Abstract
Medication-related osteonecrosis of the jaw (MRONJ) is a rare but detrimental intraoral lesion that predominantly occurs in patients with long-term use of antiresorptive agents, such as bisphosphonate and denosumab, a human anti–receptor activator of NF-κB ligand (RANKL) monoclonal antibody (Ab). Surgical intervention, such as tooth extraction, is a known risk factor for MRONJ, which is often performed to eliminate preexiting pathologic inflammatory conditions, such as periodontal diseases. Nonetheless, it remains unknown whether pre-existing periodontal disease condition exacerbates, or removal of such condition ameliorates, MRONJ development after tooth extraction. In this study, we combined the ligature-induced periodontitis and the tooth extraction mouse models under the administration of zoledronic acid (ZOL) or anti-RANKL Ab, and provide experimental evidence that a pre-existing pathologic inflammatory condition exacerbates MRONJ development after tooth extraction in mice. Under ZOL administration, tooth extraction alone induced ONJ lesions; however, extraction of a ligature-placed tooth further exacerbated ONJ development. When the ligature was removed and the inflammatory condition was deescalated, ONJ development was ameliorated. Anti-RANKL Ab administration resulted in similar outcomes. Interestingly, unlike ZOL-administered mice, anti-RANKL Ab–administered mice exhibited complete absence of osteoclasts, suggesting that physical presence of osteoclasts is not directly involved in ONJ development. Collectively, our study demonstrated that periodontal disease is a functionally linked risk factor that predisposes ONJ development after tooth extraction in the presence of bisphosphonate and denosumab.
Nitrogen-containing bisphosphonates (N-BPs) and denosumab (Dmab) are potent antiresorptive agents that are commonly prescribed to manage bone-related diseases, such as osteoporosis, hypercalcemia, or bone metastasis. Despite their proven efficacy to protect from bone loss and adverse skeletal-related events, the prevalence of N-BP use has declined over the past decade because of safety concerns that arise from adverse effects, such as atypical femur fracture or medication-related osteonecrosis of the jaw (MRONJ).1, 2 Knowing the pathophysiology of atypical femur fracture or MRONJ development, therefore, is of critical importance to prevent these unwanted adverse effects while continually using these effective antiresorptive drugs.
MRONJ is a rare but detrimental lesion that specifically occurs in the oral cavity of the long-term users of N-BPs and Dmab.1 MRONJ is defined as exposed bone or probable bone through intraoral and/or extraoral fistula that persists for >8 weeks in patients who are undergoing antiresorptive or antiangiogenic treatment without a history of radiation therapy.1 Osteonecrosis of the jaw (ONJ) occurs predominantly in patients receiving N-BPs or denosumab; hence, it is called bisphosphonate-related osteonecrosis of the jaw (BRONJ) or denosumab-related osteonecrosis of the jaw (DRONJ), respectively. However, ONJ is frequently noted in patients receiving an antiangiogenic agent, such as bevacizumab.3 Although the first observation was reported in 2003,4 the exact mechanisms of MRONJ pathophysiology still remain unclear to date.
There are several known risk factors that are associated with MRONJ development, such as duration of medication,5 administration route of medication,6 local inflammatory conditions,7 and dentoalveolar surgery.8 Among them, clinical observations clearly indicate that tooth extraction increases the likelihood of developing an ONJ lesion in the users of these medications.1, 8, 9 However, a close examination revealed that tooth extraction is typically preceded by pathologic inflammatory conditions (eg, periodontal or periapical diseases), which may compromise sustainability and integrity of the tooth and surrounding bone structures if the tooth were to remain as is. Indeed, it has been shown that oncological patients taking BP with active periodontal conditions frequently developed BRONJ, followed by extraction of the unrestorable teeth.10 Nonetheless, it is unknown as to what extent those pre-existing pathologic inflammatory conditions contribute to ONJ development, or whether removing inflammatory conditions may ameliorate ONJ lesions.
Previously, we established the mouse models for BRONJ and DRONJ and showed that tooth extraction alone induced ONJ development when administered with the high doses of N-BP and anti–receptor activator of NF-κB ligand (RANKL) antibody (Ab) intravenously in mice.11 Furthermore, we demonstrated that pre-existing periapical lesions exacerbated tooth extraction–induced BRONJ lesions in mice.12 In this study, a pathologic inflammatory periodontal lesion was induced by placing a ligature around a tooth in mice. By combining this ligature-induced periodontitis model and the tooth extraction model in mice, the effect of pre-existing periodontal diseases, as well as the effects of removing this periodontal disease condition, was examined on BRONJ and DRONJ development. Herein, we report that a pre-existing periodontal disease condition exacerbates a tooth extraction–induced ONJ lesion and that removing such a condition before tooth extraction ameliorates ONJ development in the presence of N-BPs or Dmab.
Materials and Methods
Animals
Six-week–old female C57BL/6J mice were purchased from Jackson Laboratories (Bar Harbor, ME) and housed in the vivarium at University of California, Los Angeles, Division of Laboratory Animal Medicine. All experimental protocols were approved by institutional guidelines from the Chancellor's Animal Research Committee (2011-062).
BRONJ and DRONJ Mouse Models with Ligature-Induced Periodontitis
Mouse models for BRONJ and DRONJ were used, as previously described.11 Briefly, for the BRONJ mouse model, mice (n = 10 per group unless otherwise indicated) were biweekly administered with 0.9% NaCl saline [vehicle (Veh)] or zoledronic acid (ZOL; Zometa; 125 μg/kg; Novartis Oncology, East Hanover, NJ) intravenously through the tail vein throughout the experiment. For the DRONJ mouse model, mice (n = 5 per group) were biweekly administered with 250 μg of anti-mouse RANKL monoclonal Ab (IK22-5)13 or control rat IgG (Sigma, St. Louis, MO) in phosphate-buffered saline intravenously through the tail vein throughout the experiment. To induce periodontitis, a ligature was placed 1 week after the initial administration of either BP or anti-mouse RANKL monoclonal Ab on the second maxillary molar using a 6-0 suture under general anesthesia using ketamine/xylazine (100 and 5 mg/kg, respectively). Three weeks after placing the ligature, the same tooth was atraumatically extracted under general anesthesia. To remove inflammatory conditions, the ligature was removed under general anesthesia. Three weeks after the tooth extraction, the mice were sacrificed, and maxillae were harvested. The harvested maxillae were fixed in 4% paraformaldehyde/1× phosphate-buffered saline solution overnight. The fixed tissues were subjected to further analyses.
Micro–Computed Tomography Scan
The fixed maxillae were scanned in Scanco μCT 40 (Scanco Medical, Brüttisellen, Switzerland) at a voxel size of 20 μm3 and a 0.5-mm aluminum filter at 55 kVp and 145 μA, with an integration time of 200 milliseconds using a cylindrical tube (field of view/diameter, 20.48 mm). Maxillary tissues were reconstructed and analyzed via the CTan and CTvol programs (Bruker microCT, Kontich, Belgium) to generate three-dimensional images and cross-sectional images. Bone loss was quantified by measuring the distance between cementoenamel junction and the alveolar ridge on palatal and buccal roots of the first and second molars in extraction and nonextraction periodontitis models, respectively, on Dataviewer (Bruker microCT).
Hematoxylin and Eosin Staining
The scanned tissues were decalcified with 5% EDTA and 4% sucrose in phosphate-buffered saline, pH 7.4. Decalcification continued for 2 to 3 weeks at 4°C, and the decalcification solution was changed daily. Fully decalcified issue samples were sent to the University of California, Los Angeles Translational Procurement Core Laboratory and processed for paraffin embedding. The embedded tissues were sectioned at the mesiodistal plane at 4 μm thickness. The sectioned slides (n = 4, every five cuts) were deparaffinized at 60°C, then rehydrated in ethanol with an increasing concentration of water. The rehydrated tissue slides were stained with hematoxylin for 2.5 minutes, washed with water and 95% ethanol, and then stained with eosin for 1 minute. The stained slides were dehydrated in 70%, 95%, and 100% ethanol, followed by xylene. The slides were mounted using mounting medium (Permount; Fisher Scientific, Houston, TX). Bone area quantification was measured using ImageJ software version 1.48 (NIH, Bethesda, MD; http://imagej.nih.gov/ij) on digital images taken through an Olympus microscope (model DP72; Olympus Corp., Tokyo, Japan) at ×100 magnification.
Histochemical Staining
Tartrate-resistant acid phosphatase staining was performed, as described previously.11 Briefly, the sectioned slides (n = 3, every five cuts) were incubated with tartrate-resistant acid phosphatase solution in a humidification chamber at 37°C in the dark for 1 hour. The slides were washed in water and counterstained in hematoxylin, then mounted with ImmunoHistoMount (Sigma-Aldrich, St. Louis, MO). Osteoclasts were identified by the presence of multiple nuclei (n > 5). Osteoclast number quantification and surface area were measured using ImageJ software version 1.48 on digital images taken through an Olympus microscope (model DP72) at ×100 magnification.
Real-Time Quantitative RT-PCR
Palatal tissues immediately adjacent to the ligated second maxillary molars were harvested. The harvested tissues were treated with 1 mL of TRIzol Reagent (Thermo Fisher Scientific, Waltham, MA), according to manufacturer's protocol. The quality of isolated mRNA was assessed using a NanoDrop Spectrophotometer (Thermo Fisher Scientific). cDNA was synthesized from 2.5 μg of total RNA extracted using the SuperScript First-Strand Synthesis system (Invitrogen, Carlsbad, CA) and Random Primer (Invitrogen). cDNA was amplified using SYBR Green I Master Mix (Roche Applied Science, Penzberg, Germany) with the LightCycler 480 II real-time PCR system with primers for IL-1β, IL-6, IL-17, and glyceraldehyde-3-phosphate dehydrogenase. The second derivative quantitation cycle values of the genes and glyceraldehyde-3-phosphate dehydrogenase were compared to assess the fold differences of amplification, following the manufacturer's instruction (Roche Applied Science).
Statistical Analysis
One-way analysis of variance and Tukey's post hos test were used to compare the number of osteoclasts, empty lacunae, and necrotic bone (percentage) among different groups. All of the statistical analyses were performed with SPSS software version 19.0 (IBM Corp., Armonk, NY), with a significance level of 0.05.
Results
Ligature-Induced Periodontal Inflammatory Condition Exacerbates BRONJ Lesions after Tooth Extraction in Mice
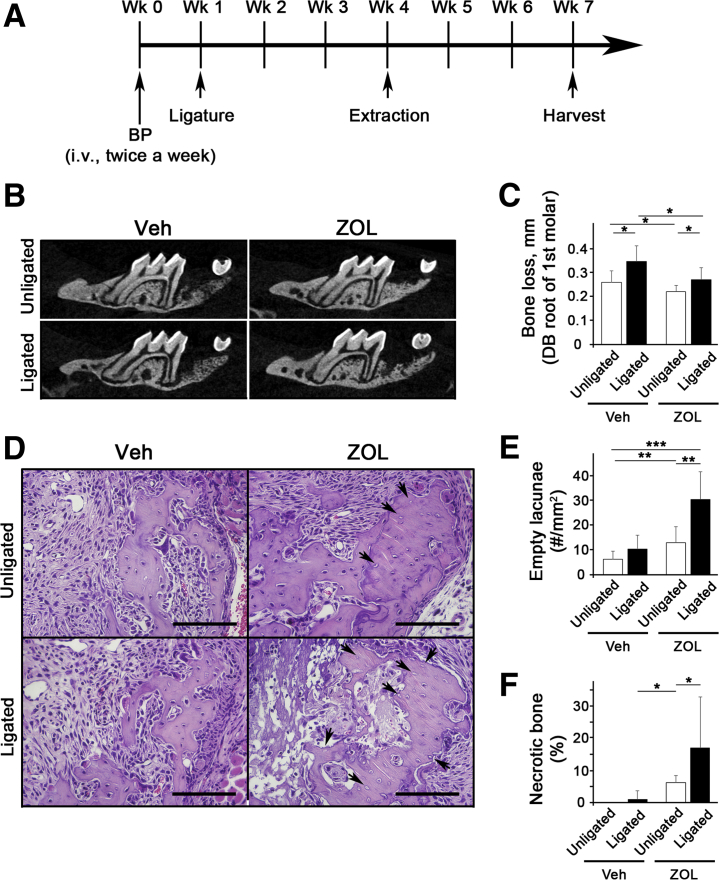
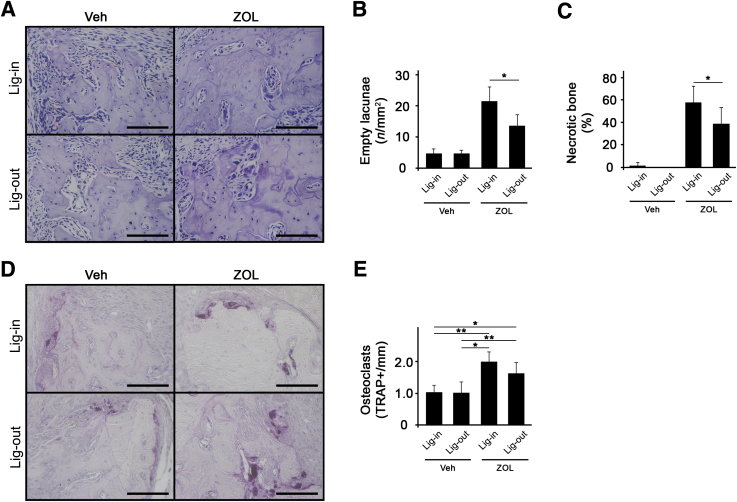
To investigate the role of periodontal disease lesions in ONJ development, a ligature-induced periodontitis model in which ligature placement induces inflammation by trapping bacteria around the ligature and causes bone resorption was used.14 Mice were first administered with ZOL for 1 week, and a ligature was placed around the second maxillary molar. After 3 weeks, the same tooth was extracted and allowed to heal for 3 additional weeks (Figure 1A). As expected, ligature placement induced significant bone loss around the tooth, and ZOL administration prevented bone loss (Figure 1, B and C). Histologic examination revealed that, in ZOL-treated mice, tooth extraction alone induced bone necrosis; however, extraction of a ligature-placed tooth further enhanced bone necrosis (Figure 1, D–F). In Veh-treated mice, tooth extraction or ligature placement did not induce bone necrosis. These data suggest that a pathologic inflammatory condition exacerbates tooth extraction–induced BRONJ lesions in mice.
Figure 1.
Ligature-induced periodontal inflammatory condition exacerbates bisphosphonate-related osteonecrosis of the jaw lesion after tooth extraction in mice. A: The schematic timeline for this study (Materials and Methods). B: Sagittal sections of micro–computed tomography scan images of the first maxillary molar. C: Alveolar bone loss measured at the distobuccal (DB) root of the maxillary first molar from cementoenamel junction to alveolar bone crest. D: Hematoxylin and eosin–stained tissues at the tooth-extracted site. Arrows indicate empty lacunae. E: Quantification of empty lacunae per mm2. F: Quantification of necrotic bone in percentage. n = 10 per group (A). ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001. Scale bars = 100 μm (D). BP, bisphosphonate; Veh, vehicle; Wk, week; ZOL, zoledronic acid.
Ligature Removal De-Escalates Inflammatory Conditions and Reduces Bone Loss
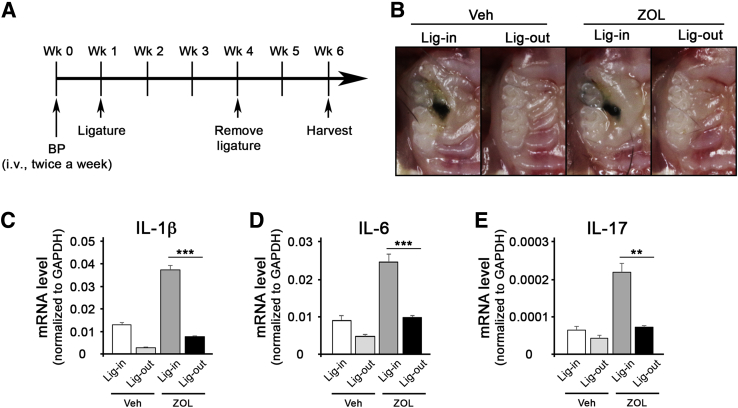
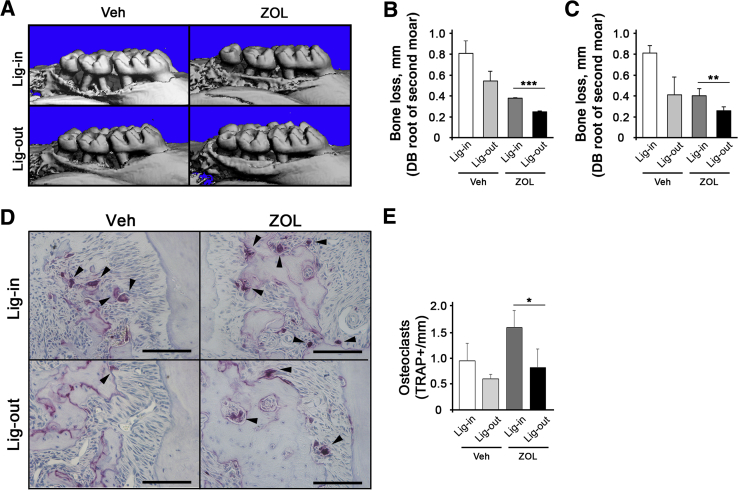
Next, it was explored whether removing the pathologic inflammatory conditions may have any effects on ONJ development. It was first examined whether simple removal of the ligature may reduce the pathologic inflammatory condition. Three weeks after ligature placement, the ligature was removed and the mice were allowed to heal for 2 weeks (Figure 2A). Visual inspection showed that, in both Veh- and ZOL-treated mice, gingival swelling was significantly diminished in ligature-removed tooth when compared with that in ligature-maintained tooth (Figure 2B). Consistent with such observations, expression of inflammatory cytokines, such as IL-1β, IL-6, and IL-17, was reduced in gingival tissues around the ligature-removed teeth, as determined by quantitative RT-PCR (Figure 2, C–E). Interestingly, ZOL-treated mice exhibited significant induction of inflammatory cytokines when compared with the respective counterparts in Veh-treated mice in both ligature-maintained and ligature-removed mice (Figure 2, C–E). Micro–computed tomography analysis showed that ZOL-treated mice exhibited reduced bone loss when compared with that in Veh-treated mice (Figure 3, A–C). Furthermore, ligature removal prevented bone loss in both Veh- and ZOL-treated mice. In line with this observation, numbers of osteoclasts were decreased when ligature was removed (Figure 3, D and E). Interestingly, the numbers of osteoclasts were higher in ZOL-treated, ligature-maintained mice, although the bone loss was significantly prevented when compared with Veh-treated, ligature-maintained mice (Figure 3, D and E), suggesting that osteoclasts in ZOL-treated, ligature-maintained mice are not functional.
Figure 2.
Ligature removal (Lig-out) deescalates inflammatory conditions in mice. A: The schematic timeline for this study. B: Clinical presentation of the ligature placement on the maxillary second molar at the end of the study. C–E: Quantitative RT-PCR for IL-1β (IL1B), IL6, and IL17 mRNA obtained from the oral mucosal tissues around the second maxillary molar. n = 3 per group (A). ∗∗P < 0.01, ∗∗∗P < 0.001. BP, bisphosphonate; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; Lig-in, ligature maintained; Veh, vehicle; Wk, week; ZOL, zoledronic acid.
Figure 3.
Ligature removal (Lig-out) prevents alveolar bone loss in mice. A: Micro–computed tomography scans of the maxillae taken from the lateral views. B and C: Alveolar bone loss measured at the distobuccal (DB) and palatal (P) roots of the maxillary second molar from cementoenamel junction to alveolar bone crest. D: Tartrate-resistant acid phosphatase (TRAP)–stained tissues at the maxillary second molar areas. Arrowheads indicate osteoclasts. E: Quantification of TRAP+ osteoclasts per mm. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001. Scale bars = 100 μm (D). Lig-in, ligature maintained; Veh, vehicle; ZOL, zoledronic acid.
Ligature Removal Ameliorates Tooth Extraction–Induced BRONJ Lesion in Mice
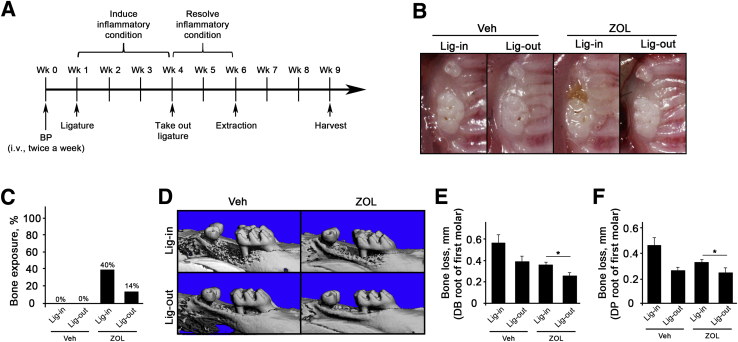
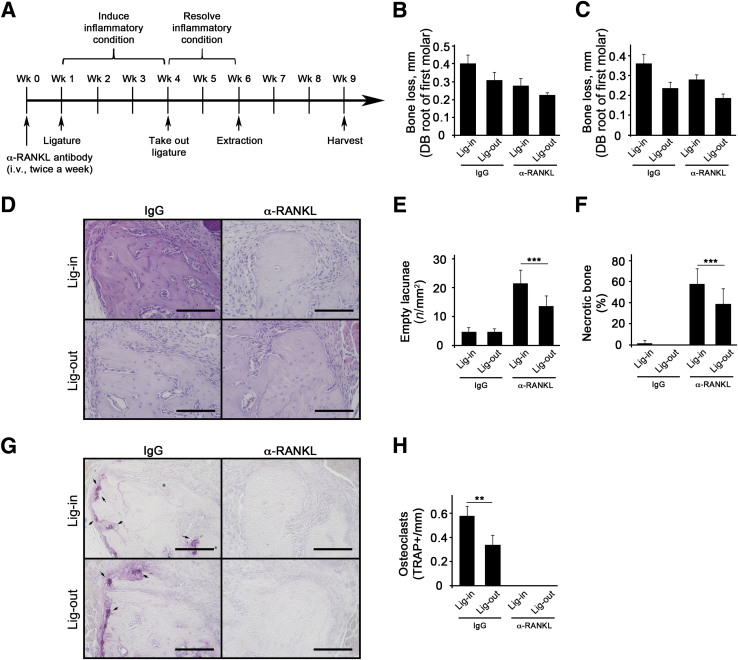
The effects of removing pathologic periodontitis conditions were next examined on tooth extraction–induced BRONJ development. After ligature placement for 3 weeks, the ligature was removed and the mice were allowed to heal for 2 weeks before tooth extraction (Figure 4A). Three weeks after tooth extraction, the mice were harvested, and it was found that all Veh-treated mice were healed, including both ligature-maintained and ligature-removed groups. On the other hand, ZOL-treated, ligature-maintained mice exhibited a significant increase in bone exposure, whereas ZOL-treated, ligature-removed mice exhibited reduction in bone exposure (Figure 4, B and C). As expected, ZOL-treated mice exhibited reduced bone loss when compared with the respective counterparts in Veh-treated mice in both ligature-maintained and ligature-removed mice (Figure 4, D–F). Histologic examination revealed a significant bone necrosis in ZOL-treated, ligature-maintained mice, whereas there was a reduced amount of bone necrosis in ZOL-treated, ligature-removed mice, as demonstrated by empty lacunae and percentage of necrotic bone (Figure 5, A–C), indicating ligature removal ameliorated BRONJ lesions. Despite the fact that bone loss was prevented in ZOL-treated mice, the numbers of osteoclasts were significantly high (Figure 5, D and E).
Figure 4.
Ligature removal (Lig-out) reduces bone exposure and bone loss in mice. A: The schematic timeline for this study (one tooth per mouse). B: Clinical presentation of the tooth-extracted sites maxillary molar at the end of the study. C: Percentage of mice with exposed bone. D: Micro–computed tomography scans of the maxillae taken from the lateral views. E and F: Alveolar bone loss measured at the distobuccal (DB) and distopalatal (DP) roots of the maxillary first molar from cementoenamel junction to alveolar bone crest. n = 10 per group (A). ∗P < 0.05. BP, bisphosphonate; Lig-in, ligature maintained; Veh, vehicle; Wk, week; ZOL, zoledronic acid.
Figure 5.
Ligature removal (Lig-out) ameliorates tooth extraction–induced bisphosphonate-related osteonecrosis of the jaw lesion in mice. A: Hematoxylin and eosin–stained tissues at the tooth-extracted site. B: Quantification of empty lacunae per mm2. C: Quantification of necrotic bone in percentage. D: Tartrate-resistant acid phosphatase (TRAP)–stained tissues at the tooth-extracted site. E: Quantification of TRAP+ osteoclasts per mm. ∗P < 0.05, ∗∗P < 0.01. Scale bars = 100 μm (A and D). Lig-in, ligature maintained; Veh, vehicle; ZOL, zoledronic acid.
Ligature Removal Ameliorates Tooth Extraction–Induced DRONJ Lesion in Mice
Next, it was further examined whether ligature removal also ameliorates tooth extraction–induced DRONJ lesions in mice. In the presence of anti-RANKL antibody administration, ligature was placed for 3 weeks, the mice were left with or without ligature for 2 weeks, and tooth extraction was performed (Figure 6A). Similar to the BRONJ model, the bone loss was significantly reduced (Figure 6, B–D). Furthermore, ligature removal was able to reduce tooth extraction–induced DRONJ lesions in mice, as demonstrated by empty lacunae and percentage of necrotic bone (Figure 6, E–G). Consistent with previous findings, no osteoclasts were found in mice treated with anti-RANKL antibody (Figure 6H).11 These data indicate that deescalating the inflammatory condition by removing ligature ameliorated tooth extraction–induced DRONJ lesions in mice.
Figure 6.
Ligature removal (Lig-Out) ameliorates tooth extraction–induced denosumab-related osteonecrosis of the jaw lesion in mice. A: The schematic timeline for this study (two teeth per mouse). B and C: Alveolar bone loss measured at the distobuccal (DB) or distopalatal (DP) roots of the first maxillary molar from cementoenamel junction to alveolar bone crest. D: Hematoxylin and eosin–stained tissues at the tooth-extracted site. E: Quantification of empty lacunae per mm2. F: Quantification of necrotic bone in percentage. G: Tartrate-resistant acid phosphatase (TRAP)–stained tissues at the tooth-extracted site. Arrows indicate osteoclasts. H: Quantification of TRAP+ osteoclasts per mm. n = 5 per group. ∗∗P < 0.01, ∗∗∗P < 0.001. Scale bars = 100 μm (D and G). Lig-In, ligature maintained; RANKL, receptor activator of NF-κB ligand; Wk, week.
Discussion
Ligature-induced periodontitis in mice is a well-established animal model that recapitulates human periodontal diseases, including the following: i) entrapment of bacteria and accumulation of dental plaque; ii) stimulation of a host response, including immune system, that causes destruction of periodontium; and iii) activation of osteoclasts and alveolar bone loss.15, 16 In this study, this ligature-induced periodontitis model was combined with the tooth extraction model, and it was shown that pre-existing pathologic inflammatory conditions exacerbate ONJ development after tooth extraction in ZOL- or anti-RANKL Ab–treated mice. Furthermore, it was also demonstrated that removing such inflammatory conditions before tooth extraction ameliorated ONJ development. Our study provides compelling experimental evidence that pre-existing pathologic inflammatory conditions, together with tooth extraction, play a causal role in inducing MRONJ development in BPs or denosumab users.
Previously, we showed that tooth extraction induced BRONJ and DRONJ lesions in mice,11 and a recent study showed that periapical disease conditions induced by pulp exposure exacerbated the development of tooth extraction–induced ONJ lesions in ZOL-treated mice.12 In the ligature-induced inflammatory condition, the presence of BPs further enhanced expression of proinflammatory cytokines, such as IL-1β, IL-6, or IL-17 (Figure 2), suggesting that BP may exacerbate inflammatory signals and significantly contribute toward ONJ pathogenesis. BPs are known to activate inflammatory signals and induce an acute phase reaction in humans,17, 18 and such a proinflammatory effect of BP was also evident in pulp-exposed inflammatory conditions.12 Therefore, a possibility exists whereby BPs potentiate local inflammatory conditions, leading to exacerbation of ONJ development.
Interestingly, periodontal disease conditions also exacerbated tooth extraction–induced ONJ lesions in mice treated with anti-RANKL Ab (Figure 6), which is known to suppress immune responses by interfering RANK-RANKL interaction that is required for immune cell activation.19 Indeed, one of the safety issues of using denosumab in clinics at the early developmental stages was immune suppression, rather than immune activation, because RANKL and its receptor RANK are abundantly expressed in multiple immune cells, such as T cells, B cells, dendritic cells, and monocytes/macrophages. However, immune suppression was later proved to be insignificant.20 Clinically, denosumab users exhibit less adverse effects, such gastrointestinal ulceration, kidney dysfunction, and acute phase reactions, when compared with ZOL users.21 Nonetheless, denosumab also causes ONJ development that is comparable, if not more, to those induced by ZOL.22, 23 Therefore, direct effects of ZOL and anti-RANKL Ab on altering inflammatory responses per se may not be an important contributing factor for ONJ development.
Deescalating pathologic inflammatory conditions by removing the ligature ameliorated ONJ development (Figures 5 and 6). Ligature removal provides opportunities not only to diminish inflammation and heal the lesions but also to stop further progressing into destructive local environments. As such, this study suggests that the degree of local damages by persistent pathologic inflammatory conditions may functionally be directly linked to ONJ development. Indeed, pathologic inflammatory conditions, such as periodontal and periapical diseases, are highly destructive to the local environments; both conditions not only induce the expression of proinflammatory cytokines but also cause the release of enzymatic proteins, such as matrix metalloproteinases that degrade collagen and extracellular matrix.24, 25, 26 Clinically, periodontal disease is exhibited by soft tissue detachment and generation of deep periodontal pockets. Therefore, persistent pathologic inflammatory conditions, such as periodontal and periapical diseases, may predispose the affected area to ONJ development after tooth extraction in the presence of ZOL or denosumab by inducing destructive structural damages at the local level.
Although both ZOL and anti-RANKL Ab primarily target osteoclasts, they have different modes of action; ZOL suppresses the resorptive functions of osteoclasts, whereas anti-RANKL Ab inhibits the differentiation of osteoclasts.21 At the clinical level, both ZOL and anti-RANKL Ab ultimately inhibit bone resorption. Bone resorption is one of the critical steps during osteomucosal healing—orchestrated but simultaneous healing of the soft and hard tissues—in the oral cavity. In particular, reinstallation of collagen fibers into the newly depositing osteoid on the resorbed alveolar bone surfaces is an important step in osteomucosal healing for full integration of soft and hard tissues at the osteomucosal interface.27 Therefore, we hypothesize that, with the prior inflammatory conditions that destroy local environments, MRONJ may develop after large structural damages (eg, tooth extraction) because of improper execution of osteomucosal wound healing with unresorbed bone surfaces in the presence of ZOL or anti-RANKL Ab.
At the clinical level, both periapical and periodontal diseases are associated with MRONJ development.1, 28 Therefore, it is tempting to speculate that any sources of pathologic inflammatory conditions (eg, poor restoration margins, recurrent decay under the existing restorations, or poor oral hygiene practices) that may have gone unnoticed for a long period of time have potential to increase the likelihood of developing ONJ in BP or Dmab users. In a previous study, cessation of ZOL treatment in the continual presence of periodontal disease conditions did not prevent ONJ development in mice.29 In sharp contrast, our study showed that the removal of periodontal disease conditions prevented ONJ development, despite the continual administration of ZOL and anti-RANKL Ab (Figures 5 and 6). Such findings signify the importance of having early interventions to reduce pathologic inflammatory conditions, rather than having drug holidays, to prevent MRONJ development in BP or Dmab users.
Although ligature removal ameliorated ONJ lesions, ligature-removed groups still exhibited considerable amounts of bone necrosis in both BRONJ and DRONJ mouse models (Figures 5, A–C, and 6, D–F). Such notion suggests that once the bone becomes necrotic, it remains as a dead bone and can only be replaced by newly forming bone. Furthermore, it underlines the detrimental effect of duration of inflammatory conditions to ONJ development; the longer the ligature is placed around the tooth, the more the necrotic bone forms because of continual local destructions.
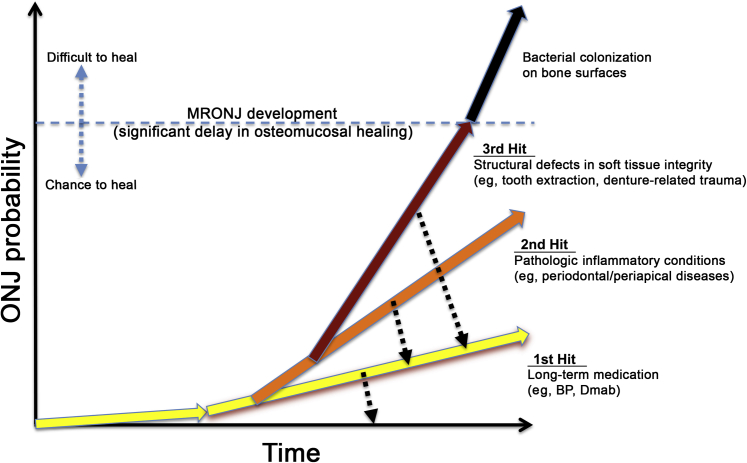
On the basis of our current study, we propose a hypothetical three-hits model for MRONJ development (Figure 7). The first hit is a long-term treatment with antiresorptive agents, such as BPs or Dmab. The second hit occurs when pathologic inflammatory conditions, such as severe periodontal or periapical diseases, persist, presumably because of improper dental care. The third hit comes when large structural defects are generated by, for example, dentoalveolar trauma (eg, tooth extraction). Any interventions that prevent these hits may provide chances to heal, although it may be delayed. However, once large structural defects are generated and bacterial colonization becomes prolonged, such lesions are difficult to heal and may mandate surgical approach.
Figure 7.
A proposed model for medication-related osteonecrosis of the jaw (MRONJ) development. A conceptual graph representing the probability of MRONJ development on the basis of three distinct but continuous hits over a period of time. The first hit is a long-term medication history. The second hit is pathologic inflammatory conditions, such as periodontal or periapical diseases. The third hit is structural defects in soft tissue integrity caused by dentoalveolar trauma, such as tooth extraction or denture-related trauma. BP, bisphosphonate; Dmab, denosumab.
In summary, our study provides compelling experimental evidence that pathologic inflammatory conditions in the presence of BP or Dmab significantly exacerbates ONJ development after tooth extraction. This study has limitations in that it is confined to the animal models and that the animal numbers are relatively small, although genetically identical mice were used. Therefore, it warrants further clinical validation and robust examination whether active therapeutic treatment for pathologic inflammatory conditions should be implemented before any surgical interventions in managing BP or Dmab users to prevent the likelihood of developing MRONJ.
Acknowledgment
We thank the University of California, Los Angeles Translational Procurement Core Laboratory for expedited and cooperative services.
Footnotes
Supported by National Institute of Dental and Craniofacial Research/NIH grants R01DE023348 (R.H.K.) and F30DE025172 (D.W.W.), and UCLA School of Dentistry Dean's Faculty Research Seed grant (R.H.K.).
Disclosures: None declared.
Supplemental material for this article can be found at https://doi.org/10.1016/j.ajpath.2018.06.019.
Supplemental Data
References
- 1.Ruggiero S.L., Dodson T.B., Fantasia J., Goodday R., Aghaloo T., Mehrotra B., O'Ryan F., American Association of Oral and Maxillofacial Surgeons American Association of Oral and Maxillofacial Surgeons position paper on medication-related osteonecrosis of the jaw: 2014 update. J Oral Maxillofac Surg. 2014;72:1938–1956. doi: 10.1016/j.joms.2014.04.031. [DOI] [PubMed] [Google Scholar]
- 2.Jha S., Wang Z., Laucis N., Bhattacharyya T. Trends in media reports, oral bisphosphonate prescriptions, and hip fractures 1996-2012: an ecological analysis. J Bone Miner Res. 2015;30:2179–2187. doi: 10.1002/jbmr.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guarneri V., Miles D., Robert N., Dieras V., Glaspy J., Smith I., Thomssen C., Biganzoli L., Taran T., Conte P. Bevacizumab and osteonecrosis of the jaw: incidence and association with bisphosphonate therapy in three large prospective trials in advanced breast cancer. Breast Cancer Res Treat. 2010;122:181–188. doi: 10.1007/s10549-010-0866-3. [DOI] [PubMed] [Google Scholar]
- 4.Marx R.E. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: a growing epidemic. J Oral Maxillofac Surg. 2003;61:1115–1117. doi: 10.1016/s0278-2391(03)00720-1. [DOI] [PubMed] [Google Scholar]
- 5.Henry D.H., Costa L., Goldwasser F., Hirsh V., Hungria V., Prausova J., Scagliotti G.V., Sleeboom H., Spencer A., Vadhan-Raj S., von Moos R., Willenbacher W., Woll P.J., Wang J., Jiang Q., Jun S., Dansey R., Yeh H. Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J Clin Oncol. 2011;29:1125–1132. doi: 10.1200/JCO.2010.31.3304. [DOI] [PubMed] [Google Scholar]
- 6.Martins A.S., Correia J.A., Salvado F., Caldas C., Santos N., Capelo A., Palmela P. Relevant factors for treatment outcome and time to healing in medication-related osteonecrosis of the jaws: a retrospective cohort study. J Craniomaxillofac Surg. 2017;45:1736–1742. doi: 10.1016/j.jcms.2017.07.014. [DOI] [PubMed] [Google Scholar]
- 7.Yamazaki T., Yamori M., Ishizaki T., Asai K., Goto K., Takahashi K., Nakayama T., Bessho K. Increased incidence of osteonecrosis of the jaw after tooth extraction in patients treated with bisphosphonates: a cohort study. Int J Oral Maxillofac Surg. 2012;41:1397–1403. doi: 10.1016/j.ijom.2012.06.020. [DOI] [PubMed] [Google Scholar]
- 8.Vahtsevanos K., Kyrgidis A., Verrou E., Katodritou E., Triaridis S., Andreadis C.G., Boukovinas I., Koloutsos G.E., Teleioudis Z., Kitikidou K., Paraskevopoulos P., Zervas K., Antoniades K. Longitudinal cohort study of risk factors in cancer patients of bisphosphonate-related osteonecrosis of the jaw. J Clin Oncol. 2009;27:5356–5362. doi: 10.1200/JCO.2009.21.9584. [DOI] [PubMed] [Google Scholar]
- 9.Saad F., Brown J.E., Van Poznak C., Ibrahim T., Stemmer S.M., Stopeck A.T., Diel I.J., Takahashi S., Shore N., Henry D.H., Barrios C.H., Facon T., Senecal F., Fizazi K., Zhou L., Daniels A., Carriere P., Dansey R. Incidence, risk factors, and outcomes of osteonecrosis of the jaw: integrated analysis from three blinded active-controlled phase III trials in cancer patients with bone metastases. Ann Oncol. 2012;23:1341–1347. doi: 10.1093/annonc/mdr435. [DOI] [PubMed] [Google Scholar]
- 10.Thumbigere-Math V., Michalowicz B.S., Hodges J.S., Tsai M.L., Swenson K.K., Rockwell L., Gopalakrishnan R. Periodontal disease as a risk factor for bisphosphonate-related osteonecrosis of the jaw. J Periodontol. 2014;85:226–233. doi: 10.1902/jop.2013.130017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williams D.W., Lee C., Kim T., Yagita H., Wu H., Park S., Yang P., Liu H., Shi S., Shin K.H., Kang M.K., Park N.H., Kim R.H. Impaired bone resorption and woven bone formation are associated with development of osteonecrosis of the jaw-like lesions by bisphosphonate and anti-receptor activator of NF-kappaB ligand antibody in mice. Am J Pathol. 2014;184:3084–3093. doi: 10.1016/j.ajpath.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song M., Alshaikh A., Kim T., Kim S., Dang M., Mehrazarin S., Shin K.H., Kang M., Park N.H., Kim R.H. Preexisting periapical inflammatory condition exacerbates tooth extraction-induced bisphosphonate-related osteonecrosis of the jaw lesions in mice. J Endod. 2016;42:1641–1646. doi: 10.1016/j.joen.2016.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kamijo S., Nakajima A., Ikeda K., Aoki K., Ohya K., Akiba H., Yagita H., Okumura K. Amelioration of bone loss in collagen-induced arthritis by neutralizing anti-RANKL monoclonal antibody. Biochem Biophys Res Commun. 2006;347:124–132. doi: 10.1016/j.bbrc.2006.06.098. [DOI] [PubMed] [Google Scholar]
- 14.Graves D.T., Fine D., Teng Y.T., Van Dyke T.E., Hajishengallis G. The use of rodent models to investigate host-bacteria interactions related to periodontal diseases. J Clin Periodontol. 2008;35:89–105. doi: 10.1111/j.1600-051X.2007.01172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Graves D.T., Kang J., Andriankaja O., Wada K., Rossa C., Jr. Animal models to study host-bacteria interactions involved in periodontitis. Front Oral Biol. 2012;15:117–132. doi: 10.1159/000329675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abe T., Hajishengallis G. Optimization of the ligature-induced periodontitis model in mice. J Immunol Methods. 2013;394:49–54. doi: 10.1016/j.jim.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muratsu D., Yoshiga D., Taketomi T., Onimura T., Seki Y., Matsumoto A., Nakamura S. Zoledronic acid enhances lipopolysaccharide-stimulated proinflammatory reactions through controlled expression of SOCS1 in macrophages. PLoS One. 2013;8:e67906. doi: 10.1371/journal.pone.0067906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adami S., Bhalla A.K., Dorizzi R., Montesanti F., Rosini S., Salvagno G., Lo Cascio V. The acute-phase response after bisphosphonate administration. Calcif Tissue Int. 1987;41:326–331. doi: 10.1007/BF02556671. [DOI] [PubMed] [Google Scholar]
- 19.Akiyama T., Shinzawa M., Akiyama N. RANKL-RANK interaction in immune regulatory systems. World J Orthop. 2012;3:142–150. doi: 10.5312/wjo.v3.i9.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferrari-Lacraz S., Ferrari S. Do RANKL inhibitors (denosumab) affect inflammation and immunity? Osteoporos Int. 2011;22:435–446. doi: 10.1007/s00198-010-1326-y. [DOI] [PubMed] [Google Scholar]
- 21.Baron R., Ferrari S., Russell R.G. Denosumab and bisphosphonates: different mechanisms of action and effects. Bone. 2011;48:677–692. doi: 10.1016/j.bone.2010.11.020. [DOI] [PubMed] [Google Scholar]
- 22.Stopeck A.T., Lipton A., Body J.J., Steger G.G., Tonkin K., de Boer R.H., Lichinitser M., Fujiwara Y., Yardley D.A., Viniegra M., Fan M., Jiang Q., Dansey R., Jun S., Braun A. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J Clin Oncol. 2010;28:5132–5139. doi: 10.1200/JCO.2010.29.7101. [DOI] [PubMed] [Google Scholar]
- 23.Fizazi K., Lipton A., Mariette X., Body J.J., Rahim Y., Gralow J.R., Gao G., Wu L., Sohn W., Jun S. Randomized phase II trial of denosumab in patients with bone metastases from prostate cancer, breast cancer, or other neoplasms after intravenous bisphosphonates. J Clin Oncol. 2009;27:1564–1571. doi: 10.1200/JCO.2008.19.2146. [DOI] [PubMed] [Google Scholar]
- 24.Nair P.N. Pathogenesis of apical periodontitis and the causes of endodontic failures. Crit Rev Oral Biol Med. 2004;15:348–381. doi: 10.1177/154411130401500604. [DOI] [PubMed] [Google Scholar]
- 25.de Paula-Silva F.W., D'Silva N.J., da Silva L.A., Kapila Y.L. High matrix metalloproteinase activity is a hallmark of periapical granulomas. J Endod. 2009;35:1234–1242. doi: 10.1016/j.joen.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sapna G., Gokul S., Bagri-Manjrekar K. Matrix metalloproteinases and periodontal diseases. Oral Dis. 2014;20:538–550. doi: 10.1111/odi.12159. [DOI] [PubMed] [Google Scholar]
- 27.James W.C., McFall W.T., Jr., Burkes E.J. Placement of free gingival grafts on denuded alveolar bone, part II: microscopic observations. J Periodontol. 1978;49:291–300. doi: 10.1902/jop.1978.49.6.291. [DOI] [PubMed] [Google Scholar]
- 28.Kim R.H., Yang P., Sung E.C. Managing intraoral lesions in oral cancer patients in a general dental practice: an overview. J Calif Dent Assoc. 2016;44:85–92. [PMC free article] [PubMed] [Google Scholar]
- 29.de Molon R.S., Shimamoto H., Bezouglaia O., Pirih F.Q., Dry S.M., Kostenuik P., Boyce R.W., Dwyer D., Aghaloo T.L., Tetradis S. OPG-Fc but not zoledronic acid discontinuation reverses osteonecrosis of the jaws (ONJ) in mice. J Bone Miner Res. 2015;30:1627–1640. doi: 10.1002/jbmr.2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.