Abstract.
The efficacies of 3-day regimens of artemether–lumefantrine (AL), artesunate–amodiaquine (AA), and dihydroartemisinin–piperaquine (DHP) were evaluated in 910 children < 5 years old with uncomplicated malaria from six geographical areas of Nigeria. Parasite positivity 1 day and Kaplan–Meier estimated risk of persistent parasitemia 3 days after therapy initiation were both significantly higher, and geometric mean parasite reduction ratio 1 day after treatment initiation (PRRD1) was significantly lower in AL-treated children than in AA- and DHP-treated children. No history of fever, temperature > 38°C, enrollment parasitemia > 75,000 μL−1, and PRRD1 < 5,000 independently predicted persistent parasitemia 1 day after treatment initiation. Parasite clearance was significantly faster and risk of reappearance of asexual parasitemia after initial clearance was significantly lower in DHP-treated children. Overall, day 42 polymerase chain reaction–corrected efficacy was 98.3% (95% confidence interval [CI]: 96.1–100) and was similar for all treatments. In a non-compartment model, declines of parasitemias were monoexponential with mean terminal elimination half-life of 1.3 hours and unimodal frequency distribution of half-lives. All treatments were well tolerated. In summary, all three treatments evaluated remain efficacious treatments of uncomplicated malaria in young Nigerian children, but DHP appears more efficacious than AL or AA.
INTRODUCTION
The development and spread of artemisinin resistance in Plasmodium falciparum in the Greater Mekong Subregion (GMS)1–7 has threatened the efficacy of artemisinin-based combination treatments (ACTs) and heightened concerns about subsequent spread to other areas of the world where ACTs are still largely efficacious treatments of uncomplicated falciparum malaria.6,8 On the African continent, many endemic sub-Saharan countries adopted and deployed ACTs in the early and mid-2000s.9 However, report of declining responsiveness in P. falciparum to ACTs on the Kenyan coast10 has heightened concerns that parasites with slow clearance phenotypes similar to those in GMS, and artemisinin-resistant parasites may exist in Africa11–13 with consequent emergence and spread of parasites with reduced susceptibility to ACTs on the continent.
In Africa, where virtually all ACTs recommended as first-line treatments of uncomplicated infections by the World Health Organization (WHO)14,15 are readily available, a commonly used ACT alongside AL and AA is dihydroartemisinin–piperaquine (DHP) which has been shown to be an efficacious treatment of uncomplicated falciparum malaria in Africa and elsewhere.16–20 The efficacies of AL, AA, and DHP in single studies have been less frequently evaluated exclusively in < 5-year-old malarious children.
In a previous study in six geographical areas of Nigeria, we showed that AL and AA are efficacious treatments of uncomplicated infections in < 5-year-old children.21 In the present study, we evaluated the therapeutic efficacies of AL, AA, and DHP in Nigerian children < 5 years old drawn from eight sentinel sites in six geographical areas. The primary aims were to compare the efficacies of these ACTs in all malaria-endemic settings in Nigeria and assess changes in P. falciparum susceptibility to these ACTs.
MATERIALS AND METHODS
Study locations.
This study was an open-label trial carried out between June 2014 and December 2015 at the following locations: Ogbia (Otuasegha), Neni, Ogwa, Numan, Ilorin, Kura, Bodinga, and Ibadan in Bayelsa, Anambra, Imo, Adamawa, Kwara, Kano, Sokoto, and Oyo States of Nigeria, respectively (Figure 1). In virtually all study locations, malaria is endemic and transmission occurs all year round; however, it is more intense during the rainy season from April to October.
Figure 1.
Map of Nigeria showing study locations.
Patients, treatments, and follow-up.
Patients were eligible to join the study if they were < 5 years of age, had symptoms compatible with acute uncomplicated falciparum malaria such as fever, anorexia, vomiting, or abdominal discomfort with or without diarrhea, with P. falciparum parasitemia between 2,000 and 200,000 asexual forms per μL, a body (axillary) temperature > 37.4°C or in the absence of measured fever, a recent history of fever in the 24–48 hours before presentation, absence of other concomitant illness, no history of antimalarial use in the 2 weeks preceding presentation, and written informed consent given by parents or guardians. Patients with severe malaria,22,23 severe malnutrition, serious underlying diseases (renal, cardiac, or hepatic), and known allergy to study drugs were excluded from the study. The study protocol was approved by National Health Research Ethics Committee, Abuja, Nigeria (NHREC/01/01/2007-22/10/2014) and registered with Pan African Clinical Trial Registry (PACTR201709002064150). The disease history, taken by the attending physician, was recorded by asking parents/guardians when the present symptomatic period started and was followed by a full physical examination by the same physician. The day of presentation was taken as day 0 (or 0 hour).
Enrolled patients were randomized to receive AL, AA, or DHP (all co-formulated) given according to body weight. The randomization was computer-generated for each site and treatment codes were sealed in individual envelopes. AL (Coartem®; Novartis, Basel, Switzerland) was given as follows: patients weighing 5–14 kg received one tablet and those weighing > 14–24 kg received two tablets at presentation (0 hour), 8 hours later, and at 24, 36, 48, and 60 hours after the first dose. Each tablet of AL contains 20 mg of artemether and 120 mg of lumefantrine. AA (Winthrop®; Sanofi Aventis, Paris, France) was given as follows: patients weighing > 4.5 to < 9 kg received one tablet, those weighing > 9 to < 18 kg received one tablet, and those weighing > 18 to < 24 kg received one tablet of the following formulations: 25/67.5 mg, 50/135 mg, 100/270 mg of fixed dose combination of artesunate/amodiaquine, respectively, daily for 3 days. Dihydroartemisinin–piperaquine (Duo-cotecxin®; Zhejiang Holley Nanhu Pharmaceutical Co. Ltd, Jiaxing City, China) was given as follows: patients weighing > 4.5 to < 10 kg received ¾ of one tablet, those weighing 10 to < 16 kg received one and a half tablet, those weighing 16 to < 24 kg received two tablets, and those weighing 24 to < 34 received two and a half tablets daily for 3 days. Each tablet of DHP contains 40 mg of dihydroartemisinin and 320 mg of piperaquine.
All drugs were given orally. In children who were not able to swallow whole tablets, the tablets were carefully crushed using a tablet crusher, dissolved in water, and administered orally. In patients treated with AA or DHP, the drug was given as single-day or single daily doses in the clinic by the physician. In patients treated with AL, the 0-, 8-, 24-, and 48-hour doses were given in the clinic by the physician, and the 36- and 60-hour doses were given by parents or guardians of the children at home. A phone call was made to remind parents/guardians of time of the second daily doses of AL and to monitor the outcome of drug administration. Parents or guardians were questioned at follow-up on the time and events after drug administration. After drug administration in the clinic, all patients waited for at least 30 minutes to ensure the drug was not vomited. If it was, the dose was repeated. If the repeated dose was vomited, the patient was excluded from the study. If necessary, the patients were provided with antipyretics (paracetamol tablets 10–15 mg/kg every 8 hours for 24 hours). Patient evaluation and follow-up after administration was performed by another physician blinded to the drug treatment. Thick and thin blood films were obtained from each child as soon as they came to the clinic and the slides were carefully labeled with the patients’ codes and air-dried before being stained.
Follow-up with clinical and parasitological evaluation was carried out daily on days 1–3 (and on day 4, 5, or 6 if parasitemia or fever was still present on day 3), and on days 7, 14, 21, 28, 35, and 42. This consists of enquiry about the patient’s well-being, presence or absence of initial presenting symptoms, presence of additional symptoms, measurement of body temperature, heart and respiratory rates, and a blood smear for quantification of parasitemia.
Adverse events were defined as symptoms and signs that first occurred or became worse after treatment was started and were checked for at every visit. Laboratory tests to further elicit adverse events were not performed routinely at every visit. Any new events occurring during treatment were also considered as side effects.
Thick and thin blood films prepared from a finger prick were stained with Giemsa and examined by light microscopy under an oil immersion objective at ×1,000 magnification by two independent assessors who did not know the drug regimen of the patients. A senior member of the study team reviewed the slides if there was any disagreement by the two microscopists. In addition, the slides of every fourth child enrolled in the study were reviewed by the senior member. Asexual parasitemia in thick films were estimated by counting asexual parasites relative to 500 leukocytes or 500 asexual forms, whichever occurred first. From this figure, the parasite density was calculated assuming a leukocyte count of 6,000/μL of blood.24 Sexual parasites were not quantified, but their presence in blood films was noted. A slide was considered parasite negative if no asexual or sexual parasite was detected after examination of 200 microscope fields.
Capillary blood, collected before and during follow-up, was used to measure hematocrit. Hematocrit was measured using a microhematocrit tube and microcentrifuge (Hawksley, Lancing, United Kingdom). Finger-pricked blood samples were spotted on 3MM Whatman® filter paper (Sigma-Aldrich Inc., St. Louis, MO) on days 0–3, 7, 14, 21, 28, 35, and 42 and at the time of treatment failure for parasite genotyping. Blood spots on filter papers were air-dried and kept in individual envelops with silica gel to avoid moisture and fungi growth that could destroy the integrity of the collected samples. Paired primary and posttreatment samples were analyzed using parasites’ polymorphic loci in selected P. falciparum genes, to distinguish recrudescence from new infections as described previously by Happi and others.25 Briefly, block 2 of the merozoite surface protein-1 (MSP-1) and the block 3 of the merozoite surface protein-2 (MSP-2) genes were amplified by two rounds of polymerase chain reaction (PCR) using specific primers.25 Three microliters of the nested PCR product were resolved by electrophoresis on a 2% agarose gel and sized against 100-bp molecular weight DNA ladder (New England Biolabs, Beverly, MA). The banding pattern of posttreatment parasite was compared with matched primary parasites in each of the patients who had parasitemia after treatment. Banding patterns were binned in 20 bp using the GBox and the genetic analyzer software. Posttreatment and primary infection parasites showing identical banding patterns at both MSP-1 and MSP-2 loci were considered as recrudescence, whereas nonidentity in banding patterns in at least one targeted locus of MSP-1 or MSP-2 was considered as newly acquired infections. To confirm the absence of recurrent parasitemia, samples obtained from one in every four patients with microscopically negative blood films were also subjected to PCR analysis.
Response to drug treatment was assessed using a modified version of the WHO 2003 and 2009 in vivo clinical classification criteria which included axillary body temperature ≥ 37.5°C or rectal/tympanic temperature ≥ 38°C as one of the inclusion criteria for enrolment and assessment of response to treatment.24,26 Because all patients were not febrile at enrollment, a temperature < 37.5°C was not an exclusion criterion for enrollment and assessment of response. The modification also involved a follow-up for 42 days in these areas of intense transmission. The clinical classification system consisted of the following categories of response: adequate clinical and parasitological response (ACPR), late parasitological failure, late clinical failure, and early treatment failure (ETF). The primary outcomes were parasite prevalence in the first 3 days posttreatment initiation, complete clearance of initial parasitemia, and the 28- and 42-day uncorrected and PCR-corrected efficacy. The secondary outcomes were the fever clearance times, gametocyte carriage and gametocyte clearance time, proportions with a hematocrit < 30% at presentation and during follow-up, and where possible anemia recovery time in those who were anemic at presentation. Asexual parasite clearance time (PCT) was defined as time elapsing from drug administration until there was no detectable parasitemia and remained so for at least 2 days. In children who were febrile at presentation (axillary temperature ≥ 37.5°C), fever clearance time was defined as time elapsing from drug administration until body temperature fell to or below 37.4°C and remained so for at least 2 days.21 Gametocyte clearance was defined as the time from microscopic detection of gametocytemia until their complete clearance from peripheral blood. Parasite reduction ratio 1 or 2 days posttreatment initiation (PRRD1 and PRRD2) was as previously defined.27
Cure rates were defined as the percentages of patients whose asexual parasitemia cleared from peripheral blood and who were free of patent asexual parasitemia on days 14, 21, 28, 35, and 42 of follow-up. The cure rates on days 14, 28, and 42 were adjusted on the basis of the PCR genotyping results of paired samples of patients with recurrent parasitemia after day 7 of starting treatment.
Kinetic evaluation of time course of parasitemia following treatment initiation.
In a subset of 57 children, to characterize the disposition of parasitemia following treatment, finger-pricked blood samples were obtained for quantification of asexual parasitemia at the following times: pretreatment (0 hour) and at 2, 4, 6, 8, 10, 12, 16, 24, 48, 72, 96, 120, 144, 168, 336, and 504 hours and on days 35 and 42 after initiation of treatment. The kinetics of the time course of the asexual parasitemia was estimated using a non-compartment model as previously described.27,28 Briefly, parasite densities (concentrations) versus time until complete clearance of parasitemia were plotted on a semilogarithmic graph. Final parasite density at the time of apparent clearance was assumed to be 0.01 asexual parasites/μL of blood, a level below microscopic detection. The apparent terminal elimination rate constant (λ) was obtained by least square regression analysis of the post peak log-linear part of the parasitemia–time curve and apparent terminal elimination half-time of parasitemia was obtained from ln2/λ (i.e., λt = 0.693).
Data analysis.
Assuming a cure rate of 100% for AL and 95% for AA or DHP and a 5% drop out rate, we estimated that a minimum of 50 patients per treatment arm in each sentinel site would provide 95% power and a 95% confidence interval (CI). Data were analyzed using version 6 of Epi-Info software29 and the statistical program SPSS for Windows version 22.0.30 Variables considered in the analysis were related to the densities of P. falciparum asexual forms. Proportions were compared by calculating χ2 using Yates’ correction, Fisher exact or Mantel–Haenszel tests as appropriate. Normally distributed, continuous data were compared by Student’s t test and analysis of variance or by paired t test. Post hoc comparisons of parameters between two treatments, where necessary, were carried out using Tukey’s honestly significant difference test. Kaplan–Meier estimator and pairwise log-rank tests were used to determine cumulative risk of recurrent parasitemia on day 42, after the initial clearance of parasitemia. Univariate analyses and stepwise multiple logistic regression models were used to test the association between demographic, clinical, parasitological, or hematological parameters and parasite positivity 1 or 2 days posttreatment initiation and independent predictors of these parameters, respectively. Data were double-entered serially using patients’ codes and were only analyzed at the end of the study. P values of < 0.05 were taken to indicate significant differences.
RESULTS
Patient characteristics.
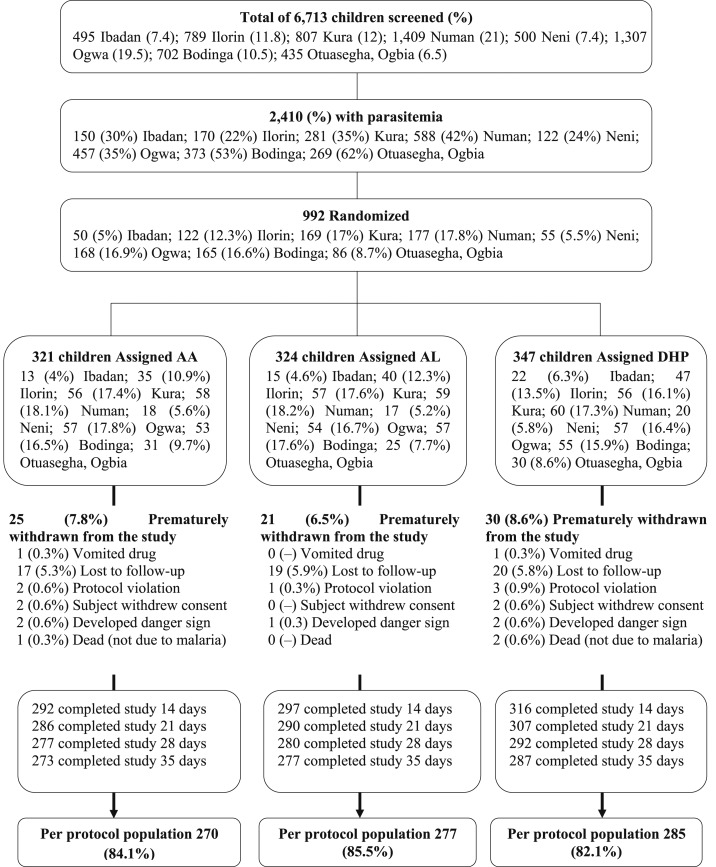
Between June 2014 and December 2015, 992 children were recruited: 321, 324, and 347 children in AA, AL, and DHP treatment groups, respectively (Figure 2). Twenty five, 21, and 30 children were excluded from data analyses in the AA, AL, and DHP treatment groups, respectively, for the following reasons: vomited drug, protocol violation, withdrawal of consent, or loss to follow-up during the first week of follow-up (Figure 2). All children with a minimum follow-up of 7 days were evaluated (N = 910). The baseline characteristics of these children are shown in Table 1. The baseline characteristics were similar for all treatment groups (Table 1).
Figure 2.
The study profile.
Table 1.
Baseline characteristics at enrolment
| Variables | AA (N = 321) | AL (N = 324) | DHP (347) | All (N = 992) | P value |
|---|---|---|---|---|---|
| Male:Female | 167:154 | 174:150 | 196:150 | 537:454 | 0.5 |
| Age (month) | |||||
| Mean | 38.5 | 39.8 | 38.1 | 38.8 | 0.41 |
| 95% CI | 36.7–40.3 | 37.9–41.6 | 36.4–39.9 | 37.8–39.8 | |
| No. ≤ 12 months (%) | 29 (9) | 23 (7.1) | 32 (9.2) | 84 (8.5) | 0.56 |
| Reported duration of illness (day) | |||||
| Mean | 4 | 3.8 | 3.8 | 3.9 | 0.65 |
| 95% CI | 3.6–4.5 | 3.3–4.2 | 3.4–4.2 | 3.6–4.1 | |
| Weight (kg) | |||||
| Mean | 13.4 | 13.4 | 12.9 | 13.2 | 0.17 |
| 95% CI | 12.9–13.8 | 12.9–13.8 | 12.4–13.3 | 12.9–13.4 | |
| Temperature (°C) | |||||
| Mean | 37.8 | 37.9 | 37.8 | 37.8 | 0.31 |
| 95% CI | 37.6–37.9 | 37.8–38 | 37.7–37.9 | 37.8–37.9 | |
| No. with | |||||
| ≥ 37.5°C (%) | 199 (62) | 211 (65) | 210 (60.5) | 620 (62.5) | 0.46 |
| ≥ 40°C (%) | 32 (10) | 23 (7.1) | 33 (9.5) | 88 (8.9) | 0.38 |
| Hematocrit (%) | |||||
| Mean | 30.5 | 30.4 | 30.8 | 30.6 | 0.48 |
| 95% CI | 29.9–31 | 29.8–31 | 30.3–31.4 | 30.3–30.9 | |
| No with anemia (%) | 121 (37.7) | 125 (38.6) | 112 (32.3) | 358 (36.1) | 0.17 |
| Mild (%) | 114 (35.5) | 113 (34.9) | 99 (28.5) | 326 (32.9) | 0.28 |
| Moderate (%) | 6 (1.9) | 11 (3.4) | 13 (3.7) | 30 (3) | 0.18 |
| Severe (%) | 1 (0.3) | 1 (0.3) | 0 (0) | 2 (0.2) | – |
| Parasitemia (μL−1) | |||||
| Geometric mean | 15,675 | 16,337 | 15,163 | 15,670 | 0.86 |
| Range | 2,000–200,000 | 2,003–200,000 | 2,000–200,000 | 2,000–200,000 | |
| Gametocytemia (%) | 20 (6.2) | 10 (3.1) | 19 (5.5) | 49 (4.9) | 0.16 |
AA = artesunate–amodiaquine; AL = artemether–lumefantrine; CI = confidence interval; DHP = dihydroartemisinin–piperaquine.
Primary outcomes.
Parasite prevalence following initiation of treatment.
Parasite prevalence on day 1.
Overall, parasite prevalence 1 day posttreatment initiation was 59.5% (541 of 910 children). In a pooled analysis of all three treatments, it was significantly higher in AL-treated children compared with AA- and DHP-treated children (195 of 301 children [64.8%] versus 173 of 292 children [59.2%] versus 173 of 317 children [54.6%], respectively; P = 0.03). In a post hoc analysis, parasite prevalence 1 day posttreatment initiation was significantly higher in AL-treated children than DHP-treated children (P = 0.01), but it was similar in AA- and DHP-treated children and in AA-treated compared with AL-treated children (P = 0.28 and 0.19, respectively). When analyzed according to study site, parasite prevalence 1 day posttreatment initiation was similar in all three treatment groups at all sites except in Numan and Kura where it was significantly higher in children treated with AL compared with AA and DHP (P = 0.04 and 0.03, respectively).
Predictors of parasite prevalence on day 1.
In a multiple logistic regression model, absence of history of fever before presentation (day 0), body temperature > 38°C, enrollment asexual parasitemia > 75,000 μL−1, and parasite reduction ratio < 5,000 1 day posttreatment initiation independently predicted residual asexual parasitemia 1 day posttreatment initiation (Table 2).
Table 2.
Predictors of residual asexual parasitemia 1 day postinitiation of artemisinin-based combination treatments in acutely malarious < 5-year-old children
| Variable | Total no. | No. with residual parasitemia on day 1 | OR (95% CI) | P value | AOR (95% CI) | P value |
|---|---|---|---|---|---|---|
| Gender | ||||||
| Female | 418 | 244 | 1 | |||
| Male | 492 | 297 | 1.1 (0.8–1.5) | 0.59 | – | – |
| Age (month) | ||||||
| > 24 | 674 | 408 | 1 | |||
| ≤ 24 | 236 | 133 | 0.8 (0.6–1.1) | 0.29 | – | – |
| History of fever at presentation | ||||||
| Present | 749 | 427 | 1 | 1 | ||
| Absent | 161 | 114 | 1.8 (1.3–2.6) | 0.002 | 3.6 (1.4–9.0) | 0.006 |
| Temperature at presentation (°C) | ||||||
| < 38 | 352 | 190 | 1 | 1 | ||
| ≥ 38 | 558 | 351 | 1.4 (1.1–1.9) | 0.009 | 1.6 (0.9–2.7) | 0.1 |
| History of fever on day 1 | ||||||
| Absent | 737 | 422 | 1 | 1 | ||
| Present | 173 | 119 | 1.6 (1.2–2.3) | 0.01 | 0.7 (0.4–1.4) | 0.35 |
| Temperature on day 1 (°C) | ||||||
| < 38 | 790 | 466 | 1 | 1 | ||
| ≥ 38 | 96 | 69 | 1.8 (1.1–2.8) | 0.02 | 3.2 (1.0–9.8) | 0.043 |
| Enrolment parasitemia | ||||||
| ≤ 75,000 | 771 | 432 | 1 | 1 | ||
| > 75,000 | 139 | 109 | 2.8 (1.9–4.4) | < 0.0001 | 37.9 (4.6–321.5) | 0.001 |
| Gametocyte carriage | ||||||
| Absent | 863 | 514 | 1 | |||
| Present | 47 | 27 | 0.9 (0.5–1.7) | 0.89 | – | – |
| Drug treatment | ||||||
| DHP | 317 | 173 | 1 | 1 | ||
| AA | 292 | 173 | 1.2 (0.9–1.7) | 0.28 | – | – |
| AL | 301 | 195 | 1.5 (1.1–2.1) | 0.01 | 1.4 (0.9–2.5) | 0.16 |
| Fever clearance time (day) | ||||||
| ≤ 2 | 541 | 338 | 1 | |||
| > 2 | 23 | 15 | 0.7 (0.5–1.2) | 0.25 | – | – |
| Parasite reduction ratio on day 1 | ||||||
| > 5,000 | 257 | 5 | 1 | 1 | ||
| < 5,000 | 653 | 536 | 230.9 (93.2–572.1) | < 0.0001 | 1,339.3 (171.2–104,780) | < 0.0001 |
AA = artesunate–amodiaquine; AL = artemether–lumefantrine; AOR = adjusted odds ratio; CI = confidence interval; DHP = dihydroartemisinin–piperaquine; OR = odds ratio.
Parasite prevalence on day 2.
Overall, parasite prevalence 2 days posttreatment initiation was 22.6% (206 of 910 children), and it was similar in all three treatment groups (70 of 292 children [24%] versus 76 of 301 children [25.3%] versus 60 of 317 children [18.9%] in AA-, AL-, and DHP-treated children, respectively; P = 0.14). At all sites, parasite prevalence 2 days posttreatment initiation was similar (P ≥ 0.2) except at Ilorin study site where it was significantly lower in DHP-treated children compared with AA- and AL-treated children (6 of 36 children [14.3%] versus 7 of 25 children [28%] versus 15 of 33 children [45.5%], respectively; P = 0.03).
Predictors of parasite prevalence on day 2.
In a univariate analysis, history of fever 1 day posttreatment initiation (odd ratio [OR] = 2.5 [95% CI: 1.8–3.6], P < 0.0001), body temperature > 38°C 1 day (OR = 2.0 [95% CI: 1.3–3.2], P = 0.003) and 2 days posttreatment initiation (OR = 3.1 [95% CI: 1.5–6.5], P = 0.003), hematocrit ≥ 29% on day 1 (OR = 1.8 [95% CI: 1.3–2.5], P = 0.001) and ≥ 30% on day 2 (OR = 1.7 [95% CI: 1.2–2.4], P = 0.002), enrollment parasitemia > 100,000 μL−1 (OR = 1.9 [95% CI: 1.2–3.0], P = 0.008), and parasite reduction ratio < 1,000 1 day (OR = 7.4 [95% CI: 4.8–11.3], P < 0.0001) and < 2,500 2 days posttreatment initiation (OR = 67.7 [95% CI: 40.5–113.2], P < 0.0001) were significantly associated with residual asexual parasitemia on day 2. In a multiple logistic regression model, body temperature > 38°C 2 days posttreatment initiation (adjusted OR [AOR] = 10.2 [95% CI: 2.1–49.8], P = 0.004), enrollment parasitemia > 100,000 μL−1 (AOR = 43.8 [95% CI: 13.8–139.4], P < 0.0001), and parasite reduction ratio < 1,000 1 day (AOR = 8.3 [95% CI: 4.4–15.6], P < 0.0001) and < 2,500 2 days posttreatment initiation (OR = 281.8 [95% CI: 91.2–871.4], P < 0.0001) independently predicted residual asexual parasitemia on day 2.
Parasite prevalence on day 3 (by microscopy).
Of 816 children with pretreatment parasitemia < 100,000 μL−1, 19 children (2.3%) had residual parasitemia on day 3 (distributed as follows: five of 261 [1.9%], nine of 271 [3.3%], and five of 284 [1.8%] in AA-, AL-, and DHP-treated children, respectively). Overall, parasite positivity 3 days posttreatment initiation was 2.1% (19 of 910 children), and it was similar in all treatment groups (five of 292 children [1.7%] versus nine of 301 children [3.0%] versus five of 317 [1.6%] in AA, AL, and DHP treatment groups, respectively [P = 0.41]). At the individual site, parasite positivity 3 days posttreatment initiation were 0 of 169 children (0%), five of 49 (10.2%), 0 of 84 children (0%), six of 158 children (3.8%), 0 of 164 children (0%), one of 94 children (0.6%), 0 of 49 children (0), and seven of 143 children (4.9%) in Numan, Neni, Otuasegha, Ogwa, Kura, Ilorin, Ibadan, and Bodinga, respectively. None of the 19 children with residual asexual parasitemia on day 3 had recurrent infections 14–42 days posttreatment initiation.
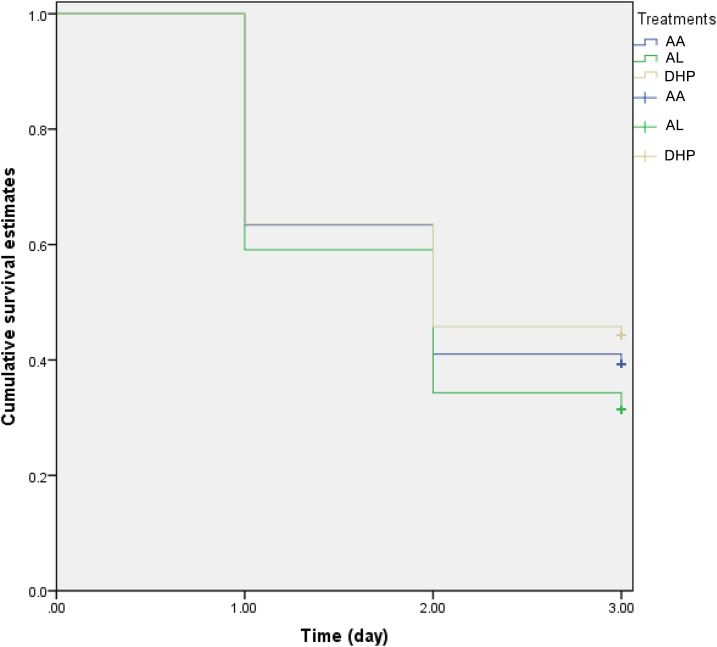
The cumulative probability of persistent parasitemia on day 3 posttreatment initiation was significantly higher in AL-treated compared with DHP- or AA-treated children (log-rank statistic = 9.18, P = 0.01; Figure 3). The clinical and parasitological features of the children with delayed clearance of parasitemia were not significantly different from those without parasitemia on day 3 (data not shown), making it impossible because of the small number of children with asexual parasite positivity on day 3 (APPD3), to evaluate the risk factors for APPD3. However, in 16 pairs of age-, gender-, sentinel site–, same day presentation–, parasitemia-, and treatment-matched children with APPD3 and those without APPD3 (mean age 46.5 ± 17.4 months [range: 10–59] versus 46.1 ± 17.5 months [range: 12–59]; geometric mean parasitemia 8,159 [range: 2,018–60,587] versus 7,984 [range: 2,016–54,721 μL−1], respectively), PCT was significantly higher, as expected, in those with APPD3 than those without APPD3 (4.1 ± 0.3 days [range: 4–5] versus 2.1 ± 0.8 days [range: 1–3], P < 0.0001) but geometric mean PRRD1 was similar (38, [range: 1.1–14,000] versus 150 [range: 0.5–28,000], respectively; P = 0.19). The mean of the ratio of PRRD1 in children with APPD3 to those of the children without APPD3 was 1:3.9. Geometric mean PRRD2 was significantly lower in children with APPD3 compared with those without APPD3 (130 [range: 4.9–160,000] versus 1,500 [range: 1.8–54,000], respectively, P = 0.02). The mean of the ratio of PRRD2 in children with APPD3 to those of the children without APPD3 was 1:7.
Figure 3.
Kaplan–Meier survival estimates of persistent parasitemia on day 3 following treatment with artesunate–amodiaquine (AA, blue line), artemether–lumefantrine (AL, green line), or dihydroartemisinin–piperaquine (DHP, gray line) in children with uncomplicated falciparum malaria. The probability of persistent parasitemia on day 3 was significantly higher in AL-treated children (log-rank statistic = 9.18, P = 0.01). This figure appears in color at www.ajtmh.org.
Polymerase chain reaction–corrected asexual parasite prevalence on day 3.
Polymerase chain reaction amplification was possible in pretreatment (on day 0) of 636 of 842 samples (76%). Polymerase chain reaction–corrected APPD3 was 12 of 636 (1.9%).
Parasite clearance time:
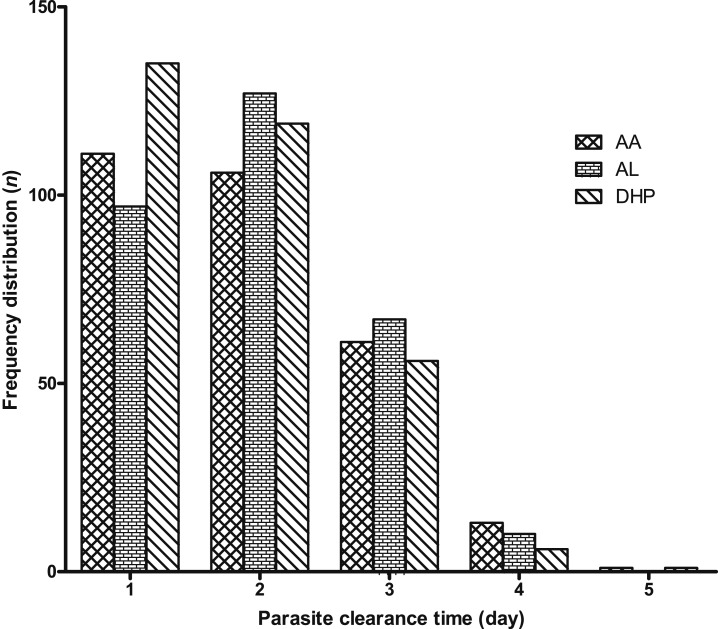
Overall, for all three treatment regimens, mean PCT was 1.9 days (95% CI: 1.8–2, N = 910). Mean PCT was significantly faster in children treated with DHP than those treated with AA and AL (1.8 days [95% CI: 1.7–1.9, N = 317] versus 1.9 days [95% CI: 1.8–2, N = 292] versus 2 days [95% CI: 1.9–2.1, N = 301], respectively, P = 0.03). In a post hoc analysis, PCT was significantly faster in DHP-treated than AL-treated children, but it was similar in AA- and DHP-treated children (P = 0.14) and in AL- and AA-treated children (P = 0.84). The frequency distribution of PCTs was unimodal for all treatments (Figure 4).
Figure 4.
Frequency distribution of parasite clearance time according to treatment groups. AA = artesunate–amodiaquine; AL = artemether–lumefantrine; and DHP = dihydroartemisinin–piperaquine.
When analyzed according to study sites, overall, mean PCTs for all three treatment regimens were 1.6 days (95% CI: 1.5–1.7, N = 169), 3 days (95% CI: 2.8–3.1, N = 49), 1.6 days (95% CI: 1.5–1.8, N = 84), 2.1 days (95% CI: 1.9–2.2, N = 158), 1.2 days (95% CI: 1.2–1.3, N = 164), 2.2 days (95% CI: 2.1–2.3, N = 94), 1.7 days (95% CI: 1.5–1.9, N = 49), and 2.5 days (95% CI: 2.3–2.6, N = 143) for children enrolled in Numan, Neni, Otuasegha, Ogwa, Kura, Ilorin, Ibadan, and Bodinga, respectively. Mean parasite clearance for all three treatment regimens was significantly faster in Kura compared with other sites (P < 0.0001). When the three treatment regimens were compared at each site, parasite clearance was similar with the three treatment regimens at all sites except at three study sites: in Numan (Adamawa State) and Kura (Kano State), where PCT was significantly longer in AL-treated children than AA- and DHP-treated children (P = 0.03 and 0.02, respectively), and in Bodinga (Sokoto State), where PCT was significantly faster in DHP-treated children than AA- and AL- treated children (P = 0.04).
Parasite reduction ratios:
Data for estimation of parasite reduction ratios were available in all 910 children.
Parasite reduction ratio 1 day posttreatment initiation.
Overall, geometric mean PRRD1 was 490 (95% CI: 450–630, range: 0.5–200,000, N = 910), and it was significantly lower in children treated with AL than in AA- and DHP-treated children (280 [95% CI: 190–400, range: 1.1–130,000, N = 301] versus 570 [95% CI: 400–820, range: 0.5–190,000, N = 292] versus 740 [95% CI: 490–1,000, range: 0.7–200,000, N = 317], respectively, P = 0.0006). However, it was similar in AA- and DHP-treated children (P = 0.33, post hoc). Overall geometric means PRRD1 for all three treatment regimens at all sentinel sites were as follows: 1,300 (95% CI: 820–2,000, range: 1.7–200,000, N = 169) in Numan; 47 (95% CI: 28–80, range: 1.4–14,000, N = 49) in Neni; 730 (95% CI: 410–1,300, range: 1.8–110,000, N = 84) in Otuasegha; 380 (95% CI: 240–590, range: 1.1–190,000, N = 158) in Ogwa; 5,700 (95% CI: 3,800–8,700, range: 1.3–180,000, N = 164) in Kura; 110 (95% CI: 68–180, range: 1–62,000, N = 94) in Ilorin; 600 (95% CI: 250–1,400, range: 0.7–98,000, N = 49) in Ibadan; and 56 (95% CI: 34–94, range: 50–32,000, N = 143) in Bodinga. PRRD1 was significantly higher in children enrolled at Kura (Kano) than in other sites (P < 0.0001). Further exploratory analysis showed that when Kura was excluded from the analysis, PRRD1 was significantly higher in Numan compared with other sites (P < 0.0001). When the three treatment regimens were compared at each study site, PRRD1 was similar with the three treatment regimens at all sites except in Numan and Neni where it was significantly lower in children treated with AL than in AA- and DHP-treated children (P = 0.01 and 0.003, respectively).
Parasite reduction ratio 2 days posttreatment initiation.
Overall, geometric mean PRRD2 was 5,400 (95% CI: 4,500–6,300, range: 1.3–200,000, N = 910) and was similar with all three treatment regimens (4,900 [95% CI: 3,600–6,700, range: 2.5–200,000] versus 4,800 [95% CI: 3,600–6,600, range: 2.2–200,000] versus 6,400 [95% CI: 4,900–8,300, range: 1.3–200,000] in AA-, AL-, and DHP-treated children, respectively, P = 0.71). Overall geometric means PRRD2 for all three treatment regimens at all sentinel sites were as follows: 2,100 (95% CI: 1,700–2,600, range: 67–200,000, N = 169) in Numan; 520 (95% CI: 280–960, range: 7–100,000, N = 49) in Neni; 6,700 (95% CI: 4,900–9,200, range: 42–110,000, N = 84) in Otuasegha; 6,000 (95% CI: 4,200–8,700, range: 2.5–190,000, N = 158) in Ogwa; 24,000 (95% CI: 20,000–29,000, range: 65–200,000, N = 164) in Kura; 6,500 (95% CI: 4,200–10,000, range: 8.3–200,000, N = 94) in Ilorin; 17,000 (95% CI: 12,000–26,000, range: 400–200,000, N = 49) in Ibadan; and 200 (95% CI: 120–320, range: 1.3–170,000, N = 143) in Bodinga. Parasite reduction ratio 2 days posttreatment initiation was significantly lower in children enrolled at Bodinga (Sokoto) than in other sites (P < 0.0001) except Neni where PRRD2 was similar (P = 0.09). When the three treatment regimens were compared at each study site, PRRD2 was similar with the three treatment regimens at all sites.
Polymerase chain reaction–uncorrected efficacy of ACTs:
Overall, five children (two, one, and two children in AA, AL, and DHP treatment groups, respectively) developed danger signs (e.g., inability to drink or breast feed, inability to sit or stand, and vomiting everything) within 1 day of starting treatment. No other children had evidences of ETF. Overall, PCR-uncorrected ACPR on day 42 was 683 of 798 children (84.8% [95% CI: 78.9–90.7]) (Table 3), and it was significantly higher in DHP-treated children than in AA- and AL-treated children (253 of 271 [92.1%] (95% CI: 85.5–98.6) versus 219 of 262 [80.9%] (95% CI: 71.5–90.3) versus 213 of 268 [80.5%] (95% CI: 73.4–87.6), respectively; P < 0.0001). In a post hoc analysis, PCR-uncorrected ACPR on day 42 was similar in AA- and AL-treated children (P = 0.27).
Table 3.
Forty two-day efficacy of AA, AL, or DHP according to study sites
| PCR-uncorrected on day 42 | PCR-corrected on day 42 | |||||||
| Study sites | ACRP_u | Failure_u | Total | % Cure rate | ACPR_c | Recrudescence | Total | % Recrudescence |
| All treatments | ||||||||
| Numan (Adamawa) | 137 | 26 | 163 | 84 | 133 | 10 | 143 | 7 |
| Neni (Anambra) | 33 | 11 | 44 | 75 | 18 | 0 | 18 | 0 |
| Otuasegha (Bayelsa) | 62 | 18 | 80 | 77.5 | 65 | 3 | 68 | 4.4 |
| Ogwa (Imo) | 113 | 22 | 135 | 83.7 | 99 | 0 | 99 | 0 |
| Kura (Kano) | 151 | 9 | 160 | 93.7 | 146 | 0 | 146 | 0 |
| Ilorin (Kwara) | 55 | 10 | 65 | 84.6 | 42 | 0 | 42 | 0 |
| Ibadan (Oyo) | 43 | 2 | 45 | 95.6 | 44 | 0 | 44 | 0 |
| Bodinga (Sokoto) | 92 | 17 | 109 | 84.4 | 47 | 1 | 48 | 2.1 |
| All sites | 683 | 115 | 798 | 85.6 | 594 | 14 | 608 | 2.3 |
| AA | ||||||||
| Numan (Adamawa) | 45 | 9 | 54 | 83.3 | 41 | 3 | 44 | 6.8 |
| Neni (Anambra) | 9 | 5 | 14 | 64.2 | 4 | 0 | 4 | 0 |
| Otuasegha (Bayelsa) | 19 | 8 | 27 | 70.4 | 19 | 2 | 21 | 9.5 |
| Ogwa (Imo) | 43 | 7 | 50 | 86 | 35 | 0 | 35 | 0 |
| Kura (Kano) | 51 | 1 | 52 | 98.1 | 47 | 0 | 47 | 0 |
| Ilorin (Kwara) | 12 | 4 | 16 | 75 | 12 | 0 | 12 | 0 |
| Ibadan (Oyo) | 12 | 1 | 13 | 92.3 | 11 | 0 | 11 | 0 |
| Bodinga (Sokoto) | 28 | 8 | 36 | 77.8 | 14 | 1 | 15 | 6.7 |
| All sites | 219 | 43 | 262 | 83.6 | 183 | 6 | 189 | 3.2 |
| AL | ||||||||
| Numan (Adamawa) | 39 | 16 | 55 | 70.9 | 42 | 6 | 48 | 12.5 |
| Neni (Anambra) | 11 | 2 | 13 | 84.6 | 7 | 0 | 7 | 0 |
| Otuasegha (Bayelsa) | 16 | 7 | 23 | 69.6 | 21 | 1 | 22 | 4.5 |
| Ogwa (Imo) | 32 | 11 | 43 | 74.4 | 26 | 0 | 26 | 0 |
| Kura (Kano) | 47 | 8 | 55 | 85.5 | 50 | 0 | 50 | 0 |
| Ilorin (Kwara) | 18 | 5 | 23 | 78.3 | 14 | 0 | 11 | 0 |
| Ibadan (Oyo) | 13 | 1 | 14 | 92.9 | 14 | 0 | 14 | 0 |
| Bodinga (Sokoto) | 37 | 5 | 42 | 88.1 | 20 | 0 | 10 | 0 |
| All site | 213 | 55 | 268 | 79.5 | 194 | 7 | 201 | 3.5 |
| DHP | ||||||||
| Numan (Adamawa) | 53 | 1 | 54 | 98.1 | 50 | 1 | 51 | 1.9 |
| Neni (Anambra) | 13 | 4 | 17 | 76.5 | 7 | 0 | 7 | 0 |
| Otuasegha (Bayelsa) | 27 | 3 | 30 | 90 | 25 | 0 | 25 | 0 |
| Ogwa (Imo) | 38 | 4 | 42 | 90.5 | 38 | 0 | 38 | 0 |
| Kura (Kano) | 52 | 1 | 53 | 98.1 | 49 | 0 | 49 | 0 |
| Ilorin (Kwara) | 25 | 1 | 26 | 96.2 | 16 | 0 | 16 | 0 |
| Ibadan (Oyo) | 18 | 0 | 18 | 100 | 19 | 0 | 19 | 0 |
| Bodinga (Sokoto) | 27 | 4 | 31 | 87.1 | 13 | 0 | 13 | 0 |
| All sites | 253 | 18 | 271 | 93.4 | 217 | 1 | 218 | 0.5 |
AA = artesunate–amodiaquine; ACPR_c = adequate clinical and parasitological response corrected; ACPR_u = adequate clinical and parasitological response uncorrected; AL = artemether–lumefantrine; DHP = dihydroartemisinin–piperaquine; Failure_u = treatment failure uncorrected; PCR = polymerase chain reaction.
Overall, PCR-uncorrected ACPR on day 28 was 790 of 849 children (92.1% [95% CI: 87.4–96.8]) (Table 4), and it was significantly higher in DHP-treated children than in AA- and AL-treated children (286 of 295 [96.4%] (95% CI: 92.9–99.9) versus 251 of 276 [87.9%] (95% CI: 78.3–97.5) versus 253 of 278 [91%] (95% CI: 86–96), respectively; P = 0.005). In a post hoc analysis, PCR-uncorrected ACPR on day 28 was similar in AA- and AL-treated children (P = 0.9).
Table 4.
Twenty eight-day efficacy of AA, AL, or DHP according to study sites
| PCR-uncorrected on day 28 | PCR-corrected on day 28 | |||||||
| Study sites | ACRP_u | Failure_u | Total | % Cure rate | ACPR_c | Recrudescence | Total | % Recrudescence |
| All treatments | ||||||||
| Numan (Adamawa) | 154 | 12 | 166 | 92.8 | 144 | 6 | 150 | 4 |
| Neni (Anambra) | 35 | 9 | 44 | 79.5 | 16 | 0 | 16 | 0 |
| Otuasegha (Bayelsa) | 76 | 7 | 83 | 91.6 | 72 | 1 | 73 | 1.4 |
| Ogwa (Imo) | 142 | 9 | 151 | 94 | 93 | 0 | 93 | 0 |
| Kura (Kano) | 155 | 5 | 160 | 96.9 | 142 | 0 | 142 | 0 |
| Ilorin (Kwara) | 66 | 5 | 71 | 93 | 34 | 0 | 34 | 0 |
| Ibadan (Oyo) | 46 | 1 | 47 | 97.9 | 44 | 0 | 44 | 0 |
| Bodinga (Sokoto) | 116 | 11 | 127 | 91.3 | 43 | 1 | 44 | 2.3 |
| All sites | 790 | 59 | 849 | 93.1 | 588 | 8 | 596 | 1.5 |
| AA | ||||||||
| Numan (Adamawa) | 52 | 3 | 55 | 94.5 | 46 | 2 | 48 | 4.2 |
| Neni (Anambra) | 8 | 5 | 13 | 61.5 | 4 | 0 | 5 | 0 |
| Otuasegha (Bayelsa) | 27 | 3 | 30 | 90 | 24 | 1 | 25 | 4 |
| Ogwa (Imo) | 49 | 5 | 54 | 90.7 | 35 | 0 | 35 | 0 |
| Kura (Kano) | 52 | 0 | 52 | 100 | 45 | 0 | 45 | 0 |
| Ilorin (Kwara) | 16 | 2 | 18 | 88.9 | 10 | 0 | 10 | 0 |
| Ibadan (Oyo) | 12 | 1 | 13 | 92.3 | 11 | 0 | 11 | 0 |
| Bodinga (Sokoto) | 35 | 6 | 41 | 85.4 | 14 | 1 | 15 | 6.7 |
| All sites | 251 | 25 | 276 | 90.9 | 189 | 4 | 193 | 2.1 |
| AL | ||||||||
| Numan (Adamawa) | 47 | 9 | 56 | 83.9 | 47 | 4 | 51 | 7.8 |
| Neni (Anambra) | 10 | 2 | 12 | 83.3 | 5 | 0 | 5 | 0 |
| Otuasegha (Bayelsa) | 21 | 2 | 23 | 91.3 | 22 | 0 | 22 | 0 |
| Ogwa (Imo) | 46 | 2 | 48 | 95.8 | 23 | 0 | 23 | 0 |
| Kura (Kano) | 50 | 5 | 55 | 90.9 | 50 | 0 | 50 | 0 |
| Ilorin (Kwara) | 20 | 3 | 23 | 87 | 9 | 0 | 9 | 0 |
| Ibadan (Oyo) | 15 | 0 | 15 | 100 | 14 | 0 | 14 | 0 |
| Bodinga (Sokoto) | 44 | 2 | 46 | 95.7 | 17 | 0 | 17 | 0 |
| All sites | 253 | 25 | 278 | 91 | 187 | 4 | 191 | 2.1 |
| DHP | ||||||||
| Numan (Adamawa) | 55 | 0 | 55 | 100 | 51 | 0 | 51 | 0 |
| Neni (Anambra) | 17 | 2 | 19 | 89.5 | 7 | 0 | 7 | 0 |
| Otuasegha (Bayelsa) | 28 | 2 | 30 | 93.3 | 26 | 0 | 26 | 0 |
| Ogwa (Imo) | 47 | 2 | 49 | 95.9 | 35 | 0 | 35 | 0 |
| Kura (Kano) | 53 | 0 | 53 | 100 | 47 | 0 | 47 | 0 |
| Ilorin (Kwara) | 30 | 0 | 30 | 100 | 15 | 0 | 15 | 0 |
| Ibadan (Oyo) | 19 | 0 | 19 | 100 | 19 | 0 | 19 | 0 |
| Bodinga (Sokoto) | 37 | 3 | 40 | 92.5 | 12 | 0 | 12 | 0 |
| All sites | 286 | 9 | 295 | 96.9 | 212 | 0 | 212 | 0 |
AA = artesunate–amodiaquine; ACPR_c = adequate clinical and parasitological response corrected; ACPR_u = adequate clinical and parasitological response uncorrected; AL = artemether–lumefantrine; DHP = dihydroartemisinin–piperaquine; Failure_u = treatment failure uncorrected; PCR = polymerase chain reaction.
Overall for all treatments, PCR-uncorrected ACPR on day 14 was 877 of 888 children (98.6% [95% CI: 97.2–100]), and it was similar in the three treatment groups (281 of 286 [97.6%] (95% CI: 95.3–99.9) versus 290 of 292 [99.3%] (95% CI: 98.1–100.5) versus 306 of 310 [98.7%] (95% CI: 96.6–100.9) in AA-, AL- and DHP-treated children, respectively; P = 0.51).
Polymerase chain reaction–corrected efficacy of ACTs:
Of 842 children with pretreatment samples, amplification was successful in 636 samples (76%). In these 636 children, the proportion of children with recurrent infections was significantly lower in DHP-treated children than in AA- and AL-treated children: 14 of 221 children (1.8%) versus 25 of 205 children (11.7%) versus 25 of 210 children (11.9%), respectively, P < 0.0001).
Overall, PCR-corrected ACPR on day 42 was 594 of 608 children (98.3% [95% CI: 96.1–100]) (Table 3), and it was similar for all three treatments (183 of 189 [97.1%] (95% CI: 93.7–100) versus 194 of 201 [97.9%] (95% CI: 94.1–100) versus 217 of 218 [99.8%] (95% CI: 99.2–100) in AA-, AL- and DHP-treated children, respectively; P = 0.08). On day 28, overall, PCR-corrected ACPR was 588 of 596 children (99.1% [95% CI: 97.8–100]) (Table 4). Polymerase chain reaction–corrected ACPR on day 28 in DHP-treated children was 100%. Polymerase chain reaction–corrected ACPR on day 28 for AA and AL was similar (189 of 193 children [98.1% (95% CI: 96.2–100)] versus 187 of 191 children [99.2% (95% CI: 95.8–100)], respectively, P = 1.0).
Survival estimates of reappearance of asexual parasitemia after initial clearance:
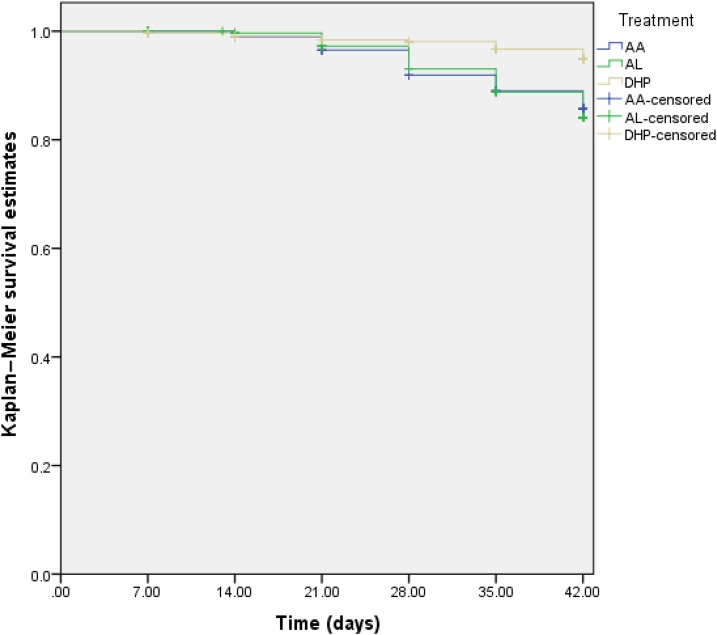
The probability of reappearance of asexual parasitemia after initial clearance was significantly lower in children treated with DHP than those treated with AA and AL (log-rank statistic = 19.21; P < 0.0001, Figure 5). When analyzed according to the study sites, the probability of reappearance of asexual parasitemia after initial clearance was significantly higher in AL-treated children enrolled in Numan (log-rank statistic = 15.54, P < 0.0001) and Kura (log-rank statistic = 8.18, P = 0.017). However, at other sentinel sites, the probability of reappearance of parasitemia after initial clearance was similar with all three treatments.
Figure 5.
Kaplan–Meier survival estimates of reappearance of asexual parasitemia after initial clearance following treatment with artesunate–amodiaquine (AA, blue line), artemether–lumefantrine (AL, green line), or dihydroartemisinin–piperaquine (DHP, yellow line). Pooled data from all sentinel sites (log-rank statistic = 19.21, P < 0.0001). The probability of reappearance of asexual parasitemia was lowest in DHP-treated children. This figure appears in color at www.ajtmh.org.
Time of recurrent parasitemia.
Of 115 children with recurrent parasitemia, 6, 15, 26, 22, and 46 children had recurrent asexual parasitemia on days 14, 21, 28, 35, and 42, respectively. The mean of time to recurrent asexual parasitemia for all three treatments was 33.3 days (95% CI: 31.7–34.9), and it was similar for all treatments (32.2 days [95% CI: 29.6–34.9, N = 43] versus 34.2 days [95% CI: 32–36.4, N = 54] versus 33.1 days [95% CI: 27.5–38.6, N = 18] for AA, AL, and DHP treatment groups, respectively, P = 0.54). Recurrent infections during the 42 days of follow-up were not related to age (data not shown).
Survival estimates of recrudescent asexual parasitemia after initial clearance:
Recrudescence after initial clearance of asexual parasitemia was observed at three sites: Numan (N = 10; three in AA-, six in AL- and one in DHP-treated children); Otuasegha (N = 3; two in AA- and one in AL-treated children); and Bodinga (N = 1 in AA-treated children) (Table 3). The cumulative probabilities of recrudescence of asexual parasitemia after initial clearance were similar with all three treatments (data not shown).
Secondary outcomes.
Fever clearance.
Overall, 564 of 910 children (62%) were febrile at presentation and distributed as follows: 181 of 292 children (62%), 194 of 301 children (64.5), and 189 of 317 (59.6%) in AA, AL, and DHP treatment groups, respectively. There was no significant difference in the proportions of children who were febrile at presentation in the three treatment groups (P = 0.46). Overall, mean fever clearance time was 1.2 days (95% CI: 1.2–1.3), and it was significantly longer in AL-treated children than in AA- and DHP-treated children (1.3 days [95% CI: 1.2–1.4] versus 1.1 days [95% CI: 1.1–1.2] versus 1.2 days [95% CI: 1.1–1.3], respectively, P = 0.002). In a post hoc analysis, fever clearance time was similar in AA- and DHP-treated children (P = 0.48).
Gametocyte carriage.
At enrolment, gametocytes were detected microscopically in the peripheral blood of 47 of 910 children (5.2%) (Table 5). The proportions of children with gametocyte carriage pretreatment were similar in all treatment groups (P = 0.08). By day 7, gametocyte carriage was significantly higher in children treated with AA than in children treated with AL or DHP, but it was similar in AL- and DHP-treated children. By day 28, gametocyte carriage was similar in all treatment groups (Table 5). Of the 47 children with gametocytemia pretreatment, 12, four, three children, and one child had persistent gametocytemia until days 7, 14, 21, and 28, respectively. Of the 12 children with persistent gametocytemia up to day 7, six, two, and four children were treated with AA, AL, and DHP, respectively. In general, gametocyte carriage posttreatment was significantly lower than pretreatment of all treatments (Table 5). None of the children who had recurrent asexual parasitemia 14–42 days posttreatment initiation had gametocytemia at the time of recurrent infections.
Table 5.
Gametocyte carriage pre- and posttreatment of malarious children with AA, AL, or DHP
| Posttreatment (%) | ||||||
| Treatment | Pretreatment (day 0) (%) | Day 7 | Day 14 | Day 21 | Day 28 | P value* |
| AA | 20/292 (6.8) | 14/283 (4.9) | 6/285 (2.1) | 3/276 (1.1) | 1/274 (0.4) | < 0.0001 |
| AL | 9/301 (3) | 5/295 (1.7) | 1/289 (0.3) | 2/280 (0.7) | 0/275 (0) | 0.001 |
| DHP | 18/317 (5.8) | 5/308 (1.6) | 1/307 (0.3) | 2/300 (0.7) | 2/297 (0.7) | < 0.0001 |
| All treatments | 47/910 (5.2) | 24/886 (2.7) | 8/881 (0.9) | 7/856 (0.8) | 3/846 (0.4) | < 0.0001 |
| P value† | 0.08 | 0.03 | 0.046 | 0.84 | 1.0‡ | – |
AA = artesunate–amodiaquine; AL = artemether–lumefantrine; DHP = dihydroartemisinin–piperaquine.
Test for trend.
Test of proportions.
Children treated with artemether–lumefantrine were not included in the comparison.
Gametocyte clearance.
Gametocyte clearance times were determined in the first 3 days posttreatment initiation. Gametocytes cleared completely from peripheral blood by day 3 of follow-up in 32 of 47 children (68.1%) distributed as follows: 13 of 20 children (65%) in AA, seven of nine children (77.8%) in AL, and 12 of 18 children (66.7%) in DHP treatment groups. The proportions of children who cleared their gametocytemia by day 3 of follow-up were similar in all treatment groups (P = 0.73). Overall, gametocyte clearance time in the 32 children was 1.6 days (range: 1–3), and it was similar in all treatment groups (1.8 days [range: 1–3] versus 1.4 days [range: 1–3] versus 1.5 days [range: 1–3] in AA, AL, or DHP treatment groups, respectively; P = 0.43).
Anemia pretreatment and during follow-up.
Hematocrit data were available in 891 of 910 children (97.9%) pretreatment initiation and in 855 (94%), 845 (92.9%), 806 (88.6%), 795 (87.4%), 778 (85.5%), and 688 of 910 children (75.6%) on days 7, 14, 21, 28, 35, and 42 posttreatment initiation, respectively. Of the 891 children, 333 children (37.4%) distributed as follows: 111 of 284 children (39.1%) in AA, 118 of 298 children (40%) in AL, and 104 of 309 children (33.7%) in DHP treatment groups were anemic. The prevalence of anemia pretreatment was similar in all treatment groups (P = 0.24, Table 6). Of the 333 children with pretreatment anemia, anemia was mild, moderate, or severe in 304 children (91.3%), 28 children (8.4%), or one child (0.3%), respectively. The prevalence of mild or moderate anemia was also similar in all treatment groups (mild: 104 of 111 children [93.7%] versus 109 of 118 children [92.4%] versus 91 of 104 children [87.5%] in AA, AL, and DHP treatment groups, respectively [P = 0.24]; moderate: six of 111 children [5.4%] versus nine of 118 children [7.6%] versus 13 of 104 children [12.5%] in AA, AL, and DHP treatment groups, respectively [P = 0.16]). Following treatment, the proportions of children with anemia in all treatment groups decreased significantly over time (Table 6). Following initiation of treatment prevalence of anemia at all times were also similar in all treatment groups (Table 6).
Table 6.
Frequency distribution of malaria-associated anemia pre- and posttreatment with AA, AL, or DHP
| Day of follow-up | |||||||||||||||||||||
| 0 | 1 | 2 | 3 | 7 | 14 | 21 | 28 | 35 | 42 | ||||||||||||
| Anemia | Anemia | Anemia | Anemia | Anemia | Anemia | Anemia | Anemia | Anemia | Anemia | ||||||||||||
| Treatment | Y | N | Y | N | Y | N | Y | N | Y | N | Y | N | Y | N | Y | N | Y | N | Y | N | P value* |
| AA | 111 | 173 | 120 | 158 | 128 | 152 | 119 | 157 | 97 | 176 | 45 | 228 | 28 | 232 | 28 | 234 | 21 | 232 | 24 | 223 | < 0.0001 |
| AL | 118 | 180 | 139 | 150 | 139 | 151 | 132 | 158 | 118 | 166 | 66 | 215 | 43 | 223 | 31 | 227 | 27 | 231 | 23 | 228 | < 0.0001 |
| DHP | 104 | 205 | 132 | 165 | 141 | 158 | 147 | 152 | 132 | 166 | 59 | 232 | 37 | 243 | 31 | 244 | 26 | 241 | 19 | 237 | < 0.0001 |
| All | 333 | 558 | 390 | 473 | 408 | 461 | 398 | 467 | 347 | 508 | 170 | 675 | 108 | 698 | 90 | 705 | 74 | 704 | 66 | 688 | < 0.0001 |
| P value† | 0.24 | 0.5 | 0.86 | 0.34 | 0.09 | 0.12 | 0.19 | 0.89 | 0.69 | 0.63 | – | ||||||||||
AA = artesunate–amodiaquine; AL = artemether–lumefantrine; DHP = dihydroartemisinin–piperaquine; N = no, anemia absent; Y = yes, anemia present.
Test for trend.
Test of proportion.
Recovery from malaria-associated anemia at presentation.
Of the 333 anemic children at presentation, 28 children (8.4%) did not recover from their anemia during the entire follow-up period (7 of 111 children [6.3%] versus 12 of 118 children [10.2%] versus nine of 104 [8.7%] in AA-, AL-, and DHP-treated children, respectively, P = 0.57). Following treatment, recovery from anemia occurred in 305 of 333 children (91.6%). Overall, 97 of 305 children (31.8%) who recovered from their anemia did so by 3 days following initiation of treatment. In these children, the proportions who achieved recovery within 3 days were similar in all treatment groups (35 of 104 children [33.7%] versus 40 of 106 children [37.7%] versus 22 of 95 children [23.2%] in AA, AL, and DHP, respectively; P = 0.08). Overall, in these 97 children, mean anemia recovery time was 1.7 days (95% CI: 1.5–1.8) and it was similar in all treatment groups (1.7 days [95% CI: 1.4–1.9] versus 1.8 days [95% CI: 1.5–2.1] versus 1.5 days [95% CI: 1.2–1.9] in AA, AL, and DHP, respectively; P = 0.56).
Kinetic evaluation of time course of parasitemia following initiation of treatment.
The demographic and other characteristics of the 57 children (N = 18 for AA, N = 17 for AL, and N = 22 for DHP) are shown in Table 7.
Table 7.
Demographic and clinical characteristics of children enrolled in kinetics of parasitemia study
| Variable | Value |
|---|---|
| Number enrolled | 57 |
| Gender (Male:Female) | 38:19 |
| Age (mean ± SD) (months) [range] | 37.6 ± 17.3 [6–59] |
| No. < 12 months | 6 |
| Reported duration of illness (mean ± SD) (day) [range] | 3.6 ± 2.6 [1–14] |
| Temperature (mean ± SD) (°C) [range] | 37 ± 1.3 [36–40] |
| No. > 37.4°C (%) | 34 (59.6) |
| > 40°C (%) | 7 (12.3) |
| Hematocrit (mean ± SD) (%) [range] | 30.4 ± 5.7 [19–46] |
| No. < 30% (%) | 20 (35.1) |
| Geometric mean parasitemia (μL−1) [range] | 20,934 [2,028–195,947] |
| No. > 100,000 μL−1 (%) | 10 (17.5) |
| Fever clearance time (mean ± SD) (day) [range] | 1.2 ± 0.4 [1–3] |
| Parasite clearance time (mean ± SD) (hour) [range] | 32.8 ± 24.1 [2–96] |
SD = standard deviation.
Area under curve of parasitemia versus time.
A rise in parasitemia occurring within 4 hours of initiating treatment was seen in seven of 57 children (12.3%) (distributed as follows: two of 18 children [11.1%], one of 17 children [5.9%], and four of 22 children [18.2%] treated with AA, AL, and DHP, respectively, P = 0.5). Overall, geometric mean area under curve of parasitemia versus time (AUCpd) was 62,745 μL.h (range: 594–2,302,237), and it was similar with all treatments (61,596 μL h [range: 1,843–1,066,873] versus 55,490 μL.h [range: 717–2,302,237] versus 71,135 μL.h [range: 594–1,079,970] in AA-, AL-, and DHP-treated children, respectively, P = 0.87).
Parasitemia half-time.
Declines of parasitemias were monoexponential (Figure 6A). , mean parasitemia elimination half-time was 1.3 hours (range: 0.1–4.6), and it was similar with all three treatments (1.4 hours [range: 0.2–4.6] versus 1.3 hours [range: 0.1–3.1] versus 1.3 hours [range: 0.1–3.1] in AA-, AL-, and DHP-treated children, respectively, P = 0.98). Parasitemia terminal elimination half-time was not related to age (1.2 hours [range: 0.2–3.1] versus 1.4 hours [range: 0.1–4.6] in children > 36 months and those ≤ 36 months, respectively, P = 0.71). The frequency distribution of parasitemia elimination half-time was unimodal (Figure 6B). Six of the 57 children (two children each from each treatment arm) had parasitemia elimination half-time > 3 hours.
Figure 6.
Semilog plots of parasitemia vs. time (A) in all children treated with AA, AL, or DHP and (B) frequency distribution of parasitemia half-times in all children. AA = artesunate–amodiaquine; AL = artemether–lumefantrine; DHP = dihydroartemisinin–piperaquine.
Adverse events.
Adverse events were carefully documented at three sites: Otuasegha, Neni, and Ibadan. Overall, 49 of 189 children (25.9%) (24 of 61 [39%] in AA, 23 of 56 [41%] in AL, and 33 of 72 [46%] in DHP) reported at least one adverse event within the first week of starting treatment. Cough and running nose were the most commonly reported adverse events. Other reported adverse events include fever, anorexia, vomiting, abdominal pain, diarrhea, weakness poor sleep, and headache.
DISCUSSION
Repeated large-scale country-wide evaluation of the efficacy of ACTs in young Nigerian children at a relatively short interval of ≤ 5 years has become imperative because of the threats posed by the development and spread of artemisinin-resistant parasites in the GMS and the increasing likelihood, historically, that its pattern of spread may follow the same routes as those of chloroquine- and sulfadoxine–pyrimethamine–resistant parasites to Africa. With the highest malaria burden in sub-Saharan Africa,31 such a spread to Nigeria will be detrimental for the use of ACTs in general and challenging for the country’s malaria elimination program. In addition, a recent report of mutations in PfK13 gene conferring artemisinin resistance in an indigenous isolate from Equatorial Guinea13 makes surveillance even more imperative.
In this relatively large population of < 5-year-old children in settings of high-intensity malaria transmission, we found, overall, approximately 60%, 23%, and 2.1% of children did not clear their parasitemias by 1, 2, and 3 days, respectively, after treatment initiation. As baseline parasitemias vary between sites and between different studies and considering that comparing absolute percentages of children in whom parasites remain to be cleared can be problematic and complex, it would appear, compared with similar parameters in < 5-year-old children from our previous study in 2009–2010,21,28 and the present results indicate some decline in the early response rate markers that are directly attributable to the artemisinin derivative components of ACTs. The worsening of early response rate markers was particularly more profound with AL where the risk of persistent parasitemia 3 days after treatment initiation was significantly higher than AA and DHP (Figure 3). Overall, although 2.1% of the children had a positive blood smear by microscopy at 72 hours, an in vivo predictor of subsequent treatment failure with ACTs,32 in order not to overestimate this in vivo predictor whose threshold is ≥ 3% in patients with a parasitemia < 100,000/μL,32 we PCR-corrected parasite positivity 3 days after treatment initiation. Overall, the PCR-corrected value of 1.9% clearly indicated that there is no significant delayed clearance of parasitemia or significant risk of treatment failure in < 5-year-old children in endemic areas of Nigeria. We suggest in other areas of intense transmission, when facilities are available, parasite positivity 3 days posttreatment initiation be PCR-corrected.
Residual parasitemias 1 or 2 days posttreatment initiation were independently predicted by a number of factors among which were presence of fever following treatment initiation, high enrollment parasite burden, and relatively low parasite reduction ratios. Collectively, these factors were much more likely encountered in AL-treated children indicating declining responsiveness in P. falciparum in these settings of intense transmission to this drug when compared with AA which was deployed at the same time with AL in Nigeria in 2005. It is intriguing some of the independent predictors of residual parasitemias, such as, fever and relatively heavy baseline parasite burden are similar to those reported from another area of seasonal intense transmission in Africa.10 As relatively heavy parasite burden are cleared more slowly than moderate or mild burden,28 these findings were not unexpected. As slow parasite and fever clearance were distinct features of AL-treated children, it was also not surprising that AL treatment was associated with residual parasitemia 1 day posttreatment initiation.
Four points may possibly help explain the significantly faster parasite clearance in DHP-treated children than the other two ACTs. First, although readily available, recognized in Nigeria’s antimalarial treatment policy as first-line ACT,33 but not officially deployed, DHP is not as widely used as AL or AA in young children. This reduces the risk of a blunted treatment response. Second, artemether component of AL and artesunate component of AA are converted to dihydroartemisinin. However, artesunate is more rapidly converted to dihydroartemisinin than artemether.34 The absence of a lag time for DHP may favor a rapid onset of parasite killing. Third, the contribution of piperaquine to the parasite killing effect of DHP in the first few days of initiating treatment may be greater than those of amodiaquine or lumefantrine to artesunate or artemether, respectively. Finally, Nigerian isolates of P. falciparum may be more sensitive in vitro and by extension, in vivo to piperaquine than lumefantrine or amodiaquine.
It is intriguing predicting which of the eight sentinel sites is likely to show significantly reduced susceptibility of P. falciparum to ACTs in the future. Based on the findings of the present study, reduction in responsiveness to AL was prominent in Numan and Neni when compared with AA and DHP at these sentinel sites. This is evidenced by significantly lower PRRD1 values at the two sites and PCR-corrected efficacy of < 90% on day 42 in Numan.
The unimodal frequency distribution of PCTs in all three treatments suggests the relative absence of parasites with slow clearance phenotypes in these endemic areas of Nigeria (Figure 4). In some countries in the GMS, for example, in southern Myanmar, and Cambodia, where parasites with slow clearance phenotypes have emerged,4,5,35 frequency distribution of parasitemia half-times and of PCTs are bimodal (see the following paragraphs).
A number of 4-aminoquinolines, including amodiaquine,36 chloroquine, and possibly also piperaquine, a bisquinoline, structurally related to but more potent than chloroquine, have potent antipyretic effect. This would partly explain the significantly faster fever clearance in children treated with AA and DHP than with AL. Based on PCR-corrected cure rates on days 28 and 42 of more than 95%, all three ACTs evaluated demonstrated high efficacy. However, in areas of intense transmission, reducing the risk of recurrent parasitemias (recrudescent or re-infection) after initial clearance may be desired as an additional advantage of antimalarial drugs. Viewed in this context, DHP significantly reduced the risk of recurrent infections compared with the other two ACTs (Figure 5). This finding is analogous to showing AA significantly reduced the risk of recurrent infections compared with AL in children from the same endemic areas.21 Thus, the prophylactic efficacy of AA over AL in our previous study21 and of DHP over AA and AL in the present study is explicable in the context of relatively long plasma terminal elimination half-lives of their partner drugs (∼10–50 days)36–39 compared with lumefantrine in malarious patients. Our finding that DHP significantly reduced the risk of recurrent parasitemia is also similar to a previous study in falciparum malaria16 and may be analogous to another showing prophylactic efficacy of piperaquine in vivax malaria.38 It is noteworthy that recrudescence was not associated with gametocyte carriage in any of the patients we evaluated.
As in previous reports,8,27,40,41 a rise in parasitemia within a few hours of initiating treatment occurred in more than 12% of children evaluated. In these children, early rise in parasitemia had no effect on the terminal elimination half-times of parasitemia, which averagely, was 1.3 hours. The unimodal frequency distribution of parasitemia half-times indicate there is no evidence of slow clearance of parasitemia in the cohort of children evaluated. This observation is in agreement with similar reports from other endemic areas of Africa.8,27,28 Frequent sampling ensured that we did not overestimate parasitemia elimination half-time or misinterpret the observed unimodal frequency distribution of parasite elimination half-times. However, a limitation of our kinetics of parasitemia study is the non-estimation of concentrations of the artemisinin components of the administered ACTs and/or their metabolites. In southern Myanmar, plasma concentrations of dihydroartemisinin in artesunate-treated malarious patients were unrelated to terminal elimination half-times of parasitemia.35 Another limitation is the non-evaluation of the many host factors that may influence PCTs such as immune status.
There are potential implications of the findings of the present study. First, the superior efficacy of DHP in clearing parasitemia and in reducing the risk of recurrent infections when compared with AL or AA would suggest DHP be formally considered as one of first-line ACTs in future review of treatment policy. In support of these findings and suggestion is the suggestion that a potentially useful strategy to delay the emergence of antimalarial drug resistance in P. falciparum is to deploy different antimalarials at the same time as first-line therapies.20,42 This would ensure that parasite escaping killing by one first-line therapy may be killed by another first-line therapy should the parasite find itself in another host.41 Second, although all three ACTs evaluated, based on PCR-corrected efficacy of ≥ 96% are efficacious treatments of uncomplicated falciparum malaria, the conduct of two large-scale country-wide efficacy studies at an interval of 5 years in 2009–201021 and in 2014–2015 affords an opportunity for a post hoc analysis of the efficacy of AA and AL. This should allow a validation of evidences of declining or reducing susceptibility in P. falciparum to these ACTs. Post hoc analyses of these two studies are currently underway.
In conclusion, based on PCR-corrected efficacy ≥ 96%, all three ACTs evaluated are efficacious treatments of uncomplicated falciparum malaria in Nigerian children < 5 years old and all are well tolerated. However, based on significantly faster parasite clearance, significantly higher PCR-uncorrected efficacy, significantly lower proportion of children with recurrent infections after initial clearance of asexual parasitemia, and a PCR-corrected efficacy of 100% 28 days posttreatment initiation, DHP is more efficacious than AA and AL.
Acknowledgments:
We thank the parents/guardians of the children and the children for their participation in the study. We also thank the medical officers at the recruiting facilities for assisting with patient recruitment, and the state government where the sentinel sites are located for providing logistic support. U.S. President’s Malaria Initiative assisted with publication expenses.
REFERENCES
- 1.Carrara VI, et al. 2009. Changes in the treatment responses to artesunate-mefloquine on the northwestern border of Thailand during 13 years of continuous deployment. PLoS One 4: e4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dondorp AM, et al. 2009. Artemisinin resistance in Plasmodium falciparum. N Engl J Med 361: 455–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Noedl H, et al. 2010. Artemisinin resistance in Cambodia: a clinical trial designed to address an emerging problem in southeast Asia. Clin Infect Dis 51: e82–e89. [DOI] [PubMed] [Google Scholar]
- 4.Phyo AP, et al. 2012. Emergence of artemisinin-resistant malaria on the western border of Thailand: a longitudinal study. Lancet 379: 1960–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Phyo AP, et al. 2016. Declining efficacy of artemisinin combination therapy against P. falciparum malaria on the Thai-Myanmar border (2003–2013): the role of parasite genetic factors. Clin Infect Dis 63: 784–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization , 2015. Status Report on Artemisinin and ACT Resistance Geneva, Switzerland: WHO. Available at: http://www.who.int/malaria/publications/atoz/update-artemisinin-resistance-sep2015/en/. Accessed September 1, 2016.
- 7.Imwong M, et al. 2017. The spread of artemisinin-resistant Plasmodium falciparum in the Greater Mekong Subregion: a molecular epidemiology observational study. Lancet Infect Dis 17: 491–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maiga AW, et al. 2012. No evidence of delayed parasite clearance after oral artesunate treatment of uncomplicated falciparum malaria in Mali. Am J Trop Med Hyg 87: 23–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bosman A, Mendis KN, 2007. A major transition in malaria treatment: the adoption and deployment of artemisinin-based combination therapies. Am J Trop Med Hyg 77 (Suppl 6): 193–197. [PubMed] [Google Scholar]
- 10.Borrmann S, et al. 2011. Declining responsiveness of Plasmodium falciparum infections to artemisinin-based combination treatments on the Kenyan coast. PLoS One 6: e26005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ashley EA, et al. Tracking Resistance to Artemisinin Collaboration (TRAC) , 2014. Spread of artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med 371: 411–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ouattara A, et al. 2015. Polymorphisms in the K13-propeller gene in artemisinin-susceptible Plasmodium falciparum parasites from Bougoula-Hameau and Bandiagara, Mali. Am J Trop Med Hyg 92: 1202–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu P, et al. 2017. Emergence of indigenous artemisinin-resistant Plasmodium falciparum in Africa. N Engl J Med 376: 991–993. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization , 2010. Guidelines for the Treatment of Malaria, 2nd edition. Geneva, Switzerland: WHO. [Google Scholar]
- 15.World Health Organization , 2015. Guidelines for the Treatment of Malaria, 3rd edition. Geneva, Switzerland: WHO. [Google Scholar]
- 16.Zwang J, et al. 2009. Safety and efficacy of dihydroartemisinin-piperaquine in falciparum malaria: a prospective multi-centre individual patient data analysis. PLoS One 4: e6358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nji AM, et al. 2015. Randomized non-inferiority and safety trial of dihydroartemisin-piperaquine and artesunate-amodiaquine versus artemether-lumefantrine in the treatment of uncomplicated Plasmodium falciparum malaria in Cameroonian children. Malar J 14: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leang R, Barrette A, Bouth DM, Menard D, Abdur R, Duong S, Ringwald P, 2013. Efficacy of dihydroartemisinin-piperaquine for treatment of uncomplicated Plasmodium falciparum and Plasmodium vivax in Cambodia, 2008 to 2010. Antimicrob Agents Chemother 57: 818–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Y, Yang Z, Yuan G, Parker D, Lee M-C, Yan G, Fan Q, Xiao Y, 2015. Clinical efficacy of dihydroartemisinin-piperaquine for the treatment of uncomplicated Plasmodium falciparum malaria at the China-Myanmar Border. Am J Trop Med Hyg 93: 577–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sagara I, et al. 2018. Pyronaridine–artesunate or dihydroartemisinin–piperaquine versus current first-line therapies for repeated treatment of uncomplicated malaria: a randomised, multicentre, open-label, longitudinal, controlled, phase 3b/4 trial. Lancet 391: 1378–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oguche S, et al. 2014. Efficacy of artemisinin-based combination treatments of uncomplicated falciparum malaria in under-five year-old Nigerian children. Am J Trop Med Hyg 91: 925–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Health Organization , 2000. Severe falciparum malaria. Trans R Soc Trop Med Hyg 94 (Suppl 1): 1–90. [PubMed] [Google Scholar]
- 23.World Health Organization , 2014. Severe malaria. Trop Med Int Health 19 (Suppl 1): 7–131. [DOI] [PubMed] [Google Scholar]
- 24.World Health Organization , 2003. Assessment and Monitoring of Antimalarial Drug Efficacy for the Treatment of Uncomplicated Falciparum Malaria. WHO/HTM/RBM/2003.50. Geneva, Switzerland: WHO. [Google Scholar]
- 25.Happi CT, Gbotosho GO, Sowunmi A, Falade CO, Akinboye DO, Gerena L, Kyle DE, Milhous W, Wirth DE, Oduola AM, 2004. Molecular analysis of Pasmodium falciparum recrudescent malaria infection in children treated with chloroquine in Nigeria. Am J Trop Med Hyg 70: 20–26. [PubMed] [Google Scholar]
- 26.World Health Organization , 2009. Methods for Surveillance for Antimalarial Drug Efficacy. Geneva, Switzerland: WHO. [Google Scholar]
- 27.Sowunmi A, Akano K, Ayede AI, Adewoye EO, Ntadom G, Fatunmbi B, Gbotosho GO, Folarin OA, Happi CT, 2017. Early rising asexual parasitaemia in Nigerian children following a first dose of artemisinin-based combination treatments of falciparum malaria. BMC Infect Dis 17: 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gbotosho GO, Sowunmi A, Happi CT, Okuboyejo TM, 2011. Therapeutic efficacies of artemisinin-based combination therapies in Nigerian children with uncomplicated falciparum malaria during five years of adoption as first-line treatments. Am J Trop Med Hyg 84: 936–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anon, 2016. Epi Info Version 7. A Word Processing Data Base and Statistics Program for Public Health on IBM-Compatible Microcomputers. Atlanta, GA: Centers for Disease Control and Prevention. [Google Scholar]
- 30.Anon, 2013. International Business Machine (IBM) Corporation 2013. SPSS for Windows Release 22.0 (Standard Version). Armonk, NY: IBM Corp. [Google Scholar]
- 31.World Health Organization , 2017. World Malaria Report. Geneva, Switzerland: WHO. [Google Scholar]
- 32.Stepniewska K, et al. 2010. In vivo parasitological measures of artemisinin susceptibility. J Infect Dis 201: 570–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Federal Ministry of Health , 2005. National Antimalarial Treatment Guideline. Abuja, Nigeria: Federal Ministry of Health. [Google Scholar]
- 34.Nosten F, White NJ, 2007. Artemisinin-based combination treatment of falciparum malaria. Am J Trop Med Hyg 77 (Suppl 6): 181–192. [PubMed] [Google Scholar]
- 35.Kyaw MP, et al. 2013. Reduced susceptibility of Plasmodium falciparum to artesunate in southern Myanmar. PLoS One 8: e57689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olliaro P, Mussano P, 2003. Amodiaquine for treating malaria. Cochrane Database Syst Rev 2: CD000016. [DOI] [PubMed] [Google Scholar]
- 37.Tarning J, Ashley EA, Lindegardh N, Stepniewska K, Phaiphun L, Day NPJ, McGready R, Ashton M, Nosten F, White NJ, 2008. Population pharmacokinetics of piperaquine after two different treatment regimens with dihydroartemisinin-piperaquine in patients with Plasmodium falciparum malaria in Thailand. Antimicrob Agents Chemother 52: 1052–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tarning J, Thana P, Phyo AP, Lwin KM, Hanpithakpong W, Ashley EA, Day NPJ, Nosten F, White NJ, 2014. Population pharmacokinetics and antimalarial pharmacodynamics of piperaquine in patients with Plasmodium vivax malaria in Thailand. CPT Pharmacometrics Syst Pharmacol 3: e132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoglund RM, et al. 2017. Population pharmacokinetic properties of piperaquine in falciparum malaria: an individual participant data meta-analysis. PLoS Med 14: e1002212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Michael OS, Gbotosho GO, Folarin OA, Okuboyejo T, Sowunmi A, Oduola AM, Happi CT, 2010. Early variations in Plasmodium falciparum dynamics in Nigerian children after treatment with two artemisinin-based combinations: implications on delayed parasite clearance. Malar J 9: 335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sowunmi A, Okuboyejo TM, Gbotosho GO, Happi CT, 2012. Early changes in Plasmodium falciparum asexual and sexual populations in children with acute infections following treatment with artemisinin-based combination drugs. Mal Chemother Ctrl Elim 1: 235498. [Google Scholar]
- 42.White NJ, 2016. Can new treatment development combat resistance in malaria? Expert Opin Pharmacother 17: 1303–1307. [DOI] [PubMed] [Google Scholar]