Abstract.
Using a decision-tree approach, we examined the cost-effectiveness of indoor residual spraying (IRS) of households with insecticide combined with insecticide-treated bed net (ITN) distribution (IRS + ITN), compared with ITN distribution alone in the programmatic context of mainland Tanzania. The primary outcome of our model was the expected economic cost to society per case of malaria averted in children ≤ 5 years of age. Indoor residual spraying of households with insecticide data came from a program implemented in northwest Tanzania from 2008 to 2012; all other data originated from the published literature. Through sensitivity and scenario analyses, the model also examined the effects of variations in insecticide resistance, malaria prevalence, and different IRS modalities. In the base case, IRS + ITN is expected to be more expensive and more effective than the ITN-only intervention (incremental cost-effectiveness ratio [ICER]: $152.36). The number of IRS rounds, IRS insecticide costs, ITN use, malaria prevalence, and the probability that a child develops symptoms following infection drove the interventions’ cost-effectiveness. Compared with universal spraying, targeted spraying is expected to lead to a higher number of malaria cases per person targeted (0.211–0.256 versus 0.050–0.076), but the incremental cost per case of malaria averted is expected to be lower (ICER: $41.70). In a scenario of increasing pyrethroid resistance, the incremental expected cost per case of malaria averted is expected to increase compared with the base case (ICER: $192.12). Tanzania should pursue universal IRS only in those regions that report high malaria prevalence. If the cost per case of malaria averted of universal IRS exceeds the willingness to pay, targeted spraying could provide an alternative, but may result in higher malaria prevalence.
INTRODUCTION
Malaria causes considerable morbidity and mortality in mainland Tanzania. Of the 47.8 million residents, 73% live in regions with high rates of malaria transmission, with 1.55 million confirmed malaria cases reported in 2013.1 Plasmodium falciparum causes almost all confirmed cases; major vectors include Anopheles gambiae sensu stricto (s.s.), Anopheles arabiensis, and Anopheles funestus (s.s.).2 In the last decade and a half, Tanzania scaled up its malaria prevention and control programming, primarily case detection and management; intermittent preventive treatment in pregnancy (IPTp); distribution of insecticide-treated bed nets (ITNs), especially long-lasting insecticidal nets; and indoor residual spraying of households with insecticide (IRS). Such intervention scale-up benefited from increased investment by the government of Tanzania and external support from donors such as the Global Fund to Fight AIDS, Tuberculosis, and Malaria; the World Bank; the UK Department for International Development; and the U.S. President’s Malaria Initiative (PMI). As a result, annual malaria morbidity has been declining in Tanzania since 2000, and the national malaria prevalence among children less than 10 years of age fell from 18.1% to 9.5% between 2008 and 2012.3
Mainland Tanzania’s National Malaria Strategic Plan 2014–2020 targets reducing the average malaria prevalence to less than 1% by 2020.2 Meeting this goal will require maintaining and even expanding current malaria prevention and control efforts, which may stress available resources—a particularly challenging undertaking given that external resources are plateauing. Resource constraints could lead policy-makers to introduce cheaper but less effective programming or to halt programming that has reduced malaria but has yet to eliminate it, which in turn could result in malaria resurgence.4–7 Interventions that require recurrent infusions of resources, as does IRS, exhibit particular vulnerability to such premature ends.
Insecticide-treated bed net distribution and IRS campaigns are important components of the malaria prevention and control efforts that will help Tanzania meet its 2020 goals. The National Malaria Control Program (NMCP) and its partners are aiming to achieve and sustain universal ITN coverage: by 2014, more than 36 million ITNs had been distributed via “catch up” mass campaigns and “keep up” distribution programs in clinics and schools,2 with 63% of households owning at least one ITN in 2010–2011, compared with 23% in 2004–2005.3 Indoor residual spraying of households with insecticide has been implemented in up to 18 districts of mainland Tanzania since 2007. To manage insecticide resistance, the insecticide used in IRS operations alternates among pyrethroids, carbamates, and organophosphates. Because IRS is only carried out once or twice per year and because it does not tend to cover entire districts, households that receive IRS may also receive ITNs through mass campaign distributions. Receiving both interventions ensures continuous household protection from mosquito bites and possible malaria infection. Furthermore, the combination of IRS and ITNs provides additional protection in areas with high levels of insecticide resistance, moderate long lasting insecticidal net use, seasonal malaria transmission, or frequent malaria epidemics.8–10
Tanzanian district health officials have cited insecticide cost as a particularly worrisome factor in sustaining IRS operations.4 The spread of insecticide resistance further increases insecticide costs as it forces programs to use newer, more expensive insecticide formulations; resistance to insecticides including deltamethrin, permethrin, dichlorodiphenyltrichloroethane (DDT), and bendiocarb occurs at varying levels throughout Tanzania.11–13 An increase in pyrethroid resistance could drive up global demand for, and therefore the cost of, bendiocarb and other non-pyrethroid insecticides. In addition, although evidence remains inconclusive regarding the effects of insecticide resistance on the efficacy and effectiveness of ITNs,14 any decline in ITN efficacy could also decrease the add-on effect of combining IRS and ITNs.
In 2012, driven by budget constraints, the Tanzania NMCP changed its implementation strategy from universal to targeted IRS: instead of spraying every house in target districts, operations focused on responding to hot spots of malaria transmission.3,4,6 Although such an approach can reduce costs, it can also leave the population more vulnerable to malaria epidemics than universal IRS would. For example, a resurgence of malaria occurred in Muleba in 2013 after the IRS approach was switched, although other gaps in the malaria control efforts such as shortages of diagnostic tests and antimalarial drugs may also have contributed to that resurgence.4
In cases of ongoing or increasing resource requirements, in a context of increasing resource constraints, assessments of the relative costs and effectiveness of different combinations of interventions become crucial for informing policy and programmatic decision-making. Economic models, which apply lessons from multiple contexts to a specific environment, can allow policy-makers to see how changes in model parameters might play out in their context without the risks of real-world trials. Unfortunately, as often happens with interventions that work well at a low cost, cost-effectiveness analyses of IRS or ITNs are often neglected in favor of adequacy evaluations. Extant cost-effectiveness studies of these interventions display a high level of heterogeneity in their findings,15 with differences in types of nets and classes of insecticides driving much of the variation in costs per person protected.16–20 Other important considerations include the cost and life expectancy of ITNs, personnel costs, the number of structures sprayed per day, and the number of annual rounds of IRS.21–25
Indoor residual spraying of households with insecticide and ITNs, both alone and in combination, have proven protective efficacy against malaria, but their relative cost-effectiveness is context specific. Given Tanzania’s goals of reducing malaria in the context of its ongoing programs and its potential resource constraints, our study used decision tree economic modeling to examine the expected incremental cost per case of malaria averted by conducting both IRS operations and ITN distribution in mainland Tanzania, compared with conducting ITN distribution alone. Through sensitivity and scenario analyses, we examine how variations in model parameters, including malaria prevalence, IRS approach, and insecticide resistance, alter the incremental expected cost per malaria case averted.
MATERIALS AND METHODS
We examined malaria prevention among children less than 5 years of age in mainland Tanzania. The data on malaria prevalence, prevention activities, and treatment-seeking behaviors of older age groups are less reliable and were, therefore, excluded from the analysis.26 The baseline scenario examines mainland Tanzania as a whole, and the sensitivity and scenario analyses describe the results of regional variations in the parameters.
Our model compares the combined intervention of both IRS and ITN distribution (IRS + ITN) with ITN distribution alone (ITN-only). The baseline IRS intervention is the IRS round conducted by RTI International (RTI) and the NMCP under the PMI-funded Tanzania Vector Control Scale-up Project (TVCSP) in mainland Tanzania in 2011. We chose 2011 because it was the fourth round of IRS carried out by the project, with costs reflecting ongoing rather than start-up costs; it was the first year of bendiocarb insecticide use after years of pyrethroid use, which required a shift from one to two rounds of IRS; and it was the last year of universal rather than targeted IRS, which is the approach from which the estimates of risk reduction were derived. Universal spraying involves spraying all structures within a geographic or administrative area (e.g., district or ward), and targeted spraying involves spraying structures only within identified transmission “hot spots” (e.g., clusters of households). The areas sprayed within household structures remain the same between universal and targeted spraying. The TVCSP IRS operations carried out between 2008 and 2012 provided the data for sensitivity analyses. The baseline ITN intervention is the Tanzanian National Voucher Scheme (TNVS), which promoted ITN ownership for pregnant women and their children with subsidized vouchers distributed through antenatal clinics (ANCs).27 We chose the TNVS because of the quality of its published costing data; we account for other distribution methods as described in the subsection on calculating ITN costs.
We defined the outcome of interest as the incremental cost per case of malaria averted by implementing a combined IRS + ITN intervention instead of the ITN-only intervention, as measured by their incremental cost-effectiveness ratio (ICER). The ICER was calculated by subtracting the expected cost of the ITN-only intervention from that of the combined intervention and dividing it by the difference in the number of expected cases in the ITN-only intervention versus the combined intervention. Malaria cases averted were chosen as the health outcome because the question involves the allocation of malaria prevention funds, not general public health or other government funds. Using cost per disability-adjusted life year averted or cost per dollar value benefit would have added complexity and required additional unnecessary—and possibly unsubstantiated—assumptions.
Cost calculations: general approach.
All costs were gathered retrospectively from the societal perspective, in line with recommendations for economic evaluations of malaria prevention and control programs.28 Costs drawn from RTI documents were recorded in U.S. dollars (USD) according to the exchange rate during the month in which they were recorded; all published costs in non-U.S. currencies were accompanied by conversions to USD. In accordance with the World Health Organization cost-effectiveness guidelines,27 all costs were converted to 2011 USD using the U.S. gross domestic product deflator.28 Capital costs were annualized over their expected useful life at a 3% discount rate. Controversy persists over the appropriateness of this rate,29 so the discount rate was varied between 0% and 5% in sensitivity analyses to assess the robustness of the findings to this assumption. The time frame and analytic horizon were 1 year.
Calculation of IRS costs.
Expenditure data for IRS operations were recorded electronically at the time of expenditure at regional TVCSP offices. Staff at the central office in Dar es Salaam reviewed the data and compiled them into a monthly report and staff at RTI headquarters in North Carolina reviewed these reports and entered the data into Cognos software (IBM Corporation, Armonk, NY). Values of in-kind contributions were estimated through in-person interviews with district, regional, and national government officials. These estimates were reviewed by RTI staff who supported the IRS operations and by government health officers. When government officials’ estimates exceeded those of RTI staff, the arithmetic mean of the estimates was used.6 Cost data from the IRS program were divided in MS Excel (Microsoft Corporation, Redmont, WA) as follows: spray operations, spray operations commodities, local administration, in-kind contributions from the government and community members, and short-term technical assistance (STTA) and support services from the United States or RTI’s regional office in Nairobi (Supplemental Table 1). None of the included ITN programs considered international support in their costing, so all U.S.- or Nairobi-based costs and STTA costs were excluded from the analyses presented here.
During IRS operations, spray teams tracked the number of people reported to reside in each household sprayed. The mean number of people per household each year was multiplied by the number of structures sprayed to estimate the number of people protected. Each component category’s cost was divided by the number of people protected each year to determine the cost per person protected of each component category for each year. Expected useful lives for capital purchases were determined through discussion with Tanzania-based RTI staff and estimates from published literature. The minimum and maximum values used for sensitivity analyses reflect the lowest and highest reported annual costs per person protected for each category.
To allow for sensitivity analyses of the number of spray rounds, a parameter representing the number of spray rounds was created. All costs directly associated with IRS operations—spray operations costs, insecticide costs, IRS commodity shipping costs, and water usage at households—were multiplied by the number of IRS rounds. This total was added to all other IRS costs to determine the cost per person protected by IRS.
Calculation of ITN costs.
Insecticide-treated bed net cost data were drawn from the published literature using the categories outlined in Supplemental Table 1. Cost data for the baseline TNVS program were extracted from Mulligan et al.27 for all categories except ITN costs. To reflect current ITN costs, an estimate of 2014 ITN prices in Tanzania was obtained from Tanzania-based RTI staff; minimum and maximum ITN costs used in the sensitivity analyses reflect the minimum and maximum 2014 global costs of ITNs reported by the United Nations Children’s Fund.30 We divided the cost of the ITN itself by three to annualize the cost over the expected 3-year life of an ITN, which we varied between 1 and 5 in the sensitivity analysis.31
To reflect differences in costs of ITN programs due to varying distribution strategies, a review of published costing studies for ITN distribution was consulted,15 with additional searches of MEDLINE, EMBASE, Web of Science, and EconLit conducted to capture studies published after the review’s time frame. Costs per net distributed were extracted according to the categories used by Mulligan et al.27 Economic costs, which include monetary valuations of volunteers’ time and account for the time value of money through measures of discounting and depreciation, were preferentially extracted over financial costs. To avoid double counting of costs, where categorizations were unclear or did not match with the Mulligan et al.27 categorizations, the costs for that category were excluded for that study. After conversion to 2011 USD, the minimum and maximum nonzero costs were used as the bounds for the sensitivity analyses. The only exception to this process was the voucher costs, for which the minimum cost was set to zero to reflect a non-voucher–based distribution system. The cost per ITN distributed was calculated by adding the costs from each category (Supplemental Table 1). The cost per ITN distributed was divided by the estimated number of people sleeping under each net to determine the total cost of ITN distribution per person protected.
In Tanzania, the NMCP works with multiple international partners, with each focusing on a portion of the overall malaria prevention and control effort. As such, separate project and administrative units conduct IRS operations and ITN distribution. The IRS + ITN arm of the decision tree, therefore, includes separate local administration costs for each intervention.
Calculation of malaria costs.
The probability of a case of malaria was calculated based on estimates from the mainland Tanzania 2011–2012 Malaria Indicator Survey (MIS). Reductions in the probability of a case because of the interventions were drawn from a review of MIS data in 17 sub-Saharan African countries.26,32 We chose this source rather than Tanzania-specific data from randomized controlled trials, such as the one conducted by West et al.,8 as the latter focused on the high-prevalence Lake Zone. Data from the broader review provide better estimates of the intervention effects as applicable to a greater variety of transmission settings and to an operational program context. The estimates of the cost of a case of malaria to society were drawn from a study by Sicuri et al.33 that examined the societal costs of a case of malaria in a child less than 5 years of age in Tanzania. The components considered in the costs of illness are presented in Supplemental Table 2. The study presents societal costs for uncomplicated malaria, defined as malaria not requiring hospitalization; malaria with severe anemia; cerebral malaria, most commonly accompanied by coma; and cerebral malaria with neurological sequelae. Uncomplicated malaria costs in Tanzania were used as the baseline for the cost of a malaria case without complications. As there were no confidence limits presented around this point, the costs were varied by 50% in both directions for the sensitivity analyses. The cost of a severe malaria case was calculated using the method from Sicuri et al.33 as the mean cost of a case with complications. The minimum value for sensitivity analyses was a case of malaria with severe anemia; the maximum value was a case of cerebral malaria with neurological sequelae.
Data analysis.
The data were analyzed using a decision tree created in TreeAge (TreeAge Software, Inc., Williamstown, MA). Supplemental File 1 describes the parameters included in the analyses and Supplemental Table 3 describes the sources of the probability estimates at each chance node in the order that they appear in the decision tree.
Insecticide cost and parasitemia prevalence were deemed programmatically important parameters to examine through sensitivity and scenario analyses. To identify other important parameters that could affect the outcome, tornado diagrams were drawn to determine the most parsimonious combination of variables that explained at least 95% of the variability in the expected costs and in the expected number of malaria cases in the combined IRS + ITN intervention. The tornado diagrams were also examined for any additional parameters that displayed a threshold effect on the costs and/or effects. One-way sensitivity analyses were performed on the effects of varying the identified parameters over their plausible ranges on the expected costs, cases, and cost per case averted for each intervention.
Scenario analyses.
Best- and worst-case scenario analyses were conducted in which the variables selected from the tornado diagram were set to the levels that made the combined IRS–ITN intervention appear as “good” and as “bad” as possible, respectively, when compared with ITNs alone. “Best” and “worst” were assessed in terms of expected cost per case averted. If variations in the parameter did not result in any changes in the expected cost per case averted, then the value that provided the highest or lowest cost for the combined intervention was selected. If varying the parameter changed neither the expected cost per case averted nor the expected cost of the intervention, the value that provided the highest or lowest expected number of malaria cases was selected. All parameters not identified in the tornado diagrams were held at their base-case level.
The potential effects of using targeted rather than universal spraying were assessed through a scenario analysis in which the IRS coverage rates and effectiveness were shifted to their minimum plausible levels. Finally, the potential effects of changes in insecticide characteristics were assessed using a scenario analysis describing an increase in pyrethroid resistance. The expected ITN effectiveness was decreased to its lowest plausible value, and the maximum insecticide cost estimates from two annual IRS rounds with bendiocarb were used. In both analyses, all other parameters were held at their base-case values.
The only primary data analyzed were cost data with no links to identifiable individuals; all other data were secondary data. Because the study did not include human subjects research, Institutional Review Board approval was not required.
RESULTS
Values.
Probability values, ranges, and sources are listed in Table 1. Non-annualized cost values, ranges, and sources are specified in Table 2. All costs are presented in terms of person protected except for ITN costs, which were recorded in terms of net distributed so that the number of people protected per net could be varied in sensitivity analyses. Other estimated values are displayed with their sources and ranges in Table 3.
Table 1.
Probability values*
| Description | Baseline | Minimum | Maximum | Source(s) |
|---|---|---|---|---|
| Probability of receiving IRS in the dwelling | 0.908 | 0.840 | 0.947 | 4,8 |
| Probability of using an ITN | 0.745 | 0.587 | 0.889 | 26 |
| Probability of infection with malaria parasite, given no intervention | 0.095 | 0.000 | 0.318 | 26,32 |
| Reduction of odds of parasitemia with ITN use | 0.130 | 0.030 | 0.220 | 32 |
| Reduction of odds of parasitemia with IRS use | 0.200 | 0.030 | 0.340 | 32 |
| Reduction of odds of parasitemia with IRS and ITN use | 0.530 | 0.370 | 0.670 | 32 |
| Probability of developing symptoms if infected | 0.890 | 0.117 | 1.000 | 41–43 |
| Probability of seeking formal treatment | 0.776 | 0.481 | 0.946 | 26 |
| Probability of treatment failure | 0.027 | 0.000 | 0.485 | 26,44,45 |
| Probability of developing complicated malaria | 0.157 | 0.104 | 0.233 | 46 |
IRS = indoor residual spraying of households with insecticide; ITN = insecticide-treated bed net.
See Supplemental Table 1 for description of parameters and assumptions.
Table 2.
Non-annualized cost values (2011 USD)
| Description | Baseline | Minimum | Maximum | Source(s) |
|---|---|---|---|---|
| IRS cost categories, per person protected | ||||
| Planning and logistics assessment activities | $0.02 | $0.02 | $0.26 | RTI expense reports |
| Environmental compliance | $0.00 | $0.00 | $0.02 | RTI expense reports |
| Training | $0.27 | $0.14 | $0.27 | RTI expense reports |
| IEC and community mobilization | $0.09 | $0.07 | $0.23 | RTI expense reports |
| Short-term labor | $0.45 | $0.40 | $0.61 | RTI expense reports |
| Transportation | $0.33 | $0.29 | $0.50 | RTI expense reports |
| Other spray operations costs | $0.44 | $0.05 | $0.44 | RTI expense reports |
| Insecticide | $0.43 | $0.34 | $0.84 | RTI expense reports |
| Spray equipment and equipment repair kits | $0.00 | $0.00 | $0.15 | RTI expense reports |
| Personal protective equipment | $0.04 | $0.00 | $0.11 | RTI expense reports |
| Shipping | $0.06 | $0.04 | $0.07 | RTI expense reports |
| Office leases, utilities, maintenance | $0.04 | $0.03 | $0.07 | RTI expense reports |
| Office furniture, equipment, supplies | $0.01 | $0.01 | $0.16 | RTI expense reports |
| Communication | $0.02 | $0.02 | $0.02 | RTI expense reports |
| Travel and transportation | $0.03 | $0.03 | $0.09 | RTI expense reports |
| Vehicle purchase | $0.00 | $0.00 | $0.06 | RTI expense reports |
| Local labor | $0.26 | $0.07 | $0.45 | RTI expense reports |
| Other local administration | $0.02 | $0.02 | $0.04 | RTI expense reports |
| Warehouse space | $0.01 | $0.01 | $0.03 | RTI expense reports |
| Office space | $0.00 | $0.00 | $0.01 | RTI expense reports |
| Government vehicles | $0.00 | $0.00 | $0.01 | RTI expense reports |
| Fuel for government vehicles | $0.00 | $0.00 | $0.00 | RTI expense reports |
| Government labor costs | $0.02 | $0.02 | $0.05 | RTI expense reports |
| Water usage at households | $0.04 | $0.03 | $0.05 | RTI expense reports |
| ITN cost categories, per net distributed | ||||
| Planning and logistics assessment activities | $0.02 | $0.02 | $0.81 | 15,17,27,47 |
| Training distributors | $0.49 | $0.01 | $1.52 | 15,17,27,47,48 |
| IEC and community mobilization | $1.29 | $0.04 | $2.16 | 15,27,49,50 |
| Warehousing/storage of nets | $0.12 | $0.01 | $0.30 | 15,27,51 |
| Distributors’ labor | $0.05 | $0.01 | $1.95 | 15,21,27,52 |
| Transportation | $0.42 | $0.05 | $3.16 | 15,27,51 |
| Nets | $3.36 | $2.50 | $4.80 | Personal communication from Centers for Disease Control and Prevention (CDC) malaria expert31 |
| Printing vouchers | $0.20 | $0.01 | $0.20 | 15,27,48 |
| Office leases, utilities, maintenance | $0.27 | $0.00 | $0.27 | 15,27,51 |
| Office furniture, equipment, supplies | $0.01 | $0.01 | $0.07 | 15,27,53 |
| Administration/other staff labor (not net distribution) | $2.04 | $0.04 | $12.65 | 15,21,27,51 |
| Cost of illness, per person | ||||
| Uncomplicated malaria | $5.36 | $2.68 | $8.03 | 33 |
| Severe malaria | $74.36 | $39.53 | $141.87 | 33 |
IEC = information, education, and communication; IRS = indoor residual spraying of households with insecticide; ITN = insecticide-treated bed net; RTI = RTI International; USD = U.S. dollars.
Table 3.
Other estimated values
| Description | Baseline | Minimum | Maximum | Source(s) |
|---|---|---|---|---|
| Useful lives (years) | ||||
| Soak pits | 3 | 1 | 5 | 19 |
| Spray equipment | 5 | 1 | 10 | 19 |
| Personal protective equipment | 3 | 1 | 4 | 19 |
| Office furniture, equipment, and supplies | 3 | 1 | 5 | 19 |
| Vehicle | 5 | 4 | 6 | 19 |
| Net | 3 | 1 | 5 | Personal communication from CDC malaria expert31 |
| Rounds and coverage estimates | ||||
| Number of rounds of IRS required | 2 | 1 | 2 | 6 |
| Number of people covered by a single net | 2 | 1 | 3 | 26 |
| Discount rate | ||||
| Discount rate | 0.03 | 0 | 0.05 | 29 |
IRS = indoor residual spraying of households with insecticide.
Base case.
As shown in Table 4, with all parameters at baseline values, the IRS + ITN intervention is more expensive but more effective than ITNs alone. The expected cost of the ITN-only intervention is $3.41 per person in the target population, with an expected 0.076 cases of malaria per person targeted; the expected cost of the IRS + ITN intervention is $7.49 per person targeted, with an expected 0.050 cases of malaria per person targeted. The expected incremental cost per case averted by the combined intervention compared with the ITN-only baseline is $152.36.
Table 4.
Results of base-case, best-case, and worst-case scenario analyses
| Scenario | IRS + ITN (combined intervention) | ITN-only | Expected incremental cost per case averted by combined intervention* | ||
|---|---|---|---|---|---|
| Expected cost per person in population* | Expected cases per person in population | Expected cost per person in population* | Expected cases per person in population | ||
| Base case | $7.49 | 0.050 | $3.41 | 0.076 | $152.36 per case averted |
| Best case | $4.55 | 0.172 | $3.07 | 0.281 | $13.58 per case averted |
| Worst case | $17.42 | 0.004 | $13.18 | 0.005 | $2,516.41 per case averted |
| Increased pyrethroid resistance | $7.94 | 0.059 | $3.45 | 0.083 | $192.12 per case averted |
| Targeted spraying | $6.55 | 0.211 | $4.68 | 0.256 | $41.70 per case averted |
IRS = indoor residual spraying of households with insecticide; ITN = insecticide-treated bed net.
2011 U.S. dollars.
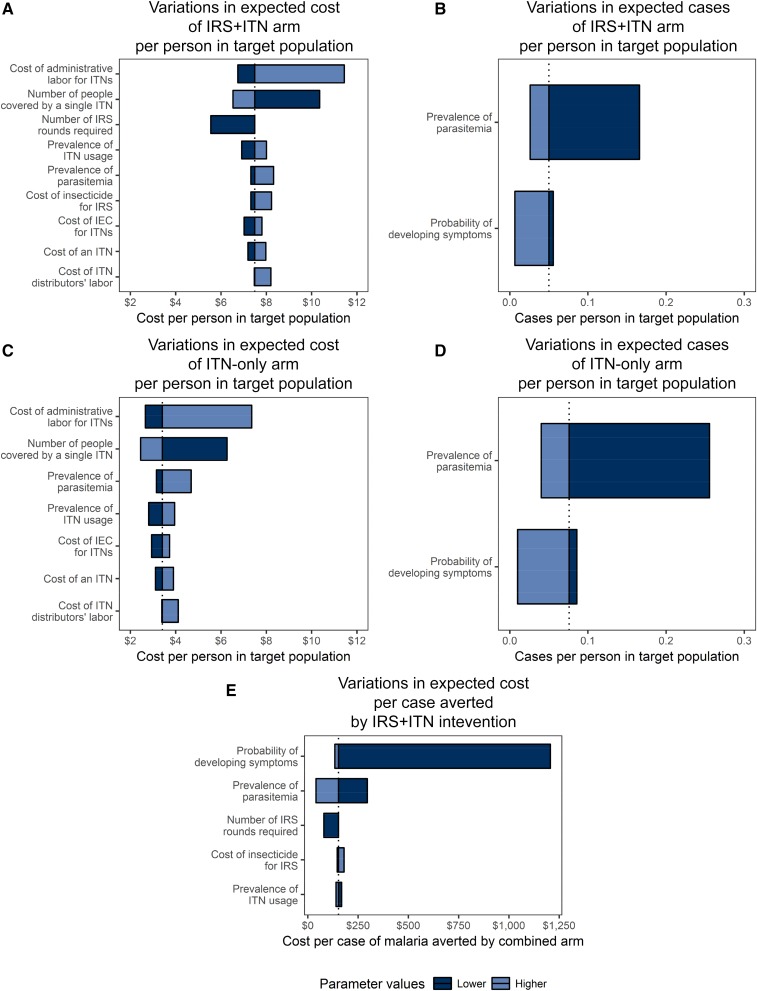
The tornado diagrams describing the variability in expected costs and cases are displayed in Figure 1. As shown in Figure 1A, the primary drivers of variability in costs of the IRS + ITN intervention from the IRS portion were the number of IRS rounds required and the insecticide costs; from the ITN portion, the cost of a net, the number of people protected by a single net, the cost of distributor labor, the cost of staff labor, the cost of information, education, and communication (IEC) materials, and the prevalence of ITN use; and from the tree as a whole, the prevalence of parasitemia. Together, these parameters accounted for more than 95% of the variability in costs of the IRS + ITN intervention. As shown in Figure 1B, the prevalence of parasitemia and the probability that an infected child develops symptomatic malaria explained more than 98% of the variability in expected cases in the combined intervention arm. No parameters displayed a threshold effect. Subsets of these parameters accounted for at least 95% of the variation in the expected costs and expected cases of the ITN-only intervention and in the expected incremental cost per case averted by the IRS + ITN intervention.
Figure 1.
Tornado diagrams of drivers of variability in expected costs and cases. These tornado diagrams show the most parsimonious combination of parameters that describe at least 95% of the variability in the expected cost and expected cases per person in the population for each intervention arm and in the expected incremental cost per case averted by the combined intervention compared with the insecticide-treated bed net (ITN)-only intervention. Parameters are listed in descending order of the proportion of variability of outcome they describe. Wider bars correspond to greater variation in outcome, and the line in each bar shows the base-case value of the outcome. Dark blue bars correspond to the lower bound of the plausible range of the parameters, and light blue bars correspond to the upper bound. Therefore, a parameter with a dark blue bar on the left and a light blue bar on the right of the base-case value is positively associated with the outcome in question, and a parameter with a dark blue bar on the right and a light blue bar on the left is negatively associated with the outcome in question. This figure appears in color at www.ajtmh.org.
Scenario analyses.
The input values used for the best- and worst-case scenario analyses, the scenario analysis of increasing pyrethroid resistance, and the scenario analysis of targeted spraying are displayed in Supplemental Tables 4–6.
In the best-case scenario, both interventions had a lower expected cost per person in the target population and a higher expected number of cases per person in the target population than the base case, and the incremental cost per case averted by the combined intervention compared with the ITN-only intervention fell to $13.58. In the worst-case scenario, both interventions had a higher expected cost per person in the target population and a lower expected number of cases per person in the target population than in the base case, and the expected incremental cost per additional case of malaria averted by the combined intervention compared with the ITN-only intervention rose to $2,516.41.
With targeted spraying, both interventions had a lower expected cost per person in the target population and a higher expected number of cases per person in the target population than in the base case with universal spraying, and the expected incremental cost of the combined intervention compared with the ITN-only intervention fell to $41.70 per case averted.
With increasing pyrethroid resistance, both interventions had a higher expected cost per person in the target population and expected number of cases per person in the target population than in the base case, and the expected incremental cost of the combined intervention compared with the ITN-only intervention rose to $192.12 per case averted.
One-way sensitivity analysis.
Of the input parameters, 10 were selected for sensitivity analysis. Of these, two affect only the IRS portion of the combined intervention, five affect the ITN portion of both interventions, and three affect the probability of developing symptomatic malaria (Figure 1). The values for each individual sensitivity analysis and figures illustrating the trends in their associations with the model outcomes are provided in Supplemental File 2.
The two parameters affecting the IRS portion of the combined intervention were the number of IRS rounds required and insecticide cost. For both of these parameters, as their value increased, the expected cost per person in the population of the combined intervention increased linearly, and the expected cost of the ITN intervention and the expected number of cases in each intervention remained constant. Driven by the changes in costs of the combined intervention, the expected incremental cost per case averted by the combined intervention increased linearly over the parameters’ ranges.
Five parameters directly affected the costs of the ITN portion of both interventions. Of these, variation in the cost of an ITN, of ITN distributors’ labor, of IEC, and of administrative labor produced similar effects. As the values of these parameters increased, the expected cost of both interventions increased linearly at the same rate, and the expected number of cases remained constant for both interventions. In addition, as the number of people covered by a single ITN increased, the expected cost per person in the target population of the intervention declined at the same rate, with the rate of decline diminishing as the number of people per net increased, and the expected number of cases remained constant. None of these parameters affected the expected cost per case averted by the IRS + ITN intervention compared with the ITN-only intervention.
Three probability parameters drove variability in the model’s outcomes: the prevalence of ITN use, the prevalence of parasitemia, and the probability that an infected child would develop symptomatic malaria. As the prevalence of ITN use increased, the expected cost per person in the population of each intervention increased linearly at the same rate. At the same time, the expected number of cases per person in the population decreased linearly for each intervention, with the decrease being steeper in the IRS + ITN intervention than in the ITN-only intervention. Consequently, as the prevalence of ITN use increased, the incremental cost per case averted by the combined intervention versus the ITN-only intervention decreased.
As the prevalence of parasitemia and of the probability that an infected child would develop symptomatic malaria increased, the expected cost of each intervention increased linearly, with a more dramatic increase in the ITN-only intervention. Concurrently, the expected number of cases increased linearly in each arm, with the increase being steeper in the ITN-only arm. As the values of these parameters increased, the expected incremental cost per case averted by the combined arm versus the ITN-only arm decreased at a diminishing rate.
DISCUSSION
In all iterations of the scenarios examined using our model, the combination of IRS and ITNs was more effective but more expensive than ITN distribution alone. Although this reduction of expected cases when compared with either intervention alone aligns with recent findings,32,34 if IRS repels mosquitoes from the household, the mosquitoes might not come into contact with the ITNs, which would undermine the ITNs’ effectiveness.35 Bendiocarb, however, has very little repellent effect when compared with other IRS insecticides, which renders this deleterious effect unlikely.35,36
To account for the different ITN distribution methods used in Tanzania, the studies used to generate ranges for ITN distribution costs included distribution of subsidized and free nets; distribution through ANCs, community groups, and the private sector; stand-alone ITN programs; and ITN programs integrated with vaccination campaigns.15,37 Any parameters that affected only the cost of the ITN portion of the interventions made no difference in the expected cost per case averted by the combined IRS + ITN intervention versus the ITN-only intervention. Together, this suggests that the results of this model are applicable to ITN programs, regardless of their distribution approach.
In the base-case scenario, which reflects a universal IRS throughout mainland Tanzania, our findings show that if the willingness to pay to avert a case of malaria is greater than or equal to $152.36, then an IRS component should be included on top of the ITN distribution component. This value falls toward the more expensive end of the range of cost per case of malaria averted in the most recent systematic review of malaria program economic evaluations.15 The broad variability in expected cost per case averted shown in the best- and worst-case scenario analyses suggests, however, that although a universal IRS across mainland Tanzania would be unwise, IRS might prove useful under certain conditions. The one-way sensitivity analysis and scenario analysis highlight important factors to consider before deciding whether to implement the combined intervention—particularly parasite prevalence, insecticide cost, the extent of insecticide resistance, and the modality of IRS.
In places with a low parasite prevalence, such as the central regions of Tanzania, the expected cost per case averted from the combined intervention versus the ITN-only intervention is almost twice as great as in the baseline scenario. Conversely, in regions with high malaria prevalence, such as the north and southeast of Tanzania, the expected cost per case averted is only about one-third as great as that in the baseline scenario. These findings support the current IRS strategy in Tanzania, which has been focusing on the regions with the highest malaria prevalence, particularly the Lake Zone.3,6,26
In Tanzania’s current programmatic context, the expansion of insecticide resistance is of increasing concern. It requires that different classes or formulations of insecticides are to be used for IRS, which generally tend to be more expensive than the ones previously used. Similarly, if resistance impacts ITN efficacy, any decline in efficacy would also decrease the add-on effect of combining IRS and ITNs. Together, these effects suggest that as pyrethroid resistance increases, the expected cost per case averted of the combined intervention versus the ITN-only intervention could increase, even as the efficacy of ITNs declines. In such a scenario, the importance of non-insecticide-based malaria prevention and control efforts, namely case management and IPTp, would also increase.
It is also important to consider the implications of the one-way sensitivity analyses for ITN use. As ITN use increases, the expected cost per case averted by the combined intervention decreases. That is, as the coverage of one component of malaria control improves, the overall cost-effectiveness of the program improves. Thus, even as pyrethroid resistance increases, sustaining and increasing ITN coverage must remain an important component of the NMCP. A combination of interventions that tackles different aspects of malaria transmission is likely to be preferable to any single intervention.26
An IRS program could respond to rising costs by switching from universal to targeted spraying. In the scenario analysis, targeted IRS led to more expected cases than in the baseline universal IRS scenario, but the expected incremental cost per case averted decreased by 73% from the base-case scenario. These findings suggest that Tanzania’s 2012 strategic change from universal to targeted IRS, which was driven by budget constraints, will indeed reduce the costs of IRS operations.3,4,6 If budget constraints make universal IRS not feasible, targeted IRS would be preferable to no IRS at all in regions with a high malaria prevalence, such as the north and southeast of Tanzania. The savings in terms of dollars of moving from a universal to a targeted spraying strategy will, however, probably require human and economic cost in terms of illness. Again, a well-rounded malaria control program could compensate for weaknesses in one intervention, particularly if the NMCP chooses a less expensive but less effective approach like targeted IRS.
Our analyses’ strengths include its use of primary data drawn from 5 years of IRS operations in mainland Tanzania, which allows the study to examine a practical policy question in a specific environment. The health outcome of cost per malaria case averted is well supported by the literature and understandable by policy-makers. As suggested by Kolaczinski and Hanson,28 costing took a societal perspective, and economic costs were considered over financial costs. The scenario and sensitivity analyses should enable policy-makers to see the effects of the assumptions underlying the model and the interplay of various variables and allow adaption of country-wide baseline findings to local settings.
Making the model specific to the choice facing mainland Tanzania, however, reduces its generalizability. The findings of this study can be applied to settings whose conditions fall within the parameters of the model in terms of malaria transmission, cost of malaria treatment, population treatment-seeking behaviors, and intervention coverage. The model only accounts for two of the possible combinations of interventions: it does not consider IRS as a stand-alone intervention, nor does it consider other interventions such as case management or IPTp. Insecticide-treated bed nets appear in both arms because of ITNs’ status as a cornerstone of Tanzania’s malaria prevention and control efforts. In a country with less mature ITN distribution programming, this study’s comparison would prove less useful. Applying a similar decision tree cost-effectiveness model to other malaria control programs and other contexts would inform policy-making, especially where real-world evidence remains weak or conflicting. Such evidence-based decision-making becomes particularly important in light of shifting malaria transmission patterns and increasing resource constraints. A more comprehensive cost-effectiveness study of all of the NMCP’s components should be conducted to enhance the country’s capacity to design the most cost-effective malaria prevention and control program possible.
From a technical perspective, our study has several limitations. First, ITN data were drawn from the published literature rather than being collected in the same manner as the IRS data. Second, the model relies on effectiveness data from an analysis of MIS data in 17 sub-Saharan African countries for comparability with other indicators in the model, but evidence regarding the combined effectiveness of IRS and ITNs remains inconclusive; the model might, therefore, overstate the effectiveness of combining IRS and ITNs. However, we note that, as described in the sensitivity analyses section, the relative effectiveness of the interventions accounts for less than 1% in the variations of costs and effectiveness of the two intervention arms. Second, the system used to record the costs of the IRS operations did not allow for an ingredients approach to costing, which is the preferred method of costing for economic studies because of its ease of use for generalization.28 Third, as our model only considers a single year of the intervention, it does not describe the potential cost savings of long-term malaria prevention and control programming reducing parasite prevalence. Indoor residual spraying of households with insecticide requires spraying at least once each year and so generates relatively high recurrent costs; ITN distribution requires only one net for several years’ protection and so generates relatively high capital costs. A focus on single-year costs rather than multiple-year costs might bias the results in favor of the combined IRS and ITN intervention versus ITNs alone. Unfortunately, the effectiveness data that would allow a multiyear analysis do not exist, and extrapolating the available single-year effectiveness data into a multiyear model would involve an unacceptable amount of guesswork. The annualization of the cost of an ITN over its useful life should help to mitigate the potential bias toward ITNs caused by the relatively short time frame and analytic horizon, but multiyear effectiveness data for IRS and ITN programs are necessary for better long-term planning. Fourth, the parameter which describes the probability that an infected individual will develop symptoms of malaria explains approximately 11% of the variability in expected cases, the second most of any parameter. Unfortunately, the empirical data on this probability’s value remain inconclusive. Many national health surveys present parasite prevalence as the prevalence of malaria without any consideration of asymptomatic cases. Because populations probably experience a nonzero rate of asymptomatic infections, future studies should examine the probability that an infected person will develop symptoms of malaria. Fifth, these data used in this model reflect costs and structures from 2008 to 2012. We acknowledge that since then, the costs of some of the inputs have changed (e.g., ITN prices have generally decreased and the Tanzanian IRS program has switched from bendiocarb to pirimiphos-methyl to manage insecticide resistance).38,39 We note that our input’s cost ranges do include some of these changes. In addition, we hope that–regardless of the time period and inputs under study–the detailed descriptions of our model inputs and structure will allow policy-makers to see how these and other changes would affect the results when applying the model to other programmatic contexts. Finally, because of a lack of evidence, this study excludes the long-term environmental, agricultural, and health effects of using insecticide for IRS. Without these considerations, any economic evaluation of IRS is incomplete. Rigorous studies must be conducted on these long-term effects. Most studies examining the long-term effects of insecticide-based vector control focus on DDT, not modern formulations, and most studies describing the relationship between malaria and agriculture focus on productivity losses due to illness, not on the potential for insecticide-based vector control to aggravate resistance to insecticides used for crop protection.40 The data are insufficient to include these considerations in the model, but the NMCP and its partners must consider the potential long-term costs of pesticide use in their programmatic decisions.
CONCLUSION
Our decision tree analysis suggests that implementing IRS in an area with high ITN coverage will be more expensive than ITN distribution alone, but it will also reduce the number of cases of malaria. As neither branch of the decision tree proved both cheaper and more effective than the other, the Tanzanian government and its partners must consider the local context and their own willingness to pay to avert a single case of malaria in their decision-making. Based on our findings, implementing IRS in the central region of Tanzania, which has a low malaria prevalence, is not recommended. Continuing universal IRS in the high-prevalence northern and southeastern regions of Tanzania, however, would be more cost-effective in terms of cost per case averted than would a nationwide IRS campaign.
If the NMCP and its partners lack sufficient financial capacity for universal spraying, targeted spraying provides an attractive alternative based on the cost per case of malaria averted. Although discussions of the political desirability of reducing malaria cases to their lowest possible prevalence exceed the scope of this study, the NMCP and its partners should weigh the importance of saving money with the potential negative repercussions of malaria resurgence if choosing targeted spraying. Compared with universal spraying throughout Tanzania, however, targeted spraying does, reduce the expected cost per case averted by about 73%. If the NMCP and its partners find that the expected cost per case averted of universal spraying exceeds their willingness to pay, they could still continue targeted spraying in high-prevalence regions.
Beyond evaluating the cost-effectiveness of the combination of IRS + ITN, our findings highlight the importance of a well-rounded malaria prevention and control program. When ITN use increases in our model, the expected cost per case averted by the combined intervention declines; if pyrethroid resistance were to undermine the effectiveness of ITNs, the expected cost per case averted by the combined intervention would rise. If this scenario comes to pass, malaria control through case management, IPTp, improved testing, and other noninsecticide-based interventions will grow in importance. In choosing a package of malaria prevention and control interventions, the NMCP and its partners should use multiple approaches: the strengths of one intervention or approach can compensate for weaknesses in and reinforce the strengths of the other.
Supplementary Material
Acknowledgments:
The authors thank the people who collected and synthesized the data on the RTI-implemented IRS program, with particular acknowledgment of Vera John for answering our programmatic questions. We also thank anonymous reviewers of the manuscript, who contributed to significantly improving it. The results presented formed part of RS’s MPH thesis.
Note: Supplemental files and tables appear at www.ajtmh.org.
Disclaimer: Neither RTI nor Emory had a role in designing the study, analyzing the data, interpreting the analyses, or writing the manuscript. The opinions expressed herein are those of the authors and do not necessarily reflect the views of the employing organizations or sources of funding.
REFERENCES
- 1.WHO Global Malaria Programme , 2014. World Malaria Report 2014 Geneva, Switzerland: World Health Organization. [Google Scholar]
- 2.Tanzania Ministry of Health and Social Welfare , 2014. National Malaria Strategic Plan, 2014–2020. Dar-es-Salaam, Tanzania: Ministry of Health and Social Welfare.
- 3.Roll Back Malaria Partnership , 2012. Progress & Impact Series: Country Reports. Focus on Mainland Tanzania. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 4.Akim I, Govere J, Gruber J, Ngasala B, 2014. Tanzania Vector Control Scale Project: Mid-Term Performance Evaluation. Arlington, VA: African Strategies for Health Project. [Google Scholar]
- 5.Cohen JM, Smith DL, Cotter C, Ward A, Yamey G, Sabot OJ, Moonen B, 2012. Malaria resurgence: a systematic review and assessment of its causes. Malar J 11: 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colaço R, Yevstigneyeva V, Lalji S, 2014. Tanzania Vector Control Scale-Up Project: Costs of Indoor Residual Spraying, 2011–2012. Unpublished Manuscript. Dar-es-Salaam, Tanzania: RTI International.
- 7.Nájera JA, González-Silva M, Alonso PL, 2011. Some lessons for the future from the global malaria eradication programme (1955–1969). PLoS Med 8: e1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.West PA, Protopopoff N, Wright A, Kivaju Z, Tigererwa R, Mosha FW, Kisinza W, Rowland M, Kleinschmidt I, 2014. Indoor residual spraying in combination with insecticide-treated nets compared to insecticide-treated nets alone for protection against malaria: a cluster randomised trial in Tanzania. PLoS Med 11: e1001630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kafy H, 2013. Combination of IRS with LLINs Versus LLINS Alone in Sudan: Results of a Very Large Randomized Trial (Abstract). 6th MIM Pan-African Malaria Conference, October 6–11, 2013, Durban, South Africa. [Google Scholar]
- 10.Hamainza B, Sikaala CH, Moonga HB, Chanda J, Chinula D, Mwenda M, Kamuliwo M, Bennett A, Seyoum A, Killeen GF, 2016. Incremental impact upon malaria transmission of supplementing pyrethroid-impregnated long-lasting insecticidal nets with indoor residual spraying using pyrethroids or the organophosphate, pirimiphos methyl. Malar J 15: 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Protopopoff N, et al. 2013. High level of resistance in the mosquito Anopheles gambiae to pyrethroid insecticides and reduced susceptibility to bendiocarb in north-western Tanzania. Malar J 12: 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kabula B, et al. 2014. Distribution and spread of pyrethroid and DDT resistance among the Anopheles gambiae complex in Tanzania. Med Vet Entomol 28: 244–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kisinza WN, et al. 2017. Multiple insecticide resistance in Anopheles gambiae from Tanzania: a major concern for malaria vector control. Malar J 16: 439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strode C, Donegan S, Garner P, Enayati AA, Hemingway J, 2014. The impact of pyrethroid resistance on the efficacy of insecticide-treated bed nets against African anopheline mosquitoes: systematic review and meta-analysis. PLoS Med 11: e1001619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.White MT, Conteh L, Cibulskis R, Ghani AC, 2011. Costs and cost-effectiveness of malaria control interventions—a systematic review. Malar J 10: 337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verlé P, Lieu TTT, Kongs A, Van Der Stuyft P, Coosemans M, 1999. Control of malaria vectors: cost analysis in a province of northern Vietnam. Trop Med Int Health 4: 139–145. [DOI] [PubMed] [Google Scholar]
- 17.Guyatt HL, Kinnear J, Burini M, Snow RW, 2002. A comparative cost analysis of insecticide-treated nets and indoor residual spraying in highland Kenya. Health Policy Plan 17: 144–153. [DOI] [PubMed] [Google Scholar]
- 18.Kroeger A, Ayala C, Lara AM, 2002. Unit costs for house spraying and bednet impregnation with residual insecticides in Colombia: a management tool for the control of vector-borne disease. Ann Trop Med Parasitol 96: 405–416. [DOI] [PubMed] [Google Scholar]
- 19.Sine J, Colaço R, Frawley H, 2011. An Economic Analysis of the Costs of Indoor Residual Spraying in 12 PMI Countries, 2008–2010. Research Triangle Park, NC: RTI International. [Google Scholar]
- 20.Abbott M, Johns B, 2013. PMI IRS Country Programs: Comparative Cost Analysis, August 11, 2011–December 31, 2012. Bethesda, MD: Africa Indoor Residual Spraying (AIRS) Project, Abt Associates Inc. [Google Scholar]
- 21.Goodman CA, Mnzava AEP, Dlamini SS, Sharp BL, Mthembu DJ, Gumede JK, 2001. Comparison of the cost and cost-effectiveness of insecticide-treated bednets and residual house-spraying in KwaZulu-Natal, South Africa. Trop Med Int Health 6: 280–295. [DOI] [PubMed] [Google Scholar]
- 22.Xu JW, Yang H, Yang ZQ, Yang GC, Ma XW, Wang WR, Gu YA, Wang LB, Yang XW, Ma J, 2002. Cost-effectiveness analysis of the current measures for malaria prevention in Yuanjiang valley, Yunnan province [in Chinese]. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi 20: 238–241. [PubMed] [Google Scholar]
- 23.Bhatia MR, Fox-Rushby J, Mills A, 2004. Cost-effectiveness of malaria control interventions when malaria mortality is low: insecticide-treated nets versus in-house residual spraying in India. Soc Sci Med 59: 525–539. [DOI] [PubMed] [Google Scholar]
- 24.Conteh L, Sharp BL, Streat E, Barreto A, Konar S, 2004. The cost and cost-effectiveness of malaria vector control by residual insecticide house-spraying in southern Mozambique: a rural and urban analysis. Trop Med Int Health 9: 125–132. [DOI] [PubMed] [Google Scholar]
- 25.Yukich JO, et al. 2008. Costs and consequences of large-scale vector control for malaria. Malar J 7: 258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tanzania Commission for AIDS, Zanzibar AIDS Commission, National Bureau of Statistics, Office of Chief Government Statistician, ICF International , 2013. Tanzania HIV/AIDS and Malaria Indicator Survey 2011–12. Dar es Salaam, Tanzania: TACAIDS, ZAC, NBS, OCGS, and ICF International.
- 27.Mulligan JA, Yukich J, Hanson K, 2008. Costs and effects of the Tanzanian national voucher scheme for insecticide-treated nets. Malar J 7: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kolaczinski J, Hanson K, 2006. Costing the distribution of insecticide-treated nets: a review of cost and cost-effectiveness studies to provide guidance on standardization of costing methodology. Malar J 5: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tan-Torres Edejer T, Baltussen R, Adam T, Hutubessy R, Acharya A, Evans DB, Murray CJL, 2003. Making Choices in Health: WHO Guide to Cost-Effectiveness Analysis. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 30.Bureau of Economic Analysis , 2018. National Economic Accounts Available at: http://www.bea.gov/national/index.htm. Accessed April 9, 2018.
- 31.UNICEF , 2014. Long Lasting Insecticidal Nets Price Data Available at: http://www.unicef.org/supply/files/LLINs_price_transparency_2006_-_2014.pdf. Accessed April 9, 2018.
- 32.Fullman N, Burstein R, Lim SS, Medlin C, Gakidou E, 2013. Nets, spray or both? The effectiveness of insecticide-treated nets and indoor residual spraying in reducing malaria morbidity and child mortality in sub-Saharan Africa. Malar J 12: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sicuri E, Vieta A, Lindner L, Constenla D, Sauboin C, 2013. The economic costs of malaria in children in three sub-Saharan countries: Ghana, Tanzania and Kenya. Malar J 12: 307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kleinschmidt I, Schwabe C, Shiva M, Segura JL, Sima V, Mabunda SJ, Coleman M, 2009. Combining indoor residual spraying and insecticide-treated net interventions. Am J Trop Med Hyg 81: 519–524. [PMC free article] [PubMed] [Google Scholar]
- 35.Yakob L, Dunning R, Yan G, 2011. Indoor residual spray and insecticide-treated bednets for malaria control: theoretical synergisms and antagonisms. J R Soc Interface 8: 799–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Okumu FO, Moore S, 2011. Combining indoor residual spraying and insecticide-treated nets for malaria control in Africa: a review of possible outcomes and an outline of suggestions for the future. Malar J 10: 208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Savigny D, Webster J, Agyepong IA, Mwita A, Bart-Plange C, Baffoe-Wilmot A, Koenker H, Kramer K, Brown N, Lengeler C, 2012. Introducing vouchers for malaria prevention in Ghana and Tanzania: context and adoption of innovation in health systems. Health Policy Plan 27 (Suppl 4): iv32–iv43. [DOI] [PubMed] [Google Scholar]
- 38.UNICEF , 2018. Long Lasting Insecticidal Nets Price Data Available at: https://www.unicef.org/supply/files/LLINs_Pricing_2018.pdf. Accessed April 13, 2018.
- 39.President’s Malaria Initiative, Tanzania Malaria Operational Plan FY18 , 2017. Available at: https://www.pmi.gov/docs/default-source/default-document-library/malaria-operational-plans/fy-2018/fy-2018-tanzania-malaria-operational-plan.pdf?sfvrsn=5. Accessed December 6, 2017.
- 40.Asenso-Okyere K, Asante FA, Tarekegn J, Andam KS, 2011. A review of the economic impact of malaria in agricultural development. Agric Econ 42: 293–304. [Google Scholar]
- 41.Njama-Meya D, Kamya MR, Dorsey G, 2004. Asymptomatic parasitaemia as a risk factor for symptomatic malaria in a cohort of Ugandan children. Trop Med Int Health 9: 862–868. [DOI] [PubMed] [Google Scholar]
- 42.Mabunda S, Aponte JJ, Tiago A, Alonso P, 2009. A country-wide malaria survey in Mozambique. II. Malaria attributable proportion of fever and establishment of malaria case definition in children across different epidemiological settings. Malar J 8: 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Strøm GEA, Tellevik MG, Fataki M, Langeland N, Blomberg B, 2013. No asymptomatic malaria parasitaemia found among 108 young children at one health facility in Dar es Salaam, Tanzania. Malar J 12: 417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mutabingwa TK, Anthony D, Heller A, Hallett R, Ahmed J, Drakeley C, Greenwood BM, Whitty CJM, 2005. Amodiaquine alone, amodiaquine+sulfadoxine-pyrimethamine, amodiaquine+artesunate, and artemether-lumefantrine for outpatient treatment of malaria in Tanzanian children: a four-arm randomised effectiveness trial. Lancet 365: 1474–1480. [DOI] [PubMed] [Google Scholar]
- 45.Shayo A, Mandara CI, Shahada F, Buza J, Lemnge MM, Ishengoma DR, 2014. Therapeutic efficacy and safety of artemether-lumefantrine for the treatment of uncomplicated falciparum malaria in north-eastern Tanzania. Malar J 13: 376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gonçalves BP, Huang CY, Morrison R, Holte S, Kabyemela E, Prevots R, Fried M, Duffy PE, 2014. Parasite burden and severity of malaria in Tanzanian children. N Engl J Med 370: 1799–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guyatt HL, Corlett SK, Robinson TP, Ochola SA, Snow RW, 2002. Malaria prevention in highland Kenya: indoor residual house-spraying vs. insecticide-treated bednets. Trop Med Int Health 7: 298–303. [DOI] [PubMed] [Google Scholar]
- 48.Yukich J, Mulligan J, Tediosi F, Hanson K, Lengeler C, 2007. Tanzania: costing and cost-effectiveness. Yukich J, Tediosi F, Lengeler C, eds. Operations, Costs and Cost-Effectiveness of Five Insecticide-Treated Net Programs (Eritrea, Malawi, Tanzania, Togo, Senegal) and Two Indoor Residual Spraying Programs (Kwa-Zulu-Natal, Mozambique) Basel, Switzerland: Swiss Tropical Institute, 47–64. [Google Scholar]
- 49.Grabowsky M, et al. 2005. Distributing insecticide-treated bednets during measles vaccination: a low-cost means of achieving high and equitable coverage. Bull World Health Organ 83: 195–201. [PMC free article] [PubMed] [Google Scholar]
- 50.Yukich J, et al. 2007. Senegal: costing and cost-effectiveness. Yukich J, Tediosi F, Lengeler C, eds. Operations, Costs and Cost-Effectiveness of Five Insecticide-Treated Net Programs (Eritrea, Malawi, Tanzania, Togo, Senegal) and Two Indoor Residual Spraying Programs (Kwa-Zulu-Natal, Mozambique) Basel, Switzerland: Swiss Tropical Institute, 83–9. [Google Scholar]
- 51.Kolaczinski JH, Kolaczinski K, Kyabayinze D, Strachan D, Temperley M, Wijayanandana N, Kilian A, 2010. Costs and effects of two public sector delivery channels for long-lasting insecticidal nets in Uganda. Malar J 9: 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Becker-Dreps SI, Biddle AK, Pettifor A, Musuamba G, Imbie DN, Meshnick S, Behets F, 2009. Cost-effectiveness of adding bed net distribution for malaria prevention to antenatal services in Kinshasa, Democratic Republic of the Congo. Am J Trop Med Hyg 81: 496–502. [PubMed] [Google Scholar]
- 53.De Allegri M, Marschall P, Flessa S, Tiendrebéogo J, Kouyaté B, Jahn A, Müller O, 2010. Comparative cost analysis of insecticide-treatment net delivery strategies: sales supported by social marketing and free distribution through antenatal care. Health Policy Plan 25: 28–38. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.