Abstract.
Antibiotics improve both weight and height gain in randomized trials of preschool children with preexisting morbidity. Here, we assess the effect of a short course of three different antibiotics (amoxicillin, azithromycin, and cotrimoxazole) on short-term linear and ponderal growth in a population-based sample of preschool children in rural Burkina Faso. We randomized households with at least two children in the Nouna district, Burkina Faso, to a 5-day course of amoxicillin, azithromycin, cotrimoxazole, or placebo. Within each antibiotic-randomized household, one child was randomly assigned to receive the antibiotic and the other to receive the placebo. Weight and height measurements were taken at baseline and 30 days following the last study medication dose. Weight-for-height Z (WHZ), height-for-age Z (HAZ), and weight-for-age Z (WAZ) scoreswere calculated based on the 2006 World Health Organization standards. Of the 124 households and 248 children enrolled, 229 had anthropometry measurements at 1 month and were analyzed. Children randomized to amoxicillin gained significantly more weight compared with both the placebo household (mean difference 317 g, 95% confidence interval [CI]: 115–519 g) and placebo sibling (mean difference 315 g, 95% CI: 147–482 g) controls. Growth velocity in g/kg/day, and WHZ and WAZ scores were higher in amoxicillin-treated children compared with placebo households and siblings. There were no differences in weight gain in children randomized to azithromycin or cotrimoxazole compared with placebo households or placebo siblings. There were no differences in height gain or HAZ across any of the study arms. Amoxicillin may have short-term growth-promoting effects in healthy children.
INTRODUCTION
Undernutrition contributes to nearly 50% of child mortality globally.1 Children with acute malnutrition have higher rates of all-cause and infectious mortality compared with non-malnourished children.2,3 Undernourished children may be both more likely to acquire infection4,5 and have poorer response to treatment.6 In regions with a high burden of child mortality, interventions that reduce the prevalence of undernutrition will likely be important to sustainably improve child survival.
In children with preexisting morbidity, including severe acute malnutrition (SAM)7,8 and diarrhea, antibiotics are generally growth-promoting.9 Although the exact mechanism of this effect is unclear, there are at least two plausible hypotheses for this effect. First, antibiotics may treat clinical or subclinical infection. During an active infection, children may not be gaining weight, and treatment of the infection may lead to more rapid weight gain as the child recovers.10 Second, antibiotics may alter the composition of the intestinal microbiome.11,12 Antibiotics lead to decreases in intestinal bacterial diversity.13 Alteration to the microbiome, which could be mediated via treatment of enteropathogens, may affect nutrient absorption or energy metabolism.11,14 In regions with high burdens of child mortality and undernutrition, there may be growth-related benefits to antibiotic use that extend beyond treatment of infection.
Here, we present 1-month nutritional outcomes in a randomized controlled trial of three commonly used antibiotics (amoxicillin, cotrimoxazole, and azithromycin) compared with placebo in a population-based sample of children in a region of rural Burkina Faso with high rates of undernutrition and child mortality. We hypothesized that a short course of antibiotics would lead to increased weight but not height gain compared with placebo.
METHODS
Ethical approval.
This study was reviewed and approved by the Comité Institutionnel d’Ethique at the Center de Recherche en Santé de Nouna in Nouna, Burkina Faso, and the Institutional Review Board at the University of California, San Francisco. Written informed consent was obtained from the caregiver of each participant.
Study setting.
This study was conducted in the Nouna Health and Demographic Surveillance Site (HDSS) in rural northwestern Burkina Faso from July to August 2017.15 The Nouna HDSS is situated in the sub-Sahel region, and inhabitants are primarily subsistence farmers and cattle keepers. The rainy season lasts from July through October, coinciding with the malaria and malnutrition season.
Participants and procedures.
Households with two or three children between the ages of 6 and 59 months at the most recent HDSS census were eligible for participation. We enrolled households with two children preferentially, and enrolled three-child households after all two-child households had been enrolled until we met the required sample size. If the household had two children, both children were enrolled and had anthropometric measurements performed. Children were evaluated at baseline before randomization and 30 days after the final antibiotic dose.
Randomization.
Households were randomized in a 1:1:1:1 fashion to a 5-day course of amoxicillin, azithromycin, cotrimoxazole, or placebo. Within each antibiotic household, one child was randomly assigned to receive placebo. In placebo households, both children received placebo. The randomization sequence was generate by TCP in R version 3.3.1 (The R Foundation for Statistical Computing) using a masked seed value.16
Interventions.
All study medications were prepared as an oral suspension. Weight-based dosing was determined from the baseline weight assessment as part of anthropometry measurements. Amoxicillin was dosed at 25 mg/kg/day twice daily for 5 days, azithromycin 10 mg/kg once on day 1 and then 5 mg/kg once daily on days 2–5, and cotrimoxazole 240 mg once daily for 5 days. Because children were not being treated for an established infection, there was no standard dose for the study medication, and we elected to use the lower end of approved dosing regimens to minimize the risk of adverse events. Amoxicillin dosing was based on the lower end of the approved dosing range for children younger than 12 years. Azithromycin dosing was based on the lower end of the range of standard pediatric dosing for mild to moderate infection. Cotrimoxazole dosing was based on prophylactic dosing for children living with human immunodeficiency virus (HIV).17–20 Placebo was prepared to be similar in appearance to the antibiotics and was a mixture of powdered milk, sugar, and bottled water prepared fresh each day. All antibiotics were prepared in orange opaque syringes with the correct dosing based on the child’s weight. Syringes were labeled with the child’s identification number. Treatment teams were not told treatment assignment, although treatment was not masked given that there were slight differences in appearance and taste, and the twice-daily dosing of amoxicillin. All doses were directly observed. Treatment was administered via a central point in each study community. A community mobilizer visited the homes of children participating in the study and instructed caregivers to bring their children to the central point. Treatment teams had a list of all study participants and recorded which children had received their allocated treatment. Before administration of each dose, caregivers of all children were asked if their child was currently receiving another antibiotic, and those who were receiving antibiotics outside the study were not treated with study medication.
Outcome assessment.
Anthropometric measurements were taken at baseline and 30 days after the last antibiotic dose. If the household had three children, two children were selected as the sentinel children (one randomly selected antibiotic-treated child and the placebo-treated child). Height, weight, and mid-upper arm circumference measurements were taken at each time point for all children. Recumbent length was measured in children who were not able to stand, and standing height in children who could stand (Shorrboard; Shorr Products, LLC, Olney, MD). Children were weighed standing if able or in the arms of a caregiver (Seca 874 flat floor scale; Seca GMBH & Co., Hamburg, Germany). Heavy garments and jewelry were removed before weight measurements. Height and weight measurements were taken in triplicate and the median of each measure was used for analysis. Weight-for-height Z (WHZ), weight-for-age Z (WAZ), and height-for-age Z (HAZ) scoreswere calculated based on the 2006 World Health Organization (WHO) standards.
Adverse events.
On each day of treatment, caregivers were asked if the child had had any symptoms since the previous day, including gastrointestinal symptoms (nausea, abdominal pain, vomiting, or diarrhea), respiratory symptoms (including wheezing, shortness of breath, or cough), a rash, or any other symptoms. Four days after the last treatment dose, caregivers were asked if their child had had three or more loose or watery stools over a 24-hour period, consistent with the WHO definition of diarrhea,21 and if the child currently had a cough or difficulty breathing. Each child’s respiratory rate was taken. A child was considered to have tachypnea if they had a respiratory rate of > 50 breaths/minute for children aged 6–11 months and > 40 breaths/minute for children aged 12–59 months. Caregivers reporting that their child had loose or watery stools were asked if the child’s diarrhea had lasted for longer than 14 days and if there was blood or mucus in the stool. Children with diarrhea lasting > 14 days, blood or mucus in the stool, or tachypnea were referred to the local health facility for treatment.
Sample size determination.
The sample size calculation was based on the primary outcome for the trial, bacterial diversity in the intestinal microbiome. A sample size of 30 children per arm was estimated to provide at least 80% power to detect a 1.5-unit difference in Simpson’s α diversity based on a previous study in Niger.13 For the secondary outcome of change in weight, a sample size of 30 children in the antibiotic arm and 60 in the placebo arm was estimated to provide at least 80% power to detect a mean 0.63 standard deviation difference in WHZ score.
Statistical methods.
Descriptive statistics were calculated with medians and interquartile ranges (IQR) for continuous variables and proportions for categorical variables. We compared anthropometric measurements in children receiving each antibiotic to two control groups: 1) children in households randomized to placebo and 2) the child randomized to placebo within households randomized to antibiotics. For each measurement comparing the antibiotic-treated child with the placebo household, we used a linear regression model with a term for treatment arm and baseline value of the outcome, with standard errors accounting for clustering within the household. For analyses comparing with the placebo-treated within-household sibling, the antibiotic-treated child was compared with their placebo-treated household-matched control using a linear mixed effects regression model. Children with implausible weight or height changes between the baseline and 1-month measurements (e.g., gained or lost more than 5 cm or 2 kg) were assumed to be data entry errors and were excluded from analyses. A sensitivity analysis was run including all children with follow-up weight and height measurements. All analyses were conducted in Stata 14.1 (StataCorp, College Station, TX).
RESULTS
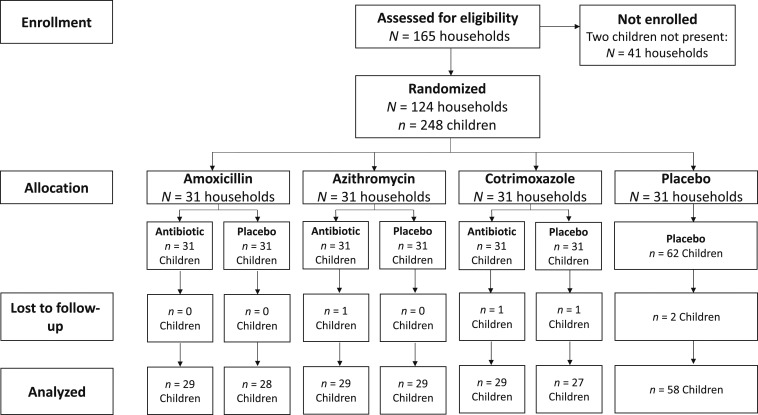
In July 2017, 248 children in 124 households were enrolled in the trial. Four weeks after the last antibiotic dose, 243 children (98.0%) from 124 households had anthropometric measurements and 229 (94.2%) had measurements that did not indicate a data entry problem (Figure 1). Median age was 37 months (IQR 25–49) and 50.4% were female (Table 1). At baseline, 10.1% of children were wasted, 21.0% stunted, and 14.1% underweight. No children had SAM in this sample. Overall antibiotic coverage was high, with > 90% of children receiving their allocated study medication dose at most time points (Table 2). Of 75 missed doses (6.0% of total expected doses), the most common reasons for not receiving the study medication dose included that the child was absent from the treatment round (56.0% of missed doses), the child was receiving another antibiotic (29.3% of missed doses), the child refused the dose (10.7% of missed doses), and the child was sick (2.7% of missed doses).
Figure 1.
CONSORT flow diagram of study participation. Of 243 children who were not lost to follow-up, 229 had valid anthropometric measurements and were included in the analysis.
Table 1.
Baseline descriptive characteristics of the study sample by randomization arm
| Amoxicillin household | Azithromycin household | Cotrimoxazole household | Placebo household | ||||
|---|---|---|---|---|---|---|---|
| Antibiotic (N = 31) | Placebo (N = 31) | Antibiotic (N = 31) | Placebo (N = 31) | Antibiotic (N = 31) | Placebo (N = 31) | N = 62 | |
| Age, months, median (IQR) | 35 (23–52) | 36 (26–53) | 29 (21–51) | 42 (30–47) | 37 (23–48) | 40 (23–50) | 38 (30–49) |
| Female gender, N (%) | 14 (45.2) | 18 (58.1) | 18 (58.1) | 12 (38.7) | 20 (64.5) | 19 (61.3) | 24 (38.7) |
| Weight, kg, median (IQR) | 12.1 (10.8–14.9) | 11.8 (10.9–14.6) | 12.2 (9.2–13.6) | 13.2 (11.3–15) | 12.9 (10.4–14.6) | 12.5 (9.7–14.5) | 13.4 (11.4–14.9) |
| Height, cm, median (IQR) | 88.4 (83–100.9) | 90.1 (82.7–98.7) | 86.6 (80.2–94.9) | 93.8 (85.3–98.9) | 90.2 (79.6–98.8) | 90 (82.6–96) | 92.5 (86.5–99.5) |
| WHZ, median (IQR) | −0.40 (−1.02 to 0.07) | −0.63 (−1.56 to 0.81) | −0.68 (−1.54 to 0) | −0.15 (−1.17 to 0.08) | −0.22 (−0.83 to 0.48) | −0.48 (−1.78 to 0.39) | −0.29 (−0.83 to 0.35) |
| HAZ, median (IQR) | −1.01 (−2.05 to −0.34) | −1.15 (−1.89 to −0.48) | −1.29 (−2.15 to −0.52) | −1.20 (−1.99 to −0.43) | −1.16 (−1.70 to −0.57) | −1.50 (−2.25 to −0.77) | −0.95 (−1.50 to −0.29) |
| WAZ, median (IQR) | −0.78 (−1.67 to −0.41) | −0.95 (−1.35 to −0.63) | −1.19 (−1.91 to −0.51) | −0.94 (−1.44 to −0.29) | −0.88 (−1.17 to −0.19) | −1.45 (−2.22 to −0.50) | −0.66 (−1.27 to −0.20) |
| MUAC, mm, median (IQR) | 150 (146–154) | 147 (143–160) | 147 (140–158) | 150 (145–159) | 150 (147–162) | 145 (140–163) | 155 (146–163) |
| Wasted, N (%)* | 2 (6.7) | 3 (9.7) | 2 (6.5) | 3 (9.7) | 2 (6.5) | 7 (22.6) | 6 (9.7) |
| Stunted, N (%)† | 8 (25.8) | 7 (22.6) | 8 (25.8) | 7 (22.6) | 4 (12.9) | 11 (35.5) | 7 (11.3) |
| Underweight, N (%)‡ | 5 (16.1) | 3 (9.7) | 4 (12.9) | 4 (12.9) | 3 (9.7) | 10 (32.3) | 6 (9.7) |
| Visited healthcare facility in past 30 days, N (%) | 6 (19.4) | 2 (6.5) | 4 (12.9) | 2 (6.5) | 6 (19.5) | 8 (25.8) | 14 (22.6) |
| Diarrhea§ | 1 (3.2%) | 0 | 0 | 0 | 0 | 0 | 1 (1.6%) |
| Cough§ | 0 | 0 | 0 | 0 | 1 (3.2%) | 0 | 0 |
| Fever§ | 0 | 0 | 0 | 1 (3.2%) | 0 | 0 | 2 (3.2%) |
cm = centimeters; HAZ = height-for-age Z score; IQR = interquartile range; kg = kilograms; mm = millimeters; MUAC = mid-upper arm circumference; WAZ = weight-for-age Z score; WHZ = weight-for-height Z score.
Defined as WHZ < −2 SD.
Defined as HAZ < −2 SD.
Defined as WAZ < −2 SD.
Assessed on first treatment day, by caregiver report.
Table 2.
Treatment coverage by study day and randomization arm
| Amoxicillin household | Azithromycin household | Cotrimoxazole household | Placebo household | |||||
|---|---|---|---|---|---|---|---|---|
| Antibiotic AM (N = 31) | Antibiotic PM (N = 31) | Placebo (N = 31) | Antibiotic (N = 31) | Placebo (N = 31) | Antibiotic (N = 31) | Placebo (N = 31) | N = 62 | |
| Day 1 | 30 (96.8%) | 29 (93.5%) | 29 (93.5%) | 29 (93.5%) | 30 (96.8%) | 30 (96.8%) | 27 (87.1%) | 58 (93.6%) |
| Day 2 | 31 (100%) | 30 (98.4%) | 30 (96.8%) | 29 (93.5%) | 31 (100%) | 30 (96.8%) | 28 (90.3%) | 55 (88.7%) |
| Day 3 | 31 (100%) | 31 (100%) | 31 (100%) | 30 (96.8%) | 31 (100%) | 31 (100%) | 27 (87.1%) | 56 (90.3%) |
| Day 4 | 31 (100%) | 31 (100%) | 28 (90.3%) | 30 (96.8%) | 29 (93.5%) | 31 (100%) | 28 (90.3%) | 56 (90.3%) |
| Day 5 | 31 (100%) | 31 (96.8%) | 27 (87.1%) | 28 (90.3%) | 30 (96.8%) | 30 (96.8%) | 28 (90.3%) | 56 (90.3%) |
At 30 days after the last antibiotic dose, children who received an antibiotic in households randomized to amoxicillin gained significantly more weight over the past month compared both with the placebo households (mean difference 317 g, 95% CI: 115–519 g) and the placebo-treated sibling control (mean difference 315 g, 95% CI: 147–482 g). Other measures of weight gain, including growth velocity, WHZ score, and WAZ score were consistently higher in the amoxicillin-treated children compared with placebo-treated children (Table 3). There was no difference in weight outcomes with azithromycin or cotrimoxazole compared with the placebo-treated household or placebo sibling controls. There were no differences in height outcomes in any antibiotics compared with either household or sibling controls. In models comparing with placebo households, the joint test of significance across all treatment groups was significant for all weight-related outcomes, and there were no significant differences in the joint test of significance for any height outcomes (Table 3).
Table 3.
Growth and anthropometric indices in each antibiotic group compared with placebo 4 weeks after last antibiotic dose
| Amoxicillin (mean difference, 95% CI) | Azithromycin (mean difference, 95% CI) | Cotrimoxazole (mean difference, 95% CI) | ||||
|---|---|---|---|---|---|---|
| Vs. Placebo household | Vs. Placebo sibling | Vs. Placebo household | Vs. Placebo sibling | Vs. Placebo household | Vs. Placebo sibling | |
| Change in weight, g* | 317 (115–519) | 315 (147–482) | 46 (−151 to 243) | −52 (−209 to 106) | −31 (−219 to 157) | −4 (−251 to 243) |
| Weight gain, g/kg/day† | 0.79 (0.33–1.22) | 0.74 (0.37–1.10) | 0.12 (−0.36 to 0.59) | −0.15 (−0.53 to 0.23) | −0.05 (−0.46 to 0.37) | −0.05 (−0.65 to 0.56) |
| Change in height, cm‡ | 0.10 (−0.21 to 0.40) | 0.02 (−0.54 to 0.58) | 0.03 (−0.42 to 0.47) | 0.06 (−0.39 to 0.51) | 0.29 (−0.13 to 0.72) | 0.38 (−0.17 to 0.93) |
| WHZ§ | 0.29 (0.10–0.47) | 0.28 (0.10–0.45) | 0.03 (−0.20 to 0.27) | −0.09 (−0.34 to 0.16) | −0.07 (−0.25 to 0.11) | −0.06 (−0.31 to 0.19) |
| HAZ‖ | 0.02 (−0.06 to 0.11) | 0.02 (−0.15 to 0.18) | −0.03 (−0.17 to 0.11) | 0.003 (−0.13 to 0.14) | 0.08 (−0.03 to 0.20) | 0.14 (−0.06 to 0.33) |
| WAZ¶ | 0.23 (0.11–0.35) | 0.21 (0.10–0.31) | 0.04 (−0.11 to 0.18) | −0.03 (−0.15 to 0.09) | −0.01 (−0.12 to 0.11) | 0.03 (−0.14 to 0.20) |
| MUAC# | 0.12 (−0.18 to 0.43) | 0.10 (−0.16 to 0.35) | −0.19 (−0.47 to 0.08) | −0.27 (−0.55 to 0.01) | 0.19 (−0.13 to 0.51) | 0.12 (−0.22 to 0.45) |
HAZ = height-for-age Z score; MUAC = mid-upper arm circumference; WAZ = weight-for-age Z score; WHZ = weight-for-height Z score. Bold indicates statistically significant difference (P <0.05).
P value for joint test of significance in models comparing to placebo-treated household: P = 0.002.
P value for joint test of significance in models comparing to placebo-treated household: P = 0.001.
P value for joint test of significance in models comparing to placebo-treated household: P = 0.57.
P value for joint test of significance in models comparing to placebo-treated household: P = 0.002.
P value for joint test of significance in models comparing to placebo-treated household: P = 0.46.
P value for joint test of significance in models comparing to placebo-treated household: P = 0.0005.
P value for joint test of significance in models comparing to placebo-treated household: P = 0.03.
Adverse events were uncommon during the course of the study (Table 4). The most common adverse event was fever, with caregivers of 5.2% of participants reporting fever on at least one treatment day. Gastrointestinal adverse events were uncommon and did not occur more frequently in the antibiotic-treated children. No adverse events were determined to be related to study medication.
Table 4.
Adverse events and signs of respiratory infection by study arm
| Amoxicillin household | Azithromycin household | Cotrimoxazole household | Placebo household | ||||
|---|---|---|---|---|---|---|---|
| Antibiotic (N = 31) | Placebo (N = 31) | Antibiotic (N = 31) | Placebo (N = 30) | Antibiotic (N = 30) | Placebo (N = 31) | N = 60 | |
| Diarrhea, days 1–5 | 0 | 0 | 0 | 1 (3.3%) | 0 | 0 | 2 (3.3%) |
| Diarrhea, day 9 | 0 | 0 | 0 | 0 | 1 (3.3%) | 1 (3.2%) | 1 (1.6%) |
| Vomiting, days 1–5 | 1 (3.0%) | 0 | 0 | 1 (3.3%) | 0 | 1 (3.2%) | 1 (1.6%) |
| Nausea, days 1–5 | 1 (3.0%) | 0 | 0 | 0 | 0 | 0 | 0 |
| Fever, days 1–5 | 3 (9.7%) | 0 | 2 (6.7%) | 4 (13.3%) | 1 (3.2%) | 0 | 3 (4.9%) |
| Cough | |||||||
| Days 1–5 | 0 | 0 | 1 (3.3%) | 1 (3.2%) | 2 (6.5%) | 2 (6.7%) | 1 (1.6%) |
| Day 9 | 1 (3.0%) | 0 | 0 | 0 | 2 (6.5%) | 2 (6.7%) | 2 (3.3%) |
| Tachypnea,* day 9 | 3 (9.1%) | 2 (6.5%) | 0 | 0 | 0 | 0 | 0 |
| Any adverse event, days 1–5 | 5 (16.1%) | 0 | 3 (9.7%) | 5 (16.7%) | 3 (10.0%) | 3 (9.7%) | 7 (11.7%) |
Defined as a respiratory rate > 50 breaths/minute for children aged 6–11 months and > 40 breaths/minute for children aged 12–59 months.
DISCUSSION
In this study of preschool children in rural Burkina Faso, children randomized to a short course of amoxicillin had significantly increased weight gain over a 1-month period compared with placebo-treated children, but there was no difference with azithromycin or cotrimoxazole. Amoxicillin has been previously shown to lead to increased weight gain among children undergoing treatment for SAM in randomized controlled trials.7,8 In high-income settings in observational studies, early life exposure to antibiotics has been associated with weight gain and obesity,22 with greater effects of macrolide antibiotics compared with non-macrolides23 or penicillins.24 An observational study in multiple low-resource settings found that children receiving macrolides but not penicillins had increased weight gain until an age of 2 years compared with children not receiving antibiotics.25 The results of the present study are broadly consistent with the hypothesis that antibiotics may lead to weight gain, but suggest that amoxicillin may have greater effects on growth than other antibiotic classes.
Our results may differ from previous studies for several reasons. First, the randomized, placebo-controlled nature of the present study prevents confounding by indication or other sources of confounding that may have affected estimates from observational studies. Second, we followed children for only a single month following the antibiotic course. There may be differences in long-term weight change in children receiving different classes of antibiotics. Third, there may be differences in study populations, for example, in studies of children with SAM or in different geographic settings with different antibiotic prescription practices. This study was conducted in rural Burkina Faso during the rainy season, which coincides with the malaria and malnutrition seasons in the Sahel and sub-Sahel regions during which child mortality peaks.26–28 The results of this study may not be generalizable outside of similar regions, because of the high rates of undernutrition and distribution of infectious organisms.29,30 However, as this region has the highest child mortality rates in sub-Saharan Africa,31 children living in the Sahel and sub-Sahel region may benefit the most from the unintended positive effects of antibiotics such as increases in weight gain.
The mechanism of action for any effect of antibiotics on weight gain remains unclear. Antibiotics have been shown to reduce bacterial diversity in the intestinal microbiome in children.13 A study in Finland found an association between penicillin and macrolide antibiotic use during early life and microbiota composition, and found that an abundance of four genera were related to obesity.32 Another study found that the association between intestinal microbiota and body mass index (BMI) outcomes was moderated by antibiotic use, with stronger effects of the intestinal microbiome on BMI outcomes in children receiving antibiotics.33 In the short term, antibiotics may lead to modifications in gut microbiota that affect nutrient absorption and weight gain.34 Antibiotics may also affect weight gain by treating clinical or subclinical infection, as infection may lead to worse nutritional outcomes.35,36 For example, repeated enteric infection may alter intestinal barrier function, which can in turn affect nutritional outcomes.37,38 Repeated infection has also been shown to be associated with growth hormone resistance, which could affect anthropometric outcomes.39
In this study, there was no effect of any antibiotics on short-term changes in height. Height and stunting are generally thought to be related to longer term nutritional status,40,41 whereas weight and wasting are thought to reflect acute nutritional status. We did not expect to see differences in height across antibiotic arms given the short follow-up period of the study and the use of only one short course of antibiotics. A previous meta-analysis of randomized controlled trials of antibiotics demonstrated a greater effect of antibiotics on weight compared with height outcomes.9 Of the studies included in the meta-analysis, the strongest effect of antibiotics on height were those among children living with HIV receiving long-term daily cotrimoxazole prophylaxis.42 Longer term or repeated antibiotic treatment may be required for height benefits.
Empiric treatment of children with antibiotics without established infection is used in several public health programs, including mass azithromycin distribution for trachoma control,43–46 amoxicillin for uncomplicated SAM,7,8 and prophylactic cotrimoxazole for children living with HIV.19,20 A large community-randomized trial recently demonstrated a significant reduction in all-cause child mortality with mass distribution of azithromycin,47 which could result in expansion of indication for mass empiric antibiotic treatment.48,49 Unintended benefits of mass azithromycin distribution for trachoma control have previously been shown for common childhood infectious illness, such as diarrhea, pneumonia, and malaria.50–53 The present study suggests that weight gain benefits of empiric antibiotic use may be observed with some antibiotic classes but not others, a hypothesis that should be tested in future studies of programs using empiric antibiotic treatment with various antibiotic classes. If true, collateral weight gain benefits of empiric antibiotic treatment could represent an opportunity for intervention in regions with high burdens of malnutrition, but such benefits may depend on the class of antibiotics used.
The results of this study must be considered in the context of several limitations. First, the sample sizes were small. This analysis was a secondary analysis of a randomized controlled trial designed to assess the effects of antibiotic use on the composition of the intestinal microbiome, and was not specifically powered for anthropometry outcomes. The duration of follow-up in this study was short and the antibiotic effects on the outcomes in this study are likely to differ in the longer term. None of the children included in this study had SAM. Children with SAM may respond differently to antibiotics than those who are well nourished. Previous randomized studies have shown a significant effect of amoxicillin on weight gain in children with SAM.7,8 Cotrimoxazole did not lead to weight gain in children who had recovered from complicated SAM.54 Although results of the present study were broadly consistent, the results of this study cannot be generalized to children with SAM. Children were not tested for HIV as part of this study, and thus, we do not know if some children were receiving cotrimoxazole prophylaxis. Caregivers were asked if their child was receiving a non-study antibiotic, and concomitant treatment with non-study antibiotics was rare. However, contamination could have arisen with non-study use of antibiotics. Although the study was placebo-controlled, because of differences in appearance and taste of each antibiotic, the study may not have been fully masked. Children randomized to amoxicillin additionally received two doses daily; no other antibiotic or placebo group received two doses of medication. Twice-daily interaction with study staff could have differentially affected children in the amoxicillin arm. However, outcome assessors at the 1-month measurement were masked, and results were remarkably consistent across two separate control groups.
In this randomized controlled trial of preschool children during the rainy season in rural Burkina Faso, we demonstrate an increase in weight gain in children randomized to amoxicillin compared with placebo-randomized household and randomized sibling controls. Although previous observational studies have noted greater weight gain outcomes with macrolides compared with penicillins, here, we document a significant effect of amoxicillin on weight gain among healthy children in a randomized controlled trial in a setting with a high burden of infectious disease and malnutrition. This may represent a positive secondary effect of routine antibiotic use in settings with high prevalence of undernutrition and child mortality, such as rural Burkina Faso. There may be a role for antibiotics in improving child health in regions with a large burden of child morbidity, although use of antibiotics must be balanced against the potential for selection for antibiotic resistance.
Acknowledgments
Clinical Trial Registration: clinicaltrials.gov NCT03187834.
REFERENCES
- 1.Black RE, et al. Maternal and Child Nutrition Study Group , 2013. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet 382: 427–451. [DOI] [PubMed] [Google Scholar]
- 2.Caulfield LE, de Onis M, Blössner M, Black RE, 2004. Undernutrition as an underlying cause of child deaths associated with diarrhea, pneumonia, malaria, and measles. Am J Clin Nutr 80: 193–198. [DOI] [PubMed] [Google Scholar]
- 3.Ginsburg AS, Izadnegahdar R, Berkley JA, Walson JL, Rollins N, Klugman KP, 2015. Undernutrition and pneumonia mortality. Lancet Glob Health 3: e735–e736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Page A-L, et al. 2013. Infections in children admitted with complicated severe acute malnutrition in Niger. PLoS One 8: e68699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Deen JL, Walraven G, von Seidlein L, 2002. Increased risk for malaria in chronically malnourished children under 5 years of age in rural Gambia. J Trop Pediatr 48: 78–83. [DOI] [PubMed] [Google Scholar]
- 6.Denoeud-Ndam L, et al. 2016. Efficacy of artemether-lumefantrine in relation to drug exposure in children with and without severe acute malnutrition: an open comparative intervention study in Mali and Niger. BMC Med 14: 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Isanaka S, et al. 2016. Routine amoxicillin for uncomplicated severe acute malnutrition in children. N Engl J Med 374: 444–453. [DOI] [PubMed] [Google Scholar]
- 8.Trehan I, Goldbach HS, LaGrone LN, Meuli GJ, Wang RJ, Maleta KM, Manary MJ, 2013. Antibiotics as part of the management of severe acute malnutrition. N Engl J Med 368: 425–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gough EK, et al. 2014. The impact of antibiotics on growth in children in low and middle income countries: systematic review and meta-analysis of randomised controlled trials. BMJ 348: g2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oldenburg CE, Guerin PJ, Berthé F, Grais RF, Isanaka S, 2018. Malaria and nutritional status among children with severe acute malnutrition in Niger: a prospective cohort study. Clin Infect Dis Epub ahead of print, 10.1093/cid/ciy207/4924398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garrett WS, 2013. Kwashiorkor and the gut microbiota. N Engl J Med 368: 1746–1747. [DOI] [PubMed] [Google Scholar]
- 12.Smith MI, et al. 2013. Gut microbiomes of Malawian twin pairs discordant for kwashiorkor. Science 339: 548–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doan T, et al. 2017. Gut microbial diversity in antibiotic-naive children after systemic antibiotic exposure: a randomized controlled trial. Clin Infect Dis 64: 1147–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Clercq NC, Groen AK, Romijn JA, Nieuwdorp M, 2016. Gut microbiota in obesity and undernutrition. Adv Nutr 7: 1080–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sie A, Louis VR, Gbangou A, Muller O, Niamba L, Stieglbauer G, Yé M, Kouyate B, Sauerborn R, Thorson A, 2010. The health and demographic surveillance system (HDSS) in Nouna, Burkina Faso, 1993–2007. Glob Health Action 3: 5284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Porco TC, Stoller NE, Keenan JD, Bailey RL, Lietman TM, 2015. Public key cryptography for quality assurance in randomization for clinical trials. Contemp Clin Trials 42: 167–168. [DOI] [PubMed] [Google Scholar]
- 17.Lockman S, et al. 2017. Effect of co-trimoxazole on mortality in HIV-exposed but uninfected children in Botswana (the Mpepu Study): a double-blind, randomised, placebo-controlled trial. Lancet Glob Health 5: e491–e500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.WHO , 2006. Guidelines on Co-Trimoxazole Prophylaxis for HIV-Related Infections among Children, Adolescents, and Adults Available at: http://www.who.int/hiv/pub/guidelines/ctxguidelines.pdf. Accessed June 12, 2018.
- 19.Chintu C, et al. CHAP Trial Team , 2004. Co-trimoxazole as prophylaxis against opportunistic infections in HIV-infected Zambian children (CHAP): a double-blind randomised placebo-controlled trial. Lancet 364: 1865–1871. [DOI] [PubMed] [Google Scholar]
- 20.Bwakura-Dangarembizi M, et al. 2014. A randomized trial of prolonged co-trimoxazole in HIV-infected children in Africa. N Engl J Med 370: 41–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aiemjoy K, et al. 2018. Defining diarrhea: a population-based validation study of caregiver-reported stool consistency in the Amhara Region of Ethiopia. Am J Trop Med Hyg 98: 1013–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scott FI, Horton DB, Mamtani R, Haynes K, Goldberg DS, Lee DY, Lewis JD, 2016. Administration of antibiotics to children before age 2 years increases risk for childhood obesity. Gastroenterology 151: 120–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gerber JS, Bryan M, Ross RK, Daymont C, Parks EP, Localio AR, Grundmeier RW, Stallings VA, Zaoutis TE, 2016. Antibiotic exposure during the first 6 months of life and weight gain during childhood. JAMA 315: 1258–1265. [DOI] [PubMed] [Google Scholar]
- 24.Saari A, Virta LJ, Sankilampi U, Dunkel L, Saxen H, 2015. Antibiotic exposure in infancy and risk of being overweight in the first 24 months of life. Pediatrics 135: 617–626. [DOI] [PubMed] [Google Scholar]
- 25.Rogawski ET, et al. MAL-ED Network Investigators , 2017. Early antibiotic exposure in low-resource settings is associated with increased weight in the first two years of life. J Pediatr Gastroenterol Nutr 65: 350–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wehner S, Stieglbauer G, Traoré C, Sie A, Becher H, Muller O, 2017. Malaria incidence during early childhood in rural Burkina Faso: analysis of a birth cohort protected with insecticide-treated mosquito nets. Acta Trop 175: 78–83. [DOI] [PubMed] [Google Scholar]
- 27.Guillebaud J, et al. 2013. Epidemiology of malaria in an area of seasonal transmission in Niger and implications for the design of a seasonal malaria chemoprevention strategy. Malar J 12: 379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kampe EOI, Muller O, Sie A, Becher H, 2015. Seasonal and temporal trends in all-cause and malaria mortality in rural Burkina Faso, 1998–2007. Malar J 14: 300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Belesova K, Gasparrini A, Sie A, Sauerborn R, Wilkinson P, 2018. Annual crop yield variation, child survival, and nutrition among subsistence farmers in Burkina Faso. Am J Epidemiol 187: 242–250. [DOI] [PubMed] [Google Scholar]
- 30.Belesova K, Gasparrini A, Sie A, Sauerborn R, Wilkinson P, 2017. Household cereal crop harvest and children’s nutritional status in rural Burkina Faso. Environ Health 16: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Golding N, et al. 2017. Mapping under-5 and neonatal mortality in Africa, 2000–15: a baseline analysis for the sustainable development goals. Lancet 390: 2171–2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Korpela K, Salonen A, Virta LJ, Kekkonen RA, Forslund K, Bork P, de Vos WM, 2016. Intestinal microbiome is related to lifetime antibiotic use in Finnish pre-school children. Nat Commun 7: 10410–10413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Korpela K, Zijlmans MAC, Kuitunen M, Kukkonen K, Savilahti E, Salonen A, de Weerth C, de Vos WM, 2017. Childhood BMI in relation to microbiota in infancy and lifetime antibiotic use. Microbiome 5: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Angelakis E, Merhej V, Raoult D, 2013. Related actions of probiotics and antibiotics on gut microbiota and weight modification. Lancet Infect Dis 13: 889–899. [DOI] [PubMed] [Google Scholar]
- 35.Page A-L, et al. 2013. Infections in children admitted with complicated severe acute malnutrition in Niger. PLoS One 8: e68699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Padonou G, Le Port A, Cottrell G, Guerra J, Choudat I, Rachas A, Bouscaillou J, Massougbodji A, Garcia A, Martin-Prevel Y, 2014. Factors associated with growth patterns from birth to 18 months in a Beninese cohort of children. Acta Trop 135: 1–9. [DOI] [PubMed] [Google Scholar]
- 37.Leo GO, et al. MAL-ED Network Investigators , 2017. Infant nutritional status, feeding practices, enteropathogen exposure, socioeconomic status, and illness are associated with gut barrier function as assessed by the lactulose mannitol test in the MAL-ED Birth Cohort. Am J Trop Med Hyg 97: 281–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Owino V, Ahmed T, Freemark M, Kelly P, Loy A, Manary M, Loechl C, 2016. Environmental enteric dysfunction and growth failure/stunting in global child health. Pediatrics 138: pii: e20160641. [DOI] [PubMed] [Google Scholar]
- 39.DeBoer M, Scharf RJ, Leite AM, Férrer A, Havt A, Pinkerton R, Lima AA, Guerrant RL, 2017. Systemic inflammation, growth factors, and linear growth in the setting of infection and malnutrition. Nutrition 33: 248–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Onis M, Branca F, 2016. Childhood stunting: a global perspective. Matern Child Nutr 12: 12–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Briend A, Khara T, Dolan C, 2015. Wasting and stunting—similarities and differences: policy and programmatic implications. Food Nutr Bull 36: S15–S23. [DOI] [PubMed] [Google Scholar]
- 42.Prendergast A, Walker AS, Mulenga V, Chintu C, Gibb DM, 2011. Improved growth and anemia in HIV-infected African children taking cotrimoxazole prophylaxis. Clin Infect Dis 52: 953–956. [DOI] [PubMed] [Google Scholar]
- 43.Schachter J, et al. 1999. Azithromycin in control of trachoma. Lancet 354: 630–635. [DOI] [PubMed] [Google Scholar]
- 44.Solomon AW, et al. 2004. Mass treatment with single-dose azithromycin for trachoma. N Engl J Med 351: 1962–1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chidambaram JD, et al. 2006. Effect of a single mass antibiotic distribution on the prevalence of infectious trachoma. JAMA 295: 1142–1146. [DOI] [PubMed] [Google Scholar]
- 46.Emerson PM, Hooper PJ, Sarah V, 2017. Progress and projections in the program to eliminate trachoma. PLoS Negl Trop Dis 11: e0005402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Keenan JD, et al. MORDOR Study Group , 2018. Mass azithromycin distribution for reducing childhood mortality in sub-Saharan Africa. N Engl J Med 378: 1583–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matheson AI, Manhart LE, Pavlinac PB, Means AR, Akullian A, Levine GA, Jacobson J, Shutes E, Walson JL, 2014. Prioritizing countries for interventions to reduce child mortality: tools for maximizing the impact of mass drug administration of azithromycin. PLoS One 9: e96658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pavlinac PB, et al. 2017. Azithromycin to prevent post-discharge morbidity and mortality in Kenyan children: a protocol for a randomised, double-blind, placebo-controlled trial (the Toto Bora trial). BMJ Open 7: e019170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fry AM, Jha HC, Lietman TM, Chaudhary JSP, Bhatta RC, Elliott J, Hyde T, Schuchat A, Gaynor B, Dowell SF, 2002. Adverse and beneficial secondary effects of mass treatment with azithromycin to eliminate blindness due to trachoma in Nepal. Clin Infect Dis 35: 395–402. [DOI] [PubMed] [Google Scholar]
- 51.Gaynor BD, et al. 2014. Impact of mass azithromycin distribution on malaria parasitemia during the low-transmission season in Niger: a cluster-randomized trial. Am J Trop Med Hyg 90: 846–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schachterle SE, Mtove G, Levens JP, Clemens E, Shi L, Raj A, Dumler JS, Munoz B, West S, Sullivan DJ, 2014. Short-term malaria reduction by single-dose azithromycin during mass drug administration for trachoma, Tanzania. Emerg Infect Dis 20: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Coles CL, Seidman JC, Levens J, Mkocha H, Munoz B, West S, 2011. Association of mass treatment with azithromycin in trachoma-endemic communities with short-term reduced risk of diarrhea in young children. Am J Trop Med Hyg 85: 691–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Berkley JA, et al. 2016. Daily co-trimoxazole prophylaxis to prevent mortality in children with complicated severe acute malnutrition: a multicentre, double-blind, randomised placebo-controlled trial. Lancet Glob Health 4: e464–e473. [DOI] [PMC free article] [PubMed] [Google Scholar]