Abstract
Corneal trauma/injury often results in serious complications including permanent vision loss or loss of visual acuity which demands corneal transplantations or treatment with allogenic graft tissues. There is currently a huge shortage of donor tissue worldwide and the need for human corneal equivalents increases annually. In order to meet such demand the current clinical approach of treating corneal injuries is limited and involves synthetic and allogenic materials which have various shortcomings when it comes to actual transplantations. In this study we introduce the newly developed, next generation of our previously established 3D self-assembled constructs, where multiple constructs are grown and stacked on top of each other without any other artificial product. This new technology brings our 3D in vitro model closer to what is seen in vivo and provides a solid foundation for future studies on corneal biology.
Lipids are known for playing a vital role during metabolism and diseased state of various tissues and Sphingolipids are one such class of lipids which are involved in various cellular mechanisms and signaling processes. The impacts of Sphingolipids that have been documented in several human diseases often involve inflammation, neovascularization, tumorigenesis, and diabetes, but these conditions are not yet thoroughly studied. There is very little information about the exact role of Sphingolipids in the human cornea and future studies aiming at dissecting the mechanisms and pathways involved in order to develop novel therapies. We believe that our novel 3D stacked model can be used to delineate the role of Sphingolipids in the human cornea and provide new insights for understanding and treating various human corneal diseases.
Keywords: 3D constructs, Cornea, Extra cellular matrix, Sphingolipids, Stacking
1. Introduction
In recent years tissue engineering applications have garnered great interests across various fields of medical science in order to treat various diseased conditions. The vast implication of tissue engineering using different biomaterials has been a great success, yet there are various limitations when it comes to actual applications due to a number of contributory factors such as immune response to foreign body or material, synthetic materials fail to respond to the changing physiological loads or biochemical stimuli which limit the lifetime of artificial body parts, graft rejections, infection, glaucoma, retinal detachment and extrusion [1, 2]. The application of tissue engineering in treating ocular dystrophies has also stimulated great interest and has been a great success over the past years [3–7].
Wound healing is one of the major challenges when it comes to treating ocular injuries. It often leads to scarring resulting in either partial vision loss or permanent blindness. The process of corneal wound healing is complex; it involves interactions between the wound-healing epithelium, a temporary “provisional matrix,” and cells present in the extracellular matrix (ECM) [8]. During this process the wounds either tend to heal in a regenerative manner, where the tissue returns to its original state, or in a fibrotic manner, where a scar is produced.
Being able to treat corneal injuries without scarring and be able to mimic the actual in vivo process remains elusive. In vitro there have been a number of models investigated and proposed [2, 8–17]. 3D in vitro models are of great interest due to their potential of mirroring cellular and physiological events that are very important during fibrosis and wound healing [8, 9, 18, 19]. In the cornea, the elucidation of using 3D in vitro systems is imperative in order to improve treatments and lead us to the identification of new therapeutic approaches. Our original 3D in vitro model has been well studied and has shown the impeccable ability of recapitulating in vivo events in vitro [18–20] but one of the biggest limitations of our model is that the cells have limited proliferative potential and can only assemble a certain amount of ECM. Such limitations have been partially overcome by stimulating with various growth factors, mainly transforming growth factor-β (TGF-β) isoforms which aid the cells in stimulating, secreting, and assembling double or triple the amount of ECM [18, 21, 22]. Even with TGF-β stimulation, however, the ECM assembled does not exceed 120–150 μm over 4 weeks, when a human corneal stroma is approximately triple in thickness [18,21,23]. Thus, a 3D in vitro model that closely mimics the corneal stroma in size would lead to more accurate results and a better understanding of cellular and ECM mechanisms. The herein described 3D self-assembled stacked model represents the latest generation of our promising in vitro model.
Sphingolipids (SPLs) are known to be involved in human diseases associated with inflammation, neovascularization, tumorigenesis, and diabetes; however, their roles associated with these diseases remain understudied and not fully understood [24, 25]. Bioactive SPLs such as Sphingosine-1-phosphate (S1P) and Ceramide (Cer) have been acknowledged as being essential mediators of many basic cellular processes such as cell migration, survival, contraction, proliferation, gene expression, and cell–cell interactions [26]. S1P and Cer actions/levels are regulated by Ceramidase enzymes; their ability to regulate diverse cellular processes has grasped the attention and interest of researchers due to their capabilities of regulating tissue fibrosis in various organ systems by utilizing S1P and/or Cer [24,27, 28]. Among the fields ofinterest pertaining to SPLs, the cornea remains one of the most scarcely studied. There are currently only a few publications that reported the presence of SPLs in the cornea. Swaney et al. [28] reported the presence of Sphingosine kinase-1 (SphK1), Sphingosine kinase-2 (SphK2), and S1P1-3,5 receptor proteins in cultured human primary corneal fibroblasts (HCFs). Watsky et al. [29] observed expression of S1P receptor’s mRNA in cultured corneal epithelial cells which mimicked wound healing responses in vivo. In a recent study, our group showed significant differences in total composition and specific SPL subspecies in the healthy cornea compared to the diabetic cornea [30].
The 3D in vitro model described in detail here can be used in order to investigate the role of SPLs in the healthy and the diseased human cornea while providing new insights in treating ocular dystrophies with better clinical results.
2. Materials
Corneal samples obtained should only be used for scientific purposes and ethical permission must be obtained prior conducting any further experiments. The corneal tissue samples should be from donors with no history of ocular trauma or systemic disease. All reagents and media used should be completely sterile and all the protocols must be initiated in a sterile Laminar flow hood. The storage temperature of the media should be at 4 °C. Waste material should be disposed as per the proper disposal regulations.
2.1. Cell Isolation and Culture
Healthy corneal tissue samples from donors with no ocular trauma or systemic disease.
Dulbecco’s Phosphate Buffered Solution (1×).
Sterile forceps.
Single edge razor blades and sterile surgical scalpel blades No. 10.
Eagle’s Minimum Essential Medium (American Type Culture Collection, Manassas, VA, USA) containing 10% FBS and 1% antibiotic. 6.0.05% Trypsin-EDTA (1×).
2.2. 3D Constructs Assembly
Polycarbonate membrane inserts with 0.4-μm pores (Corning Costar; Corning Incorporated, Corning, NY, USA).
Eagle’s Minimum Essential Medium containing 10% FBS and 1% Antibiotic.
0.5 mM 2-O-α-Dglucopyranosyl-l-ascorbic acid (Vitamin C).
2.3. Stacked Constructs
Sterile forceps.
Sterile Spatula.
Wax block.
Dulbecco’s Phosphate Buffered Solution (1×).
2.4. S1P Stock Preparation
S1P stock solution was prepared at a concentration of 125 μM for all S1P treatments by dissolving S1P powder in 4 mg/ml of BSA in water at 37 °C in a glass vessel.
SphKI2 is a selective inhibitor of SphK1 [31] and a stock solution was made at a concentration of 5 mM by dissolving the powder in DMSO.
3. Methods
3.1. Cell Isolation
On receipt of the corneal tissue samples, the tissues should be transferred into a petri dish containing DPBS (1×).
The corneal epithelium and endothelium should be removed from the stroma by scraping with a razor blade.
The corneal stromal tissues are further cut into small pieces of size ~2 × 2 mm and placed into T25 culture flaks.
Explants then should be allowed to adhere to the bottom of the flask at 37 °C for about 30–40 min and then EMEM media containing 10% fetal bovine serum and 1% antibiotic needs to be added carefully without disturbing the explants.
The explants should be left undisturbed until the cells begin isolating and migrating through the flask and further they require passage into T75 culture flasks upon 100% confluence after 1–2 weeks of cultivation at 37 °C, 5% CO2.
3.2. Culture of Primary Human Corneal Fibroblast Cells and Assembly of 3D Constructs
HCF cells isolated from explants are cultured in Eagle’s Minimum Essential Medium containing 10% fetal bovine serum and 1% antibiotic.
Fresh media needs to be supplied every other day for the entire duration of culture. The cultures need to be passaged upon 80–100% confluence.
For assembly of 3D constructs about 1 × 106 cells/well of HCF cells need to be counted and seeded on polycarbonate membrane inserts with 0.4-μm pores (Fig. 1) (see Note 1).
The constructs need to be grown in Eagle’s Minimum Essential Medium containing 10% fetal bovine serum and 1% antibiotic and after 24 h of cell seeding the cultures need to be stimulated with 0.5 mM 2-O-α-Dglucopyranosyl-l-ascorbic acid (Vitamin C) (see Note 2).
The cultures should be maintained for 2 weeks time point and fresh media should be supplied every other day during the entire study period.
Fig. 1.
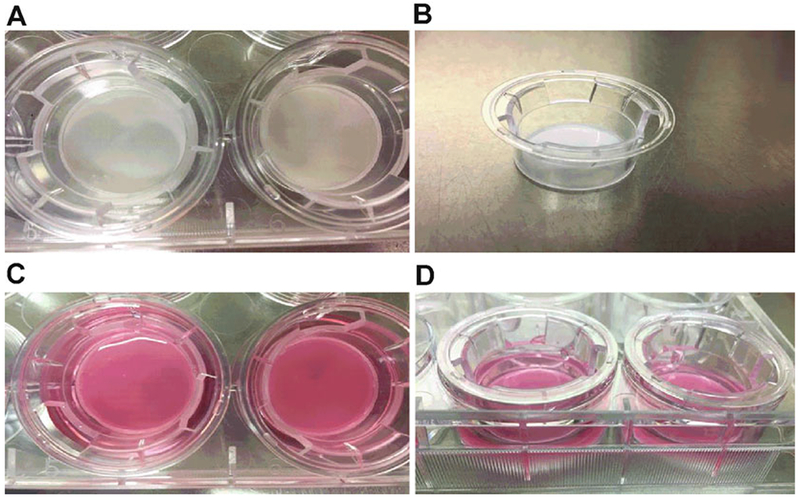
3D constructs assembly using polycarbonate membrane inserts. (a, b) Polycarbonate membrane plate and respective inserts with 0.4-μm pores. (c) Human corneal fibroblast grown in EMEM media containing 10% fetal bovine serum and 1% antibiotic. (d) Cells stimulated with media containing 0.5 mM 2-O-α-Dglucopyranosyl-l-ascorbic acid (Vitamin C)
3.3. Stacking of 3D Constructs
3D constructs maintained for 2 weeks are used for stacking. Firstly, the media needs to be aspirated and the constructs should be washed with sterile DPBS (1×) twice.
Further remove the constructs on to a wax block with sterile forceps and gently detach the edges of the membrane from the plastic with the help of a sterile spatula.
Now, slowly peel the ECM secreted inwards from one edge of the membrane without any breakage and transfer to a 21.5 cm2 petri dish containing 2 ml of sterile DPBS (1×) (see Note 3).
Aspirate media from another construct well designated to be stacked upon and wash it with DPBS (1×) twice. Transfer the detached construct on top of the second designated construct well using forceps and ensure the transferred construct is folded inwards (see Note 4). Spread the construct gently with the help of forceps and spatula ensuring even attachment covering the entire construct well (see Notes 4 and 5).
Incubate the construct for about 15–20 min in about 150–200 μl culture media at 37 °C to allow proper attachment to the base construct and also to prevent floating. Gently add media to the stacked constructs without disturbing or detaching the top construct from the bottom layer.
Repeat stacking every other day until 8 constructs are stacked all together and change the media 3 times per week (Fig. 2).
Fig. 2.
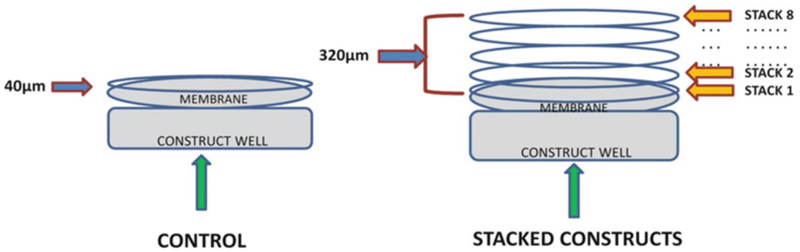
Stacking of 3D constructs. In controls the cells seeded on the polycarbonate membrane secrete an ECM of about 40 μm thickness whereas in the stacked constructs the total thickness of the ECM makes about 320 μm
3.4. Sphingolipid Analysis
Lipid extraction from human corneas needs to follow our previously optimized protocol [30].
Samples should be analyzed using targeted LC MS/MS methods. Using targeted lipidomics analysis the changes in SPLs profile in the samples should be identified.
Acknowledgement
This work was supported by NIH/NEI EY025256.
4 Notes
During assembly of constructs make sure to have an even cell suspension without any cell lumps and distribute evenly throughout each well by pipetting up and down in order to avoid construct contractions.
While preparing the Vitamin C (0.5 mM 2-O-α-Dglucopyranosyl-l-ascorbic acid) media, take about 12 ml of media to which dissolve 0.5 mM 2-O-α-Dglucopyranosyl-l-ascorbic acid and incubate it for about 15 min in order to ensure even dissolving of Vitamin C. Further filter this Vitamin C solution to the entire bottle of culture media.
While peeling off the matrix from the membrane try to slowly roll one edge of the matrix initially in order to avoid breakage. When reached half way through the membrane can be slowly peeled off with forceps in one stroke.
When the matrix is transferred to the petri dish containing PBS it spreads out flat, exactly the way it’s peeled, which clearly gives an idea about the matrix initial orientation and making it easier to identify the top and bottom of the matrix. Ensure spreading the construct the same manner as that of its initial orientation.
Ensure even spreading of the corneal matrix without any creases, this would help in avoiding contraction of the corneal matrix.
References
- 1.Akpek EK, Alkharashi M, Hwang FS, Ng SM, Lindsley K (2014) Artificial corneas versus donor corneas for repeat corneal transplants. Cochrane Database Syst Rev CD009561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chen FM, Liu X (2016) Advancing biomaterials of human origin for tissue engineering. Prog Polym Sci 53:86–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karamichos D, Brown RA, Mudera V (2007) Collagen stiffness regulates cellular contraction and matrix remodeling gene expression. J Biomed Mater Res A 83:887–894 [DOI] [PubMed] [Google Scholar]
- 4.Griffith LG, Naughton G (2002) Tissue engineering—current challenges and expanding opportunities. Science 295:1009–1014 [DOI] [PubMed] [Google Scholar]
- 5.Ruberti JW, Zieske JD (2008) Prelude to corneal tissue engineering—gaining control of collagen organization. Prog Retin Eye Res 27:549–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guo X, Hutcheon AE, Melotti SA, Zieske JD, Trinkaus-Randall V et al. (2007) Morphologic characterization of organized extracellular matrix deposition by ascorbic acid-stimulated human corneal fibroblasts. Invest Ophthalmol Vis Sci 48:4050–4060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Karamichos D (2015) Ocular tissue engineering: current and future directions. J Funct Biomater 6:77–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zieske JD (2001) Extracellular matrix and wound healing. Curr Opin Ophthalmol 12:237–241 [DOI] [PubMed] [Google Scholar]
- 9.Karamichos D, Guo XQ, Hutcheon AE, Zieske JD (2010) Human corneal fibrosis: an in vitro model. Invest Ophthalmol Vis Sci 51:1382–1388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Priyadarsini S, Sarker-Nag A, Rowsey TG, Ma JX, Karamichos D (2016) Establishment of a 3D in vitro model to accelerate the development of human therapies against corneal diabetes. PLoS One 11:e0168845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karamichos D, Hjortdal J (2014) Keratoconus: tissue engineering and biomaterials. J Funct Biomater 5:111–134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wilson SL, Yang Y, el Haj AJ (2014) Corneal stromal cell plasticity: in vitro regulation of cell phenotype through cell-cell interactions in a three-dimensional model. Tissue Eng A 20:225–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Proulx S, Uwamaliya JD, Carrier P, Deschambeault A, Audet C et al. (2010) Reconstruction of a human cornea by the self-assembly approach of tissue engineering using the three native cell types. Mol Vis 16:2192–2201 [PMC free article] [PubMed] [Google Scholar]
- 14.Gonzalez-Andrades M, Alonso-Pastor L, Mauris J, Cruzat A, Dohlman CH et al. (2016) Establishment of a novel in vitro model of stratified epithelial wound healing with barrier function. Sci Rep 6:19395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hopkins AM, DeSimone E, Chwalek K, Kaplan DL (2015) 3D in vitro modeling of the central nervous system. Prog Neurobiol 125:1–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schulz S, Beck D, Laird D, Steinberg T, Tomakidi P et al. (2014) Natural corneal cell-based microenvironment as prerequisite for balanced 3D corneal epithelial morphogenesis: a promising animal experiment-abandoning tool in ophthalmology. Tissue Eng Part C Meth 20:297–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghezzi CE, Rnjak-Kovacina J, Kaplan DL (2015) Corneal tissue engineering: recent advances and future perspectives. Tissue Eng Part B Rev 21:278–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karamichos D, Hutcheon AE, Zieske JD (2011) Transforming growth factor-beta3 regulates assembly of a non-fibrotic matrix in a 3D corneal model. J Tissue Eng Regen Med 5: e228–e238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karamichos D, Lakshman N, Petroll WM (2009) An experimental model for assessing fibroblast migration in 3-D collagen matrices. Cell Motil Cytoskeleton 66:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karamichos D, Zareian R, Guo X, Hutcheon AE, Ruberti JW et al. (2012) Novel in vitro model for Keratoconus disease. J Funct Biomater 3:760–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karamichos D, Rich CB, Zareian R, Hutcheon AE, Ruberti JW et al. (2013) TGF-β3 stimulates stromal matrix assembly by human corneal keratocyte-like cells. Invest Ophthalmol Vis Sci 54:6612–6619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karamichos D, Hutcheon AE, Zieske JD (2014) Reversal of fibrosis by TGF-beta3 in a 3d in vitro model. Exp Eye Res 124:31–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saika S (2006) TGFbeta pathobiology in the eye. Lab Invest 86:106–115 [DOI] [PubMed] [Google Scholar]
- 24.Coant N, Sakamoto W, Mao C, Hannun YA (2016) Ceramidases, roles in sphingolipid metabolism and in health and disease. Adv Biol Regul 63:122–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brush RS, Tran JT, Henry KR, McClellan ME, Elliott MH et al. (2010) Retinal sphingolipids and their very-long-chain fatty acid-containing species. Invest Ophthalmol Vis Sci 51:4422–4431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shea BS, Brooks SF, Fontaine BA, Chun J, Luster AD et al. (2010) Prolonged exposure to sphingosine 1-phosphate receptor-1 agonists exacerbates vascular leak, fibrosis, and mortality after lung injury. Am J Respir Cell Mol Biol 43:662–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shea BS, Tager AM (2012) Sphingolipid regulation of tissue fibrosis. Open Rheumatol J 6:123–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Swaney JS, Moreno KM, Gentile AM, Sabbadini RA, Stoller GL (2008) Sphingosine-1-phosphate (S1P) is a novel fibrotic mediator in the eye. Exp Eye Res 87:367–375 [DOI] [PubMed] [Google Scholar]
- 29.Watsky MA, Weber KT, Sun Y, Postlethwaite A (2010) New insights into the mechanism of fibroblast to myofibroblast transformation and associated pathologies. Int Rev Cell Mol Biol 282:165–192 [DOI] [PubMed] [Google Scholar]
- 30.Priyadarsini S, McKay TB, Sarker-Nag A, Allegood J, Chalfant C et al. (2016) Complete metabolome and lipidome analysis reveals novel biomarkers in the human diabetic corneal stroma. Exp Eye Res 153:90–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.French KJ, Schrecengost RS, Lee BD, Zhuang Y, Smith SN et al. (2003) Discovery and evaluation of inhibitors of human sphingosine kinase. Cancer Res 63:5962–5969 [PubMed] [Google Scholar]


