Abstract.
Angiostrongyliasis is a food-borne parasitic disease caused by the nematode Angiostrongylus cantonensis that can lead to eosinophilic meningitis (EM) or meningoencephalitis in humans. Angiostrongylus cantonensis is prevalent in the Pacific Islands. In recent years, a large number of outbreaks and severe cases have occurred. Several species of mollusk, such as snails and slugs, act as intermediate and paratenic hosts of A. cantonensis. In this study, two cases of EM were found to have been caused by infection with A. cantonensis due to consumption of raw centipedes. To survey the A. cantonensis infections acquired through centipedes that the patients had bought at a vegetable market, we performed etiological examinations and polymerase chain reaction amplification of A. cantonensis genes. Third-instar larvae of A. cantonensis were detected in the centipedes, and specific genes from A. cantonensis were detected in all the specimens. This indicates that the centipede may act as a competent host for the transmission of A. cantonensis. To our knowledge, this is the first report of A. cantonensis infection through the consumption of centipedes.
INTRODUCTION
Angiostrongyliasis is an important food-borne parasitic and zoonotic disease. The pathogen Angiostrongylus cantonensis was first observed in the pulmonary arteries of a Rattus norvegicus specimen captured in the Guangzhou suburbs by Chen Xintao in 1934.1 Angiostrongylus cantonensis is common in more than 30 countries, including China, South Korea, Thailand, the Philippines, and Vietnam. Thailand, mainland China, and Taiwan are the most prevalent areas.2 Humans, as nonpermissive hosts, become infected mainly through eating raw or poorly cooked snails, slugs, monitor lizards, frogs, and fish,3–11 or by eating vegetables and salads contaminated with these hosts.12–14 There is no confirmed report of angiostrongyliasis due to eating centipedes. Here we present two cases of eosinophilic meningitis (EM) contracted by ingesting raw centipedes.
CASE REPORTS
Case 1.
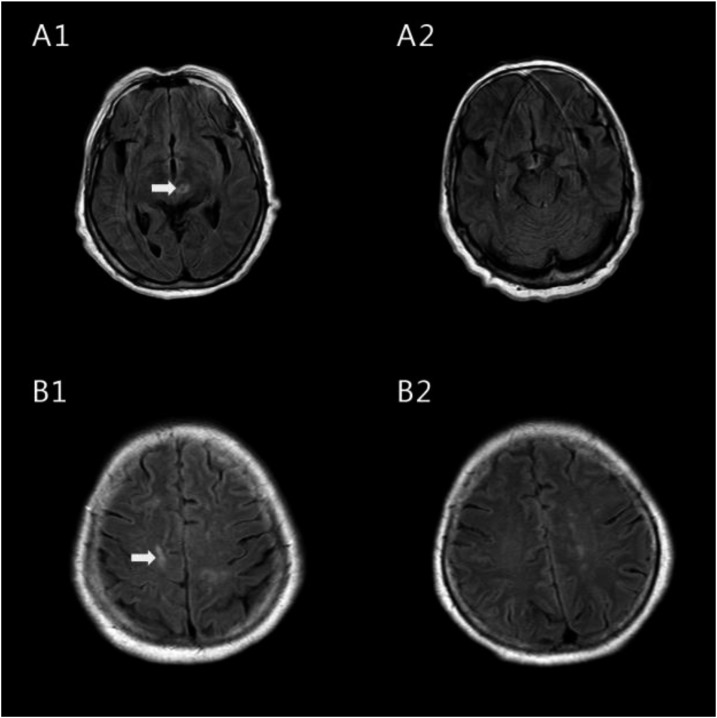
A 78-year-old woman was admitted to Zhujiang Hospital, Guangzhou, China, on November 22, 2012. She had been suffering from a moderate headache, somnolence, and cognitive impairment for several weeks, with no fever or vomiting. The patient said she had not sustained any recent trauma, been exposed to toxins, or consumed raw seafood or aquatic products. Physical examination revealed slight neck stiffness. Her cranial nerves, muscle strength, and sensation were normal. A cerebrospinal fluid (CSF) examination was performed (Table 1). Her cerebrospinal pressure was 26 cm H2O. The CSF appeared light yellow and opaque. The protein level was 137 mg/dL and the glucose was 52.2 mg/dL. Cerebrospinal fluid analysis revealed 600 cells/μL white blood cells (WBCs) and 40 cells/μL eosinophils (EOS) by Wright and Giemsa staining. She had a peripheral blood leukocyte count of 12.42 × 10 E3/μL, of which 3.69 × 10 E3/μL (29.7% of total leukocyte count) were EOS. The peripheral blood lymphocytes were 1.77 10 E3/μL (12.7% of total leukocyte count) (Table 1). Here, the erythrocyte sedimentation rate was 15 mm/hour (normal, 0–20 mm/hour). Her liver function test and chest X-ray results were normal. A magnetic resonance imaging (MRI) Fluid attenuated inversion recovery (FLAIR) sequence showed a high signal in the left midbrain and right frontal lobe (Figure 1 A1, B1). The results of enzyme-linked immunosorbent assay (ELISA) revealed that both the serum and the CSF were positive for antibodies (immunoglobulin G [IgG] and immunoglobulin M [IgM]) against A. cantonensis. Further questions about the patient’s history showed that she had eaten centipedes without cooking them on several occasions. The patient was diagnosed with A. cantonensis meningoencephalitis. She was also diagnosed with eosinophilic meningoencephalitis. The patient was treated with albendazole (40 mg/day) for 21 days and dexamethasone (10 mg/day) for 15 days. The patient was conscious. Her headache and cognitive impairment were relieved after the treatment. According to the follow-up examination on December 27, 2012, the CSF was colorless and transparent, containing 10 cells/μL WBCs, 0 cells/μL EOS, 63 mg/dL protein, and 64.8 mg/dL glucose. Her cerebrospinal pressure dropped down to 15 cm H2O. No EOS were detected in her CSF. The anti–A. cantonensis IgG and IgM antibodies turned out to be negative in both the serum and the CSF after treatment based on ELISA results. The signal in the MRI FLAIR sequence disappeared after the treatment (Figure 1 A2, B2).
Table 1.
Biochemical analysis of two patients’ CSF and hematological analysis
| Case No. | Gender | Date | CSF | Date | Blood | ||||
|---|---|---|---|---|---|---|---|---|---|
| Protein (mg/dL) (15–45) | Glucose (mg/dL) (45–80) | WBCs (cells/μL) (0–15) | EOS (cells/μL) (0) | WBCs (10 E3/μL) (4–10 × 10 E3/μL) | EOS (10 E3/μL) (0.0 −0.7 × 10 E3/μL) | ||||
| 1 | Female | November 26, 2012 | 137 | 52.2 | 770 | N | November 22, 2012 | 12.42 | 3.69 |
| December 6, 2012 | 163 | 55.8 | 600 | 40 | December 4, 2012 | 13.97 | 6.01 | ||
| December 13, 2012 | 83 | 73.8 | 90 | 11 | – | N | N | ||
| December 21, 2012 | 65 | 84.6 | 10 | 0 | December 21, 2012 | 10.93 | 0.01 | ||
| December 27, 2012 | 63 | 64.8 | 10 | 0 | – | N | N | ||
| 2 | Male | December 14, 2012 | 89 | 45 | 125 | 21 | December 10, 2012 | 12.18 | 5.12 |
| December 31, 2012 | 37 | 50.4 | 20 | 0 | December 31, 2012 | 11.94 | 0.13 | ||
CSF = cerebrospinal fluid; EOS = eosinophils; N = no test; WBCs = white blood cells.
Figure 1.
Flow alternating inversion recovery-magnetic resonance imaging of the female patient’s brain before and after treatment. The arrows indicate the hyperintense signal that suggests multiple inflammatory foci in specific areas before treatment (A1, B1). They disappeared after treatment (A2, B2).
Case 2.
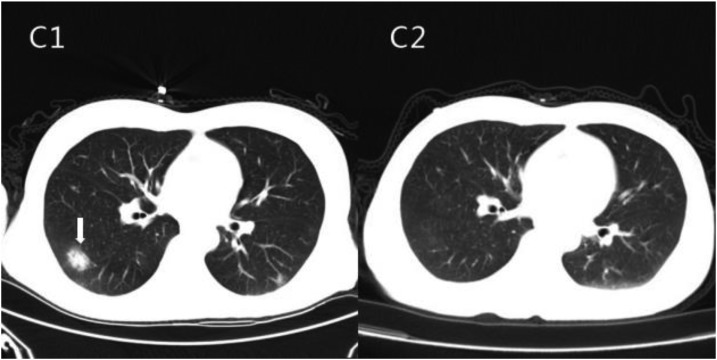
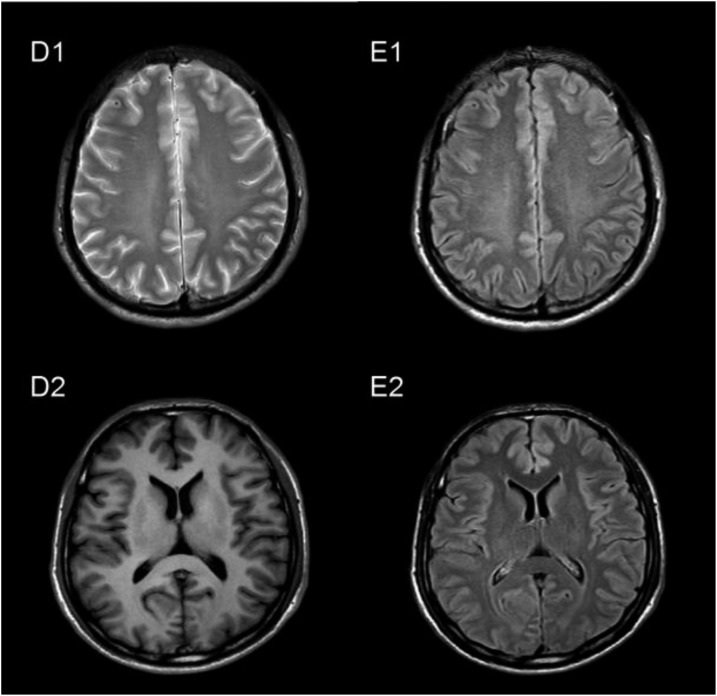
A 46-year-old male was admitted to Zhujiang Hospital in Guangzhou, China, on December 14, 2012. The patient’s main complaint was a mild headache that had lasted for more than 20 days. He experienced no seizures, changes in consciousness, paralysis, vomiting, or fever. The sole obvious focal neurologic sign was neck rigidity. He had also consumed raw centipedes. A CSF examination was carried out (Table 1). The protein level was 89 mg/dL and the glucose concentration was 40 mg/dL. The CSF pressure was elevated at 30.3 cm H2O. He had a peripheral blood leukocyte count of 12.18 × 10 E3/μL, of which 5.12 × 10 E3/μL (42% of total leukocyte count) were EOS (normal, 0.0–0.7 10 E3/μL). The peripheral blood lymphocyte count was 2.03 10 E3/μL (16.7% of total leukocyte count). His erythrocyte sedimentation rate was 33 mm/hour (normal, 0–15 mm/hour). Liver function test results were normal. Computed tomographic lung scans showed a high-intensity nodule located in the posterior lateral segment of the right-lung lower lobe, and the boundary of that nodule was clear (Figure 2 C1). His cranial MRI showed general normal signals in the brain parenchyma without dural enhancement (Figure 3 D1, D2). Enzyme-linked immunosorbent assay indicated that both the serum and the CSF were positive for antibodies (IgG and IgM) against A. cantonensis. The patient was diagnosed with A. cantonensis meningitis and EM. The patient was treated with albendazole (40 mg/day) for 21 days and dexamethasone (10 mg/day) for 16 days. The patient’s headache was relieved after the treatment, and he was discharged from the hospital. According to the follow-up examination on December 31, 2012, the CSF was colorless and transparent, containing 20 cells/μL WBCs, 0 cells/μL EOS, 36.6 mg/dL protein, and 50.4 mg/dL glucose. His cerebrospinal pressure dropped down to 13 cm H2O, and no EOS were detected in the CSF. Enzyme-linked immunosorbent assay indicated that anti–A. cantonensis IgG and IgM antibodies were absent in both the serum and the CSF. The nodule in the lung disappeared after the treatment (Figure 2, C2), and the signals of the MRI were also normal (Figure 3, E1, E2).
Figure 2.
Computed tomography of the male patient’s lungs before and after treatment. The arrow indicates a high-density lesion that suggests inflammatory foci before treatment (C1). It disappeared after treatment (C2).
Figure 3.
Flow alternating inversion recovery-magnetic resonance imaging of the male patient’s brain before and after treatment. There was no abnormal signal before treatment (D1, D2) or after treatment (E1, E2).
Pathogen detection.
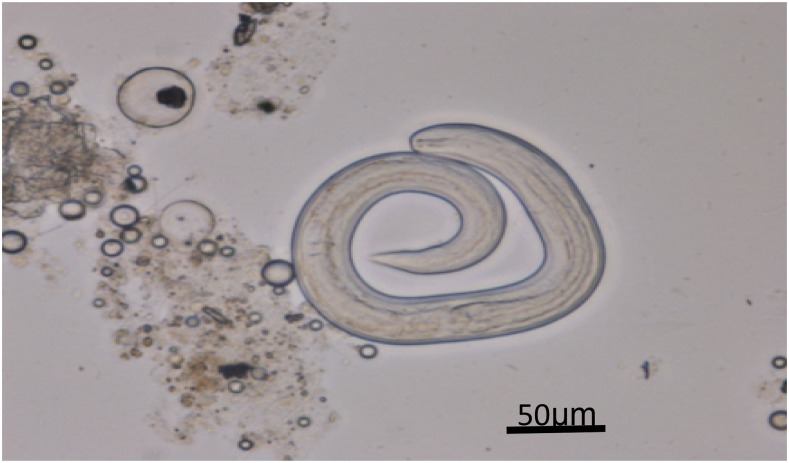
To determine whether centipedes can serve as hosts for A. cantonensis, we performed etiological examinations and PCR analysis. We purchased 20 centipedes from the Qingping Market in Guangzhou where the patients had acquired their centipedes. These centipedes were originally caught in the wild in Guangxi Province, China. The centipedes were homogenized and digested in pepsin-HCl (pH 2.0, 500 IU pepsin/gram tissue) below 37°C for 2 hours. The cellular debris were removed using a natural precipitation method.15 The supernatant was centrifuged at 2,500 rpm for 5 minutes. We detected third-stage larvae of A. cantonensis in the sediments of seven specimens of the 20 centipedes (Figure 4). The larvae were similar to the infective larvae (L3) of A. cantonensis. An average of 56 third-stage larvae of A. cantonensis was recovered from each centipede.
Figure 4.
The third-stage larvae of Angiostrongylus cantonensis recovered in the centipede under optical microscope (magnified ×400). This figure appears in color at www.ajtmh.org.
PCR analysis.
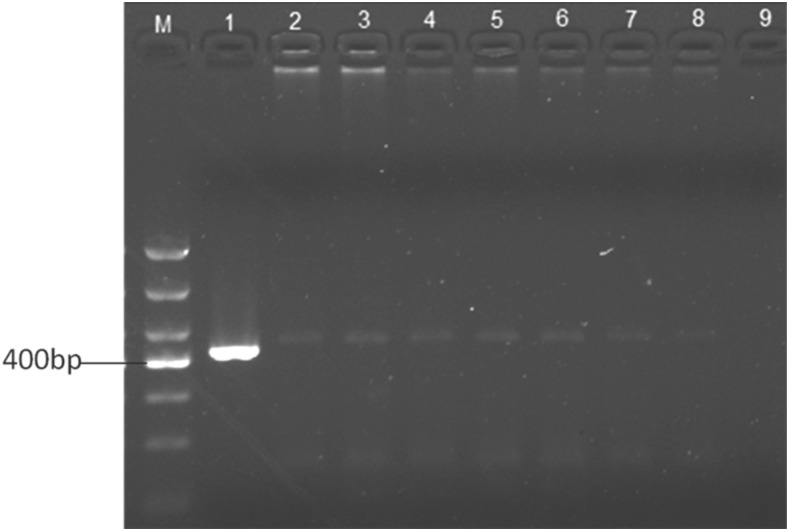
The DNA of the centipedes was extracted to serve as the template using an Omega Tissue DNA kit (D3396-01; Omega Bio-tek, Norcross, GA) according to the instructions of the manufacturer. Primers 5′-TGATGCTTGTGCGGTTTTCG-3′ and 5′-TTCCCTTGGCTGTGGTTTCG-3′ were designed according to the fragment of the large-subunit rRNA gene of A. cantonensis (421 bp), and DNA amplification was completed by the method described by Wei.16 Amplification was carried out with a Biometra T-Professional thermocycler (Applied Biometra, Jena, Germany). PCR was performed as follows: initial denaturation at 94°C for 10 minutes followed by 35 cycles at 94°C/30 seconds, 48.5°C/30 seconds, and 72°C/1 minute. Final extension was at 72°C/10 minutes and 16°C/pause. Amplified DNA products were assessed on 1.5% agarose gel and stained with ethidium bromide. We observed obvious bands in seven centipedes comparable to the positive control (Figure 5). Positive PCR products were purified and ligated into the plasmid pBack-Zero-T for cloning. The plasmid was sent for sequencing. The sequence was confirmed to be 99% identical to the corresponding region of the A. cantonensis large-subunit rRNA gene sequence. We found only three mutations in the sequence compared with the corresponding region of A. cantonensis (Figure 6).
Figure 5.
Agarose gel electrophoresis of PCR. The expected bands were amplified from all seven centipedes. M: DNA molecular weight marker DL1000; lane 1: DNA from Angiostrongylus cantonensis adults (positive control); lanes 2–8: DNA from seven centipedes; 9: ddH2O (negative control).
Figure 6.
Sequencing results for Angiostrongylus cantonensis large-subunit rRNA gene sequences.
DISCUSSION
Humans infected with A. cantonensis usually experience mild symptoms, predominantly headache, vomiting, photophobia, fever, hyperesthesia, and neck stiffness. For some patients, these symptoms resolve without treatment. Angiostrongylus cantonensis meningitis is usually diagnosed by a typical clinical presentation, a history of exposure, CSF containing EOS, and serological detection. The symptoms of these two patients were very light, manifesting mainly as headache, neck stiffness, and other mild symptoms of intracranial hypertension. Changes in the CSF are not typical of this condition, so the diagnosis could not be confirmed without etiological detection (such as the presence of antibody in the CSF and serum). Although there are no previously reported cases of infection with A. cantonensis by eating centipedes, we observed the third-instar larvae of A. cantonensis from the centipedes we purchased from the same market. The results show specific bands after PCR amplification. Because of the low detection rate of pathogens in laboratory diagnosis, antibody detection has become an important auxiliary method of diagnosing this disease. A previous study confirmed the utility of positive serological testing with ELISA.17 Here, ELISA was used for diagnosis. The sera were positive for IgG and IgM antibodies in the two cases, and they became negative after treatment.
The optimal treatment for EM caused by A. cantonensis has not been established. A recent study showed that albendazole and prednisolone are effective in the treatment of EM.18 The combination of albendazole and dexamethasone has been shown to be effective,19 and the patients described here responded well to it. Dehydration treatment was also given to the patients, who recovered after 15 days of treatment. Angiostrongylus cantonensis should be suspected in patients who have signs of EM after eating centipedes.20
The increase in angiostrongyliasis cases is thought to be due to changes in human dietary patterns. In these two cases, the patients might have become infected with A. cantonensis by ingesting raw centipedes. Up to now, more than 140 species of mollusks have been reported as natural or experimental intermediate hosts for A. cantonensis.21
Epidemiologic studies have indicated that the African giant land snail (Achatina fulica) and the apple snail (Pomacea canaliculata) are the main vectors for angiostrongyliasis in China.17 Centipedes, however, have never been reported to be hosts for this parasite. In this case, 20 centipedes were collected from the same location where the patients had obtained them, and an average of 56 larvae were detected per centipede. DNA was extracted from the centipede and amplified with species-specific PCR primers following the method described by Wei Jiling.16 The sequence was confirmed to be 99% identical to the corresponding region of the purpose fragment. The results of both the etiological examination and the PCR analysis suggested that centipedes might be the hosts of A. cantonensis. We bought 30 centipedes from the same market and divided these centipedes into two groups: group 1 (including 10 centipedes) was used as the control and group 2 (including 20 centipedes) was infected with the first-stage larvae (L1) of A. cantonensis. The results of the etiological examination and PCR analysis of the control group were negative; however, the centipedes in group 2 died after infection with L1. For these reasons, we remain uncertain whether the centipede is an intermediate host of A. cantonensis.
REFERENCES
- 1.Chen HT, 1935. Un nouveau n{\'e}matodepulmonaire, Pulmonemacantonensis ng, n. sp., des rats de Canton. Ann Parasitol Hum Comp 13: 312–314. [Google Scholar]
- 2.Vitta A, Polsut W, Fukruksa C, Yimthin T, Thanwisai A, Dekumyoy P, 2016. Levels of infection with the lungworm Angiostrongylus cantonensis in terrestrial snails from Thailand, with Cryptozona siamensis as a new intermediate host. J Helminthol 90: 737–741. [DOI] [PubMed] [Google Scholar]
- 3.Kwon E, Ferguson TM, Park SY, Manuzak A, Qvarnstrom Y, Morgan S, Ciminera P, Murphy GS, 2013. A severe case of Angiostrongylus eosinophilic meningitis with encephalitis and neurologic sequelae in Hawa’i. Hawaii J Med Public Health 72 (Suppl 2): 41–45. [PMC free article] [PubMed] [Google Scholar]
- 4.Liu IH, Chung YM, Chen SJ, Cho WL, 2006. Necrotizing retinitis induced by Angiostrongylus cantonensis. Am J Ophthalmol 141: 577–579. [DOI] [PubMed] [Google Scholar]
- 5.Panackel C, Vishad, Cherian G, Vijayakumar K, Sharma RN, 2006. Eosinophilic meningitis due to Angiostrongylus cantonensis. Indian J Med Microbiol 24: 220–221. [PubMed] [Google Scholar]
- 6.Kanpittaya J, Jitpimolmard S, Tiamkao S, Mairiang E, 2000. MR findings of eosinophilic meningoencephalitis attributed to Angiostrongylus cantonensis. AJNR Am J Neuroradiol 21: 1090–1094. [PMC free article] [PubMed] [Google Scholar]
- 7.Nalini A, Ramakrishna A, Dekumoy P, Kumar RR, Pakdee W, Saini J, Hegde VS, 2013. Severe form of radiculo—myelo—neuropathy with meningo—encephalitis secondary to Angiostrongylus cantonensis infection: unusual corpus callosal lesions and serial magnetic resonance imaging findings. Neurol India 61: 414–418. [DOI] [PubMed] [Google Scholar]
- 8.Tsai HC, Lai PH, Sy CL, Lee SS, Yen CM, Wann SR, Chen YS, 2011. Encephalitis caused by Angiostrongylus cantonensis after eating raw frogs mixed with wine as a health supplement. Intern Med 50: 771–774. [DOI] [PubMed] [Google Scholar]
- 9.Lai CH, Yen CM, Chin C, Chung HC, Kuo HC, Lin HH, 2007. Eosinophilic meningitis caused by Angiostrongylus cantonensis after ingestion of raw frogs. Am J Trop Med Hyg 76: 399–402. [PubMed] [Google Scholar]
- 10.Luessi F, Sollors J, Torzewski M, Müller HD, Siegel E, Blum J, Sommer C, Vogt T, Thömke F, 2009. Eosinophilic meningitis due to Angiostrongylus cantonensis in Germany. J Travel Med 16: 292–294. [DOI] [PubMed] [Google Scholar]
- 11.Ali AB, Van den Enden E, Van Gompel A, Van Esbroeck M, 2008. Eosinophilic meningitis due to Angiostrongylus cantonensis in a Belgian traveller. Travel Med Infect Dis 6: 41–44. [DOI] [PubMed] [Google Scholar]
- 12.Fuller AJ, Munckhof W, Kiers L, Ebeling P, Richards MJ, 1993. Eosinophilic meningitis due to Angiostrongylus cantonensis. West J Med 159: 78–80. [PMC free article] [PubMed] [Google Scholar]
- 13.Maretić T, Perović M, Vince A, Lukas D, Dekumyoy P, Begovac J, 2009. Meningitis and radiculomyelitis caused by Angiostrongylus cantonensis. Emerg Infect Dis 15: 996–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lim JM, Lee CC, Wilder-Smith A, 2004. Eosinophilic meningitis caused by Angiostrongylus cantonensis: a case report and literature review. J Travel Med 11: 388–390. [DOI] [PubMed] [Google Scholar]
- 15.Zhang X, Liu M, Wu Y, Mo Z, Shen H, Chen D, Li H, 2012. Analysis of larval excretory-secretory antigen and its immunodiagnosis of Angiostrongyliasis cantonensis infection [in Chinese]. Nan Fang Yi Ke Da Xue Xue Bao 32: 477–481. [PubMed] [Google Scholar]
- 16.Wei JL, Zhou WC, Shao BY, She SS, Wang SK, Chen WB, Chen SL, 2008. Establishment of a PCR assay for detection of snails infected with Angiostrongylus cantonensis. Chinese J Zoonoses 12: 1136–1140. [Google Scholar]
- 17.Wang QP, Chen XG, Lun ZR, 2007. Invasive fresh water snail, China. Emerg Infect Dis 13: 1119–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang TB, Wu ZD, Lun ZR, 2013. The apple snail Pomacea canaliculata, a novel vector of the rat lungworm, Angiostrongylus cantonensis: its introduction, spread, and control in China. Hawaii J Med Public Health 72 (Suppl 2): 23–25. [PMC free article] [PubMed] [Google Scholar]
- 19.Chen SN, 1986. Enzyme-linked immunosorbent assay (ELISA) for the detection of antibodies to Angiostrongylus cantonensis. Trans R Soc Trop Med Hyg 80: 398–405. [DOI] [PubMed] [Google Scholar]
- 20.Chotmongkol V, Wongjitrat C, Sawadpanit K, Sawanyawisuth K, 2004. Treatment of eosinophilic meningitis with a combination of albendazole and corticosteroid. Southeast Asian J Trop Med Public Health 35: 172–174. [PubMed] [Google Scholar]
- 21.Kim JR, Hayes KA, Yeung NW, Cowie RH, 2014. Diverse gastropod hosts of Angiostrongylus cantonensis, the rat lungworm, globally and with a focus on the Hawaiian Islands. PLoS One 9: e94969. [DOI] [PMC free article] [PubMed] [Google Scholar]