Abstract
Introduction
To report an unusual case of CMV retinitis in Primary Immune Deficiency Disease (PIDD).
Case Report
13-year-old child with combined T and B cell deficiencies was diagnosed of bilateral zone 1 CMV retinitis. Intravitreal injections were unable to be given in a regular and timely manner under general anaesthesia due to her underlying systemic disease. The child was treated with intravenous ganciclovir for 8 weeks until eventual resolution of the retinitis. However, visual acuity deteriorated due to progressive optic nerve involvement.
Conclusion
Paediatric patients often do not notice subtle symptoms of CMV retinitis. Although ocular manifestations are uncommon in PIDD, recognition and high index of suspicion will allow for timely referral, diagnosis, and treatment to be instituted for better visual outcomes.
1. Introduction
Primary Immune Deficiency Disease (PIDD) is a group of genetic disorders that affect the innate and adaptive immune system [1], causing recurrent chronic infections. Common ocular manifestations in combined immunodeficiency include CMV retinitis followed by chorioretinitis and optic neuritis [2]. Cytomegalovirus (CMV) retinitis is a sight threatening condition which is more frequently reported in children with acquired immunodeficiency syndrome compared to other immunosuppressed condition such as post bone marrow or renal transplantation, PIDD, or chemotherapy for leukaemia. As such, present treatment recommendations focus mainly on CMV retinitis in HIV-infected children [3]. Unfortunately, optimal treatment for CMV retinitis in children with PIDD remains unknown. We found limited published reports describing CMV retinitis in PIDD [2, 4, 5]. Here, we present our case findings and treatment approach of CMV retinitis in a child with combined T and B cell deficiencies.
2. Case Report
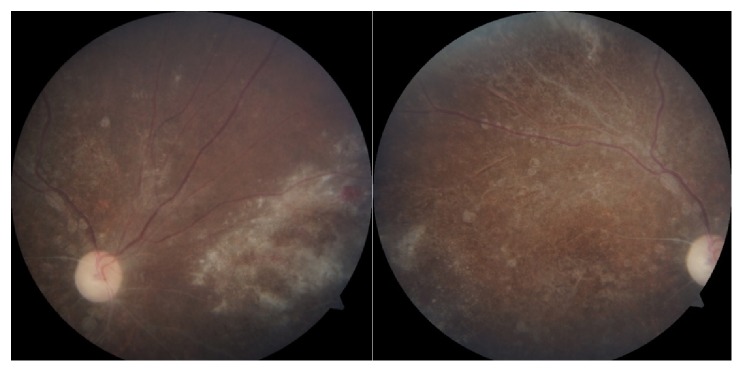
A 13-year-old child with learning disability was referred for ophthalmic assessment as she complained of bilateral blurring of vision. At presentation, her visual acuity was 1/60 in the right eye and 6/18 in the left eye. Relative afferent pupillary defect (RAPD) was present in the right eye. Both eyes anterior segment examination findings were normal. Grade 1 vitritis was noted in the right eye. Funduscopic examination of the right eye revealed a pale optic disc and pigmented scar over the macula with salt and pepper appearance. Intense retinitis with focal areas of haemorrhage was present nasally (Figure 1). There was mild pallor of the left optic disc with macula and peripheral granular retinitis (Figure 2).
Figure 1.
Right eye prior to treatment.
Figure 2.
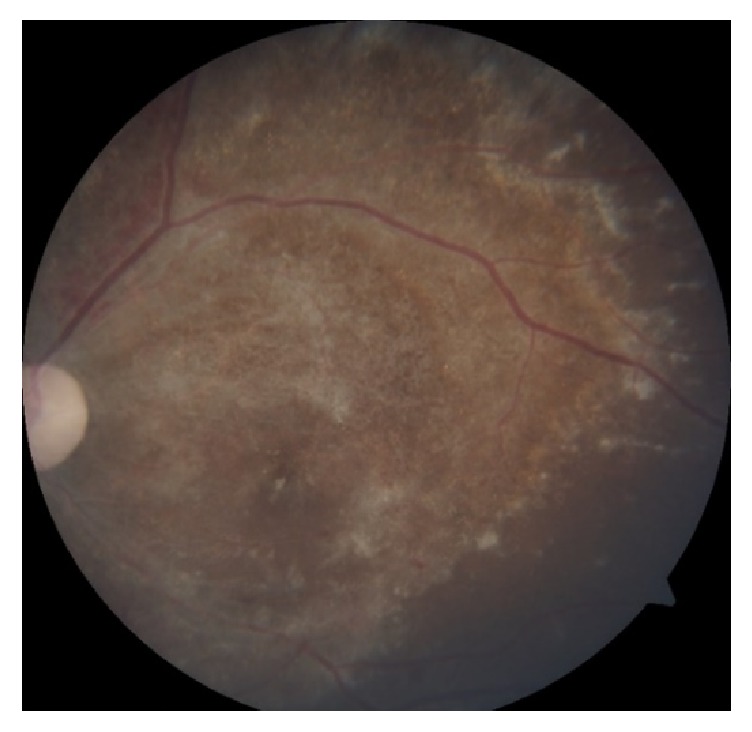
Left eye prior to treatment.
Systematically, she was diagnosed with combined T and B cell deficiencies by the immunologist at the age of 11. At that time, she presented with high fever, recurrent episodes of diarrhoea, oral thrush, and failure to thrive, with the weight of only 12kg. PIDD screening showed low T cell, very low B cell counts, and low immunoglobulin levels (Table 1). Her systemic therapy consisted of 3 weekly intravenous immunoglobulin, sulfamethoxazole, and trimethoprim prophylaxis as well as empirical therapy for fungal infection which include syrup fluconazole 6mg/kg/day and syrup nystatin 1ml QID. She was also treated for CMV colitis as HPE of the colon showed CMV inclusion bodies. She completed 6 weeks of intravenous ganciclovir 3 months prior to presentation of her ophthalmic symptoms.
Table 1.
PIDD screening.
(a).
| Lymphocytes Subset | Normal range for age 7-17 | |||
|---|---|---|---|---|
| % | [x10 (6)L] | % | [x10 (6)L] | |
| Total T cells | 38 | 802 | 66-76 | 1400-2000 |
| Total B cells | 0 | 7 | 12-22 | 300-500 |
| Th cells (CD4) | 10 | 176 | 33-41 | 700-1000 |
| Ts cells (CD8) | 24 | 440 | 27-35 | 600-900 |
| NK cells | 59 | 1428 | 9-16 | 200-600 |
(b).
| Immunoglobulins | Result | Reference range |
|---|---|---|
| IgG | 613 | 931-1916 |
| IgA | 134 | 70-473 |
| IgM | 117 | 34-265 |
| IgE | 121 | <165 |
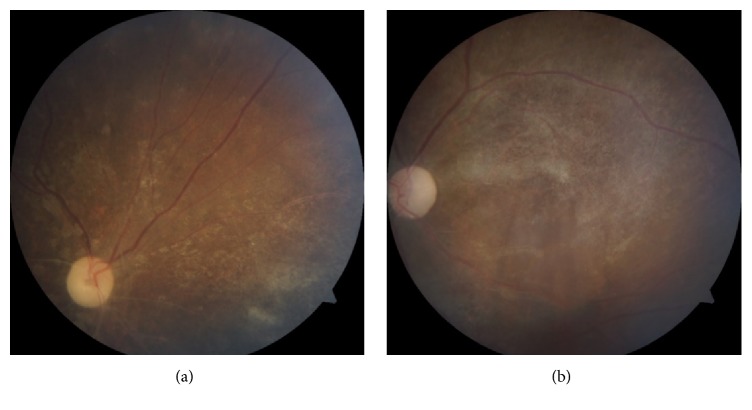
We diagnosed the child of having bilateral eye CMV retinitis based on typical fundus features and history of treated CMV colitis. She was planned for right eye intravitreal ganciclovir injection in view of poor visual function with posterior pole involvement. However, she was deemed unfit to undergo general anaesthesia due to concomitant hospital acquired pneumonia. IV ganciclovir 75mg (6mg/kg) 12 hourly was started and good response was noted after 2 weeks of therapy (Figure 3). The treatment was continued for 8 weeks until the retinitis lesions had healed with scarring (Figure 4). However, the right visual acuity reduced to light perception and improved to 6/9 in the left eye.
Figure 3.
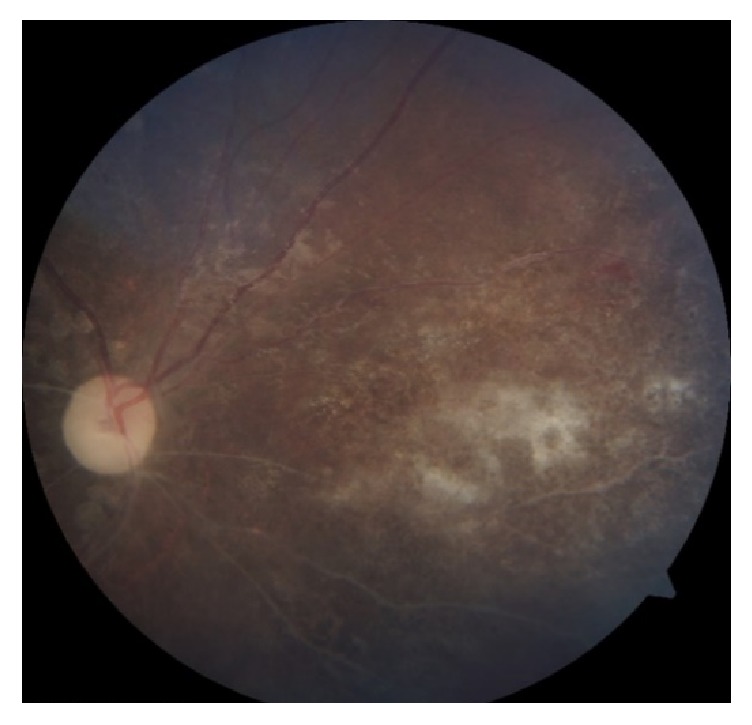
Right eye 2 weeks after IV ganciclovir.
Figure 4.
Right eye after completion of 8 weeks of IV ganciclovir (a). Left eye after completion of 8 weeks of IV ganciclovir (b).
3. Discussion
CMV retinitis can occur in patients with impaired T- cell response such as in solid organ transplant, bone marrow transplant, PIDD, AIDS, or those on immunosuppressive therapy [6]. Although anti-CMV antibody is produced initially as a result of CMV infection, CD4+ T cells and CD8+ T cells play the most important role [6]. The classical fundus appearance of CMV retinitis varies in early and late presentations. In early presentation, it occurs in the peripheral retina with less intense white retinitis, which may not have focal areas of retinal haemorrhages. As the disease progresses, dense white areas of retina necrosis involving the posterior pole, spreading along the vascular arcades, may be seen [7]. The diagnosis of CMV retinitis is mainly clinical, based on classical fundus findings. It can be further confirmed with aqueous CMV polymerase chain reaction (PCR) analysis [6].
The diagnosis of CMV retinitis in this child was established late as evident by bilateral and zone 1 involvement. Patients, especially children with early peripheral CMV retinitis often do not notice the subtle symptoms such as mild blurring of vision, loss of peripheral vision, floaters, or scotoma. Significant visual loss in her right eye is mainly attributed to aggressive extension of retinitis to the optic nerve and macula. Despite achieving resolution of retinitis with antiviral therapy, her visual outcome remained poor.
In AIDS patients, extraocular CMV disease seems to be a strong predisposing factor for developing CMV retinitis. A study has shown that 85% AIDS patients with extraocular CMV disease subsequently developed CMV retinitis after a mean of 6.4 months [8]. The child was diagnosed with CMV colitis and was treated with 6 weeks of IV ganciclovir. She never had any ophthalmic evaluation prior to this. This posed a diagnostic dilemma on whether the initial presentation was due to partial treatment, treatment failure, or relapse of CMV retinitis. In these circumstances, we recommend that such patient should be referred for ophthalmic evaluation before commencement of treatment for extraocular CMV disease.
Treatment strategies which include choice of antiviral and its duration for CMV retinitis in human immunodeficiency virus (HIV) negative patients are vague, particularly in the paediatric group. We have extrapolated the treatment approach used in HIV-infected children with CMV disease in this case. IV ganciclovir, IV foscarnet, IV cidofovir and oral valganciclovir are choices of antiviral available for CMV retinitis in both adults and children. Ganciclovir sustained-release intraocular implant is no longer being manufactured. The treatment guidelines from the Centers for Disease Control and Prevention, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America recommended IV ganciclovir 6 mg/kg body weight/dose administered 12 hourly as initial treatment for HIV-infected infants with CMV disease [3].
Valganciclovir, the oral prodrug of ganciclovir, has been well established to be as effective as IV ganciclovir for induction and maintenance therapy of CMV retinitis in HIV adult patients [9]. Data for the usage of oral valganciclovir for initial treatment in CMV retinitis in children is still lacking. However, oral valganciclovir can be considered for chronic maintenance therapy [3]. Recommended dosage for valganciclovir in paediatric patients (one daily milligram dose) is 7 x body surface area x creatinine clearance (calculated using a modified Schwartz formula). If the calculated Schwartz creatinine clearance exceeds 150 mL/min/1.73m2, then a maximum value of 150 mL/min/1.73m2 should be used [10].
For Zone 1 disease, intensive administration of intravitreal ganciclovir and/or foscarnet given concomitantly with systemic antiviral has been suggested [11]. Repeated intravitreal ganciclovir results in high intraocular antiviral levels could significantly reduce the amount of CMV in CMV retinitis [12]. However, intraocular therapy when administered alone, does not give protection to the fellow eye or additional cytomegalovirus disease, and thus should be combined with systemic antiviral [13]. Meanwhile, small peripheral lesions usually resolve with systemic therapy alone without local treatment [3]. As the usage of cidofovir has not been well established in children, it is considered only if other antivirals failed. Combination therapy of ganciclovir with foscarnet, although has been shown to delay progression of retinitis, should be weighed carefully against its potential risk of nephrotoxicity and neurotoxicity [3].
The severity and location of retinitis, patient's systemic condition, and complications and response to treatment need to be tailored accordingly. IV ganciclovir 6 mg/kg body weight/dose administered 12 hourly was started in this case. We intended for intravitreal injection in this case but, after discussion with the paediatricians and anaesthetists, biweekly intravitreal injection was not feasible due to her multiple systemic comorbidities. Within 2 weeks of treatment with IV ganciclovir, we noted substantial regression of retinitis in the child. Hence, additional antiviral agents were not considered. After complete regression of retinitis with IV ganciclovir, oral valganciclovir as a maintenance is preferred due to its more convenient administration. Unfortunately, it was not available in our setting. As all current antiviral therapies for CMV retinitis are virostatic [14], the definitive treatment in her condition with underlying primary immunodeficiency disease would be bone marrow transplantation with a related matched donor [15]. Unless immune recovery ensues, she will require lifelong monitoring and antiviral therapy for prevention of CMV reactivation.
4. Conclusion
In this case, zone 1 CMV retinitis was diagnosed late and suboptimal treatment was given due to her underlying systemic disease. Hence, although there was eventual resolution of the retinitis after 8 weeks of intravenous therapy, the visual acuity deteriorated due to progressive optic nerve involvement from the retinitis. This highlights the importance of a high index of suspicion with timely referral to prevent irreversible visual loss in these patients. The importance of good teamwork between the Paediatrician, Anaesthetist, and Ophthalmologist may ensure optimal treatment, thus preventing blinding complications of CMV retinitis in children with PIDD.
Consent
Consent to publish was not obtained. No information in this case report that could lead to identification of the patient.
Conflicts of Interest
All authors report no conflicts of interest.
References
- 1.Geha R. S., Notarangelo L. D., Casanova J. L., et al. The International Union of Immunological Societies (IUIS) primary immunodeficiency diseases (PID) classification committee. The Journal of Allergy and Clinical Immunology. 2007;120(4):776–794. doi: 10.1016/j.jaci.2007.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hosseinverdi S., Hashemi H., Aghamohammadi A., Ochs H. D., Rezaei N. Ocular involvement in primary immunodeficiency diseases. Journal of Clinical Immunology. 2014;34(1):23–38. doi: 10.1007/s10875-013-9974-2. [DOI] [PubMed] [Google Scholar]
- 3.Siberry G. K., Abzug M. J., Nachman S., et al. Guidelines for the prevention and treatment of opportunistic infections in HIV-exposed and HIV-infected children: Recommendations from the national institutes of health, centers for disease control and prevention, the HIV medicine association of the infectious diseases society of America, the pediatric infectious diseases society, and the american academy of pediatrics. The Pediatric Infectious Disease Journal. 2013;32(02):i–KK4. doi: 10.1097/01.inf.0000437856.09540.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baumal C. R., Levin A. V., Read S. E. Cytomegalovirus retinitis in immunosuppressed children. American Journal of Ophthalmology. 1999;127(5):550–558. doi: 10.1016/S0002-9394(99)00031-8. [DOI] [PubMed] [Google Scholar]
- 5.Aghamohammadi A., Abolhassani H., Hirbod-Mobarakeh A., et al. The uncommon combination of common variable immunodeficiency, macrophage activation syndrome, and cytomegalovirus retinitis. Viral Immunology. 2012;25(2):161–165. doi: 10.1089/vim.2011.0060. [DOI] [PubMed] [Google Scholar]
- 6.Carmichael A. Cytomegalovirus and the eye. Eye. 2012;26(2):237–240. doi: 10.1038/eye.2011.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henderly D. E., Freeman W. R., Causey D. M., Rao N. A. Cytomegalovirus Retinitis and Response to Therapy with Ganciclovir. Ophthalmology. 1987;94(4):425–434. doi: 10.1016/S0161-6420(87)33454-2. [DOI] [PubMed] [Google Scholar]
- 8.Verbraak F. D., Van Den Horn G. J., Van Der Meer J. T. M., et al. Risk of developing CMV retinitis following non-ocular CMV end organ disease in AIDS patients. British Journal of Ophthalmology. 1998;82(7):748–750. doi: 10.1136/bjo.82.7.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin D. F., Sierra-Madero J., Walmsley S., et al. A controlled trial of valganciclovir as induction therapy for cytomegalovirus retinitis. The New England Journal of Medicine. 2002;346(15):1119–1126. doi: 10.1056/NEJMoa011759. [DOI] [PubMed] [Google Scholar]
- 10.Genetech. Valganciclovir Full Prescribing Information. https://www.gene.com/download/pdf/valcyte_prescribing.pdf. [Google Scholar]
- 11.Vishnevskia-Dai V., Shapira Y., Rahav G., Shimoni A., Somech R., Moisseiev J. Cytomegalovirus retinitis in HIV-negative patients: A practical management approach. Ophthalmology. 2015;122(4):866–868.e3. doi: 10.1016/j.ophtha.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 12.Miao H., Tao Y., Jiang Y.-R., Li X.-X. Multiple intravitreal injections of ganciclovir for cytomegalovirus retinitis after stem-cell transplantation. Graefe's Archive for Clinical and Experimental Ophthalmology. 2013;251(7):1829–1833. doi: 10.1007/s00417-013-2368-6. [DOI] [PubMed] [Google Scholar]
- 13.Martin D. F., Kuppermann B. D., Wolitz R. A., Palestine A. G., Li H., Robinson C. A. Oral ganciclovir for patients with cytomegalovirus retinitis treated with a ganciclovir implant. The New England Journal of Medicine. 1999;340(14):1063–1070. doi: 10.1056/NEJM199904083401402. [DOI] [PubMed] [Google Scholar]
- 14.Biron K. K. Antiviral drugs for cytomegalovirus diseases. Antiviral Research. 2006;71(2-3):154–163. doi: 10.1016/j.antiviral.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 15.Ahuja A. Immune System and Immunodeficiency. Encyclopedia of Infant and Early Childhood Development. 2008:137–146. [Google Scholar]