Abstract
Toll like receptor (TLR) ligands are important adjuvant candidates, causing antigen presenting cells to release inflammatory mediators, leading to the recruitment and activation of other leukocytes. The aim of this study was to define the response of human blood derived dendritic cells and macrophages to three TLR ligands acting singly or in combination, Poly I:C (TLR3), GLA (TLR4) and R848 (TLR7/8). Combinations of TLR agonists have been shown to have a synergistic effect on individual cytokines, here we look at the global inflammatory response measuring both cytokines and chemokines. Using a custom Luminex assay we saw dose responses in several mediators including CCL3 (MIP1α), IL-1α, IL-1β, IL-12, CXCL10 (IP-10) and IL-6, all of which were significantly increased by the combination of R848 and GLA, even when low dose GLA was added. The synergistic effect was inhibited by specific MAP kinase inhibitors blocking the kinases p38 and JNK but not MEK1. Combining TLR adjuvants also had a synergistic effect on cytokine responses in human mucosal tissue explants. From this we conclude that the combination of R848 and GLA potentiates the inflammatory profile of antigen presenting cells. Since the pattern of inflammatory mediators released can alter the quality and quantity of the adaptive immune response to vaccination, this study informs vaccine adjuvant design.
1. Introduction
New vaccine adjuvants are required to improve immune responses in patients with impaired immunity, in particular the elderly, the newborn and the immunocompromised [1]. Adjuvants may be beneficial in vaccines to emerging pandemics where rapid immune induction is required, and may also enable dose sparing when using costly or difficult to manufacture recombinant proteins. Adjuvants can modulate antigen specific immune responses through a number of different mechanisms [2]. One key mechanism is the activation of pattern recognition receptors leading to the release of inflammatory mediators and subsequent immune cell recruitment and activation. Toll-like receptor (TLR) agonists are attractive molecular adjuvants due to their ability to directly activate cells of the innate and adaptive immune systems, leading to enhanced humoral and cellular responses [3]. Although TLRs as a group have a certain degree of functional redundancy, with common signaling pathways, responses to individual TLRs can be modulated across different cell populations by differences in receptor expression [4].
Combining TLR ligands could potentially be beneficial, but it is unclear whether combining ligands that utilise the same intracellular signalling pathways would have synergistic or antagonistic effects [5, 6]. The effect of multiple TLR agonists on the response of individual cytokines, particularly IL-12p70 has been previously demonstrated [7–9]. But more understanding about the mechanism and global pattern of response to TLR adjuvants, acting alone or in combination, is required for a number of reasons. If combinations are additive or synergistic, it could potentially reduce the dose of adjuvant required, which has cost and safety implications. Adjuvants can affect the ‘flavour’ of the downstream immune response [10], affecting whether vaccine responses are protective or ineffective, with different cytokines affecting different aspects of the immune response. Since adjuvants alter the inflammatory profile of vaccines, they may therefore alter the balance between reactogenicity and safety [11]. Greater understanding of how adjuvants activate different cell populations will enable vaccinologists to tailor vaccines to safely promote different types of immune responses.
In this study we sought to understand the interaction of three TLR ligands considered as potential adjuvant candidates and to profile the mediator response in two immune sentinel cells, dendritic cells (DC) and macrophages. We tested the TLR3 agonist Poly I:C, the synthetic TLR4 agonist Glucopyranosyl Lipid Adjuvant (GLA), and the TLR7/8 agonist R848 (Resiquimod). We observed that stimulation of antigen presenting cells with TLR4 plus TLR7/8 agonists enhanced inflammatory mediator release. Using specific MAP Kinase inhibitors we determined that this response was predominantly mediated through the transcription factor c-Fos/APl pathway and not NF-κB. The response in individual cells of the immune system was recapitulated in human tissue explants.
2. Material and Methods
2.1. Preparation of cells
Human peripheral blood mononuclear cells (PBMC) were obtained from single donor buffy coats (NHS Blood and transplant, Colindale, UK) of healthy, HIV-seronegative donors by Hystopaque 1077 (Sigma Aldrich) density gradient centrifugation, resuspended in RPMI + 10% FCS and used immediately. Monocyte-derived macrophages (MDM) were isolated from buffy coat-derived PBMC by adherence, and matured by 5-7 days culture in AIM-V medium (Invitrogen) + 20ng/ml GM-CSF (R&D). Monocyte derived dendritic cells (DC) were isolated from buffy coat-derived PBMC by separation of CD14+ cells using CD14 MicroBeads (Miltenyi Biotec) according to manufacturer’s instructions. Monocytes were differentiated into DC by culturing them in RPMI + 10 FCS% in presence of 50 ng/ml GM-CSF (R&D) and 10 ng/ml IL-4 (R&D) for 6 days. Prior to use GM-CSF and/or IL-4 were removed from culture medium. Three independent donors were used per study.
Penile tissue was obtained following gender reconstruction surgery at Charing Cross Hospital, London, UK. All subjects had ceased oestrogen therapy four to six weeks prior to surgery. Written informed consent was obtained from all donors. Approval for this collection was granted by the Imperial College Healthcare Tissue Bank, under their HTA research licence, and ethics thus conveyed through this process by the Multi Research Ethics Committee (MREC), Wales. Penis was cut into 2–3 mm3 explants comprising both epithelium and stroma. Tissue explants were cultured in RPMI 1640 medium supplemented with glutamax, 10% FCS, penicillin and streptomycin.
2.2. Reagents
TLR ligands used in this study were R848 (Invivogen), GLA (Infectious Disease Research Institute, Seattle), Poly I:C (Invivogen).
Pharmacological inhibitors used were PD98059 (specific MEK1 inhibitor used at 10 μM) from Merk Calbiochem and SB202190 (specific p38 inhibitor 10 μM) and SP600125 (specific stress-activated protein kinase/JNK inhibitor 10 μM) and pyrrolidine dithiocarbamate (PDTC; specific NF-κB inhibitor 100 μM) from Enzo Lifescience.
Peptide inhibitors of adaptor proteins (InvivoGen) were used at 5 μM. The MyD88 inhibitor contains a sequence from the MyD88 TIR homodimerization domain (RDVLPGT) [12] preceeded by a protein transduction sequence (RQIKIWFQNRRMKWKK) derived from antennapedia which enables the peptide to translocate through the cell membrane. The TRIF inhibitor contains the 14 aa that correspond to the sequence of the BB loop of TRIF (FCEEFQVPGRGELH) [13] linked to the cell-penetrating segment of the antennapedia homoedomain (RQIKIWFQNRRMKWKK).
2.3. Cell and tissue stimulation with TLR ligands and pharmacological inhibitors
DC (2.5×105/well) were plated in U bottom 96 well plates just before stimulation with TLR ligands. 105 MDM per well were prepared by adherence of monocytes to flat bottom 96 well plates. One explant of penile tissue was used per well of a 96 well plate in 200 μl media, performed in triplicate for each donor.
TLR ligand stimulation. Cells and tissue were incubated with TLR ligands singly or in combination, using a concentration range from 10 μg/ml to 0.01μg/ml in a total volume of 200 μl depending upon the study. Cells and tissues were incubated for 24 hours at 37°C. Supernatants were collected for mediator quantification as described below.
Inhibitors were added to cells 30 minutes prior to stimulation with TLR ligands; inhibitors and TLR ligands were then left in culture for the following 24 hours before collecting supernatants.
2.4. Mediator quantification
Cell supernatant collected following treatment with single or multiple TLR ligands was tested for inflammatory mediator accumulation. The quantification was performed by in house multiplex bead immunoassay as previously described [14]. Culture supernatants were simultaneously assessed for the presence of the following molecules: IL-1α, IL-1β, IL-2, IL-4, IL-6, IL-12, IL-16, GM-CSF, IFN-β, IFN-γ, CXCL10 (IP-10), CCL7 (MCP-2), CXCL9 (MIG), CCL3 (MIP-1α), CCL4 (MIP-1β), CCL5 (RANTES), CXCL12 (SDF-1), TGF-β and TNF. In this assay microbeads (R&D Systems) dyed with different concentrations of fluorophores were used in order to create distinct sets. Each set was then coated with an antibody specific for one of the analytes (R&D Systems) and the captured analyte was detected with a biotinylated antibody followed by incubation with Streptavidin-phycoerithrin (S-PE). Plates were read using the Luminex 100 system (Luminex Corp., USA) and data analysed by Bioplex Manager Version 4.0 software (BioRad, UK). Lower detection limits for this assay were 5.3 pg/ml (IFN-β, IFN-γ, IL-1α, IL-1β, IL-2, IL-4, IL-16, CXCL10, CCL8, CXCL9, CCL3, CCL4, CCL5, CXCL12, TNF), 2.6 pg/ml (IL-12 and TGF-β), 5.6 pg/ml (IL-6) and 2.8 pg/ml (GM-CSF).
2.5. Flow cytometric analysis
DC from three donors were analysed by flow cytometry (FC500, Beckman Coulter) for surface expression of different maturation and activation markers. Cells were treated with TLR ligands or left untreated for 24 hours, comparisons were made with with untreated cells, fixed and stained at 0 hours after isolation. Cells were pre-incubated with PBS+10% human serum for 15 min at 37°C. 3×105 cells/sample were resuspended with the relevant antibody (all mouse anti-Human, BD Pharmingen) for 30 min at 4°C, prior fixing. The antibodies used were: anti-CD40 PE, anti-CCR7 FITC, anti-HLA-DR FITC, anti-CDllc APC, anti-CD80 FITC, anti-CD86 APC. All levels were compared to isotype controls.
2.6. Pathway Mapping
KEGG pathway mapping was performed using VANTED V2.6.1 [15], with additional information from InnateDB (www.innateDB.com).
2.7. Statistical analysis
Calculations as described in figure legends were performed using Prism 6 (GraphPad Software Inc., La Jolla, CA, USA).
3. Results
3.1. DC and Macrophages release more chemokines than cytokines in response to TLR stimulation
More understanding about the mechanism of action of TLR ligands is required to fine tune their potential use as adjuvants. One important mechanism of adjuvants is activation of antigen presenting cells (APC) leading to the release of cytokines and chemokines. Monocyte derived dendritic cell (Fig 1) and macrophage (Fig S1) cultures were treated with three putative TLR-adjuvants: Poly I:C (TLR3), GLA (TLR4) and R848 (TLR7/8) for 24 hours. TLR ligands were used at a range of concentrations spanning from 10 to 0.01 μg/ml, prior to assessing the inflammatory mediators released by the two cell types. Of the 19 cytokines tested, several were undetectable even after stimulation with 10 μg/ml ligand – IL-2, IL-16, IFN-β, IFN-γ, CCL7, CXCL12 and TGF-β and were not included in subsequent analysis.
Figure 1. Inflammatory mediator production by Human monocyte derived Dendritic cells in response to stimulation with TLR ligands.
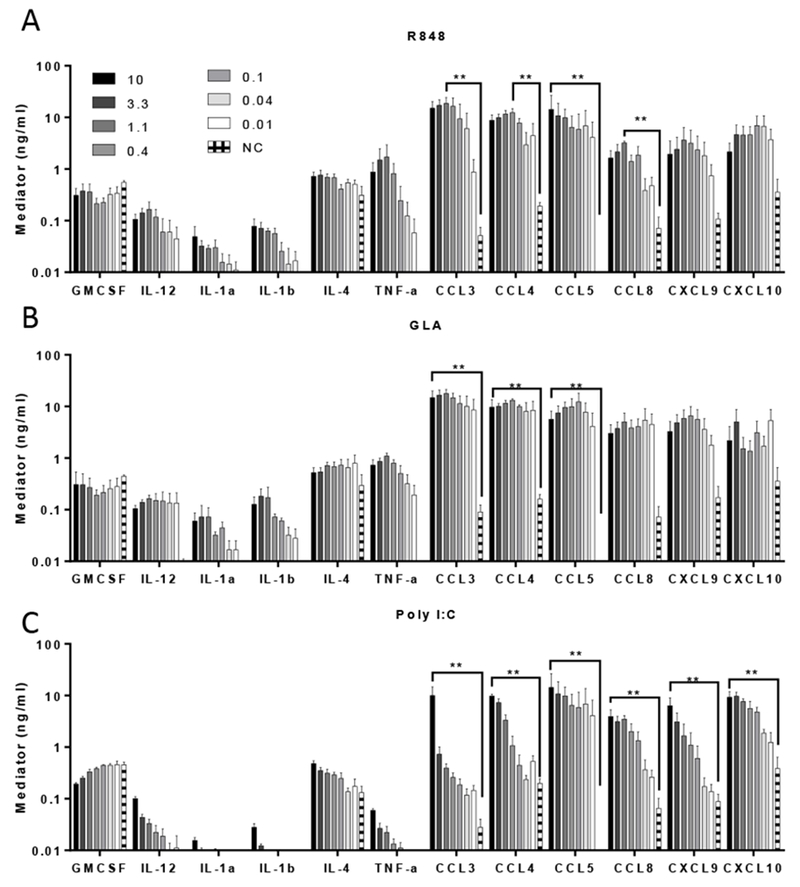
DC cultures were stimulated with R848 (A), GLA (B) and Poly-IC (C) (concentration range: 10-0.014 μg/ml indicated in legend) for 24 hours and supernatant cytokine levels subsequently tested by multiplex bead immunoassay (Luminex). Bars represent mean of n=3 donors +/− SEM. ** p<0.01 measured by two way ANOVA and post test.
After exposing dendritic cells to TLR ligands (Fig 1), levels of the cytokines (GM-CSF, IL-1α, IL-1β, IL-4 and TNFα) were in the range 0.1-1μg/ml approximately a log lower than the chemokines (CCL3, CCL4, CCL5, CCL8, CXCL9 and CXLC10) which were in the range 1-10μg/ml. The pattern of response to R848 (Fig 1A) and GLA (Fig 1B) were similar, reflecting the common use of the MyD88 signalling pathway. The pattern of response to Poly I:C (Fig 1C) was slightly different to GLA and R848. Poly I:C led to lower levels of the pro-inflammatory cytokines IL-1α, IL-1β and TNFα than the other ligands. When macrophages were treated with the same ligands, a similar response pattern was seen (Fig 2 and S1). When used alone, the TLR ligands, R848, GLA and Poly I:C are therefore able to induce the production of both chemokines and cytokines in DC and macrophages.
Figure 2. Comparison of TLR responses in MDM and DC.
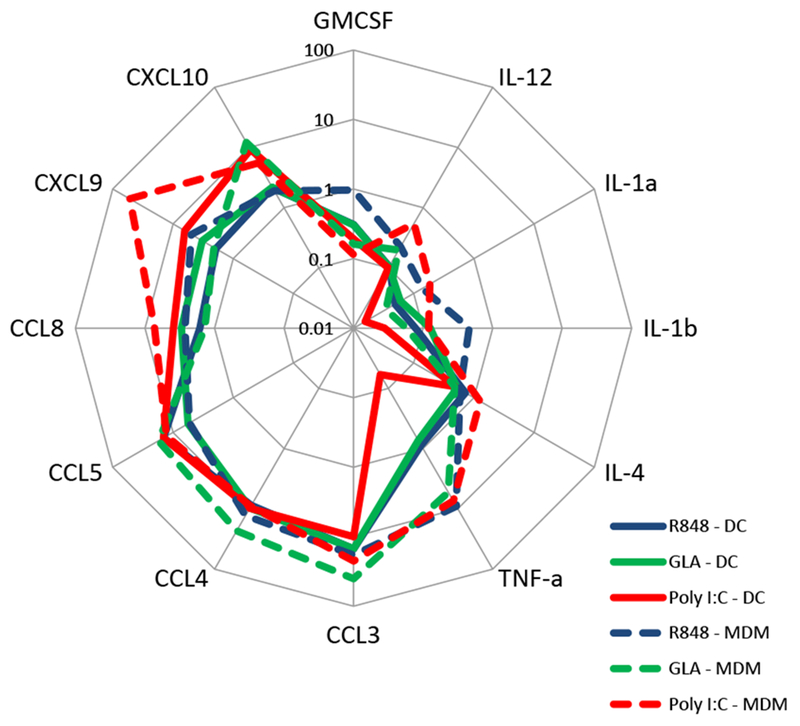
Luminex data from MDM or DC cultures stimulated with 10 μg/ml R848, GLA and Poly-IC for 24 hours re-plotted on a spider plot for comparison. Points represent mean of n=3 donors.
3.2. TLR ligands in combination boost responses.
We wished to determine whether the combination of TLR ligands could change the responses when compared to individual ligands. We compared the responses to ligands administered individually or in combination to DC (Fig 3) or Macrophages (Fig S2) and measured the responses using the same multiplex array. Co-stimulation of DC and MDM with R848 (TLR7/8) and GLA (TLR4) resulted in enhanced secretion of the cytokines IL-1α, IL-1β, IL-6, IL-12 and CCL8 compared to stimulation with single TLR ligands for both cell types. No synergy was observed in IL-4, TNF, CCL4 or CXCL9 (data not shown). Furthermore, there was a significant additive effect for GMCSF, CCL3 and CCL4 responses comparing GLA or R848 alone with R848 and GLA together. The addition of Poly I:C to R848 had a less marked effect, except for CCL8. Comparing the responses, the largest magnitude effect was on IL-6, CCL3 and CCL4 (Fig 3B). Reducing the amount of GLA added from 0.5 to 0.02 μg/ml also had additive effects on the response, in some cases by more than that seen with R848 plus 0.5 μg/ml of GLA. When MDM were stimulated with a combination of TLR ligands, potentiated responses were observed for IL-1α, IL-1β, IL-12, CCL4 and GMCSF. There was a striking increase in CCL3 response when 0.02μg GLA was added to R848 (Fig S2). It was of note that the combination of TLR ligands working through the same MyD88 pathway (R848 and GLA) enhanced responses and was not antagonistic.
Figure 3. Impact of TLR ligand combinations on induced inflammatory mediator secretion profiles of Dendritic cells.
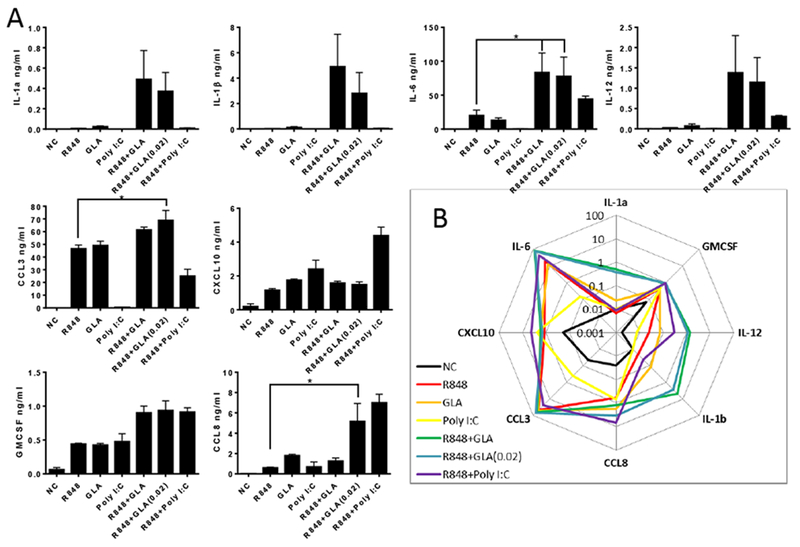
A. DC were stimulated with 0.5 μg/ml of the relevant TLR ligand (R848, GLA and Poly I:C) or a combination for 24 hours. Mediators were then measured in the culture supernatant. In each graph, cells treated with TLR agonists and untreated samples (NC) are indicated. Bars represent mean +/− SEM of n=3 individual donors. B. Summary of data plotted as spider plot. * p<0.05, measured by one way ANOVA and post test.
3.3. Use of inhibitors suggests p38 and JNK but not MEK leads to TLR ligand induced chemokine release
To determine the influence of different intracellular signalling pathways on TLR-dependent cytokine expression, DC were pre-treated with signalling pathway inhibitors 30 minutes prior to stimulation with R848, GLA or Poly I:C alone or in combination. The cells were treated with the inhibitors, SB202190, SP600125 and PD98059 which are all mitogen-activated protein (MAP) kinase inhibitors that block p38, JNK pathway and MEK1 respectively, or PDTC which inhibits the NF-κB pathway. Distinct patterns of inhibition were observed for different cytokines (Fig 4). As seen before, for the cytokines IL-1α (Fig 4A), IL-1β (Fig 4B), IL-12 (Fig 4C) and IL-6 (Fig 4D) the combination of R848 and GLA led to a significantly enhanced response compared to the ligands alone. Pre-treatment of the cells with the p38 inhibitor (SB20) or the JNK inhibitor (SP60) significantly reduced the level of IL-1α, IL-1β, IL-6 and IL-12 detected, but pre-treatment with the MEK1 inhibitor (PD98) had no effect. For the chemokine CCL3 (Fig 4E), the response to R848 and GLA alone or together was dependent upon p38 and JNK. A similar pattern was seen for CCL5 (Fig 4F), but was not significant. For CXCL10 (Fig 4G), the inhibitors SB20 (p38) and SP60 (JNK) blocked the effect of R848 and GLA. For CCL8 (Fig 4H) only SP60 (JNK) had an inhibitory effect. For any of the combinations tested PD98 (MEK1 inhibitor) had no effect on cytokine or chemokine release. PDTC had no effect on most of the mediators, with a limited effect on IL-1α, CCL3 and CXCL10. As seen in Fig 3, the addition of Poly I:C had little additive effect to the responses induced by R848 and the effect of the inhibitors on the CCL3 response to the Poly I:C/R848 combination can be presumed to be acting on R848 stimulation and not the Poly I:C. From these studies we conclude that in this system R848 (TLR7/8) and GLA (TLR4) exert their effect through p38 and JNK but not MEK1, with limited action through NF-κB.
Figure 4. Effect of R848 and GLA are p38 and JNK dependent.
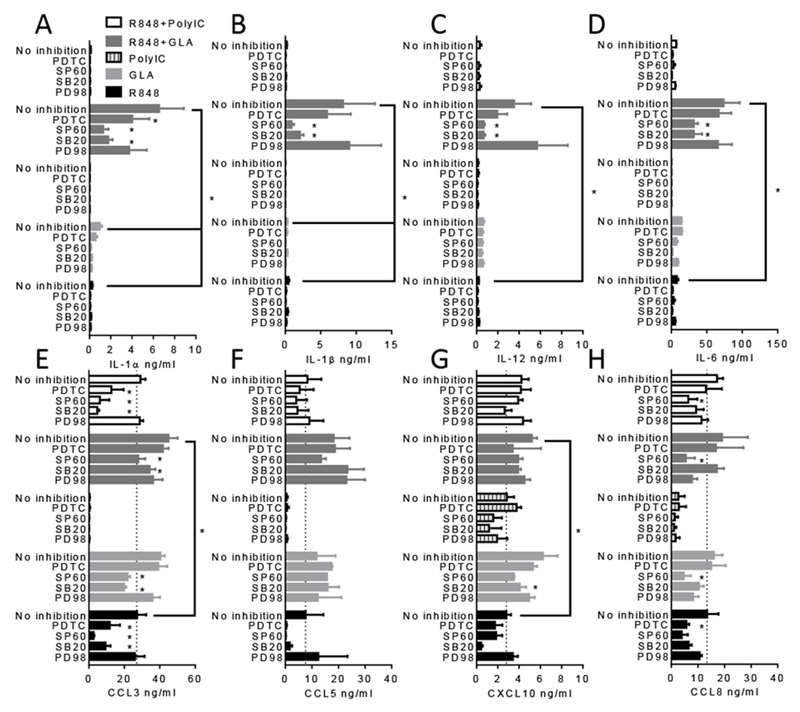
DC were stimulated with 0.5 μg/ml of R848, GLA, Poly I:C or a combination for 24 hours in the presence of signalling inhibitors: PDTC (NF-κB blocker), SP60 (JNK blocker), SB20 (p38 blocker) and PD98 (MEK1 blocker). Inflammatory mediators were then measured in the culture supernatant. In each graph, cells treated with TLR agonists and untreated samples are indicated. * pμ0.01 measured by two way ANOVA and post test.
We also tested which adaptor proteins played a role downstream of the TLR molecules (Fig 5). Dendritic cells were pretreated with inhibitory peptides to 5μM MyD88 or TRIF singly in combination, prior to exposure with 0.5 μg/ml R848 or GLA singly or in combination. MyD88 inhibition significantly reduced the IL-6 (Fig 5A) and IL-1β (Fig 5B) response to R848, it also inhibited the IL-1β response to GLA. There was no significant effect on the IL-6 response to GLA of either inhibitor. Combining R848 and GLA significantly enhanced the IL-6 and IL-1β response, but the inhibitors did not significantly reduce the response.
Figure 5. Effect of R848 and GLA are MyD88 and TRIF dependent.
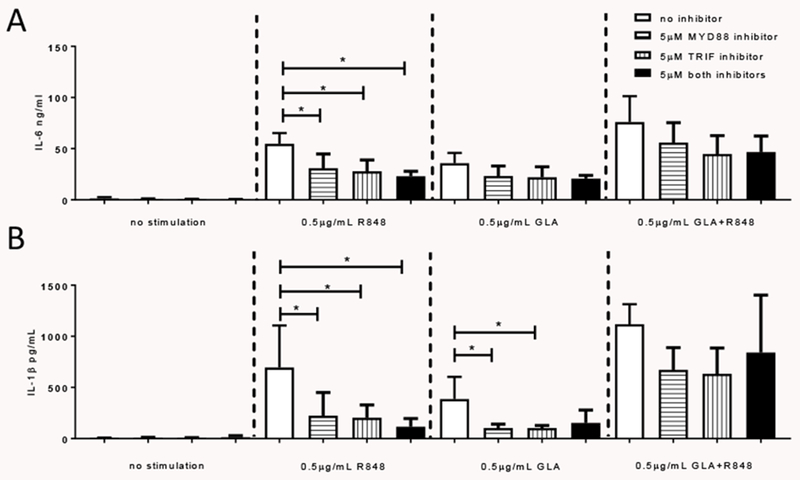
DC were stimulated with 0.5 μg/ml of R848, GLA, Poly I:C or a combination for 24 hours in the presence of MyD88 and TRIF inhibitors. IL-6 (A) and IL-1β (B) were then measured in the culture supernatant. * p<0.05 measured by two way ANOVA and post test.
3.4. Impact of TLR7/8 and TLR4 agonists alone and in combination on expression of DC activation markers.
A second mechanism of action of adjuvants is to change cell surface markers affecting cell-cell interactions and cell migration. We focussed on R848 and GLA in combination as they gave the greatest effect on cytokine release. DC were analysed by flow-cytometry following treatment with R848 and GLA alone or in combination to determine their impact on the expression of cell surface activation markers. HLA-DR and CDllc were highly expressed on resting cells and unmodified by TLR stimulation (Fig 6A, B). The combination of TLR ligands had a less marked additive effect on cell surface markers than inflammatory mediators. Significantly increased expression of CD40, CD80, CD86 and CCR7 (Fig 6C-F) was induced by GLA alone or R848 or GLA in combination compared to untreated cells. R848 alone had less effect on cell surface markers of activation, and only led to the significant upregulation of CD86 compared to controls (Fig 6E). Thus we show that the exposure of cells to TLR ligands induces both inflammatory mediator release and upregulation of cell surface markers.
Figure 6. Impact of TLR ligand combinations on DC surface molecule expression profiles.
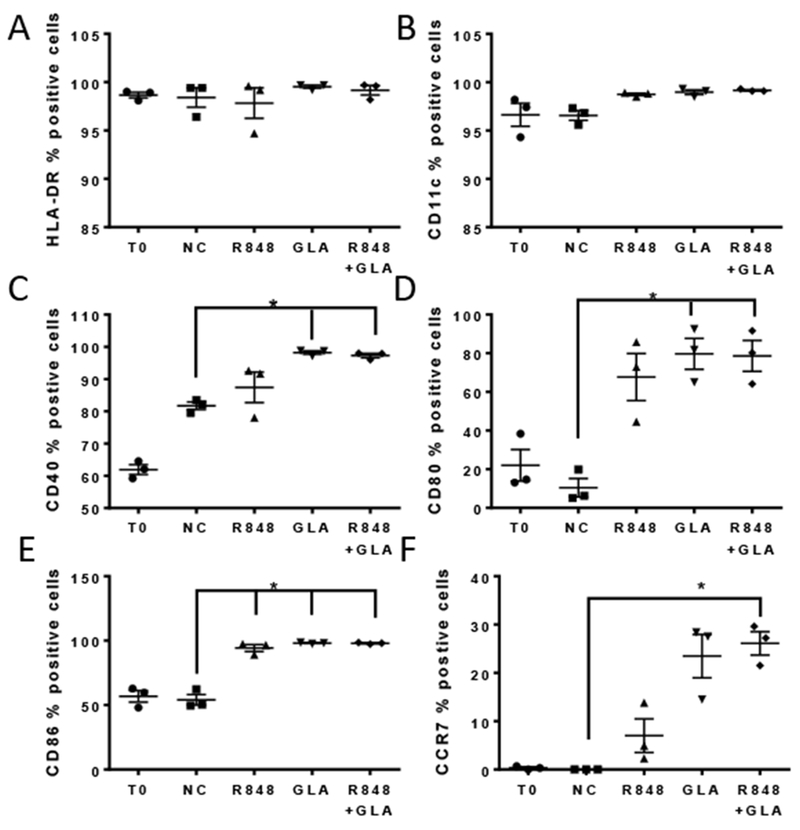
DC were treated for 24 hours with R848 or GLA alone and in combination, or untreated (Negative Control, NC). Responses were also compared with untreated cells fixed at 0 hours after isolation (T0). Cells were stained and levels of surface expression of multiple surface molecules assessed by flow cytometry: HLA-DR (A), CD11c (B), CD40 (C), CD80 (D), CD86 (E) and CCR7 (F). Each graph shows the mean and SEM of the % of positive cells for each surface molecule, as indicated. ** p<0.01 measured by One way ANOVA and post test.
3.5. TLR ligands R848 and GLA can induce inflammatory mediator release in human tissue.
Mucosally delivered vaccines may be beneficial for some of the more difficult to target pathogens [16]. But the mucosal immune system behaves differently to the systemic immune system and furthermore, leukocytes in the context of tissue may behave differently to when they are isolated from blood. To test the effect of potential TLR based adjuvants on human mucosal tissue, we used our established human penile glans explant model [17]. 2–3 mm3 tissue explants, comprising both epithelium and stroma, were treated with GLA and R848 alone or in combination. Cytokines and chemokines were measured by Luminex in supernatants after 24 hours (Fig 7). There was a pattern towards IL-1β, IL-4, GMCSF, TNF, CCL8 and CXCL10 release by R848 alone and IL-4, GMCSF, CCL3, CCL4 and CCL5 by GLA. The combination of R848 and GLA induced a significantly greater CCL3 response than either ligand alone and more CCL4 than untreated samples. From this we conclude that responses by individual cells in isolation reflect the response in tissue and that the compounds tested – GLA and R848 may be effective mucosal adjuvants.
Figure 7. Inflammatory mediator production in human mucosal tissue explant.
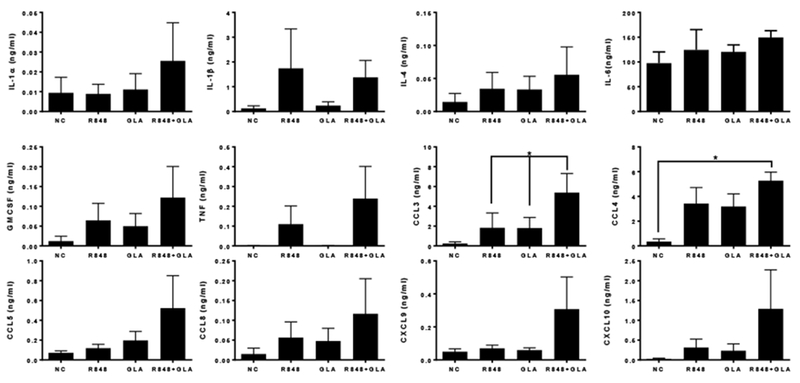
Human penile tissue was stimulated with 5 μg/ml of R848 or GLA or a combination for 24 hours. Inflammatory mediators were then measured in the culture supernatant. In each graph, cells treated with TLR agonists and untreated samples (NC) are indicated. ** p<0.01 measured by One way ANOVA and post test.
4. Discussion
In this study, we compared the inflammatory response to three TLR ligands: Poly I:C (TLR3), GLA (TLR4) and R848 (TLR7/8) in two cell types, dendritic cells and macrophages, and in human tissue. We observed a similar pattern of response to all three ligands in both individual cells and in the tissue explant model, with a larger chemokine than cytokine response. The difference in the levels of mediator measured – with greater chemokine than cytokine responses may reflect the different biological mechanisms of action – chemokines need to diffuse away from the site of infection in order to recruit cells, cytokines act more locally to modulate function. However, relative bioactivity data is needed to confirm this. As seen in other studies [7–9], when the ligands were administered in combination, synergistic effects were seen for some but not all the mediators measured. Interestingly, there was no synergistic effect on the level of DC cell surface markers, either because maximal responses were achieved by the dose of individual ligands used or because cell surface activation has a lower threshold of activation. The presumption was that because TLR3 works through TRIF, combining Poly I:C with MyD88 engaging ligands would have a larger potentiating effect, but this was not observed. Combining R848 and GLA, which both work through MyD88, had a potentiating effect, but TLR4 also works through TRIF which may also contribute. Synergy was also seen with lower doses of GLA, it will be of interest to see if adjuvant dose sparing could be achieved by combinations of lower concentrations.
Specific MAP kinase inhibitors were used to investigate which pathways were important in the action of the TLR ligands when used singly or in combination. GLA works through TLR4 and R848 through TLR7/8 both of which engage MyD88 (Fig 8). MyD88 signals through IRAK1/IRAK4 in combination with TRAF6 to engage a number of MAP kinases including P38 and JNK. Both are downstream of TRAF6 and work through the transcription factor API (c-FOS) leading to the expression of the inflammatory cytokines IL-1β, IL-12 and IL-6, and the chemotactic chemokines CCL3 and CCL5. The MAP kinase inhibitors had a significant effect on the response to GLA and R848. The inhibitor experiments suggested that p38 and JNK, but not MEK1 are important in transducing the response to GLA and R848. From this we conclude that the APl/c-FOS pathways predominates the DC cytokine response. This work is supported by other studies, blocking p38 and JNK, but not ERK reduced TLR ligand synergy on cytokine release [18]; likewise blocking p38 reduced the synergy of LPS and R848 on IL-12p70 [9]. By contrast, in mouse Flt3L induced dendritic cells, the combination of two TLR agonists had mixed effects, and for CCL2 (not measured in the current study) and CCL5, JNK and P38 had no effect on expression [19]. The NF-κB inhibitor PDTC had only a modest effect on cytokine release after R848 and GLA co-administration. This was unexpected given the central role NF-κB has in the inflammatory response and its predicted interaction with the genes encoding the cytokines we were measuring. The signalling pathways downstream of the TLR are complex, with multiple redundancies, for example TLR4 signals through both TRIF and MYD88. Blocking signalling through MYD88 reduced the response to GLA and R848.
Figure 8. Signalling pathways in the TLR response.
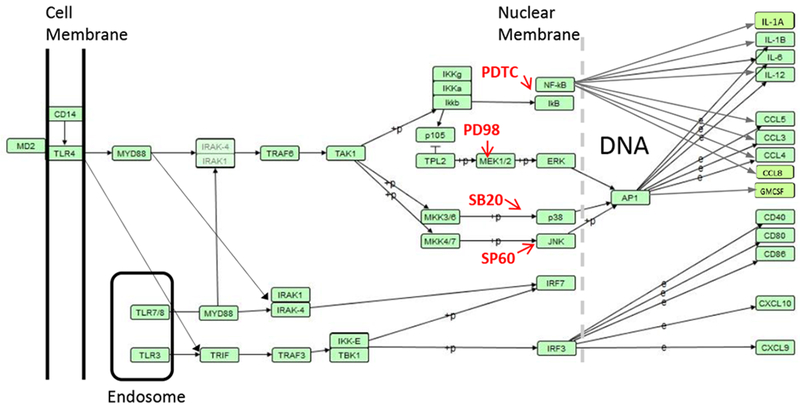
Signalling cascade from KEGG pathway has04620, redrawn using VANTED software.
Although we observed production of mediators with the potential to recruit and activate other cells, we did not assess the functional effect of the response. Ligand induced chemokines and cytokines are associated with Thl (IL-4), Th2 (IL-12), Thl7 (IL-6) responses, however in the absence of cocultured lymphocytes, it is not possible to dissect whether the ligands would alter the nature of the T or B cell response. Previous studies, using human peripheral blood monocyte derived macrophages, have demonstrated synergy between TLR4 and TLR7/8 stimulation leading to enhanced T and B cell responses to a range of antigens including Plasmodium vivax [20] and encapsulated influenza antigen vaccine [21]. Other combinations may also be effective, TLR4/9 combinations can boost responses to tuberculosis [22] or HIV antigens [23]. Combining TLR ligands can also modify the quality of the T cell response: co-culturing allogenic T cell with DCs stimulated with LPS alone induced a TH1 phenotype, whereas combining LPS and R848 induced a TH17 phenotype [24].
Stimulation with GLA and R848 have had mixed results in animals, likely reflecting differences in TLR expression patterns. In mice, GLA and MPLA (both TLR4 agonists) were more effective than R848 as mucosal adjuvants [25, 26], admixing GLA and R848 GLA also had no effect in mice [27]; but mice lack a functional copy of TLR8. Likewise, R848 and GLA administered individually or together had limited effects in cattle [28]. In minipigs, the addition of GLA to R848 significantly boosted the antibody response to the M. tuberculosis antigen ESAT-6 when administered intradermally [29]. In macaques, R848 was effective when delivered intranasally or systemically [30]. Using human mucosal tissue we observed significant additive effects of GLA and R848 on the CCL3 and CCL4 response, indicating the functionality of TLR4 and TLR 7/8 in human tissue.
R848, GLA and Poly I:C have been tested in clinical trials and shown to be safe and well tolerated. Topical R848 has been used in a phase I clinical trial for a cancer vaccine [31], and boosted responses to injected influenza vaccine when delivered topically in a clinical trial [32]. The addition of GLA led to substantially improved antibody responses to haemagglutinin in a clinical study [33]. Poly I:C has been used safely with live attenuated influenza vaccine, but there was no antigen alone control, so it is hard to determine whether it boosted responses [34]. Based on data presented here on the cytokine and chemokine release induced by R848 and GLA together on both cells and tissue and our experience in macaques and minipigs we would suggest that the combination of the two TLR ligands could be an effective adjuvant for vaccines against infectious diseases.
Supplementary Material
Acknowledgments
This work was funded by a grant to RJS by the Center for HIV/AIDS Vaccine Immunology (CHAVI) # U19 AI067854-05 and support from the Innovative Medicines Initiative Joint Undertaking under grant agreement n° [115308] Biovacsafe, resources of which are composed of financial contribution from the European Union’s Seventh Framework Programme (FP7/2007-2013) and EFPIA members’ in kind contribution. We gratefully acknowledge Dormeur Investment Service Ltd for providing funds to purchase equipment used in these studies. We also wish to thank Naomi Armanasco and Abbey Evans for coordinating human tissue collection. GLA was kindly supplied by Dr Darrick Carter, Infectious Disease Research Institute (IDRI), Seattle, USA.
References
- [1].Leroux-Roels G, Unmet needs in modern vaccinology: adjuvants to improve the immune response, Vaccine 28 Suppl 3 (2010) C25–36. [DOI] [PubMed] [Google Scholar]
- [2].Awate S, Babiuk LA, Mutwiri G, Mechanisms of action of adjuvants, Frontiers in immunology 4 (2013) 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Maisonneuve C, Bertholet S, Philpott DJ, De Gregorio E, Unleashing the potential of NOD- and Toll-like agonists as vaccine adjuvants, Proceedings of the National Academy of Sciences of the United States of America 111(34) (2014) 12294–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Akashi-Takamura S, Miyake K, TLR accessory molecules, Current opinion in immunology 20(4) (2008) 420–5. [DOI] [PubMed] [Google Scholar]
- [5].Bagchi A, Herrup EA, Warren HS, Trigilio J, Shin HS, Valentine C, Hellman J, MyD88-dependent and MyD88-independent pathways in synergy, priming, and tolerance between TLR agonists, Journal of immunology 178(2) (2007) 1164–71. [DOI] [PubMed] [Google Scholar]
- [6].Tan RS, Ho B, Leung BP, Ding JL, TLR cross-talk confers specificity to innate immunity, Int Rev Immunol 33(6) (2014) 443–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Napolitani G, Rinaldi A, Bertoni F, Sallusto F, Lanzavecchia A, Selected Toll-like receptor agonist combinations synergistically trigger a T helper type 1-polarizing program in dendritic cells, Nat Immunol 6(8) (2005) 769–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Mäkelä SM, Strengell M, Pietilä TE, Österlund P, Julkunen I, Multiple signaling pathways contribute to synergistic TLR ligand-dependent cytokine gene expression in human monocyte-derived macrophages and dendritic cells, Journal of Leukocyte Biology 85(4) (2009) 664–672. [DOI] [PubMed] [Google Scholar]
- [9].Bohnenkamp HR, Papazisis KT, Burchell JM, Taylor-Papadimitriou J, Synergism of Toll-like receptor-induced interleukin-12p70 secretion by monocyte-derived dendritic cells is mediated through p38 MAPK and lowers the threshold of T-helper cell type I responses, Cellular Immunology 247(2) (2007) 72–84. [DOI] [PubMed] [Google Scholar]
- [10].Knudsen NP, Olsen A, Buonsanti C, Follmann F, Zhang Y, Coler RN, Fox CB, Meinke A, U DO, Casini D, Bonci A, Billeskov R, De Gregorio E, Rappuoli R, Harandi AM, Andersen P, Agger EM, Different human vaccine adjuvants promote distinct antigen-independent immunological signatures tailored to different pathogens, Scientific reports 6 (2016) 19570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lewis DJ, Lythgoe MP, Application of “Systems Vaccinology” to Evaluate Inflammation and Reactogenicity of Adjuvanted Preventative Vaccines, J Immunol Res 2015 (2015) 909406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Loiarro M, Sette C, Gallo G, Ciacci A, Fantò N, Mastroianni D, Carminati P, Ruggiero V, Peptide-mediated Interference of TIR Domain Dimerization in MyD88 Inhibits Interleukin-1-dependent Activation of NF-κB, Journal of Biological Chemistry 280(16) (2005) 15809–15814. [DOI] [PubMed] [Google Scholar]
- [13].Toshchakov VU, Basu S, Fenton MJ, Vogel SN, Differential involvement of BB loops of toll-IL-1 resistance (TIR) domain-containing adapter proteins in TLR4- versus TLR2-mediated signal transduction, Journal of immunology 175(1) (2005) 494–500. [DOI] [PubMed] [Google Scholar]
- [14].Fichorova RN, Richardson-Harman N, Alfano M, Belec L, Carbonneil C, Chen S, Cosentino L, Curtis K, Dezzutti CS, Donoval B, Doncel GF, Donaghay M, Grivel JC, Guzman E, Hayes M, Herold B, Hillier S, Lackman-Smith C, Landay A, Margolis L, Mayer KH, Pasicznyk JM, Pallansch-Cokonis M, Poli G, Reichelderfer P, Roberts P, Rodriguez I, Saidi H, Sassi RR, Shattock R, Cummins JE Jr., Biological and technical variables affecting immunoassay recovery of cytokines from human serum and simulated vaginal fluid: a multicenter study, Analytical chemistry 80(12) (2008) 4741–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Junker BH, Klukas C, Schreiber F, VANTED: A system for advanced data analysis and visualization in the context of biological networks, BMC Bioinformatics 7(1) (2006) 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Neutra MR, Kozlowski PA, Mucosal vaccines: the promise and the challenge, Nature Reviews Immunology 6(2) (2006) 148–158. [DOI] [PubMed] [Google Scholar]
- [17].Fischetti L, Barry SM, Hope TJ, Shattock RJ, HIV-1 infection of human penile explant tissue and protection by candidate microbicides, Aids 23(3) (2009) 319–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hirata N, Yanagawa Y, Ebihara T, Seya T, Uematsu S, Akira S, Hayashi F, Iwabuchi K, Onoe K, Selective synergy in anti-inflammatory cytokine production upon cooperated signaling via TLR4 and TLR2 in murine conventional dendritic cells, Molecular immunology 45(10) (2008) 2734–42. [DOI] [PubMed] [Google Scholar]
- [19].Mitchell D, Olive C, Regulation of Toll-like receptor-induced chemokine production in murine dendritic cells by mitogen-activated protein kinases, Molecular immunology 47(11–12) (2010) 2065–2073. [DOI] [PubMed] [Google Scholar]
- [20].Wiley SR, Raman VS, Desbien A, Bailor HR, Bhardwaj R, Shakri AR, Reed SG, Chitnis CE, Carter D, Targeting TLRs expands the antibody repertoire in response to a malaria vaccine, Sci Transl Med 3(93) (2011) 93ra69. [DOI] [PubMed] [Google Scholar]
- [21].Kasturi SP, Skountzou I, Albrecht RA, Koutsonanos D, Hua T, Nakaya HI, Ravindran R, Stewart S, Alam M, Kwissa M, Villinger F, Murthy N, Steel J, Jacob J, Hogan RJ, Garcia-Sastre A, Compans R, Pulendran B, Programming the magnitude and persistence of antibody responses with innate immunity, Nature 470(7335) (2011) 543–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Orr MT, Beebe EA, Hudson TE, Moon JJ, Fox CB, Reed SG, Coler RN, A dual TLR agonist adjuvant enhances the immunogenicity and protective efficacy of the tuberculosis vaccine antigen ID93, PloS one 9(1) (2014) e83884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Moody MA, Santra S, Vandergrift NA, Sutherland LL, Gurley TC, Drinker MS, Allen AA, Xia SM, Meyerhoff RR, Parks R, Lloyd KE, Easterhoff D, Alam SM, Liao HX, Ward BM, Ferrari G, Montefiori DC, Tomaras GD, Seder RA, Letvin NL, Haynes BF, Toll-like receptor 7/8 (TLR7/8) and TLR9 agonists cooperate to enhance HIV-1 envelope antibody responses in rhesus macaques, Journal of virology 88(6) (2014) 3329–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lombardi V, Van Overtvelt L, Horiot S, Moingeon P, Human dendritic cells stimulated via TLR7 and/or TLR8 induce the sequential production of Il-10, IFN-gamma, and IL-17A by naive CD4+ T cells, Journal of immunology 182(6) (2009) 3372–9. [DOI] [PubMed] [Google Scholar]
- [25].Arias MA, Van Roey GA, Tregoning JS, Moutaftsi M, Coler RN, Windish HP, Reed SG, Carter D, Shattock RJ, Glucopyranosyl Lipid Adjuvant (GLA), a Synthetic TLR4 Agonist, Promotes Potent Systemic and Mucosal Responses to Intranasal Immunization with HIVgpl40, PloS one 7(7) (2012) e41144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Buffa V, Klein K, Fischetti L, Shattock RJ, Evaluation of TLR Agonists as Potential Mucosal Adjuvants for HIV gpl40 and Tetanus Toxoid in Mice, PloS one 7(12) (2012) e50529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Schwenk R, DeBot M, Porter M, Nikki J, Rein L, Spaccapelo R, Crisanti A, Wightman PD, Ockenhouse CF, Dutta S, IgG2 antibodies against a clinical grade Plasmodium falciparum CSP vaccine antigen associate with protection against transgenic sporozoite challenge in mice, PloS one 9(10) (2014) elll020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Jones GJ, Steinbach S, Clifford D, Baldwin SL, Ireton GC, Coler RN, Reed SG, Vordermeier HM, Immunisation with ID83 fusion protein induces antigen-specific cell mediated and humoral immune responses in cattle, Vaccine 31(45) (2013) 5250–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].McKay PF, King DFL, Mann JFS, Barinaga G, Carter D, Shattock RJ, TLR4 and TLR7/8 Adjuvant Combinations Generate Different Vaccine Antigen-Specific Immune Outcomes in Minipigs when Administered via the ID or IN Routes, PloS one 11(2) (2016) e0148984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Veazey RS, Siddiqui A, Klein K, Buffa V, Fischetti L, Doyle-Meyers L, King D, Tregoning JS, Shattock RJ, Evaluation of mucosal adjuvants and immunisation routes for the induction of systemic and mucosal humoral immune responses in macaques., Human Vaccines and Immunotherapeutics In Press (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Dhodapkar MV, Sznol M, Zhao B, Wang D, Carvajal RD, Keohan ML, Chuang E, Sanborn RE, Lutzky J, Powderly J, Kluger H, Tejwani S, Green J, Ramakrishna V, Crocker A, Vitale L, Yellin M, Davis T, Keler T, Induction of Antigen-Specific Immunity with a Vaccine Targeting NY-ESO-1 to the Dendritic Cell Receptor DEC-205, Science Translational Medicine 6(232) (2014) 232ra51–232ra51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Hung IF, Zhang AJ, To KK, Chan JF, Li P, Wong TL, Zhang R, Chan TC, Chan BC, Wai HH, Chan LW, Fong HP, Hui RK, Kong KL, Leung AC, Ngan AH, Tsang LW, Yeung AP, Yiu GC, Yung W, Lau JY, Chen H, Chan KH, Yuen KY, Topical imiquimod before intradermal trivalent influenza vaccine for protection against heterologous non-vaccine and antigenically drifted viruses: a single-centre, double-blind, randomised, controlled phase 2b/3 trial, The Lancet infectious diseases 16(2) (2016) 209–18. [DOI] [PubMed] [Google Scholar]
- [33].Treanor JJ, Essink B, Hull S, Reed S, Izikson R, Patriarca P, Goldenthal KL, Kohberger R, Dunkle LM, Evaluation of safety and immunogenicity of recombinant influenza hemagglutinin (H5/Indonesia/05/2005) formulated with and without a stable oil-in-water emulsion containing glucopyranosyl-lipid A (SE+GLA) adjuvant, Vaccine 31(48) (2013) 5760–5. [DOI] [PubMed] [Google Scholar]
- [34].Overton ET, Goepfert PA, Cunningham P, Carter WA, Horvath J, Young D, Strayer DR, Intranasal seasonal influenza vaccine and a TLR-3 agonist, rintatolimod, induced cross-reactive IgA antibody formation against avian H5N1 and H7N9 influenza HA in humans, Vaccine 32(42) (2014) 5490–5495. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


