Abstract.
It has been believed that concomitant bacteremia is uncommon in adults hospitalized with falciparum malaria. Accordingly, the World Health Organization treatment guidelines presently only recommended additional antibacterial therapy in these patients if they have a clinical syndrome compatible with serious bacterial infection. Admission blood cultures were collected from 20 consecutive adults in Myanmar, hospitalized with a positive immunochromatographic test and blood film, suggesting a diagnosis of falciparum malaria; four (20%) had bacteremia with a clinically significant pathogen. These case series’ data were pooled with a previously published multicenter study from Myanmar which had also collected blood cultures in adults hospitalized with a diagnosis of falciparum malaria. Among 87 patients in the two studies, 13 (15%) had clinically significant bacteremia on admission, with Gram-negative organisms in 10 (77%) and Staphylococcus aureus in the remaining three (23%). Bacteremic patients had more severe disease than non-bacteremic patients (median [interquartile range] respiratory coma acidosis malaria score 2 [1–4] versus 1 [1–2], P = 0.02) and were more likely to die (2/13 [15%] versus 1/74 [1%], P = 0.01). However, bacterial coinfection was suspected clinically in a minority of bacteremic patients (5/13 [38%] compared with 13/70 [19%] of non-bacteremic patients, P = 0.11). Concomitant bacteremia in adults diagnosed with falciparum malaria may be more common than previously believed and is difficult to identify clinically in resource-poor settings. Death is more common in these patients, suggesting that clinicians should have a lower threshold for commencing empirical antibacterial therapy in adults diagnosed with falciparum malaria in these locations than is presently recommended.
INTRODUCTION
Between 4.6% and 16% of African children with a positive film for Plasmodium falciparum in whom severe malaria is diagnosed are also bacteremic.1–6 The case fatality rate in these children with coinfection is more than three times greater than the rate in non-bacteremic cases.1,5 It is not possible to identify the coinfected children clinically or with basic laboratory tests at presentation,7 and so, the current World Health Organization (WHO) management guidelines recommend that all children diagnosed with severe falciparum malaria in malaria-endemic areas also receive empirical broad-spectrum antibacterial therapy.8
It is unclear what proportion of these coinfections may actually represent a primary bacterial infection, with the circulating parasites seen on blood film representing an incidental finding.9 A recent study performed in West African children showed that coinfected children presenting to hospital with serious bacterial infections had a lower parasite density. Meanwhile, 15% of children in the local community were parasitemic and yet asymptomatic; these asymptomatic children were also more likely to have had a lower parasite density suggesting that a proportion of the children presenting with coinfection may have been asymptomatic, if not for the bacterial infection supervening.6,10 However, from a practical perspective, clinicians caring for critically ill children that require hospitalization in these locations are still obliged to treat both the malaria and bacterial infection, as either can be rapidly fatal without appropriate therapy.
Far fewer series have examined the frequency of bacteremia in adults diagnosed with falciparum malaria, and until recently, it has been believed that concomitant bacteremia is far less common than in children.8 Accordingly, the current WHO management guidelines only strongly recommend antibacterial therapy in adults diagnosed with severe malaria if they present with a clinical syndrome compatible with serious bacterial infection (such as hypotension, meningitis, malnutrition, or severe pneumonia).8
However, a recent multicenter study from Myanmar identified a much higher rate of significant bacteremia in adults hospitalized with malaria than had been reported previously.11 There were significant pathogens in the blood cultures of 13% of the patients, and, as in African children, there was a predominance of Gram-negative organisms and an association with more severe illness. Furthermore, most of the bacteremic adults in the series were not suspected of having bacterial coinfection, calling into question the WHO treatment guidelines that recommend empirical antibacterial therapy only in patients with clear clinical evidence of a complicating bacterial disease.
Although there was little clinical evidence of a bacterial infection in many of the patients in the Myanmar series, chart review revealed that local clinicians—either unaware of the WHO recommendations or ignoring them—prescribed antibacterial therapy to most of the patients. More than 75% of the cases in this series received intravenous antibiotics—usually a third-generation cephalosporin—in addition to their antimalarial therapy.11 The fact that there was not a single death in the study among the 67 adults hospitalized with falciparum malaria—35 (52%) of whom satisfied criteria for high dependency unit admission12—was presented as circumstantial evidence to suggest that empirical antibacterial therapy may improve outcomes in adults with a diagnosis of severe falciparum malaria, particularly the more critically ill.11
The present study (ANtibiotic THErapy in adults with Malaria [ANTHEM]) was devised to address this question prospectively. The dramatic fall in malaria incidence in Myanmar13 resulted in premature termination of the study; however, the data are presented here—both separately and pooled with the results of the previously cited study—to inform clinicians working in malaria-endemic regions. It may also assist clinicians working in the Greater Mekong Region where malaria remains endemic and where artemisinin-resistant P. falciparum has the potential to lead to resurgence of the disease.14,15
MATERIALS AND METHODS
This prospective cohort study was performed at the Insein General Hospital, a tertiary referral hospital in Yangon, the largest city in Myanmar. In Myanmar, malaria is a disease of low endemicity that is transmitted predominantly during the May–October wet season. The study enrolled patients for two successive malaria seasons from May 1, 2016, to October 31, 2017.
All nonpregnant patients admitted to the adult medical ward (age ≥ 16 years) were eligible for enrollment. Consecutive patients with symptoms of malaria were screened with an immunochromatographic rapid diagnostic test (RDT) detecting the P. falciparum–specific antigen, histidine-rich protein 2 (HRP2), and the Plasmodium vivax–specific antigen, PvLDH (SD Bioline Malaria Ag P.f/P.v; Standard Diagnostics, Yongin-si, Geonggi-do, South Korea). Hospital laboratory technicians confirmed the diagnosis of falciparum malaria using thick and thin blood films and recorded a semiquantitative parasite count (1+ [1–10 parasites in 100 fields], 2+ [11–100 parasites in 100 fields], 3+ [1–10 parasites in one field], and 4+ [> 10 parasites in one field]).
After confirmation that eligible patients had received intravenous artesunate, written, informed consent was sought from patients or attending family members. Prior antibiotic therapy was not an exclusion criterion for this study. Once enrolled, patients had a standardized history and physical examination performed. Baseline hematology and simple biochemistry tests were collected. Ten milliliters of blood were collected into BACTEC-plus (Becton Dickinson, Franklin Lakes, NJ) aerobic bottles percutaneously using a sterile technique. All positive cultures were subcultured, and the isolates’ identity and antibiotic sensitivity were determined using the Vitek system (BioMérieux, Marcy-l’Étoile, France). Urinalysis, a chest X-ray, and HIV serology were performed in all patients.
After the collection of blood cultures, all patients received antibacterial therapy. They received targeted therapy if they had a clinical syndrome consistent with bacterial infection (chest X-ray consistent with pneumonia and hypotension (mean arterial pressure [MAP] < 65 mm Hg) not responsive to 30 minutes of saline boluses or unequivocal clinical evidence of serious infection including peritonism, meningism, or visible skin infection). All other patients in the study received empirical intravenous levofloxacin at a dose of 750 mg daily; this was ceased after 48 hours (two doses) if there was no laboratory, radiological, or clinical evidence of bacterial infection. Levofloxacin was chosen as the empirical therapy as it is generally well tolerated, dosed once a day, and has a broad spectrum with activity against most of the pathogens isolated in the previous multicenter study. All patients had their initial antibacterial therapy modified on the basis of culture results.
Patients otherwise received supportive care as per the WHO treatment guidelines,16 although with recent developments in the fluid management of adults with malaria, a more conservative intravenous fluid regimen was administered than was recommended in those guidelines.17,18 When able to tolerate oral medications, the patient was switched from intravenous artesunate to oral artemether–lumefantrine. Disease severity was classified using the RCAM score which has been validated previously in patients in Myanmar,19 with a score of ≥ 2 used to define severe disease.20 The likelihood of a concurrent bacterial infection was assessed at the time of hospitalization by a local consultant physician (Ne Myo Aung), with more than 10 years’ postgraduate experience. With the marked decline in malaria incidence in Myanmar,13,21 there was also a profound fall in malaria admissions to the Insein Hospital. Therefore, it was decided to terminate the ANTHEM study, as it was unlikely to be able to enroll enough additional patients to answer the study question. These data—that effectively represent a case series—are presented here. The data have also been combined with the data from the previous prospectively collected and published Myanmar-based multicenter study,11 to increase the statistical power of that study’s observations.
The Ethical Review Committees of the University of Medicine 2, Yangon, and the Menzies School of Health Research, Darwin, approved both studies. Groups were analyzed using the Kruskal–Wallis, χ2, and Fisher’s exact tests, where appropriate, with statistical software (Stata 14.0; StataCorp, College Station, TX). The ANTHEM study was registered at clinical trials.gov (registration number NCT03224052).
RESULTS
Over the 18 months of the ANTHEM study, 220 patients were screened. There were 26 malaria cases, 20 with P. falciparum and six with P. vivax. The 20 patients with falciparum malaria were enrolled in the study, their median (interquartile range [IQR]) age was 28 (19–35) years; 19 (95%) were male. Their clinical characteristics are presented in Table 1.
Table 1.
Baseline characteristics and subsequent clinical course of the 20 patients in the antibiotic therapy in adults with malaria study
| All patients N = 20 | Pathogen on blood culture N = 4 | No pathogen on blood culture N = 16 | P* | |
|---|---|---|---|---|
| Age (years) | 28 (19–35) | 37 (23–68) | 26 (18–31) | 0.13 |
| Gender (male) | 19 (95%) | 4 (100%) | 15 (94%) | 1.0 |
| Temperature on admission (°C) | 39 (38.1–40) | 39.5 (38.3–40.1) | 39 (38.1–40) | 0.39 |
| Suspected bacterial coinfection | 7 (35%) | 2 (50%) | 5 (31%) | 0.59 |
| HIV-positive | 0 | 0 | 0 | – |
| Antibiotic therapy before enrollment | 7 (35%) | 0 | 7 (44%) | 0.25 |
| Glasgow coma scale | 15 (13–15) | 13 (12–15) | 15 (14–15) | 0.06 |
| Impaired consciousness | 7 (35%) | 3 (75%) | 4 (25%) | 0.10 |
| Coma | 1 (5%) | 0 | 1 (6%) | 1.0 |
| Respiratory rate (breaths/minute) | 32 (26–36) | 36 (27–36) | 32 (26–35) | 0.36 |
| Heart rate (beats/minute) | 102 (97–110) | 106 (100–126) | 102 (87–110) | 0.27 |
| MAP (mm of Hg) | 80 (68–83) | 74 (62–88) | 80 (70–83) | 0.56 |
| MAP < 65 mm Hg | 3 (15%) | 1 (35%) | 2 (13%) | 0.51 |
| Systolic blood pressure (mm of Hg) | 100 (83–110) | 90 (80–108) | 100 (90–110) | 0.41 |
| Parasite count† | 1+ (1+ to 2+) | 1+ (1+ to 1+) | 2+ (1+ to 3+) | 0.03 |
| Hemoglobin (g/dL) | 11.0 (9.7–12.7) | 10.4 (5.6–13.4) | 11.4 (9.7–12.7) | 0.51 |
| White blood cell count 109/L) | 9.4 (4.6–11.9) | 11.0 (6.7–21.8) | 8.7 (4.2–11.4) | 0.26 |
| Neutrophil count (×109/L) | 4.6 (2–8.1) | 6.4 (2.6–15.6) | 4.6 (2.0–7.6) | 0.45 |
| Lymphocyte count (×109/L) | 3.4 (2.3–4.2) | 3.8 (3.2–5.7) | 2.9 (2.1–4.2) | 0.20 |
| Neutrophil/lymphocyte ratio | 1.1 (1.0–2.3) | 1.7 (0.7–2.8) | 1.1 (1.0–2.1) | 0.67 |
| Platelet count (×109/L) | 44 (24–134) | 145 (140–245) | 36 (23–116) | 0.006 |
| Creatinine (μmol/L)‡ | 105 (75–170) | 118 (55–295) | 105 (76–159) | 0.62 |
| Acute kidney injury‡ | 4/19 (21%) | 1 (25%) | 3/15 (20%) | 1.0 |
| RCAM score§ | 1 (1–2) | 2 (1–2) | 1 (1–2) | 0.15 |
| High RCAM score‖ | 7 (35%) | 3 (75%) | 4 (25%) | 0.10 |
| Clinical jaundice | 8 (40%) | 3 (75%) | 5 (31%) | 0.26 |
| Abnormal bleeding | 3 (15%) | 2 (50%) | 1 (6%) | 0.09 |
| Acute respiratory distress syndrome | 0 | 0 | 0 | – |
| Significant comorbidities | 4 (20%) | 2 (50%) | 12 (13%) | 0.16 |
| Died | 3 (15%) | 2 (50%) | 1 (6%) | 0.09 |
HIV = human immunodeficiency virus; MAP = mean arterial pressure.
Fisher’s exact or Kruskal–Wallis test.
Parasite counts recorded semiquantitatively (1+ to 4+).
One patient did not have an admission creatinine recorded.
Calculated using respiratory rate and Glasgow coma scale.12
RCAM score ≥ 2.
Before enrollment, seven (35%) of the patients had received antibiotics: six had received intravenous therapy (two levofloxacin monotherapy, two levofloxacin and ceftriaxone, one levofloxacin and metronidazole, and one ceftriaxone monotherapy) and one had received oral therapy (cefixime monotherapy).
There were five patients with a positive blood culture on admission: one was felt to be a contaminant (Staphylococcus epidermidis), whereas four were felt to represent true pathogens (one Escherichia coli, one Salmonella typhi, one Staphylococcus aureus, and one mixed growth of Acinetobacter baumannii and Enterobacter cloacae). None of the patients with a pathogen in their blood culture had received antibiotics before enrollment.
Bacterial coinfection was suspected clinically in only 2/4 (50%) cases of bacteremia. With only 20 patients, the study lacked statistical power, but there was a significant difference in the peripheral parasite counts between the bacteremic and non-bacteremic (1+ [1+ to 1+] versus 2+ [1+ to 3+], P = 0.03). However, the trend to more serious illness in bacteremic patients failed to reach statistical significance: three (75%) of the four bacteremic patients had a RCAM score ≥ 2 compared with four (25%) of the 16 non-bacteremic patients (P = 0.10). There were three (15%) deaths in the study, all of whom had significant comorbidities before enrollment (Table 2). Two (50%) of the four bacteremic patients died compared with one (6%) of the 16 non-bacteremic patients (P = 0.09).
Table 2.
Clinical characteristics at presentation of the three patients that subsequently died in the antibiotic therapy in adults with malaria study
| Age/gender | Parasite count* | RCAM score† | Leukocytes (×109/L) | Neutrophils (×109/L) | Comorbidity | Bacterial coinfection clinically suspected | Blood pressure (mean arterial pressure) | Antibiotic | Pathogen in blood culture | Cause of death |
|---|---|---|---|---|---|---|---|---|---|---|
| 35, male | 2+ | 2 | 9.7 | 4.6 | Cirrhosis | Yes | 100/70 (80) | Ceftriaxone before enrollment changed to levofloxacin | No | Liver failure on day 8 of hospitalization |
| 77, male | 1+ | 2 | 25 | 18 | Cirrhosis, atrial fibrillation and congestive cardiac failure | Yes | 80/60 (67) | Levofloxacin | Acinetobacter baumannii and Enterobacter cloacae | Hematemesis and melena on day 11 of hospitalization |
| 35, male | 1+ | 2 | 12 | 8 | Cirrhosis | No | 80/50 (60) | Levofloxacin | Escherichia coli | Hematemesis and melena on day 12 of hospitalization |
Clinical and laboratory findings recorded at presentation.
Parasite counts recorded semiquantitatively (1+ to 4+).
Calculated using respiratory rate and Glasgow coma scale.12
The data from the ANTHEM study were pooled with the data from the previously published multicenter study (Table 3).11 The clinical characteristics of the patients from the two studies were similar and disease severity was comparable: 7/20 (35%) had a high RCAM score compared with 32/64 (50%, P = 0.24) and 4/19 (21%) had acute kidney injury (AKI) versus 13/64 (20%, P = 0.94). The rates of bacteremia in the two studies were also comparable (4/20 [20%] versus 9/67 [13%], P = 0.47). In the multicenter study, there were only 17/67 (25%) in whom a semiquantitative parasite count could be retrieved. All patients in that study had a positive qualitative film however.
Table 3.
Clinical characteristics of falciparum malaria patients with pathogens detected on blood culture
| Patient | Study | Age/gender | Parasite count* | RCAM score† | Leukocytes (×109/L) | Neutrophils (×109/L) | Comorbidities | Bacterial coinfection suspected clinically | Blood pressure on admission (MAP mm of Hg) | Organism(s) identified in blood culture | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Multicenter | 26, male | NR | 4 | 12.8 | 9.4 | No | No | 120/80 (93) | Staphylococcus aureus‡ | Survived |
| 2 | Multicenter | 32, male | NR | 4 | 6.6 | 4.9 | HIV | Yes | 100/80 (87) | Salmonella typhi | Survived |
| 3 | Multicenter | 42, male | NR | 4 | 10.8 | 8 | No | No | 80/50 (60) | Salmonella typhimurium | Survived |
| 4 | Multicenter | 36, female | NR | 3 | 6.5 | 4.2 | No | No | 90/50 (63) | Escherichia coli | Survived |
| 5 | Multicenter | 52, male | NR | 4 | 8.6 | 7.9 | No | No | 130/90 (103) | Salmonella paratyphi | Survived |
| 6 | ANTHEM | 35, male | 1+ | 2 | 12 | 8 | Cirrhosis | No | 80/50 (60) | E. coli | Died day 12 of admission |
| 7 | ANTHEM | 77, male | 1+ | 2 | 25 | 18 | Cirrhosis, atrial fibrillation and congestive cardiac failure | Yes | 80/60 (67) | Acinetobacter baumannii§ Enterobacter cloacae | Died day 11 of admission |
| 8 | Multicenter | 20, male | 1+ | 2 | 8.4 | 6.5 | No | No | 90/60 (70) | E. coli‖ | Survived |
| 9 | ANTHEM | 19, male | 1+ | 2 | 5.6 | 2 | No | Yes | 100/70 (83) | S. typhi | Survived |
| 10 | Multicenter | 23, male | NR | 1 | 7.3 | 6.5 | No | No | 110/80 (90) | S. paratyphi | Survived |
| 11 | ANTHEM | 39, male | 1+ | 1 | 9.9 | 4.5 | No | No | 110/80 (90) | S. aureus | Survived |
| 12 | Multicenter | 19, female | NR | 1 | 12.8 | 9.8 | No | Yes | 100/70 (83) | S. aureus | Survived |
| 13 | Multicenter | 50, male | NR | 1 | 10.4 | 8 | Diabetes mellitus | Yes | 140/100 (113) | E. coli‖ | Survived |
ANTHEM = antibiotic therapy in adults with malaria; MAP = mean arterial pressure; NR = not recorded. Clinical and laboratory findings recorded at presentation.
Parasite counts recorded semiquantitatively (1+ to 4+). Note that only 17 of the 67 patients in the multicenter study were able to have a semiquantitative parasite count recorded. All patients in the multicenter study had a positive qualitative blood film.
Calculated using respiratory rate and Glasgow coma scale.12
Community-acquired methicillin-resistant S. aureus.
Intrinsically resistant organism, but this isolate was sensitive to cefepime and carbapenems.
Extended spectrum β-lactamase producer.
Of the 87 patients in the two studies, 13 (15%) had a pathogen isolated from admission blood cultures. The pathogens were Gram-negative organisms in 10 (77%) of the cases. Five of these were Salmonella species (two typhi, two paratyphi, and one typhimurium), four were E. coli, and in one case, there was a mixed growth of A. baumannii and E. cloacae. The remaining three pathogens were S. aureus (Table 4, Supplemental File 1).
Table 4.
Pooled data from both studies presenting the baseline characteristics and subsequent clinical course of the patients
| Pathogen in blood culture N = 13 | No pathogen in blood culture N = 74 | P* | |
|---|---|---|---|
| Age (years) | 35 (22–46) | 32 (21–43) | 0.53 |
| Gender (male) | 11/13 (85%) | 62/74 (84%) | 0.94 |
| Temperature on admission (°C) | 39.8 (39.0–40.0) | 38.9 (38.3–39.6) | 0.044 |
| Suspected bacterial coinfection† | 5/13 (38%) | 13/70 (19%) | 0.11 |
| HIV-positive‡ | 1/13 (8%) | 0/69 (0%) | 0.02 |
| Glasgow coma scale | 13 (10–15) | 15 (13–15) | 0.03 |
| Impaired consciousness | 9/13 (69%) | 28/74 (38%) | 0.04 |
| Coma | 4/13 (31%) | 11/74 (15%) | 0.16 |
| Respiratory rate (breaths/minute) | 36 (33–44) | 32 (24–36) | 0.01 |
| Heart rate (beats/minute) | 112 (100–118) | 100 (88–110) | 0.01 |
| MAP (mm of Hg)§ | 83 (65–92) | 80 (70–86) | 0.68 |
| MAP < 65 mm Hg§ | 3/13 (23%) | 10/71 (14%) | 0.41 |
| Systolic blood pressure (mm of Hg)§ | 100 (85–115) | 100 (90–120) | 0.64 |
| Parasite count‖ | 1+ (1+ to 1+) | 2+ (1+ to 2+) | 0.047 |
| Hemoglobin (g/dL) | 9.4 (5.3–10.8) | 10.5 (8.3–12.6) | 0.1 |
| White blood cell count (×109/L) | 9.9 (7.0–12.4) | 6.6 (4.7–9.6) | 0.008 |
| Neutrophil count (×109/L)¶ | 7.9 (4.7–8.8) | 4.0 (2.6–6.4) | 0.007 |
| Lymphocyte count (×109/L)¶ | 2.1 (1.2–3.4) | 1.6 (1.2–2.8) | 0.66 |
| Neutrophil/lymphocyte ratio¶ | 3.6 (2.6–5.1) | 2.2 (1.2–4.4) | 0.07 |
| Platelet count (×109/L)# | 98 (67–163) | 87 (32–124) | 0.12 |
| Creatinine (μmol/L)** | 146 (86–256) | 106 (83–143) | 0.21 |
| Acute kidney injury** | 5/12 (42%) | 12/71 (17%) | 0.049 |
| RCAM score†† | 2 (1–4) | 1 (1–2) | 0.02 |
| High RCAM score‡‡ | 9/13 (69%) | 30/71(42%) | 0.07 |
| Clinical jaundice | 8/13 (62%) | 31/74 (42%) | 0.19 |
| Abnormal bleeding | 2/13 (15%) | 4/74 (5%) | 0.19 |
| Acute respiratory distress syndrome | 0/13 | 1/74 (1%) | 0.67 |
| Significant comorbidities | 4/13 (31%) | 10/74 (14%) | 0.12 |
| Died | 2/13 (15%) | 1/74 (1%) | 0.01 |
MAP = mean arterial pressure.
χ2 or Kruskal–Wallis test.
Four patients in the multicenter trial had insufficient data to determine whether bacterial coinfection was suspected.
Five patients in the multicenter trial did not have HIV serology performed.
Three patients in the multicenter trial did not have their blood pressure on admission recorded.
Only 37 patients had a semiquantitative parasite count recorded (five with a pathogen on blood culture and 32 without a pathogen on blood culture).
Two patients in the multicenter trial did not have a white cell differential count performed.
Three patients in the multicenter trial did not have an admission platelet count recorded.
Four patients did not have an admission creatinine recorded; acute kidney injury defined as a creatinine > 176 μmol/L.
Calculated using respiratory rate and Glasgow coma scale.12
RCAM score ≥ 2.
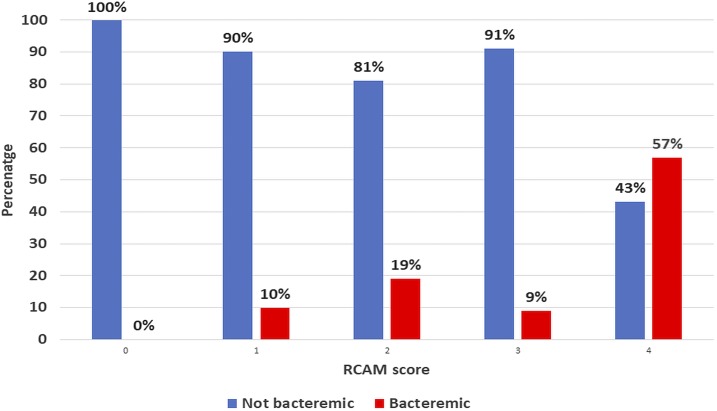
On admission to the hospital, bacterial coinfection was suspected in the minority of bacteremic patients (5/13 [38%] compared with 13/70 [19%] of non-bacteremic patients, P = 0.11) and their blood pressure was no lower (median [IQR] MAP 83 [65–92] versus 80 [70–86] in non-bacteremic patients, P = 0.68). However, bacteremic patients had more severe disease than non-bacteremic patients (P = 0.02) (Figure 1) and were more likely to have impaired consciousness (P = 0.04) and AKI (P = 0.049). Bacteremic patients were more likely to die than non-bacteremic patients (2/13 [15%] versus 1/74 [1%], P = 0.01). Bacteremic patients had a higher leukocyte count than non-bacteremic patients (median [IQR]: 9.9 [7.0–12.4] × 109/L versus 6.6 [4.7–9.6] × 109/L, P = 0.008) and higher neutrophil counts (median [IQR]: 7.9 [4.7–8.8] × 109/L versus 4.0 [2.6–6.4] × 109/L, P = 0.007) (Table 3). The median (IQR) admission leukocyte count in the three patients who died was higher than that in the survivors (12.0 [9.7–25] versus 7.1 [5.1–9.7], P = 0.02). Although there were only 37 patients in whom a semiquantitative parasite count was recorded, the median (IQR) parasite count was lower in the bacteremic than that in the non-bacteremic patients (1+ [1+ to 1+] versus 2+ [1+ to 2+], P = 0.047) (Figure 2).
Figure 1.
Relationship between disease severity (RCAM score) and the presence of bacteremia on admission. Percentage of bacteremic and non-bacteremic patients, stratified by RCAM score. This figure appears in color at www.ajtmh.org.
Figure 2.
Relationship between peripheral parasite density and frequency of concomitant bacteremia. This figure appears in color at www.ajtmh.org.
Across the two studies, 71/87 (82%) of patients received empirical antibacterial therapy on admission to the hospital. In the original multicenter study, 51/67 (76%) patients received empirical therapy, including all nine of the bacteremic patients. The ANTHEM study’s protocol required that all 20 patients received antibacterial therapy.
DISCUSSION
This small prospective tertiary hospital study supports the findings of a previous multicenter study that demonstrated a higher rate of concurrent bacteremia than has been previously identified—or thought to be present—in adults hospitalized with a diagnosis of falciparum malaria. Across the two studies, 15% of patients were bacteremic, a rate similar to that seen in series of African children.5 As in African pediatric series, the bacteremic adult patients had more severe disease and a higher case fatality rate than the non-bacteremic patients, and the isolated pathogens were most commonly enteric Gram-negative organisms.5
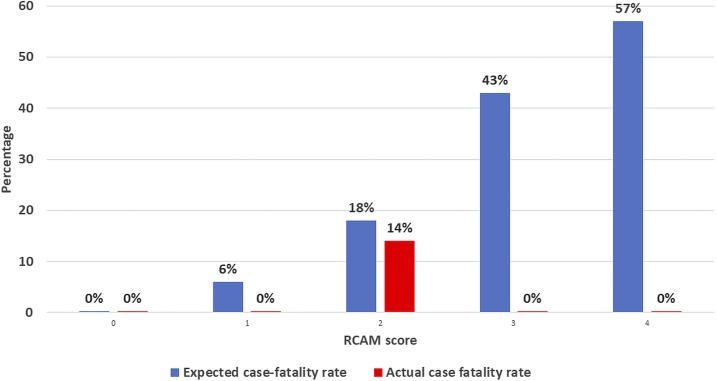
However, despite the high prevalence of bacteremia in the study and the fact that almost half the patients were at high risk for death (with a RCAM score ≥ 2), the case fatality rate was only 3% across the two studies. It is possible that as the patients were enrolled in a clinical trial, they may have received closer attention than may have otherwise been the case. However, the case fatality rate was still much lower than that seen in other clinical trials performed in Asia (Figure 3).22,23 Given the studies’ high rates of bacteremia and recognizing that only a minority of patients with significant bacterial coinfection are bacteremic,24,25 it is reasonable to suggest that the coadministration of antibacterial therapy to more than 80% of patients on admission to hospital is at least partially responsible for the good outcomes in this series.
Figure 3.
Actual case fatality rate seen in the two series compared with the expected case fatality rate, stratified by disease severity.12 (Expected case fatality rate based on a previous series of Asian adults with severe falciparum malaria who were managed with artesunate).23 This figure appears in color at www.ajtmh.org.
The present WHO guidelines suggest that empirical antibacterial therapy should be prescribed in addition to antimalarials to all children with severe falciparum malaria in high and moderate malaria transmission areas, as bacteremia cannot be identified with confidence by bedside examination or simple laboratory tests. Meanwhile, antibacterial therapy is only suggested in adults with a clinical syndrome compatible with serious bacterial infection.8 However, in these two studies, experienced treating clinicians suspected bacterial coinfection in the minority of the bacteremic adults, suggesting that a lower threshold for empirical antibacterial therapy may also be appropriate in adults, particularly the more critically ill.
The few series to explore concomitant bacteremia in adults with malaria have suggested that it may be less common than in children,8 but it is unclear why this should be the case. Although the pathophysiology of bacterial coinfection in children with falciparum malaria is incompletely understood, many of the proposed mechanisms are also present in adults. The fundamental pathological process underlying falciparum malaria in adults and children is microvascular obstruction from parasitized red blood cells26–28 with associated endothelial dysfunction.19,29 This leads to impaired blood flow and tissue ischemia, which in the gastrointestinal tract is believed to impair mucosal barrier function, increasing bacterial translocation.30–32 Indeed, intestinal ulceration has been demonstrated at autopsy in adults with falciparum malaria dying from Gram-negative bacteremia.33 Meanwhile, malaria infection results in a state of relative immune dysfunction, making the host more susceptible to bacterial infection.31 Impaired neutrophil function,34,35 macrophage function,36 and antibody production37 have all been demonstrated in patients with malaria, increasing the likelihood of complicated bacterial disease and poorer outcomes. If these features are present in both children and adults, and the pathophysiology of these aspects of disease in adults and children is felt to be very similar,26 it is reasonable to believe that adults may also be at increased risk of bacterial coinfection.
There are other clinical features of adults diagnosed with falciparum malaria that suggest that concomitant bacterial infection may be underdiagnosed. Shock in malaria—previously termed “algid malaria”—has been recognized for decades, although there is little consensus on the underlying mechanism. It is known that shock is more common in adults than in children, the incidence rising with age.8 In the largest series of adults with severe falciparum malaria, almost 12% of patients were in shock on admission22 and it is a strong independent predictor of increased mortality.20 Studies with detailed hemodynamic testing suggest that cardiac function is preserved in adults with severe malaria.33,38 Although hypovolemia may contribute to hypotension, in one series, there was no patient with shock despite the fact that every patient was hypovolemic, some profoundly so,39 whereas in another, fluid loading did not reduce the incidence of shock developing.40 Meanwhile, a French study which examined 14 adults with falciparum malaria who developed shock during their illness confirmed bacteremia in five (36%) and significant bacterial infection in two others.33 In that study, pulmonary artery occlusion pressures were normal or elevated (suggesting adequate intravascular filling), whereas the systemic vascular resistance was decreased in all the patients in whom it was measured,33 a pattern consistent with bacterial sepsis.
It has also long been recognized that leukocytosis is associated with increased mortality in adults and children with severe malaria.41,42 A higher leukocyte count has been shown to be associated with bacteremia in children with falciparum malaria1 and was more common in the bacteremic adults in these two studies. An elevated white cell count again predicted outcome in this series.
The higher rates of bacteremia seen in the Myanmar studies might be explained by the use of more sensitive, modern microbiological techniques.43 Previous negative studies have been performed in resource-limited settings where there is less sophisticated diagnostic support.44 Meanwhile, higher rates of bacterial coinfection have been identified in locations where there is advanced laboratory support. In one large French series, 30 of 400 (8%) cases of severe falciparum malaria had microbiologically proven bacterial coinfections, including 10 (2.5%) with bacteremia, most of which were enteric organisms.45
Unfortunately, a minority of the patients in these two Myanmar series were able to have semiquantitative parasite counts, and none had plasma HRP2 levels measured. Although this represents the studies’ real-world location—malaria is almost universally diagnosed in Myanmar with RDT alone—it prevents definitive statements about the pathophysiological basis of the findings.10 It is possible that the bacteremia is a complication of the falciparum infection, with sequestration leading to tissue ischemia and bacterial translocation with immune dysfunction, as outlined earlier. Alternatively, it is possible that the bacteremia is the primary pathology and that the parasitemia is simply an incidental finding.9 It is notable that the spectrum of pathogens seen in the two studies was similar to that seen to a large series that examined the etiology of blood stream infections in the unselected inpatient and outpatient population in Yangon.46
However, from a practical perspective, whether this bacterial infection is complicating the malaria or actually the primary pathology, it would not change patient management. The critically ill patient with a positive RDT and film must receive antimalarial therapy: even with treatment, an adult with severe malaria is at high risk of death.22 But if bacterial coinfection is common and its clinical identification is difficult, a lower threshold for empirical antibacterial therapy would be appropriate.
Of course, the suggestion to increase the prescription of empirical antibacterial therapy must be balanced against the tendency of this approach to drive antimicrobial resistance, which has implications for both the individual patient and the community. Antimicrobial resistance is an enormous problem in the South East Asian countries where malaria remains endemic and inappropriate use of broad-spectrum therapy is an important contributing factor.47 However, if a strategy of prescribing empirical antibacterial was only used in the seriously ill patients (RCAM ≥ 2) with a positive malaria film, at this tertiary referral hospital only seven patients (with an expected case fatality rate of > 23%)12 would have received this therapy over an 18-month period. It may be coincidence, but an RCAM score of ≥ 2 would usually translate to a qSOFA score of ≥ 2, which would identify patients at high risk for death from sepsis.48
Ideally, specific clinical or laboratory features could identify the patients with bacterial coinfection and this requires further exploration. In this series, bacteremic patients had a higher white cell count, although it should be noted that most bacteremic patients in the studies still had a white cell count within the normal range. C-reactive protein or procalcitonin might be hypothesized to have utility; however, algorithms using these indices to identify a higher risk of bacterial infection have been disappointing,49,50 and in malaria-endemic areas, access to these more sophisticated tests is usually limited.
These data need to be interpreted very cautiously. They are pooled from two small series with different methodologies and require confirmation in larger, prospective series. However, the findings have the potential to alter the approach to the clinical management of adults diagnosed with falciparum malaria in the resource-limited setting significantly. They suggest that, when modern microbiological techniques are used, concomitant bacteremia may be present in adults diagnosed with severe falciparum malaria more commonly than previously believed. It is unclear whether in malaria-endemic settings the bacterial infection is a complication of the malaria or, in fact, the primary diagnosis. However, as the patients with serious bacterial coinfection appear difficult to identify in these locations and at greater risk of death, a lower threshold for commencing antibacterial therapy in adults diagnosed with falciparum malaria would seem appropriate.
Supplementary Material
Acknowledgments:
The authors thank the doctors and nursing staff who cared for the patients.
Note: Supplemental table appears at www.ajtmh.org.
Disclaimer: The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
REFERENCES
- 1.Berkley J, Mwarumba S, Bramham K, Lowe B, Marsh K, 1999. Bacteraemia complicating severe malaria in children. Trans R Soc Trop Med Hyg 93: 283–286. [DOI] [PubMed] [Google Scholar]
- 2.Were T, Davenport GC, Hittner JB, Ouma C, Vulule JM, Ong’echa JM, Perkins DJ, 2011. Bacteremia in Kenyan children presenting with malaria. J Clin Microbiol 49: 671–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bronzan RN, et al. 2007. Bacteremia in Malawian children with severe malaria: prevalence, etiology, HIV coinfection, and outcome. J Infect Dis 195: 895–904. [DOI] [PubMed] [Google Scholar]
- 4.Prada J, Alabi SA, Bienzle U, 1993. Bacterial strains isolated from blood cultures of Nigerian children with cerebral malaria. Lancet 342: 1114. [DOI] [PubMed] [Google Scholar]
- 5.Church J, Maitland K, 2014. Invasive bacterial co-infection in African children with Plasmodium falciparum malaria: a systematic review. BMC Med 12: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hogan B, et al. Fever Without Source Study Group , 2018. Malaria coinfections in febrile pediatric inpatients: a hospital-based study from Ghana. Clin Infect Dis 66: 1838–1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nadjm B, et al. 2010. WHO guidelines for antimicrobial treatment in children admitted to hospital in an area of intense Plasmodium falciparum transmission: prospective study. BMJ 340: c1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization , 2014. Severe malaria. Trop Med Int Health 19 (Suppl 1): 7–131. [DOI] [PubMed] [Google Scholar]
- 9.Lindblade KA, Steinhardt L, Samuels A, Kachur SP, Slutsker L, 2013. The silent threat: asymptomatic parasitemia and malaria transmission. Expert Rev Anti Infect Ther 11: 623–639. [DOI] [PubMed] [Google Scholar]
- 10.Hendriksen IC, et al. 2013. Defining falciparum-malaria-attributable severe febrile illness in moderate-to-high transmission settings on the basis of plasma PfHRP2 concentration. J Infect Dis 207: 351–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nyein PP, Aung NM, Kyi TT, Htet ZW, Anstey NM, Kyi MM, Hanson J, 2016. High frequency of clinically significant bacteremia in adults hospitalized with falciparum malaria. Open Forum Infect Dis 3: ofw028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hanson J, et al. 2010. A simple score to predict the outcome of severe malaria in adults. Clin Infect Dis 50: 679–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mu TT, Sein AA, Kyi TT, Min M, Aung NM, Anstey NM, Kyaw MP, Soe C, Kyi MM, Hanson J, 2016. Malaria incidence in Myanmar 2005–2014: steady but fragile progress towards elimination. Malar J 15: 503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Imwong M, et al. 2017. The spread of artemisinin-resistant Plasmodium falciparum in the Greater Mekong subregion: a molecular epidemiology observational study. Lancet Infect Dis 17: 491–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Imwong M, Hien TT, Thuy-Nhien NT, Dondorp AM, White NJ, 2017. Spread of a single multidrug resistant malaria parasite lineage (PfPailin) to Vietnam. Lancet Infect Dis 17: 1022–1023. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization , 2012. Management of Severe Malaria: A Practical Handbook, 3rd edition. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 17.Aung NM, Kaung M, Kyi TT, Kyaw MP, Min M, Htet ZW, Anstey NM, Kyi MM, Hanson J, 2015. The safety of a conservative fluid replacement strategy in adults hospitalised with malaria. PLoS One 10: e0143062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hanson J, Anstey NM, Bihari D, White NJ, Day NP, Dondorp AM, 2014. The fluid management of adults with severe malaria. Crit Care 18: 642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaung M, Kyi TT, Aung NM, Kyaw MP, Min M, Htet ZW, Anstey NM, Kyi MM, Hanson J, 2015. The prognostic utility of bedside assessment of adults hospitalized with malaria in Myanmar: a retrospective analysis. Malar J 14: 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hanson J, et al. 2014. Rapid clinical assessment to facilitate the triage of adults with falciparum malaria, a retrospective analysis. PLoS One 9: e87020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Health Organization , 2016. World Malaria Report. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 22.Dondorp A, Nosten F, Stepniewska K, Day N, White N; South East Asian Quinine Artesunate Malaria Trial (SEAQUAMAT) Group , 2005. Artesunate versus quinine for treatment of severe falciparum malaria: a randomised trial. Lancet 366: 717–725. [DOI] [PubMed] [Google Scholar]
- 23.Hanson J, et al. 2011. Laboratory prediction of the requirement for renal replacement in acute falciparum malaria. Malar J 10: 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gilman RH, Terminel M, Levine MM, Hernandez-Mendoza P, Hornick RB, 1975. Relative efficacy of blood, urine, rectal swab, bone-marrow, and rose-spot cultures for recovery of Salmonella typhi in typhoid fever. Lancet 1: 1211–1213. [DOI] [PubMed] [Google Scholar]
- 25.Davis JS, Cheng AC, McMillan M, Humphrey AB, Stephens DP, Anstey NM, 2011. Sepsis in the tropical top end of Australia’s northern territory: disease burden and impact on indigenous Australians. Med J Aust 194: 519–524. [DOI] [PubMed] [Google Scholar]
- 26.White NJ, Turner GD, Day NP, Dondorp AM, 2013. Lethal malaria: Marchiafava and Bignami were right. J Infect Dis 208: 192–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dorovini-Zis K, Schmidt K, Huynh H, Fu W, Whitten RO, Milner D, Kamiza S, Molyneux M, Taylor TE, 2011. The neuropathology of fatal cerebral malaria in Malawian children. Am J Pathol 178: 2146–2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.MacPherson GG, Warrell MJ, White NJ, Looareesuwan S, Warrell DA, 1985. Human cerebral malaria. A quantitative ultrastructural analysis of parasitized erythrocyte sequestration. Am J Pathol 119: 385–401. [PMC free article] [PubMed] [Google Scholar]
- 29.Yeo TW, Lampah DA, Kenangalem E, Tjitra E, Weinberg JB, Granger DL, Price RN, Anstey NM, 2014. Decreased endothelial nitric oxide bioavailability, impaired microvascular function, and increased tissue oxygen consumption in children with falciparum malaria. J Infect Dis 210: 1627–1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilairatana P, Meddings JB, Ho M, Vannaphan S, Looareesuwan S, 1997. Increased gastrointestinal permeability in patients with Plasmodium falciparum malaria. Clin Infect Dis 24: 430–435. [DOI] [PubMed] [Google Scholar]
- 31.Takem EN, Roca A, Cunnington A, 2014. The association between malaria and non-typhoid Salmonella bacteraemia in children in sub-Saharan Africa: a literature review. Malar J 13: 400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Church JA, Nyamako L, Olupot-Olupot P, Maitland K, Urban BC, 2016. Increased adhesion of Plasmodium falciparum infected erythrocytes to ICAM-1 in children with acute intestinal injury. Malar J 15: 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bruneel F, Gachot B, Timsit JF, Wolff M, Bedos JP, Regnier B, Vachon F, 1997. Shock complicating severe falciparum malaria in European adults. Intensive Care Med 23: 698–701. [DOI] [PubMed] [Google Scholar]
- 34.Cunnington AJ, Njie M, Correa S, Takem EN, Riley EM, Walther M, 2012. Prolonged neutrophil dysfunction after Plasmodium falciparum malaria is related to hemolysis and heme oxygenase-1 induction. J Immunol 189: 5336–5346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dasari P, Reiss K, Lingelbach K, Baumeister S, Lucius R, Udomsangpetch R, Bhakdi SC, Bhakdi S, 2011. Digestive vacuoles of Plasmodium falciparum are selectively phagocytosed by and impair killing function of polymorphonuclear leukocytes. Blood 118: 4946–4956. [DOI] [PubMed] [Google Scholar]
- 36.Mabey DC, Brown A, Greenwood BM, 1987. Plasmodium falciparum malaria and Salmonella infections in Gambian children. J Infect Dis 155: 1319–1321. [DOI] [PubMed] [Google Scholar]
- 37.Cunnington AJ, Riley EM, 2010. Suppression of vaccine responses by malaria: insignificant or overlooked? Expert Rev Vaccines 9: 409–429. [DOI] [PubMed] [Google Scholar]
- 38.Hanson JP, et al. 2013. Fluid resuscitation of adults with severe falciparum malaria: effects on acid-base status, renal function, and extravascular lung water. Crit Care Med 41: 972–981. [DOI] [PubMed] [Google Scholar]
- 39.Hanson J, et al. 2012. Relative contributions of macrovascular and microvascular dysfunction to disease severity in falciparum malaria. J Infect Dis 206: 571–579. [DOI] [PubMed] [Google Scholar]
- 40.Nguyen HP, et al. 2011. A retrospective analysis of the haemodynamic and metabolic effects of fluid resuscitation in Vietnamese adults with severe falciparum malaria. PLoS One 6: e25523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Warrell DA, Looareesuwan S, Warrell MJ, Kasemsarn P, Intaraprasert R, Bunnag D, Harinasuta T, 1982. Dexamethasone proves deleterious in cerebral malaria. A double-blind trial in 100 comatose patients. N Engl J Med 306: 313–319. [DOI] [PubMed] [Google Scholar]
- 42.Modiano D, Sirima BS, Konate A, Sanou I, Sawadogo A, 2001. Leucocytosis in severe malaria. Trans R Soc Trop Med Hyg 95: 175–176. [DOI] [PubMed] [Google Scholar]
- 43.Fiori B, et al. 2014. Performance of two resin-containing blood culture media in detection of bloodstream infections and in direct matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS) broth assays for isolate identification: clinical comparison of the BacT/Alert Plus and Bactec Plus systems. J Clin Microbiol 52: 3558–3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McNerney R, 2015. Diagnostics for developing countries. Diagnostics (Basel) 5: 200–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bruneel F, et al. Severe Imported Malaria in Adults (SIMA) Study Group , 2010. Severe imported falciparum malaria: a cohort study in 400 critically ill adults. PLoS One 5: e13236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Myat TO, Prasad N, Thinn KK, Win KK, Htike WW, Zin KN, Murdoch DR, Crump JA, 2014. Bloodstream infections at a tertiary referral hospital in Yangon, Myanmar. Trans R Soc Trop Med Hyg 108: 692–698. [DOI] [PubMed] [Google Scholar]
- 47.Dondorp AM, Limmathurotsakul D, Ashley EA, 2018. What’s wrong in the control of antimicrobial resistance in critically ill patients from low- and middle-income countries? Intensive Care Med 44: 79–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Freund Y, et al. French Society of Emergency Medicine Collaborators Group , 2017. Prognostic accuracy of sepsis-3 criteria for in-hospital mortality among patients with suspected infection presenting to the emergency department. JAMA 317: 301–308. [DOI] [PubMed] [Google Scholar]
- 49.Andriolo BN, Andriolo RB, Salomao R, Atallah AN, 2017. Effectiveness and safety of procalcitonin evaluation for reducing mortality in adults with sepsis, severe sepsis or septic shock. Cochrane Database Syst Rev 1: CD010959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pierrakos C, Vincent JL, 2010. Sepsis biomarkers: a review. Crit Care 14: R15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.