Abstract
Purpose
The brains of patients with epilepsy may exhibit various morphological abnormalities, which are often not directly visible on structural MR images, as they may be focally subtle or related to a more large-scale inconspicuous disorganization of brain structures. To explore the relation between structural brain organization and epilepsy characteristics, including severity and cognitive co-morbidity, we determined structural covariance networks (SCNs). SCNs represent interregional correlations of morphologic measures, for instance in terms of cortical thickness, between various large-scale distributed brain regions.
Methods
Thirty-eight patients with focal seizures of all subtypes and 21 healthy controls underwent structural MRI, neurological, and IQ assessment. Cortical thickness was derived from the structural MRIs using FreeSurfer. Subsequently, SCNs were constructed on a group-level based on correlations of the cortical thicknesses between various brain regions. Individual SCNs for the epilepsy patients were extracted by adding the respective patient to the control group prior to the SCN construction (i.e. add-one-patient approach). Calculated network measures, i.e. path length, clustering coefficient and betweenness centrality were correlated with characteristics related to the severity of epilepsy, including seizure history and age at onset of epilepsy, and cognitive performance.
Results
Stronger clustering in the individual SCN was associated with a higher number of focal to bilateral tonic-clonic seizures during life time, a younger age at onset, and lower cognitive performance. The path length of the individual SCN was not related to the severity of epilepsy or cognitive performance. Higher betweenness centrality of the left cuneus and lower betweenness centrality of the right rostral middle frontal gyrus were associated with increased drug load and younger age at onset, respectively.
Conclusions
These results indicate that the correlations between interregional variations of cortical thickness reflect disease characteristics or responses to the disease and deficits in patients with epilepsy with focal seizures.
Keywords: Magnetic resonance imaging, Cortical thickness, Structural covarience networks, Epilepsy, Seizures, Cognition
Abbreviations: SCN, Structural covariance network; AOP, Add one patient; LOO, Leave one out; L, Characteristic path length; C, Clustering coefficient; γ, Normalized characteristic path length; λ, Normalized clustering coefficient; TIV, Total intracranial volume
Highlights
-
•
Interregional cortical thicknesses relations reflect the severity of epilepsy
-
•
Stronger clustering is associated with more seizures in patients with epilepsy
-
•
Stronger clustering is associated with younger onset age in patients with epilepsy
-
•
Stronger clustering is associated with lower IQ in patients with epilepsy
-
•
Betweenness centrality is associated with drug load and age at onset
1. Introduction
The epileptic brain often has morphological abnormalities including characteristic lesions and other subtle deviations which are not visible at radiological inspection. To understand these abnormalities, several previous studies investigated the cortical thickness in various brain regions in children and adults with epilepsy (Besseling et al. 2014; Overvliet et al. 2013; Widjaja et al. 2011). More recently, morphological brain measurements, especially cortical thickness, of various brain regions have been shown to correlate across subjects. These correlations are thought to be part of an underlying anatomical network reflecting interregional correlations of cortical thicknesses, the structural covariance network (SCN). However, whether these lesion-unspecific morphological abnormalities are related to disease characteristics of the epilepsy remains largely unknown.
The biological concept of a SCN relies on the assumption that axonally connected regions have trophic, developmental, and maturational concordances, resulting in similar variation patterns of morphology (Bernhardt et al. 2011). Since epilepsy has previously been associated with morphological changes, also distant to the seizure onset zone (Bernhardt et al. 2017; Overvliet et al. 2013), these type of group-level SCNs can be especially valuable in epilepsy studies, providing unique knowledge on interregional cortical associations (Bernhardt et al. 2013; Curwood et al. 2015; Yasuda et al. 2015). However, since the correlations between cortical thickness values of different brain regions are usually obtained by correlating the thickness values over a group of subjects, individual changes cannot be obtained directly from the group-level SCNs.
Recently, Saggar et al. presented a method for calculating individual contribution on the group-level SCN (Saggar et al. 2015). By adding one patient (AOP) to a group of healthy controls prior to the SCN construction, the individual contribution of patients on the SCN can be measured. In the current study, this individualized method will be used to obtain individual measures of the SCN.
The current study aims to assess whether there are associations between individual SCNs and characteristics related to the severity of epilepsy, including the seizure history, age at onset, drug load, and the most common comorbidity, cognitive deterioration. To our knowledge, this is the first study that relates individual SCNs based on cortical thickness to individual epilepsy characteristics.
2. Materials and Methods
2.1. Participants
We included 59 participants in this study, of which 38 were clinically diagnosed with epilepsy with focal seizures of various subtypes and 21 were healthy controls. These participants have already been investigated in a number of prior studies with different MRI modalities and study objectives (Jansen et al., 2014, Jansen et al., 2008; Vaessen et al. 2012; Vlooswijk et al., 2011a, Vlooswijk et al., 2011b, Vlooswijk et al., 2010). All subjects gave written informed consent before participation, and had no significant clinical MR abnormalities, as assessed by a board certified neuroradiologist. Furthermore, the following epilepsy characteristics were recorded: age at onset, drug load, seizure focus (i.e. frontal, temporal, or frontotemporal and left, right or bilateral), and the number of focal seizures during lifetime. The latter was calculated using patient records and seizure diaries (Vlooswijk et al. 2011a). The lifetime number of focal seizures with and without impairment of awareness was expressed in eight categories (0, 1–10, 11–20, 21–30, 31–40, 41–50, 51–100, and > 100 seizures), since they were more likely to occur unperceived and were, therefore, less accurately reported (Vlooswijk et al. 2011a). The lifetime number of focal to bilateral tonic-clonic seizures could be extracted more accurately from the seizure diaries. Seizure focus was estimated based on EEG and seizure semiology. For all patients, no focal to bilateral tonic-clonic seizures were reported in the last two weeks before the MRI acquisition, and there were no obvious differences in the behavioral markers of patients across the different seizure foci. Drug load was calculated by using the ratio of prescribed daily dose to defined daily dose (Lammers et al. 1995). For all the participants, a full-scale IQ (FSIQ) was determined using the Wechsler Adult Intelligence Scale third edition (WAIS-III) (Wechsler 1997). A summary of the subject characteristics is provided in Table 1.
Table 1.
Subject characteristics.
| Epilepsy | Controls | |
|---|---|---|
| #Subjects | 38 | 21 |
| Age (in years) | 40 ± 12 | 40 ± 14 |
| Sex (Male/Female) | 20/18 | 15/6 |
| Age at onset (in years) | 22 ± 13 | NA |
| #FBTCS during lifetime | 5 (21) | NA |
| #FSIA during lifetime | 5 (7) | NA |
| Drug load | 1.8 ± 1.1 | NA |
| Seizure focus (F/T/FT) | 14/12/12 | NA |
| Seizure focus (L/R/Bi) | 15/9/14 | NA |
| Intelligence (FSIQ) | 96 ± 15** | 114 ± 15 |
Characteristics of patients with focal epilepsy and healthy controls. Variables are summarized as means ± standard deviations for normal data or median (interquartile range) for non-normally distributed data. FSIQ, full-scale IQ; FBTCS, focal to bilateral tonic-clonic seizures; FSIA, focal seizures with and without impairment of awareness; F, frontal; T, temporal; FT, frontotemporal; L, left; R, right; Bi, bilateral; NA, not applicable. Statistically significant differences between groups is denoted by ** p < .01.
2.2. MRI acquisition
Magnetic resonance imaging (MRI) was performed on a 3.0-Tesla scanner (Philips Achieva, Best, the Netherlands). T1-weighted 3D fast gradient echo images were acquired for all the participants with the following parameters: repetition time (TR) 9.91 ms, echo time (TE) 4.6 ms, inversion time (TI) 3 s, flip angle 8°, voxel size 1 × 1 × 1 mm, and matrix 256 × 256 × 200.
2.3. Analysis
2.3.1. Preprocessing
From the T1-weighted images, the cortical thickness was determined using Freesurfer (version 5.1 (Fischl et al. 2000)). The brain was parcellated into 68 cortical regions using the Desikan-Killiany atlas (Desikan et al. 2006) and the mean cortical thickness was calculated for each region.
2.3.2. Group-based SCNs
For both patients and controls, adjacency matrices were obtained by calculating the Pearson's correlation coefficient between the cortical thicknesses of each region pair. The resulting adjacency matrix elements were represented in binary values by using a threshold to select the strongest correlations. To avoid that the statistical analysis would be driven by the total number of connections (edges) in the network, the threshold was chosen such that the networks exhibit an equal number of strongest correlations, i.e. the networks are equally sparse (van Wijk et al. 2010). Negative correlations occurred fewer than 5% of the total number of correlations and were set to zero, since it is still unclear what their involvement in the network is (Gong et al. 2012). The resulting binary adjacency matrix represents the SCN, where a value of ‘1’ denotes a connection between two regions and a ‘0’ in the absence of a connection.
2.3.3. Individual SCNs
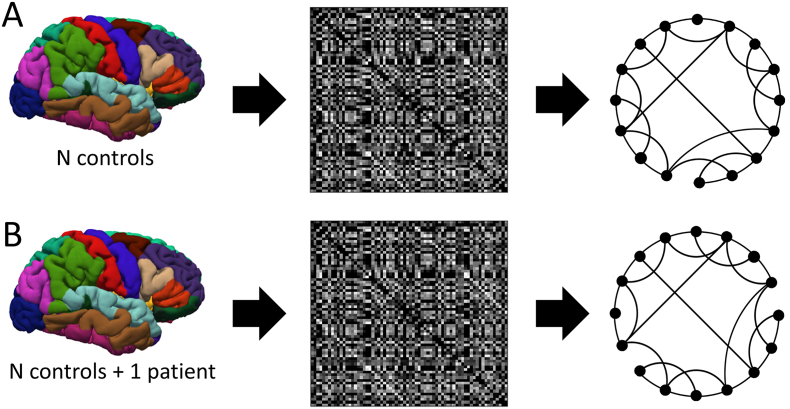
To extract the individual contribution of a patient on the SCN of a healthy control group, Saggar et al. introduced a so-called distance-based method (Saggar et al. 2015), in which the individual contribution of a patient can be assessed by adding one patient (AOP) to the control group before calculating the adjacency matrix. With this procedure, the SCN exhibits alterations compared to the SCN obtained from just the healthy control group, which are specific for the added patient. The SCN properties are quantified in terms of graph theoretical metrics (Fig. 1).
Fig. 1.
A) SCN estimation procedure for healthy controls, from the control subjects an adjacency matrix is obtained using Pearson's correlation. The adjacency matrix represents a graph. B) by adding a specific patient to the control group, the resulting adjacency matrix and graph exhibit some patient-specific alterations.
2.3.4. Network analysis
The SCNs are quantitatively described by two of the most robust and widely applied global graph metrics, the characteristic path length (L), clustering coefficient (C) (Watts and Strogatz 1998) as well as a regional measure, the betweenness centrality (BC) (Freeman 1977). L gives insight into how well information can spread throughout a network, while the C provides a measure of local information processing. A network with strong local clustering and a short path length between nodes is considered efficiently organized. The BC is a nodal graph metric that measures the number of shortest paths passing through a node, ignoring alternative paths, thereby providing insight into the influence of a node on the flow of information throughout the network. Network analysis is highly dependent on the network topology (van Wijk et al. 2010). Therefore, to obtain normalized global graph measures, the global graph metrics are determined relative to the average of 1000 random networks with similar degree distribution (λ = L/Lrand and γ = C/Crand), and only networks with the same number of nodes and edges (i.e. the networks are equally sparse) will be compared. Furthermore, to decrease the occurrence of false positives and false negatives in the network, only the nodes and edges present in the network reconstructed from the healthy controls are considered in the graph analysis (i.e. group thresholding) (De Reus and Van Den Heuvel, 2013). The reconstructed network is obtained by calculating the Pearson correlation coefficients for the control group and selecting only the statistically significant positive correlations (p < .05) as edges. Subsequently, since the optimal number of edges in a SCN is unknown and to prevent that weakly correlated connections are included (Type I errors) and that those with a high correlation are excluded (Type II errors), the number of edges in each network is varied such that the SCNs are 60–90% sparse, with intervals of 1%. Networks sparser than 90% would lead to disconnected nodes, hindering the graph analysis.
2.3.5. Statistical analysis
Previously, cortical thickness has been shown to decrease with age (Magnotta et al. 1999), to differ between males and females (Sowell et al. 2007), and to scale with the total intracranial volume (TIV) (Im et al. 2008). Therefore, for each of the 68 regions, the cortical thickness values were corrected for the effects of age, sex and TIV via linear regression models (Sanabria-Diaz et al. 2010). Differences in age, FSIQ, and corrected regional cortical thickness values were assessed with the independent samples t-test. Differences in sex were assessed with the chi-squared test of independence.
To investigate whether the cortical thickness values or the global graph metrics (λ and γ) are dependent on the location of the seizure focus (frontal, temporal or frontotemporal as well as left, right or bilateral), one-way ANOVA tests are performed. The regional corrected cortical thickness and the graph metrics are used as dependent variables in the multivariate regression analysis. Two separate sets of groups are defined as: 1) patients with a frontal, a temporal or a frontotemporal seizure focus, and 2) patients with a left, a right or a bilateral seizure focus. The false discovery rate (FDR) is used to correct for comparisons over multiple brain regions.
The Pearson's correlation coefficient was used to correlate λ and γ with FSIQ and drug load, while the correlation of the non-normally distributed variables focal to bilateral tonic-clonic seizures during lifetime, age at onset and focal seizures with and without impairment of awareness during lifetime with λ and γ was assessed using the non-parametric Spearman's ρ. Thereafter, a multivariate forward stepwise regression was performed to predict λ and γ from the variables that previously showed a significant association in the univariate analysis. Furthermore, to assess whether the relation was significantly associated with the seizure focus, four covariates (frontal focus yes/no, temporal focus yes/no, left focus yes/no and right focus yes/no) were added to the regression model.
Multivariate forward stepwise regression analysis was performed to investigate if BC was associated with the FSIQ, drug load, focal to bilateral tonic-clonic seizures during lifetime, age at onset and focal seizures with and without impairment of awareness during lifetime, while accounting for the different seizure foci. To this end, the BC of each node was predicted from one of the clinical characteristics and four added seizure focus covariates (frontal focus yes/no, temporal focus yes/no, left focus yes/no and right focus yes/no). The rate FDR was used to correct for comparisons over multiple brain regions.
The non-normally distributed variables were log-transformed prior to the regression analysis.
To assess differences between groups, the non-parametric permutation test (Yasuda et al. 2015), with 1000 permutations, was used.
Statistical significance was inferred at p < .05.
The individual SCN analysis, the graph analysis and the statistical analysis are all performed using MATLAB (version R2014b) software. All associations investigated between graph metrics and epilepsy variables were evaluated with regard to robustness to network variations by varying the sparsity value (60–90%).
3. Results
3.1. Patient characteristics
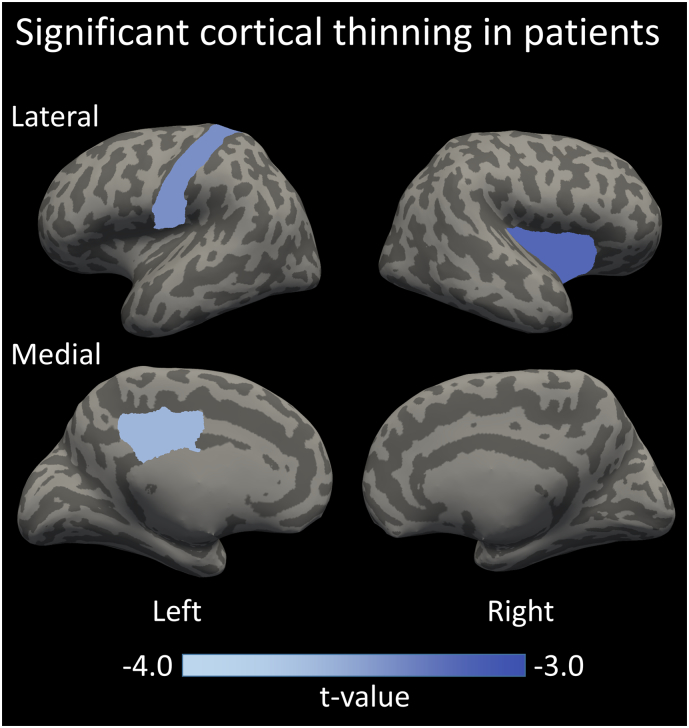
The patients with epilepsy had significantly lower FSIQ scores compared to the healthy controls (96 ± 15 vs 114 ± 15, p < .01). The patients showed cortical thinning in the postcentral gyrus and posterior cingulate in the left hemisphere, and the insula in the right hemisphere (respectively, mean ± SD in mm, 2.79 ± 0.16 vs 2.65 ± 0.15, pFDR,corrected = 0.039, 2.79 ± 0.14 vs 2.65 ± 0.13, pFDR,corrected = 0.047 and, 2.50 ± 0.12 vs 2.39 ± 0.13, pFDR,corrected = 0.048). These three regions are highlighted on an inflated cortical surface in Fig. 2. Cortical thickness did not differ significantly for the various seizure focus localizations (pFDR,corrected > 0.14).
Fig. 2.
The three cortical regions, the postcentral gyrus and posterior cingulate in the left hemisphere, and the insula in the right hemisphere, that show a significantly decreased cortical thickness in patients compared to healthy controls.
3.2. Severity of epilepsy and cognitive comorbidity
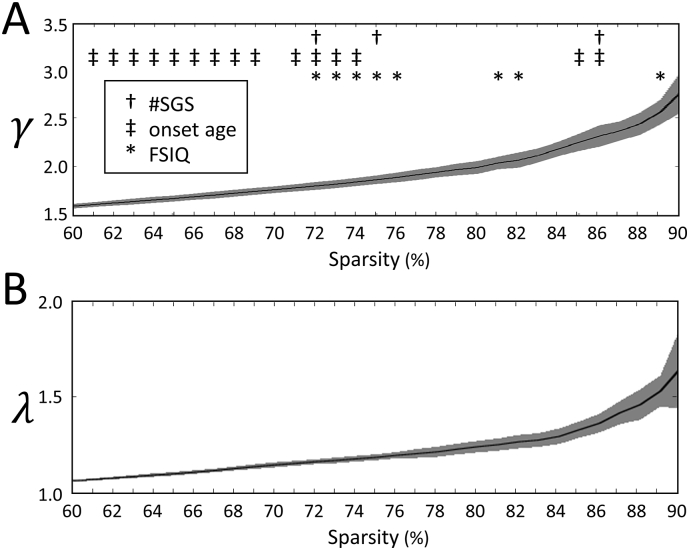
In Fig. 3, γ and λ are shown as a function of sparsity and significant associations between the two graph metrics and the clinical characteristics can be denoted.
Fig. 3.
The mean and standard deviation of the network measures (A) γ and (B) λ are shown as a function of sparsity. Significant correlations between network measures and epilepsy characteristics: †positive correlation with number of focal to bilateral tonic-clonic seizures during lifetime ‡negative correlation with onset age *negative correlation with FSIQ.
A positive correlation was found in the patient group for γ and the number of focal to bilateral tonic-clonic seizures during lifetime over the analyzed sparsity range, indicating that an increased γ is associated with more seizures.
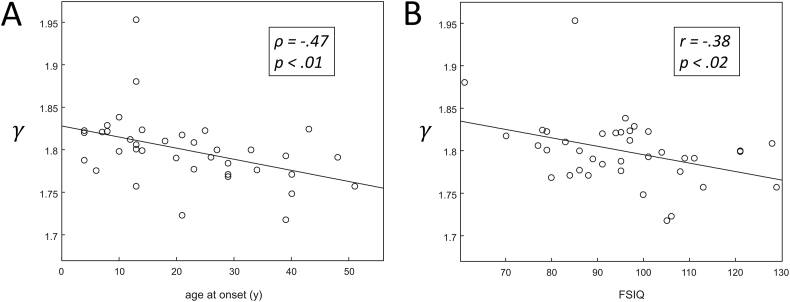
A negative correlation was found in the patient group between γ and age at onset of epilepsy over the analyzed sparsity range, indicating that an increased γ is associated with a younger age at onset of epilepsy. A scatter plot showing the relation between γ and age at onset of epilepsy for networks that are 72% sparse is shown in Fig. 4A.
Fig. 4.
Scatter plot of γ vs (A) age at onset and (B) FSIQ for a sparsity level of 72% (see Fig. 3). Linear least squares lines are fitted through the data points for visualization.
Neither consistent, nor significant associations were found between the network metrics γ and λ and either the focal seizures with and without impairment of awareness or the drug load.
A negative correlation was found for γ and FSIQ over the analyzed sparsity range, indicating that an increased γ is associated with a lower FSIQ. A scatter plot showing the relation between γ and FSIQ for networks that are 72% sparse is shown in Fig. 4B.
The correlation between λ and either the number of focal to bilateral tonic-clonic seizures during lifetime, age at onset or the FSIQ showed no such consistent correlation over the analyzed sparsity range.
Since the correlations between γ and focal to bilateral tonic-clonic seizures during lifetime, age at onset as well as FSIQ were all significant for 72% sparse networks, a multivariate stepwise linear regression analysis was performed to predict the γ for 72% sparse networks using focal to bilateral tonic-clonic seizures during lifetime, age at onset, FSIQ and two variables representing the seizure focus. As a result, both focal to bilateral tonic-clonic seizures during lifetime (β = 0.37, p < .05) and FSIQ (β = −0.31, p < .05) were entered into the regression model (F(2,35) = 5.48, p < .01, R2 = 0.24), and both added significantly to the prediction. Age at onset and the seizure focus variables (p > .05) were excluded from the regression model.
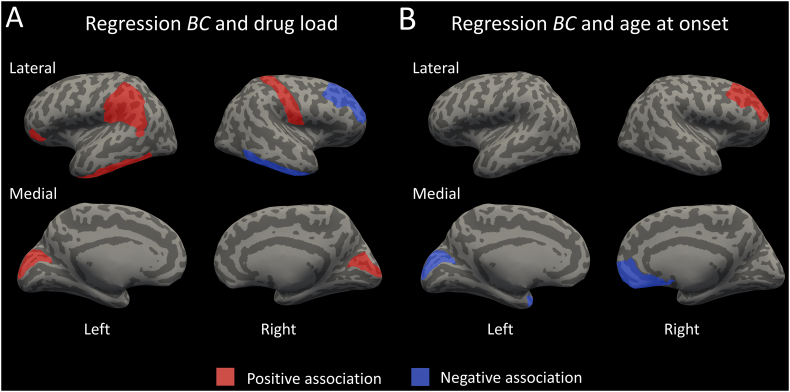
The multivariate regression analyses predicting BC from the clinical characteristics revealed significant associations (pFDR_corrected < 0.05) between drug load and the BC of the orbitalis, inferior temporal gyrus, cuneus and supramarginal gyrus of the left hemisphere and the postcentral gyrus, inferior temporal gyrus, cuneus and rostral middle frontal gyrus of the right hemisphere (Fig. 5A). Moreover, age at onset of epilepsy was associated with the BC of the cuneus and temporal pole of the left hemisphere and the medial orbitofrontal cortex and rostral middle frontal gyrus of the right hemisphere (Fig. 5B). The seizure foci variables were not added to any of the regression models using the stepwise approach (pFDR_corrected > 0.05). Higher BC of the left cuneus and lower BC of the right rostral middle frontal gyrus was associated with both increased drug load and younger age at onset of epilepsy.
Fig. 5.
The brain regions that show a significant (positive/negative) relation between BC and either drugload (A) or age at onset (B) are highlighted. The BC of the left cuneus and right rostral middle frontal gyrus are related to both drug load and age at onset.
3.3. Group-level differences
Using the non-parametric permutation test, no significant differences were found in any of the graph metrics between the patients and controls (p > .10).
4. Discussion
4.1. Current findings
The current study aimed to investigate the association between network measures of cortical thickness, in terms of individual SCNs, and clinical characteristics of patients with epilepsy with focal seizures. For the first time, it was shown that properties of SCN are correlated to characteristics of epilepsy severity. Stronger clustering within the SCN was found to be associated with (i) an increased number of focal to bilateral tonic-clonic seizures during lifetime, (ii) a younger age at onset of epilepsy and (iii) lower cognitive performance scores. Furthermore, an increased number of focal to bilateral tonic-clonic seizures during lifetime and a lower cognitive performance score were found to have the strongest association with clustering. No significant differences in terms of path length were found between the patient and control group. However, a lower BC of the left cuneus and right rostral middle frontal gyrus was shown to be associated with increased drug load and a younger age at onset of epilepsy.
4.2. Severity of epilepsy and the SCN
Our results show a relation between stronger clustering of the cortical network and higher number of focal to bilateral tonic-clonic seizures during lifetime. Previously, an increased seizure frequency was reported to be related to widespread decrease of functional connectivity in hot-water epilepsy (Bharath et al. 2015) and also functional disintegration of the default mode network in temporal lobe epilepsy (TLE) (Douw et al. 2015). Prior cohort studies in children with epilepsy suggested that early changes of functional brain organization are followed by changes of structural connectivity (Besseling et al. 2014; Overvliet et al. 2013). Therefore, the decrease (or disintegration) of functional connectivity might lead to changes in the cortical network in epilepsy. Furthermore, the notion of early changes in brain organization is in agreement with the relation with age at onset as found in the current study. In this study, stronger clustering is associated with a younger age at onset, indicating that the younger brain might already start to develop morphological adaptions in a large-scale network. This is further supported by a relation found in TLE between an increased number of seizures and neocortical atrophy (Coan et al. 2014). However, based on the current cross-sectional study design no such causal relation can be drawn formally. Alternatively, segregation of the brain network into several clusters has been implied to be a containment mechanism for activity, such as epileptic seizures (Douw et al. 2015). Therefore, the reported association could also be a compensatory mechanism, attempting to limit the effect of the seizures.
Increased drug load and younger age at onset of epilepsy are related to a lower BC of the right rostral middle frontal gyrus. Possibly, this locally decreased BC is part of a containment mechanism to isolate seizures with a frontal focus. Additionally, a higher BC of the left cuneus was found to be associated to increased severity of epilepsy (higher drug load and younger age at onset). Previously, the cortex of the cuneus was found to be thinner in patients with TLE and stronger cognitive impairment (Dabbs et al. 2009). Therefore, the observed effect might be related to changes in the cortical thickness of epilepsy patients. However, since no relation was found for a frontal or temporal seizure focus, this interpretation and its relation to the seizure focus should be investigated in more detail with other studies.
4.3. Cognitive performance and the SCN
In this study, decreased cognitive performance was found to be associated with an increase in clustering of the cortical network. Two previous studies, focusing on altered network efficiency with other MRI techniques, were performed with the same (sub)set of subjects as the current study (Vaessen et al. 2012; Vlooswijk et al. 2011b). Using diffusion tensor imaging (DTI) to construct structural networks, Vaessen et al. found that both weaker clustering and longer path lengths were related to lower cognitive performance. Furthermore, using functional MRI (fMRI) Vlooswijk et al. reported a significant association between weaker clustering and lower cognitive performance. Interestingly, the results reported in this study show an opposite relation between cognitive performance and clustering in the cortical network. Previously, SCNs were found to only partly (35–40%) reflect the fiber connections obtained using DTI (Gong et al. 2012). Furthermore, since epilepsy has been associated with morphological changes (Bernhardt et al. 2017; Overvliet et al. 2013), the SCNs might provide unique knowledge on interregional cortical associations in epilepsy, and therefore provide information which is intrinsically different from DTI and fMRI. The relationship between the network measures derived from different MRI techniques (SCN, fMRI and DTI) still needs to be elucidated in epilepsy.
4.4. Group-level differences
Cortical thinning in patients with epilepsy was observed in several cortical areas. Reduced cortical thickness was previously reported in TLE (Bernhardt et al. 2010; Keller and Roberts 2008), and is potentially related to excitotoxic damage from seizures (Kemmotsu et al. 2011). The non-parametric permutation test did not yield any differences for either the degree of clustering or the path length between patients with epilepsy and controls. The lack of differences between the two groups implies that the global characteristics of the SCN of patients with epilepsy is not significantly different from the healthy control group, which might be due to variations in the SCN between patients. This is in contrast to previous SCN studies in epilepsy (Bernhardt et al. 2013; Curwood et al. 2015; Yasuda et al. 2015), which might be explained by differences in patient populations, analysis method and sample size between our study and those studies. The current study shows that these variations in SCN between epilepsy patients reflect to some extent the epilepsy characteristics.
4.5. Study considerations
The complex interactions between morphological features (e.g. cortical thickness) are still not completely understood at a cellular level. Furthermore, whether the SCN resemble patterns of white matter connections or are more related to functional connectivity still needs to be resolved. However, in the current and previous studies it has been shown that the SCNs can be a valuable metric in epilepsy research (Bernhardt et al. 2013; Curwood et al. 2015; Yasuda et al. 2015). The location of the epileptic focus seems not of influence in the current study, however, larger study samples for the different foci are prompted for to be more definite on this matter.
4.6. Concluding remarks
The clustering of the cortical SCN is associated with the severity of epilepsy in terms of a higher number of focal to bilateral tonic-clonic seizures during lifetime, younger age at onset and lower cognitive performance. These results indicate that the correlations between interregional variations of cortical thickness reflect disease characteristics or responses to the disease and deficits in patients with epilepsy with focal seizures.
Acknowledgements
Dr. Anton de Louw is acknowledged for stimulating discussions.
This work was supported by the National Epilepsy Foundation, the Netherlands (NEF), Zeist, The Netherlands grant number 06-02.
References
- Bernhardt B.C., Bernasconi N., Concha L., Bernasconi A. Cortical thickness analysis in temporal lobe epilepsy: reproducibility and relation to outcome. Neurology. 2010;74:1776–1784. doi: 10.1212/WNL.0b013e3181e0f80a. [DOI] [PubMed] [Google Scholar]
- Bernhardt B.C., Chen Z., He Y., Evans A.C., Bernasconi N. Graph-theoretical analysis reveals disrupted small-world organization of cortical thickness correlation networks in temporal lobe epilepsy. Cereb. Cortex. 2011;21:2147–2157. doi: 10.1093/cercor/bhq291. [DOI] [PubMed] [Google Scholar]
- Bernhardt B.C., Hong S., Bernasconi A., Bernasconi N. Imaging structural and functional brain networks in temporal lobe epilepsy. Front. Hum. Neurosci. 2013;7 doi: 10.3389/fnhum.2013.00624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt B.C., Fadaie F., De Wael R.V., Hong S.-J., Liu M., Guiot M.C., Rudko D.A., Bernasconi A., Bernasconi N. Preferential susceptibility of limbic cortices to microstructural damage in temporal lobe epilepsy: A quantitative T1 mapping study. Neuroimage. 2017:1–9. doi: 10.1016/j.neuroimage.2017.06.002. [DOI] [PubMed] [Google Scholar]
- Besseling R.M.H., Jansen J.F.A., Overvliet G.M., van der Kruijs S.J.M., Ebus S.C.M., de Louw A.J.A., Hofman P.A.M., Aldenkamp A.P., Backes W.H. Delayed convergence between brain network structure and function in rolandic epilepsy. Front. Hum. Neurosci. 2014;8:1–9. doi: 10.3389/fnhum.2014.00704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharath R.D., Sinha S., Panda R., Raghavendra K., George L., Chaitanya G., Gupta A., Satishchandra P. Seizure frequency can alter brain connectivity: evidence from resting-state fmri. Am. J. Neuroradiol. 2015;36:1890–1898. doi: 10.3174/ajnr.A4373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coan A.C., Campos B.M., Yasuda C.L., Kubota B.Y., Bergo F.P.G., Guerreiro C.A.M., Cendes F. Frequent seizures are associated with a network of gray matter atrophy in temporal lobe epilepsy with or without hippocampal sclerosis. PLoS One. 2014;9 doi: 10.1371/journal.pone.0085843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curwood E.K., Pedersen M., Carney P.W., Berg A.T., Abbott D.F., Jackson G.D. Abnormal cortical thickness connectivity persists in childhood absence epilepsy. Ann. Clin. Transl. Neurol. 2015;2:456–464. doi: 10.1002/acn3.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabbs K., Jones J., Seidenberg M., Hermann B. Neuroanatomical correlates of cognitive phenotypes in temporal lobe epilepsy. Epilepsy Behav. 2009;15:445–451. doi: 10.1016/j.yebeh.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Reus M.A., Van Den Heuvel M.P. Estimating false positives and negatives in brain networks. NeuroImage. 2013;70:402–409. doi: 10.1016/j.neuroimage.2012.12.066. [DOI] [PubMed] [Google Scholar]
- Desikan R.S., Ségonne F., Fischl B., Quinn B.T., Dickerson B.C., Blacker D., Buckner R.L., Dale A.M., Maguire R.P., Hyman B.T., Albert M.S., Killiany R.J. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Douw L., Desalvo M.N., Tanaka N., Cole A.J., Liu H., Reinsberger C., Stufflebeam S.M. Dissociated multimodal hubs and seizures in temporal lobe epilepsy. Ann. Clin. Transl. Neurol. 2015;2:338–352. doi: 10.1002/acn3.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B., Fischl B., Dale a M., Dale a M. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc. Natl. Acad. Sci. U. S. A. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman L. A Set of measures of Centrality based on Betweenness. Sociometry. 1977;40:35–41. [Google Scholar]
- Gong G., He Y., Chen Z.J., Evans A.C. Convergence and divergence of thickness correlations with diffusion connections across the human cerebral cortex. NeuroImage. 2012;59:1239–1248. doi: 10.1016/j.neuroimage.2011.08.017. [DOI] [PubMed] [Google Scholar]
- Im K., Lee J.M., Lyttelton O., Kim S.H., Evans A.C., Kim S.I. Brain size and cortical structure in the adult human brain. Cereb. Cortex. 2008;18:2181–2191. doi: 10.1093/cercor/bhm244. [DOI] [PubMed] [Google Scholar]
- Jansen J.F.A., Vlooswijk M.C.G., Majoie H.J.M., de Krom M.C.T.F.M., Aldenkamp A.P., Hofman P.A.M., Backes W.H. White matter lesions in patients with localization-related epilepsy. Investig. Radiol. 2008;43:552–558. doi: 10.1097/RLI.0b013e31817e90d2. [DOI] [PubMed] [Google Scholar]
- Jansen J.F.A., Van Der Kruijs S.J.M., Vlooswijk M.C.G., Majoie H.J.M., Hofman P.A.M., Aldenkamp A.P., Backes W.H. Quantitative MR and cognitive impairment in cryptogenic localisation-related epilepsy. Epileptic Disord. 2014;16:318–327. doi: 10.1684/epd.2014.0665. [DOI] [PubMed] [Google Scholar]
- Keller, S.S., Roberts, N., 2008. Voxel-based morphometry of temporal lobe epilepsy : An introduction and review of the literature 49, 741–757. doi: 10.1111/j.1528-1167.2007.01485.x. [DOI] [PubMed]
- Kemmotsu N., Girard H.M., Bernhardt B.C., Bonilha L., Lin J.J., Tecoma E.S., Iraqui V.J., Hagler D.J., Jr., Halgren E., McDonald C.C. MRI Analysis in Temporal Lobe Epilepsy: Cortical Thinning and White Matter Disruptions are Related to Side of Seizure Onset. Epilepsia. 2011;52:2257–2266. doi: 10.1111/j.1528-1167.2011.03278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammers M.W., Hekster Y.A., Keyser A., Meinardi H., Renier W.O., Lier H. Monotherapy or Polytherapy for Epilepsy Revisited: a Quantitative Assessment. Epilepsia. 1995;36:440–446. doi: 10.1111/j.1528-1157.1995.tb00484.x. [DOI] [PubMed] [Google Scholar]
- Magnotta V.A., Andreasen N.C., Schultz S.K., Harris G., Cizadlo T., Heckel D., Nopoulos P., Flaum M. Quantitative in vivo measurement of gyrification in the human brain: changes associated with aging. Cereb. Cortex. 1999;9:151–160. doi: 10.1093/cercor/9.2.151. [DOI] [PubMed] [Google Scholar]
- Overvliet G.M., Besseling R.M.H., Jansen J.F.A., Van Der Kruijs S.J.M., Vles J.S.H., Hofman P.A.M., Ebus S.C.M., De Louw A., Aldenkamp A.P., Backes W.H. Early onset of cortical thinning in children with rolandic epilepsy. NeuroImage Clin. 2013;2:434–439. doi: 10.1016/j.nicl.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggar M., Hosseini S.M.H., Bruno J.L., Quintin E.-M., Raman M.M., Kesler S.R., Reiss A.L. Estimating individual contribution from group-based structural correlation networks. NeuroImage. 2015;120:274–284. doi: 10.1016/j.neuroimage.2015.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanabria-Diaz G., Melie-García L., Iturria-Medina Y., Alemán-Gómez Y., Hernández-González G., Valdés-Urrutia L., Galán L., Valdés-Sosa P. Surface area and cortical thickness descriptors reveal different attributes of the structural human brain networks. NeuroImage. 2010;50:1497–1510. doi: 10.1016/j.neuroimage.2010.01.028. [DOI] [PubMed] [Google Scholar]
- Sowell E.R., Peterson B.S., Kan E., Woods R.P., Yoshii J., Bansal R., Xu D., Zhu H., Thompson P.M., Toga A.W. Sex differences in Cortical Thickness Mapped in 176 healthy individuals between 7 and 87 years of Age. Cereb. Cortex. 2007;17:1550–1560. doi: 10.1093/cercor/bhl066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaessen M.J., Jansen J.F.A., Vlooswijk M.C.G., Hofman P.A.M., Majoie H.J.M., Aldenkamp A.P., Backes W.H. White matter network abnormalities are associated with cognitive decline in chronic epilepsy. Cereb. Cortex. 2012;22:2139–2147. doi: 10.1093/cercor/bhr298. [DOI] [PubMed] [Google Scholar]
- van Wijk B.C.M., Stam C.J., Daffertshofer A. Comparing Brain Networks of different size and Connectivity Density using Graph Theory. PLoS One. 2010;5 doi: 10.1371/journal.pone.0013701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlooswijk M.C.G., Jansen J.F.A., Majoie H.J.M., Hofman P.A.M., de Krom M.C.T.F.M., Aldenkamp A.P., Backes W.H. Functional connectivity and language impairment in cryptogenic localization-related epilepsy. Neurology. 2010;75:395–402. doi: 10.1212/WNL.0b013e3181ebdd3e. [DOI] [PubMed] [Google Scholar]
- Vlooswijk M.C.G., Jansen J.F.A., Jeukens C.R.L.P.N., Marian Majoie H.J., Hofman P.A.M., de Krom M.C.T.F.M., Aldenkamp A.P., Backes W.H. Memory processes and prefrontal network dysfunction in cryptogenic epilepsy. Epilepsia. 2011;52:1467–1475. doi: 10.1111/j.1528-1167.2011.03108.x. [DOI] [PubMed] [Google Scholar]
- Vlooswijk M.C.G., Vaessen M.J., Jansen J.F.A., de Krom M.C.F.T.M., Majoie H.J.M., Hofman P.A.M., Aldenkamp A.P., Backes W.H. Loss of network efficiency associated with cognitive decline in chronic epilepsy. Neurology. 2011;77:938–944. doi: 10.1212/WNL.0b013e31822cfc2f. [DOI] [PubMed] [Google Scholar]
- Watts D.J., Strogatz S.H. Collective dynamics of ‘small-world’ networks. Nature. 1998;393:440–442. doi: 10.1038/30918. [DOI] [PubMed] [Google Scholar]
- Wechsler D. 1997. Manual for the Wechsler Adult Intelligence Scale. (thrid edition) [Google Scholar]
- Widjaja E., Mahmoodabadi S.Z., Snead O.C., Almehdar A., Smith M. Lou. Widespread cortical thinning in children with frontal lobe epilepsy. Epilepsia. 2011;52:1685–1691. doi: 10.1111/j.1528-1167.2011.03085.x. [DOI] [PubMed] [Google Scholar]
- Yasuda C.L., Chen Z., Beltramini G.C., Coan A.C., Morita M.E., Kubota B., Bergo F., Beaulieu C., Cendes F., Gross D.W. Aberrant topological patterns of brain structural network in temporal lobe epilepsy. Epilepsia. 2015;56:1992–2002. doi: 10.1111/epi.13225. [DOI] [PubMed] [Google Scholar]