More L, Craft AW. Neuroblastoma: changing incidence and survival in young people aged 0-24 years. A report from the North of England Young Persons' Malignant Disease Registry. Med Pediatr Oncol. 2001;36:231–234. doi: 10.1002/1096-911X(20010101)36:1<231::AID-MPO1056>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 4.Cotterill SJ, Pearson AD, Pritchard J, Foot AB, Roald B, Kohler JA, Imeson J. Clinical prognostic factors in 1277 patients with neuroblastoma: results of The European Neuroblastoma Study Group 'Survey' 1982-1992. Eur J Cancer. 2000;36:901–908. doi: 10.1016/s0959-8049(00)00058-7. [DOI] [PubMed] [Google Scholar]
- 5.Schönthal AH. Role of serine/threonine protein phosphatase 2A in cancer. Cancer Lett. 2001;170:1–13. doi: 10.1016/s0304-3835(01)00561-4. [DOI] [PubMed] [Google Scholar]
- 6.Seshacharyulu P, Pandey P, Datta K, Batra SK. Phosphatase: PP2A structural importance, regulation and its aberrant expression in cancer. Cancer Lett. 2013;335:9–18. doi: 10.1016/j.canlet.2013.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yeh E, Cunningham M, Arnold H, Chasse D, Monteith T, Ivaldi G, Hahn WC, Stukenberg PT, Shenolikar S, Uchida T. A signalling pathway controlling c-Myc degradation that impacts oncogenic transformation of human cells. Nat Cell Biol. 2004;6:308–318. doi: 10.1038/ncb1110. [DOI] [PubMed] [Google Scholar]
- 8.Salas A, Ponnusamy S, Senkal CE, Meyers-Needham M, Selvam SP, Saddoughi SA, Apohan E, Sentelle RD, Smith C, Gault CR. Sphingosine kinase-1 and sphingosine 1-phosphate receptor 2 mediate Bcr-Abl1 stability and drug resistance by modulation of protein phosphatase 2A. Blood. 2011;117:5941–5952. doi: 10.1182/blood-2010-08-300772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shouse GP, Nobumori Y, Liu X. A B56gamma mutation in lung cancer disrupts the p53-dependent tumor-suppressor function of protein phosphatase 2A. Oncogene. 2010;29:3933–3941. doi: 10.1038/onc.2010.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jackson J, Meisinger J, Patel S, Lim ZC, Vellody K, Metz R, Young MR. Protein phosphatase-2A associates with the cytoskeleton to maintain cell spreading and reduced motility of nonmetastatic Lewis lung carcinoma cells: the loss of this regulatory control in metastatic cells. Invasion Metastasis. 1997;17:199–209. [PubMed] [Google Scholar]
- 11.Pandey P, Seshacharyulu P, Das S, Rachagani S, Ponnusamy MP, Yan Y, Johansson SL, Datta K, Fong Lin M, Batra SK. Impaired expression of protein phosphatase 2A subunits enhances metastatic potential of human prostate cancer cells through activation of AKT pathway. Br J Cancer. 2013;108:2590–2600. doi: 10.1038/bjc.2013.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li M, Makkinje A, Damuni Z. The myeloid leukemia-associated protein SET is a potent inhibitor of protein phosphatase 2A. J Biol Chem. 1996;271:11059–11062. doi: 10.1074/jbc.271.19.11059. [DOI] [PubMed] [Google Scholar]
- 13.Schweigerer L, Breit S, Wenzel A, Tsunamoto K, Ludwig R, Schwab M. Augmented MYCN expression advances the malignant phenotype of human neuroblastoma cells: evidence for induction of autocrine growth factor activity. Cancer Res. 1990;50:4411–4416. [PubMed] [Google Scholar]
- 14.Beierle EA, Trujillo A, Nagaram A, Kurenova EV, Finch R, Ma X, Vella J, Cance WG, Golubovskaya VM. N-MYC regulates focal adhesion kinase expression in human neuroblastoma. J Biol Chem. 2007;282:12503–12516. doi: 10.1074/jbc.M701450200. [DOI] [PubMed] [Google Scholar]
- 15.Sobral LM, Sousa LO, Coletta RD, Cabral H, Greene LJ, Tajara EH, Gutkind JS, Curti C, Leopoldino AM. Stable SET knockdown in head and neck squamous cell carcinoma promotes cell invasion and the mesenchymal-like phenotype in vitro, as well as necrosis, cisplatin sensitivity and lymph node metastasis in xenograft tumor models. Mol Cancer. 2014;13:32. doi: 10.1186/1476-4598-13-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Estrada-Bernal A, Palanichamy K, Chaudhury RA, Van Brocklyn JR. Induction of brain tumor stem cell apoptosis by FTY720: a potential therapeutic agent for glioblastoma. Neuro Oncol. 2012;14:405–415. doi: 10.1093/neuonc/nos005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Szymiczek A, Pastorino S, Larson D, Tanji M, Pellegrini L, Xue J, Li S, Giorgi C, Pinton P, Takinishi Y. FTY720 inhibits mesothelioma growth in vitro and in a syngeneic mouse model. J Transl Med. 2017;15:58. doi: 10.1186/s12967-017-1158-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim HS, Yoon G, Ryu JY, Cho YJ, Choi JJ, Lee YY, Kim TJ, Choi CH, Song SY, Kim BG. Sphingosine kinase 1 is a reliable prognostic factor and a novel therapeutic target for uterine cervical cancer. Oncotarget. 2015;6:26746–26756. doi: 10.18632/oncotarget.4818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chou TC. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010;70:440–446. doi: 10.1158/0008-5472.CAN-09-1947. [DOI] [PubMed] [Google Scholar]
- 20.Cristóbal I, González-Alonso P, Daoud L, Solano E, Torrejón B, Manso R, Madoz Gúrpide J, Rojo F, García-Foncillas J. Activation of the tumor suppressor PP2A emerges as a potential therapeutic strategy for treating prostate cancer. Mar Drugs. 2015;13:3276–3286. doi: 10.3390/md13063276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Q, Zhao X, Frissora F, Ma Y, Santhanam R, Jarjoura D, Lehman A, Perrotti D, Chen CS, Dalton JT. FTY720 demonstrates promising preclinical activity for chronic lymphocytic leukemia and lymphoblastic leukemia/lymphoma. Blood. 2008;111:275–284. doi: 10.1182/blood-2006-10-053884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang Y, Huang Q, Lu Y, Li X, Huang S. Reactivating PP2A by FTY720 as a novel therapy for AML with C-KIT tyrosine kinase domain mutation. J Cell Biochem. 2012;113:1314–1322. doi: 10.1002/jcb.24003. [DOI] [PubMed] [Google Scholar]
- 23.Azuma H, Takahara S, Horie S, Muto S, Otsuki Y, Katsuoka Y. Induction of apoptosis in human bladder cancer cells in vitro and in vivo caused by FTY720 treatment. J Urol. 2003;169:2372–2377. doi: 10.1097/01.ju.0000064938.32318.91. [DOI] [PubMed] [Google Scholar]
- 24.Shen Y, Cai M, Xia W, Liu J, Zhang Q, Xie H, Wang C, Wang X, Zheng S. FTY720, a synthetic compound from Isaria sinclairii, inhibits proliferation and induces apoptosis in pancreatic cancer cells. Cancer Lett. 2007;254:288–297. doi: 10.1016/j.canlet.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 25.Garner EF, Williams AP, Stafman LL, Aye JM, Mroczek-Musulman E, Moore BP, Stewart JE, Friedman GK, Beierle EA. FTY720 decreases tumorigenesis in group 3 medulloblastoma patient-derived xenografts. Sci Rep. 2018;9:6913. doi: 10.1038/s41598-018-25263-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang L, Wang H, Zhu J, Ding K, Xu J. FTY720 reduces migration and invasion of human glioblastoma cell lines via inhibiting the PI3K/AKT/mTOR/p70S6K signaling pathway. Tumour Biol. 2014;35:10707–10714. doi: 10.1007/s13277-014-2386-y. [DOI] [PubMed] [Google Scholar]
- 27.Rincón R, Cristóbal I, Zazo S, Arpí O, Menéndez S, Manso R, Lluch A, Eroles P, Rovira A, Albanell J. PP2A inhibition determines poor outcome and doxorubicin resistance in early breast cancer and its activation shows promising therapeutic effects. Oncotarget. 2015;6:4299–4314. doi: 10.18632/oncotarget.3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Agarwal A, MacKenzie RJ, Pippa R, Eide CA, Oddo J, Tyner JW, Sears R, Vitek MP, Odero MD, Christensen DJ. Antagonism of SET using OP449 enhances the efficacy of tyrosine kinase inhibitors and overcomes drug resistance in myeloid leukemia. Clin Cancer Res. 2014;20:2092–2103. doi: 10.1158/1078-0432.CCR-13-2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Farrell AS, Allen-Petersen B, Daniel CJ, Wang X, Wang Z, Rodriguez S, Impey S, Oddo J, Vitek MP, Lopez C. Targeting inhibitors of the tumor suppressor PP2A for the treatment of pancreatic cancer. Mol Cancer Res. 2014;12:924–939. doi: 10.1158/1541-7786.MCR-13-0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim MO, Choe MH, Yoon YN, Ahn J, Yoo M, Jung KY, An S, Hwang SG, Oh JS, Kim JS. Antihelminthic drug niclosamide inhibits CIP2A and reactivates tumor suppressor protein phosphatase 2A in non–small cell lung cancer cells. Biochem Pharmacol. 2017;144:78–89. doi: 10.1016/j.bcp.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 31.Saddoughi SA, Gencer S, Peterson YK, Ward KE, Mukhopadhyay A, Oaks J, Bielawski J, Szulc ZM, Thomas RJ, Selvam SP. Sphingosine analogue drug FTY720 targets I2PP2A/SET and mediates lung tumour suppression via activation of PP2A-RIPK1–dependent necroptosis. EMBO Mol Med. 2013;5:105–121. doi: 10.1002/emmm.201201283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lucas CM, Harris RJ, Giannoudis A, Copland M, Slupsky JR, Clark RE. Cancerous inhibitor of PP2A (CIP2A) at diagnosis of chronic myeloid leukemia is a critical determinant of disease progression. Blood. 2011;117:6660–6668. doi: 10.1182/blood-2010-08-304477. [DOI] [PubMed] [Google Scholar]
- 33.Chao A, Tsai CL, Wei PC, Hsueh S, Chao AS, Wang CJ, Tsai CN, Lee YS, Wang TH, Lai CH. Decreased expression of microRNA-199b increases protein levels of SET (protein phosphatase 2A inhibitor) in human choriocarcinoma. Cancer Lett. 2010;291:99–107. doi: 10.1016/j.canlet.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 34.Jiang Q, Zhang C, Zhu J, Chen Q, Chen Y. The set gene is a potential oncogene in human colorectal adenocarcinoma and oral squamous cell carcinoma. Mol Med Rep. 2011;4:993–999. doi: 10.3892/mmr.2011.526. [DOI] [PubMed] [Google Scholar]
- 35.Khanna A, Kauko O, Böckelman C, Laine A, Schreck I, Partanen JI, Szwajda A, Bormann S, Bilgen T, Helenius M. Chk1 targeting reactivates PP2A tumor suppressor activity in cancer cells. Cancer Res. 2013;73:6757–6769. doi: 10.1158/0008-5472.CAN-13-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brodeur GM, Maris JM, Yamashiro DJ, Hogarty MD, White PS. Biology and genetics of human neuroblastomas. J Pediatr Hematol Oncol. 1997;19 doi: 10.1097/00043426-199703000-00001. [DOI] [PubMed] [Google Scholar]
- 37.Mukhopadhyay A, Tabanor K, Chaguturu R, Aldrich JV. Targeting inhibitor 2 of protein phosphatase 2A as a therapeutic strategy for prostate cancer treatment. Cancer Biol Ther. 2013;14:962–972. doi: 10.4161/cbt.25943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yokoyama N, Reich NC, Miller WT. Determinants for the interaction between Janus kinase 2 and protein phosphatase 2A. Arch Biochem Biophys. 2003;417:87–95. doi: 10.1016/s0003-9861(03)00333-3. [DOI] [PubMed] [Google Scholar]
- 39.Cristóbal I, Garcia-Orti L, Cirauqui C, Alonso MM, Calasanz MJ, Odero MD. PP2A impaired activity is a common event in acute myeloid leukemia and its activation by forskolin has a potent anti-leukemic effect. Leukemia. 2011;25:606–614. doi: 10.1038/leu.2010.294. [DOI] [PubMed] [Google Scholar]
- 40.Kappos L, Antel J, Comi G, Montalban X, O'Connor P, Polman CH, Haas T, Korn AA, Karlsson G, Radue EW. Oral fingolimod (FTY720) for relapsing multiple sclerosis. N Engl J Med. 2006;355:1124–1140. doi: 10.1056/NEJMoa052643. [DOI] [PubMed] [Google Scholar]
- 41.Kappos L, Radue EW, O'Connor P, Polman C, Hohlfeld R, Calabresi P, Selmaj K, Agoropoulou C, Leyk M, Zhang-Auberson L. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med. 2010;362:387–401. doi: 10.1056/NEJMoa0909494. [DOI] [PubMed] [Google Scholar]
- 42.Azuma H, Takahara S, Horie S, Muto S, Otsuki Y, Katsuoka Y. Marked prevention of tumor growth and metastasis by a novel immunosuppressive agent, FTY720, in mouse breast cancer models. Cancer Res. 2002;62:1410–1419. [PubMed] [Google Scholar]
- 43.Lu Z, Wang J, Zheng T, Liang Y, Yin D, Song R, Pei T, Pan S, Jiang H, Liu L. FTY720 inhibits proliferation and epithelial-mesenchymal transition in cholangiocarcinoma by inactivating STAT3 signaling. BMC Cancer. 2014;14:783. doi: 10.1186/1471-2407-14-783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang L, Wang H, Ding K, Xu J. FTY720 induces autophagy-related apoptosis and necroptosis in human glioblastoma cells. Toxicol Lett. 2015;236:43–59. doi: 10.1016/j.toxlet.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 45.Wallington-Beddoe CT, Hewson J, Badstock KF, Bendall LJ. FTY720 produces caspase-independent cell death of acute lymphoblastic leukemia cells. Autophagy. 2011;7:707–715. doi: 10.4161/auto.7.7.15154. [DOI] [PubMed] [Google Scholar]
- 46.Pereira FV, Arruda DC, Figueiredo CR, Massaoka MH, Matsuo AL, Bueno V, Rodrigues EG. FTY720 induces apoptosis in B16F10-NEX2 murine melanoma cells, limits metastatic development in vivo, and modulates the immune system. Clinics (Sao Paulo) 2013;68:1018–1027. doi: 10.6061/clinics/2013(07)21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee JW, Ryu JY, Yoon G, Jeon HK, Cho YJ, Choi JJ, Song SY, Do IG, Lee YY, Kim TJ. Sphingosine kinase 1 as a potential therapeutic target in epithelial ovarian cancer. Int J Cancer. 2015;137:221–229. doi: 10.1002/ijc.29362. [DOI] [PubMed] [Google Scholar]
- 48.Rosa R, Marciano R, Malapelle U, Formisano L, Nappi L, D'Amato C, D'Amato V, Damiano V, Marfè G, Del Vecchio S. Sphingosine kinase 1 overexpression contributes to cetuximab resistance in human colorectal cancer models. Clin Cancer Res. 2013;19:138–147. doi: 10.1158/1078-0432.CCR-12-1050. [DOI] [PubMed] [Google Scholar]
- 49.Li MH, Hla T, Ferrer F. FTY720 inhibits tumor growth and enhances the tumor-suppressive effect of topotecan in neuroblastoma by interfering with the sphingolipid signaling pathway. Pediatr Blood Cancer. 2013;60:1418–1423. doi: 10.1002/pbc.24564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lange I, Espinoza-Fuenzalida I, Ali MW, Serrano LE, Koomoa DT. FTY-720 induces apoptosis in neuroblastoma via multiple signaling pathways. Oncotarget. 2017;8:109985–109999. doi: 10.18632/oncotarget.22452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou C, Ling MT, Kin-Wah Lee T, Man K, Wang X, Wong YC. FTY720, a fungus metabolite, inhibits invasion ability of androgen-independent prostate cancer cells through inactivation of RhoA-GTPase. Cancer Lett. 2006;233:36–47. doi: 10.1016/j.canlet.2005.02.039. [DOI] [PubMed] [Google Scholar]
- 52.Motyl J, Przykaza Ł, Boguszewski PM, Kosson P, Strosznajder JB. Pramipexole and Fingolimod exert neuroprotection in a mouse model of Parkinson's disease by activation of sphingosine kinase 1 and Akt kinase. Neuropharmacology. 2018;135:139–150. doi: 10.1016/j.neuropharm.2018.02.023. [DOI] [PubMed] [Google Scholar]
- 53.Zhang L, Ding K, Wang H, Wu Y, Xu J. Traumatic brain injury-induced neuronal apoptosis is reduced through modulation of PI3K and autophagy pathways in mouse by FTY720. Cell Mol Neurobiol. 2016;36:131–142. doi: 10.1007/s10571-015-0227-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figures
Data Availability Statement
No data sets were generated in the current studies.