Abstract.
Although falciparum malaria is an important risk among travelers to sub-Saharan Africa, many travelers remain unaware of this risk. In October 2015, we found a cluster of imported Plasmodium falciparum malaria cases among Thai gem mine workers in Nigeria; none had received malaria chemoprophylaxis or information regarding malaria risk. The index case developed fever and visited our hospital on arrival day in Thailand after his 3-week stay in Nigeria. Plasmodium falciparum was found in his blood. He recovered completely 3 days post-admission. After we requested he contact his colleagues in Nigeria regarding malaria risk, we found that three of his five colleagues currently had fever, were diagnosed with malaria, and were being treated in a local hospital. Two were successfully treated in Nigeria. Although their blood films were negative for malaria, we could confirm that they recently had malaria because the polymerase chain reaction (PCR) was still positive for P. falciparum. Tragically, the last febrile case died in Nigeria 6 days post-admission, after developing jaundice and alteration of consciousness. The two colleagues without fever symptoms were also tested by PCR, which was negative for malaria. In conclusion, we found that four of six workers had malaria in this cluster, which was equal to 66.7% attack rate. There is an urgent need to raise awareness of malaria among workers in highly endemic areas. Clinical practice for travelers who are ill on their return should not only focus on individual cases but also consider potential disease clusters.
INTRODUCTION
Malaria is an important life-threatening infectious disease caused by Plasmodium species. It is also the most common cause of fever in travelers returning from sub-Saharan Africa.1 Long-term travelers, including expatriates and guest workers, are particularly at high risk compared with tourists because they frequently stay longer in endemic areas. Moreover, several studies2–4 have shown that their awareness of malaria risk, adherence to mosquito protective measures, and chemoprophylaxis were suboptimal.
We describe a cluster of imported malaria cases among Thai workers in Nigeria. In this cluster, four of six workers had malaria. None had received malaria risk advice nor chemoprophylaxis against malaria before their trip. Measures to increasing awareness of malaria risk among workers in Africa are urgently needed.
CASE REPORT
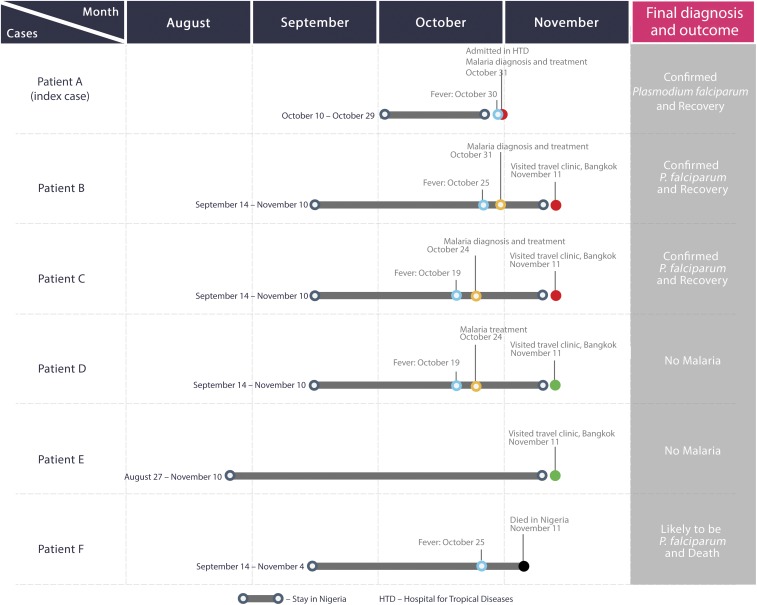
A group of six Thai workers had been working in a gem mine near Gembu city, in southeastern Taraba state, Nigeria, near the border with Cameroon. Some of the workers developed fever and chills without organ-specific symptoms at the same point during their stay (Table 1 and Figure 1). The index case flew back to Thailand and visited our hospital, the Hospital for Tropical Diseases, Bangkok, Thailand.
Table 1.
Characteristics of the cluster of malaria cases among Thai workers in Gembu, Nigeria
| Case | Gender, age (years) | Date of fever onset | Events in Nigeria | Seen in HTD | Diagnosis in Bangkok | Outcome |
|---|---|---|---|---|---|---|
| A | M, 46 | 30 October | None—symptoms began after returning to Bangkok | 31 October | Plasmodium falciparum (blood smear) | Recovered |
| B | M, 32 | 25 October | Fever 3 days, oral antimalarial drugs | 11 November | P. falciparum (PCR positive) | Recovered |
| C | M, 23 | 19 October | Fever 5 days, jaundice, admitted to hospital for 3 days | 11 November | P. falciparum (PCR positive) | Recovered |
| D | F, 49 | 29 October | No fever, blood test positive, got treatment in Nigeria | 11 November | Negative (blood smear, RDT, and PCR) | Recovered |
| E | F, 45 | 24 October | No fever, blood test negative, no treatment | 11 November | Negative (blood smear, RDT, and PCR) | Recovered |
| F | M, 54 | 25 October | Fever, jaundice, admitted to hospital for 6 days | Not seen | Suspected P. falciparum malaria | Died on 4 November in Nigeria |
F = female; HTD = Hospital for Tropical Diseases; M = male; PCR = polymerase chain reaction; RDT = rapid diagnostic test.
Figure 1.
Timeline of the clinical onset of malaria in the group of Thai workers in Nigeria. This figure appears in color at www.ajtmh.org.
Case A (index case).
A 46-year-old previously healthy man presented to our hospital on October 31, 2015 with a 1-day history of high fever, chills, and generalized malaise after returning from Nigeria, where he was working in a gem mine during the period October 10–29, 2015.
At initial presentation in our hospital, his body temperature was 38°C and physical examination was normal. Malaria parasites were detected by the thick blood film method. Parasitemia was 840 parasites/μL, showing the ring stage. The patient was diagnosed with uncomplicated Plasmodium falciparum malaria infection and admitted to our hospital for 5 days. He had completely recovered after oral administration of artesunate (4 mg/kg/day) for 3 days, followed by mefloquine (25 mg/kg/day) for 2 days. Both thick and thin blood smears were negative on the date of discharge. During his admission, we asked him to contact and warn his colleagues who were in Nigeria that anyone who developed a fever should urgently seek for medical attention to rule out malaria.
Cases B and C.
A 32- and a 23-year-old man worked at the same mine as Case A (the index case). They experienced fever with chill few days before the index case. Both attended a local hospital in Nigeria and were informed that their blood tests were positive for malaria infection; they could not remember the type of diagnostic test used. Antimalarial medication was administered. The patients recovered and their fever subsided 3 days thereafter. Doxycycline (100 mg once daily) was prescribed as chemoprophylaxis at the hospital in Nigeria. Both flew back to Thailand and visited our hospital 2 weeks after treatment in Nigeria.
At initial presentation in our hospital, neither of the cases had fever. Physical examinations were unremarkable. Laboratory investigation revealed normal white blood cell and platelet counts. Thick and thin blood smears showed no malaria parasites. However, a malaria rapid diagnostic test specific for P. falciparum–induced antigen (Paracheck Pf®; Orchid Biomedical Systems, Verna, Goa, India) was positive. Their blood samples (taken 13 days after the start of treatment) were sent to confirm malaria infection by the polymerase chain reaction (PCR) method, as described elsewhere.5,6 The PCR results were positive for P. falciparum and negative for other human malaria species (Plasmodium vivax, Plasmodium malariae, Plasmodium ovale, and Plasmodium knowlesi).
Cases D and E.
Cases D and E were a 49- and a 45-year-old woman, respectively, who worked in the same gem mine as Cases A–C. Neither had fever, but they did develop malaise. Malaria infection was suspected in Nigeria, although only one of them underwent antimalarial treatment. They presented to the Hospital for Tropical Diseases on November 11, 2015 for malaria blood tests after their return from Nigeria.
At initial presentation in our hospital in Bangkok, neither cases had fever. Thick and thin blood smears for malaria showed no parasites. Rapid tests for malaria were negative (Paracheck Pf®; Orchid Biomedical Systems). Polymerase chain reaction tests of blood samples were negative for human malarial infection (P. falciparum, P. vivax, P. malariae, P. ovale, and P. knowlesi). We, therefore, confirmed that neither Case D nor Case E had been infected with malaria.
Case F.
A 54-year-old man reported that he had developed fever and chills on 25th October in Gembu, Nigeria. He was diagnosed with malaria and exhibited severe symptoms that included progressive jaundice and impaired consciousness. He was admitted to the local hospital in Nigeria for 6 days, after which he died on November 4, 2015. His colleague informed us that he had been diagnosed with malaria in Nigeria.
DISCUSSION
Malaria is a major health problem in sub-Saharan Africa, especially Nigeria, where the disease accounts for more cases and deaths than other countries in the world.7 Although most travel-associated malaria cases occur as single, sporadic cases, some clusters of imported malaria among travelers to Africa have been reported. Most of these reports were of travelers from European countries and North America.8–11 In Asia, a familial cluster of P. ovale infections among three of four Japanese travelers was reported after a 6-week tour of Kenya.12 In other regions, a cluster of imported P. vivax cases was reported among travelers returning from Peru, where vivax malaria predominates.13 To our knowledge, no previous study has reported a cluster of imported malaria infection in Thailand.
Guest workers are at a high risk of contracting malaria. Previous studies have demonstrated that guest workers generally lack awareness and possess poor knowledge of malaria risk.2–4 Studies in China (where the growth in investment and exported labor is strong) also reported that exported workers in Africa were at high risk of P. falciparum malaria infection.14 Although P. falciparum malaria in China has become an imported disease, local transmission has reduced because of the National Malaria Elimination Program.15
Similarly, the prevalence of malaria within Thailand declined gradually, from 83.54 cases per 100,000 people in 2000 to 4.52 cases per 100,000 people in 2017.16 Migrant workers were more likely to be infected with P. falciparum than local Thai people.17 This may suggest the transition in Thailand from P. falciparum malaria as primarily a domestic problem to primarily a foreign imported disease.
According to the Royal Thai Embassy in Abuja, Nigeria, the number of Thai nationals resident in the country was estimated to be around 150. They were distributed across Abuja, Lagos, and the Cross River states. Most Thai residents are short-term workers, and so their numbers are constantly changing because of dynamic migration.18 They usually rotate as a group, dependent on the contracts made by their agency. The group reported in this study worked in a gem mine near Gembu city, Taraba state, Nigeria, from September to November 2015. These laborers worked outdoors during the day and lived in shared container housings near the site, sleeping under unimpregnated mosquito bed nets. Before working in Nigeria, they did not visit a travel medicine clinic to ascertain the risk of contracting malaria in Nigeria. Therefore, no one in the group took malaria chemoprophylaxis or had adequate protection against mosquito bites. Similar reports of inadequate preparation resulting in clusters of P. falciparum malaria cases have been made regarding European and U.S. travelers to malaria-endemic regions.9,10
In our cluster, four of the six cases had a confirmed diagnosis of P. falciparum malaria; unfortunately one had died. The high attack rate in this cluster (66.7%) demonstrates the need for increased awareness of the risk of malaria infection among guest workers within malaria-endemic areas to ensure they take adequate measures to protect themselves. Such measures are particularly important in regions such as sub-Saharan Africa, which might have limited facilities for malaria diagnosis and treatment.
Finally, it is important to note that we would have never spotted this particular cluster of cases if we had focused on treating only the index case. Clinicians who see patients with travel-related diseases should be aware that other travelers in the same group might be exposed to the same pathogen. Informing them to be aware of symptoms and reminding them to seek medical care are very important, especially when dealing with life-threatening diseases such as malaria.
REFERENCES
- 1.Wilson ME, Weld LH, Boggild A, Keystone JS, Kain KC, von Sonnenburg F, Schwartz E; GeoSentinel Surveillance Network , 2007. Fever in returned travelers: results from the GeoSentinel Surveillance Network. Clin Infect Dis 44: 1560–1568. [DOI] [PubMed] [Google Scholar]
- 2.Fegan D, Glennon J, 1993. Malaria prophylaxis in long-term expatriate mineworkers in Ghana. Occup Med (Lond) 43: 135–138. [DOI] [PubMed] [Google Scholar]
- 3.Jute S, Toovey S, 2007. Knowledge, attitudes and practices of expatriates towards malaria chemoprophylaxis and personal protection measures on a mine in Mali. Travel Med Infect Dis 5: 40–43. [DOI] [PubMed] [Google Scholar]
- 4.Hamer DH, Ruffing R, Callahan MV, Lyons SH, Abdullah AS, 2008. Knowledge and use of measures to reduce health risks by corporate expatriate employees in western Ghana. J Travel Med 15: 237–242. [DOI] [PubMed] [Google Scholar]
- 5.Snounou G, Singh B, 2002. Nested PCR analysis of Plasmodium parasites. Methods Mol Med 72: 189–203. [DOI] [PubMed] [Google Scholar]
- 6.Singh B, Kim Sung L, Matusop A, Radhakrishnan A, Shamsul SS, Cox-Singh J, Thomas A, Conway DJ, 2004. A large focus of naturally acquired Plasmodium knowlesi infections in human beings. Lancet 363: 1017–1024. [DOI] [PubMed] [Google Scholar]
- 7.US Embassy , 2011. Nigeria Malaria Fact Sheet December 2011 Available at: http://photos.state.gov/libraries/nigeria/231771/Public/December-MalariaFactSheet2.pdf. Accessed April 6, 2018.
- 8.Jelinek T, Schade Larsen C, Siikamäki H, Myrvang B, Chiodini P, Gascon J, Visser L, Kapaun A, Just-Nübling G, 2008. European cluster of imported falciparum malaria from Gambia. Euro Surveill 13: 19077. [PubMed] [Google Scholar]
- 9.Valve K, Ruotsalainen E, Karki T, Pekkanen E, Siikamaki H, 2008. Cluster of imported malaria from Gambia in Finland—travellers do not listen to given advice. Euro Surveill 13: 19068. [PubMed] [Google Scholar]
- 10.Rodger AJ, Cooke GS, Ord R, Sutherland CJ, Pasvol G, 2008. Cluster of falciparum malaria cases in UK airport. Emerg Infect Dis 14: 1284–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bisoffi Z, Matteelli A, Aquilini D, Guaraldi G, Magnani G, Orlando G, Gaiera G, Jelinek T, Behrens RH, 2003. Malaria clusters among illegal Chinese immigrants to Europe through Africa. Emerg Infect Dis 9: 1177–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kimura M, Wataya Y, 2000. Inappropriate use of mosquito bed nets in the prevention of malaria: lessons from a familial cluster of ovale malaria. J Travel Med 7: 338–339. [DOI] [PubMed] [Google Scholar]
- 13.Weitzel T, Labarca J, Cortes CP, Rosas R, Balcells ME, Perret C, 2015. Cluster of imported vivax malaria in travelers returning from Peru. J Travel Med 22: 415–418. [DOI] [PubMed] [Google Scholar]
- 14.Zhang X, Yao L, Sun J, Pan J, Chen H, Zhang L, Ruan W, 2018. Malaria in southeastern China from 2012 to 2016: analysis of imported cases. Am J Trop Med Hyg 98: 1107–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feng J, Xiao H, Zhang L, Yan H, Feng X, Fang W, Xia Z, 2015. The Plasmodium vivax in China: decreased in local cases but increased imported cases from southeast Asia and Africa. Sci Rep 5: 8847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bureau of Epidemiology, Department of Disease Control, Ministry of Public Health, Thailand , 2017. National Disease Surveillance (Report 506) [in Thai]. Available at: http://www.boe.moph.go.th/boedb/surdata/disease.php?dcontent=situation&ds=30. Accessed May 20, 2018.
- 17.Sriwichai P, Karl S, Samung Y, Kiattibutr K, Sirichaisinthop J, Mueller I, Cui L, Sattabongkot J, 2017. Imported Plasmodium falciparum and locally transmitted Plasmodium vivax: cross-border malaria transmission scenario in northwestern Thailand. Malar J 16: 258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Royal Thai Embassy Nigeria , 2018. General Information Abuja, Nigeria [in Thai] Available at: http://www.thaiembassy.org/abuja/contents/files/thai-people-20120702-123154-580347.pdf. Accessed April 8, 2018.