Abstract
Perinatal Bisphenol-A (BPA) exposure reduces fertility and fecundity in mice. This study examined effects of early BPA exposure on activation of gonadotropin releasing hormone (GnRH) neurons in conjunction with a steroid-induced luteinizing hormone (LH) surge, characterized patterns of estrous cyclicity and fertility over time, and assessed the ovarian follicular reserve to further explore factors responsible for the reduced fertility we previously described in this model. The percent activated GnRH neurons was reduced in BPA-exposed females at 3–6 months, and periods of persistent proestrus were increased. These data suggest that perinatal exposure to BPA reduces GnRH neuronal activation required for the generation of the LH surge and estrous cyclicity. Assessments of anti-Müllerian hormone (AMH) levels failed to suggest a decline in the follicular reserve at the BPA exposure levels examined.
1. Introduction
Reproductive success is central to the propagation of species and hence it is not surprising that the processes maintaining reproduction are robust as exemplified by the existence of more than one path to ovulation. Some mammals, including humans and rodents, experience spontaneous ovulatory cycles that are temporally mediated by estrogen positive feedback. Multiple species also experience postpartum estrus in which ovulation is triggered by dynamic changes in the hormones of pregnancy [1], and may also involve the vagino-cervical stimulation of labor [2]. Other species are induced to ovulate as a direct neural response to genital somatosensory stimuli during mating and are termed ‘reflex ovulators’ [3]. In some circumstances spontaneous ovulators can also manifest reflex ovulation [3,4]. In all cases, ovulation is dependent on the release of the neuropeptide gonadotropin releasing hormone (GnRH) from the hypothalamus to trigger a preovulatory surge of luteinizing hormone (LH) from the anterior pituitary. During pre- and postnatal development, gonadal steroids modulate sexual differentiation of the neural components of the hypothalamic-pituitary-gonadal (HPG) axis that regulate cyclic GnRH secretion [5].
Perinatal and adult exposures to endocrine-disrupting chemicals have been associated with adverse reproductive outcomes on timing of puberty, estrous cyclicity, fertility, birth, and premature reproductive senescence (reviewed by [6,7]). Bisphenol A (BPA) is a synthetic estrogen that is one of the highest volume chemicals manufactured globally, and it has become a widespread environmental contaminant due to its pervasive use in plastics, epoxy resins, food-contact materials and other common items. Data from the Centers for Disease Control and Prevention reveal that BPA is detected in the urine of 93% of a representative cross section of the U.S. population [8]. A review of recent biomonitoring studies suggest that the level of detection of unconjugated or free BPA in human blood is in the range of 0–1 ng/ml [9]. BPA is able to cross the human placenta and has been detected in its unconjugated form at relatively high levels in the human maternal-fetal placental unit [10,11]. In our model of maternal exposure to BPA during gestation or gestation and lactation, unconjugated BPA was below the detectability of the assay (< 0.3 ng/ml) in animals exposed to up to 250 μg BPA/kg BW/day [12]; hence, the BPA internal dose in animals in the current study should be well within the range reported in humans.
It should be noted that available studies do not always agree on the persistent effects of early BPA exposure; however, that is not surprising given the differences in routes, doses and time of exposure, the endpoints measured, and the species and strains examined. Despite these multiple variables, several studies using different BPA doses, different routes of administration and different rodent models have revealed that developmental exposure to environmentally relevant levels of BPA affect the hypothalamic-pituitary-ovarian axis, the female reproductive tract and reproductive tissues. In many cases, the reproductive effects of BPA appear to be non-monotonic and thus do not reveal a pattern of increasing impact with increasing dose. Alterations reported include impaired estrous cyclicity [13,14], and a cessation of estrous cyclicity at an earlier age [15,16]. Previous work in our laboratory demonstrated that offspring born to mothers exposed to 250 ng BPA/kg BW/d showed evidence of altered sexual differentiation of the anteroventral periventricular (AVPV) nucleus of the hypothalamus [17]. The AVPV is a sexually dimorphic brain region essential for cyclical LH release, triggering the LH surge and ovulation. Furthermore, functional and anatomical alterations of the female gonad [18–21] and genital tract [22,23] were also observed in developmentally-exposed females. Our previous study in CD1 mice showed altered follicular development that included the appearance of blood-filled ovarian bursae, typically associated with cessation of cyclical activity, in BPA-exposed females by 6 months of age [16]. There was also a significant increase of the area occupied by antral follicles and a concomitant decrease in the area occupied by corpora lutea, consistent with impaired ovulation.
We have recently reported decreased fertility and fecundity in female CD-1 mice exposed perinatally to BPA and then subjected to a forced-breeding regime beginning at 2 months of age [24]. Reproductive capacity was significantly reduced over time in two of the three BPA exposure groups examined. In the current study, we examine female siblings from those same litters at 3, 6 and 9 months of age; however, the siblings in this study were bred only once. Patterns of estrous cyclicity and a marker of the ovarian follicular reserve were assessed to further explore the likely targets of BPA exposure leading to decreased reproductive outcomes. In addition, in a separate group of animals, we examined the activation of GnRH neurons in conjunction with a steroid-induced LH surge.
2. Materials and methods
2.1. Animals
Sexually mature, female CD-1 mice (8 weeks of age; Charles River, MA) were maintained in temperature (20–24° C), humidity (30–70%) and light controlled (14hr light; 10hr dark, lights on at 0400 h) conditions at the Tufts New England Medical Center Animal Facility. All the experimental procedures were approved by Tufts University New England Medical Center Animal Research Committee and are in accordance with the Guide for Care and Use of Laboratory Animals. Food (Harlan-Teklad 2018; Harlan, Indianapolis, IN, USA) and water (in glass bottles with rubber stoppers and stainless steel sippers) were supplied ad libitum and animals were housed in static polysulfone cages. The cages, bedding, food and water tested negligible for estrogenicity using the E-SCREEN assay [25]. Animals were monitored weekly by veterinary staff and the laboratory staff monitored the animals daily for 4 days after any surgical procedures. Sentinel mice were present in the housing room to monitor the health status of the animals. Throughout the study, the animals were housed in open air cages on static racks instead of ventilated cage systems to allow for appropriate air exchange including transmission of pheromones between male and female mice located in adjacent cages.
2.2. Experimental design
Female mice were housed with males and the morning that a vaginal plug was observed was designated gestational day 1 (GD1). On GD8, the dams were briefly anesthetized with isofluorane, and then implanted subcutaneously with Alzet osmotic mini-pumps. We chose this method of exposure to ensure precise delivery of low doses of BPA to the dam. The pumps were prepared according to the manufacturer’s instructions (Alza Corp, Palo Alto, CA) to deliver vehicle alone (50% Dimethyl sulfoxide in water, as recommended by the manufacturer), a positive estrogenic control (10 ng diethylstilbestrol (DES)/kg BW/day), or BPA through day 16 of lactation. The dose of DES administered was the lowest effective dose tested in a forced -breeding regimen [26]. BPA doses of 25 ng and 250ng/kg BW/day were used in all protocols. An additional higher dose of 25 μg/kg BW/day was also examined for some endpoints. These treatment groups will be referred to as 25 ng BPA, 250 ng BPA, 25 μg BPA and 10 ng DES from here on. It should be noted that in previous studies utilizing this same BPA exposure paradigm, blood was collected from pregnant dams and their fetuses at GD18 and sent to the Centers for Disease Control and Prevention (CDC) for measurement of total and unconjugated BPA. Blood from the two highest doses, 25 μg and 250 μg BPA had detectable total BPA levels; however, unmetabolized BPA was not detectable. The levels of total BPA in the dams exposed to 25 μg BP A/were 0.7 +/− 1.14 and 0.6ng/ml in the pooled blood from their fetuses. In both cases, the mean levels of unconjugated BPA were below 0.3 ng/ml which was the level of detectability of the assay. For dams exposed to 250 μg BPA, mean total BPA levels measured were 1.48 +/− 2.33 ng/ml and 4.0 ng/ml in the pooled blood from their fetuses. Again, measurements of unconjugated BPA were below the detectability of the assay [12]. Dams were allowed to deliver naturally, and the litters were culled to 8 pups per mother one day after birth. Litters were weaned on postnatal day (PND) 21. For further clarification of animal generation and distribution for various endpoints see Fig. 1. Please note, fertility and fecundity in forced-bred females were initially reported in Cabaton et al. [24] but these findings are expanded on in this manuscript with additional endpoints and calculations for comparison at specific timepoints with their single-bred female siblings, initially reported herein. The unit of exposure is the litter and therefore only one animal from a litter was included in each time point or endpoint. The female offspring were examined to assess the effects of perinatal BPA exposure on GnRH neuronal activation, estrous cyclicity, fertility and estimates of the ovarian follicular reserve at 3, 6 and 9 months of age. Due to the importance of pheromonal exposure on estrous cyclicity in mice [27,28], cages of female mice were placed between cages of male mice. Females were housed 2 per cage during assessment of vaginal smears.
Fig. 1.
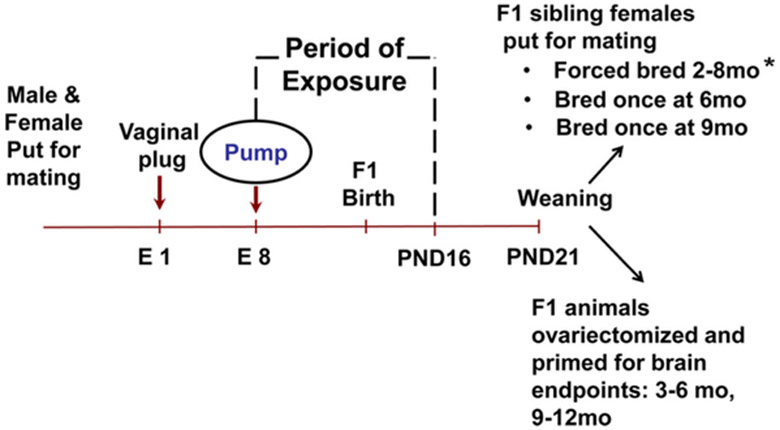
Experimental Design. Generation of animals and their distribution into various endpoints. Embryonic day (E), postnatal day (PND). * please note, some related data from the forced bred animals was reported in a previous study [24] but has been reanalyzed to compare with single bred siblings at similar ages.
2.3. Ovariectomy and steroid priming/perfusions
An ovariectomy, steroid-primed model was chosen for the study of GnRH neuronal activation in order to override potential changes in circulating ovarian hormone levels in BPA-exposed animals and to reduce variability in cyclicity and the impact of long vs short cycles on the timing of the LH surge. In brief, young female offspring (3–6 months of age; n = 11–16/treatment) and older females (9–12 months of age; n = 6–8/treatment) that had been exposed perinatally to vehicle, 25 ng BPA, 250 ng BPA or 10 ng DES were examined. These two BPA doses were specifically chosen to follow up on our previous findings in the brain [17].
The steroid priming paradigm was adapted from the work of Bronson and vom Saal [29]. All females were ovariectomized and implanted subcutaneously with a silastic capsule (0.040″ inner diameter and 0.085″ outer diameter and 10 mm in length) containing estradiol-17β (1 μg diluted in 8 ml sesame oil) to provide a continuous low level of estradiol. Ovariectomies were performed under isofluorane anesthesia and buprenorphine was provided as an analgesic. Six days after ovariectomy, animals were acutely primed for LH surge induction via a single injection of estradiol benzoate (2 μg) followed 24 h later by an injection of progesterone (400 μg) [29]. Animals were perfused intra-cardially via the left ventricle with a freshly made solution of 4% paraformaldehyde that included 2.5% acrolein. Perfusions were performed during a 90 min time period beginning 1 h prior to lights out and continuing through 30 min after lights out as preliminary studies confirmed that this time period coincided with the peak of the steroid-induced LH surge in our control mice. Brains were removed and stored in phosphate buffered saline at 4 °C until they were sectioned at 35 μm on a vibratome (Technical Products International, Earth City MO).
2.4. Immunocytochemistry
Double-labeled immunocytochemistry (ICC) was performed as previously described [30] using anti-GnRH (Immunostar, Inc. Husdson, WI) and antiserum to cFos (Oncogene, La Jolla, CA) as the primary antisera. Briefly, 35 μm sections were collected from the organum vasculosum of the lamina terminalis through the medial basal hypothalamus. This region encompassed the area ranging from interaural 5.22 mm (Bregma-1.42 mm) through interaural 1.74 mm (Bregma-2.06 mm) of the Paxinos and Franklin Atlas [31]. Every 3rd section through this region was included in the chemistry for the examination of GnRH and cFos immunoreactivity. After pretreatment to remove residual aldehydes and decrease nonspecific background, tissues were incubated with cFos antiserum for 48 h at 4°C (1:30,000). After washing, tissues were incubated first with biotinylated immunoglobulin G and then with avidin and biotinylated horseradish peroxidase molecular complex (Vectastain Elite, Vector Labs, Burlingame, CA). Nickel-intensified diaminobenzidine (Vector) was the chromogen used for the detection of nuclear Fos protein and appeared as a black reaction product restricted to the nucleus of various forebrain neurons. After an overnight wash at 4°C, tissue sections were incubated with GnRH antiserum (1:10,000) for 48 h at 4°C. Tissues were washed, incubated with biotinylated immunoglobulin G, and then with avidin and biotinylated horseradish peroxidase molecular complex. Diaminobenzidine (Sigma Chemical Co., St Louis, MO) was used as the chromogen to reveal GnRH, which appeared as a brown reaction product. The number of double-labeled (cFos/GnRH) and single-labeled GnRH neurons were counted and the percent of activated GnRH neurons was calculated.
2.5. LH assays
Blood was collected from the left ventricle by cardiac puncture from each animal at the time of perfusion or at the time of sacrifice if not perfused. Serum LH levels were measured using the mouse reference preparation and anti-LH antibody obtained through the National Institute for Diabetes and Digestive and Kidney Disease (Bethesda, MD) and Dr. Parlow at the National Pituitary Program. Iodinated LH was purchased from Amersham/GE (Pittsburgh PA). All LH measurements were done in a single LH Assay. The assay sensitivity was 0.03 ng LH/tube. Samples were run in duplicate, the interassay CV as determined from mouse pool samples was 3%.
2.6. Determination of estrous cyclicity
To determine the effect of perinatal BPA exposure on estrous cyclicity, vaginal smears were examined daily for 14–15 consecutive days in females perinatally exposed to vehicle, BPA or DES beginning at 5.5 months (n = 17–21/treatment) and 8.5 months of age (n = 20–27/treatment). Females were assessed for length of cycle (normal or extended), evidence of anovulation (a cycle that remains in proestrus for at least 2 consecutive days and does not resolve to estrus), or acyclicity (no cycles evident within the observation period). A 4–6 day estrous cycle was considered ‘normal’ and a cycle ≥ 7 days was considered ‘extended.’ Proestrus and estrus cycle stages were also classified as ‘persistent’ if they lasted for 3 or more days. As mentioned, due to the importance of pheromones for cyclicity in mice, female cages were placed between cages of male mice.
2.7. Anti-Müllerian hormone assay
Circulating levels of anti-Müllerian hormone (AMH), produced by the granulosa cells of preantral follicles, are considered an indirect measure of ovarian reserve in mice [32] and humans [33]. Serum AMH levels were measured using the Active AMH Gen II Elisa kit from Diagnostic Systems Laboratories, Inc. (Webster, TX) in intact females at 3, 6, and 9 months of age (n = 12–22 dams/treatment group/time point) in order to obtain a readout of the ovarian follicular reserve over time. AMH was also measured in blood samples from their forced-bred siblings for comparison. The AMH samples were run in duplicate and quantified with a plate reader. The LOD for the assay was 0.08 ng/ml and the LOQ was 1.6 ng/ml.
2.8. Fertility and fecundity
At 3, 6, and 9 months of age, one female offspring (F1) was arbitrarily chosen from each litter in each treatment group and individually housed to breed once with a non-exposed young male (n = 16–37 dams/treatment group/timepoint). The number of days from mating to delivery, number of pups delivered (fecundity), and numbers of mice having litters (fertility) were recorded. On the day of delivery sex and weight of the F2 pups were also recorded and no treatment group differences were observed.
2.9. Statistics
All analyses were conducted by experimenters blind to treatment groups. Differences in cyclicity parameters, AMH and LH levels, number of live pups delivered, length of gestation, and number of days to delivery from male pairing were analyzed by ANOVA with the Least Significant Difference (LSD) test for post hoc comparisons. Chi Square analysis was used to evaluate the incidence of animals showing altered cyclicity patterns and fertility. Comparisons of serum AMH levels between 9 month-old virgin females and their forced-bred siblings utilized the Students t-test. Due to the small sample size and level of variability, comparisons of the percent activated GnRH neurons across treatment groups used the Kruskal-Wallis test followed by Mann Whitney U. The alpha level for all tests was set at p < 0.05. Graphed data is expressed as mean ± SEM.
3. Results
3.1. Activation of GnRH neurons
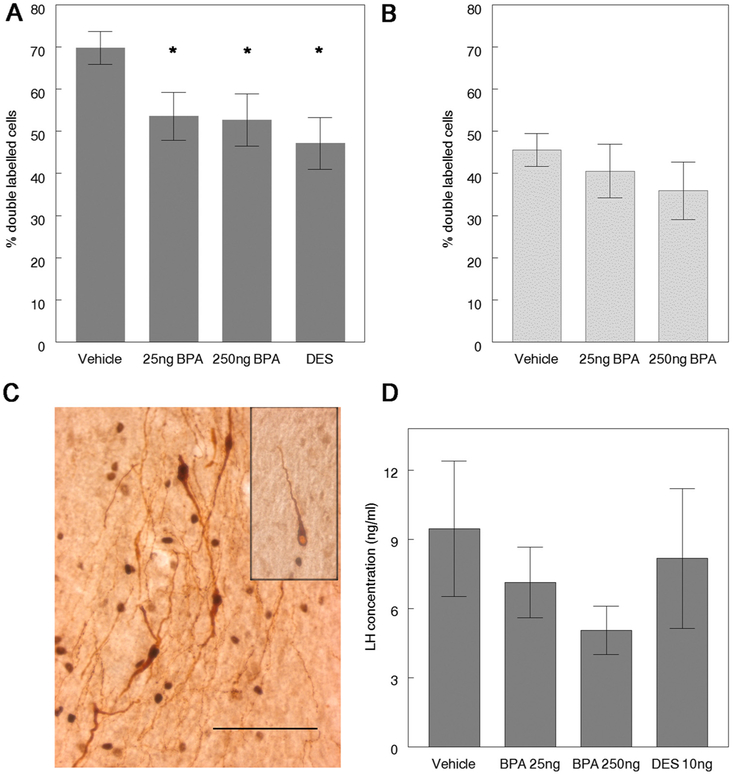
Single-labeled GnRH and double-labeled GnRH/cFos neurons were counted in coronal sections through the hypothalamus. GnRH neurons with increased levels of immunoreactive-Fos in the nucleus contained a brown reaction product in the cytoplasm and a dense black reaction product in the nucleus while single-labeled GnRH neurons had a pale nucleus (Fig. 2C). The presence of cFos in the nucleus of the GnRH neuron was considered an indicator of neuronal activation. The total number of immunoreactive GnRH neurons (single-labeled plus double-labeled GnRH neurons) did not differ between treatment groups; at 3–6 months of age the number was 147.21 ± 12.25 in controls, 142.5 ± 7.66 in 25 ng BPA, 150.93 ± 7.30 in 250 ng BPA, and 142.55 ± 11.89 in the DES group. Similarly, the cell numbers did not differ among groups at 9 months of age: 149.88 ± 10.82 in the control, 161.86 ± 15.08 in the 25 ng BPA, 129.88 ± 11.63 in the 250 ng BPA group. In contrast, the assessment of the percentage of activated GnRH neurons revealed significant differences in 3–6 month-old female offspring of dams exposed to either of the two doses of BPA examined (25 ng or 250 ng BPA), or 10 ng DES (Fig. 2A) relative to controls. The difference between BPA treated animals and controls was no longer significant at 9–12 months of age as controls showed the expected age-related reduction in activation (Fig. 2B). The mean LH levels measured in conjunction with the steroid-induced LH surge in 250 ng BPA females were reduced relative to controls at 3–6 months (Fig. 2D). However, the differences were not statistically significant which may be due to individual variations in the exact timing of the LH peak.
Fig. 2.
Females exposed perinatally to BPA revealed evidence of decreased activation of GnRH neurons in conjunction with a steroid-induced LH surge. Perinatal BPA exposure decreased activation of GnRH neurons. [A] The percent of double labeled GnRH neurons (GnRH+cFos) were significantly reduced in BPA and DES–exposed females relative to controls at 3–6 months of age (Kruskal Wallis: p=0.009); * p < 0.05 by Mann Whitney U compared to vehicle treated animals, n = 11–16/treatment. [B] By 9–12 months of age, the percent of double-labeled GnRH neurons in BPA exposed females did not differ from controls, n = 6-8/treatment. [C] Double-labeled GnRH neurons are characterized by brown cytoplasm (GnRH immunoreactivity) and black nuclei, denoting the presence of cFos protein (box inset: single-labeled GnRH neuron characterized by brown cytoplasm and a pale nucleus). Scale bar=100 μm. [D] No significant differences in mean serum LH concentrations were observed in relation to treatment; however, LH levels in 250 ng BPA females were reduced in 3–6 month old animals relative to controls, n = 11-16/treatment.
3.2. Estrous cyclicity
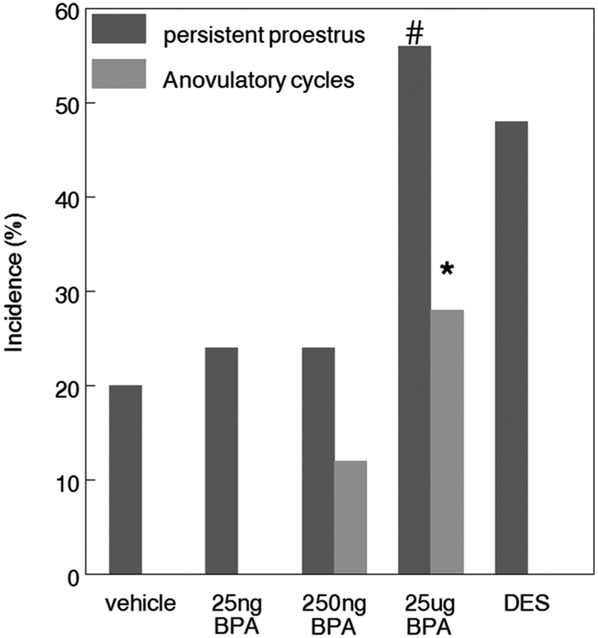
To determine the effects of perinatal BPA exposure on estrous cyclicity, we assessed the number of normal, extended, or anovulatory cycles, as well as evidence of acyclicity or persistent cycle stages at 6 and 9 months of age in virgin females. By 6 months, females exposed to 25 μg BPA showed a significant increase in the incidence of anovulatory cycles compared to controls, consistent with an increased incidence of persistent proestrus in these animals (Fig. 3; Table 1).
Fig. 3.
In utero BPA exposure resulted in a dose-dependent alteration in estrous cyclicity. By 6 months of age, females exposed to 25 μg BPA showed a significant increase in the incidence of anovulatory cycles assessed over a 14 day period compared to controls (Chi Sq * p < 0.05) In addition, the incidence of persistent proestrus is also increased but does not reach statistical significance, (# p=0.053) n = 17–21/treatment.
Table 1.
Patterns of estrous cyclicity of exposed mice at 6 and 9 months of age.
| −6 month |
9 month |
|||||
|---|---|---|---|---|---|---|
| Incidence of normal cycling |
Incidence of persistent proestrus |
Incidence of persistent estrus |
Incidence of normal cycling |
Incidence of persistent proestrus |
Incidence of persistent estrus |
|
| Vehicle | 15/20 (75%) | 4/20 (20%) | 3/20 (15%) | 13/25 (52%) | 8/25 (32%) | 3/25 (12%) |
| 25 ng BPA | 8/17 (47%) | 4/17 (24%) | 7/17(41%) | 8/20 (40%) | 5/20 (25%) | 1/20 (5%) |
| 250 ng BPA | 11/17 (65%) | 4/17 (24%) | 4/17(24%) | 15/27 (56%) | 4/27 (15%) | 8/27 (30%) |
| 25 μg BPA | 8/18 (44%) | 10/18 (56%) | 2/18(11%) | 15/22 (68%) | 4/22 (18%) | 4/22 (18%) |
| 10 ng DES | 12/21 (57%) | 10/21 (48%) | 1/21(5%) | 13/26 (50%) | 10/26 (38%) | 1/26 (4%) |
3.3. Estimates of ovarian reserve
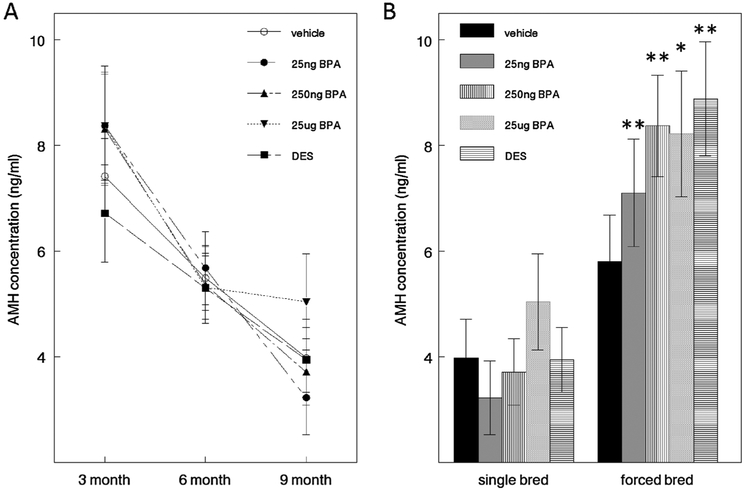
Serum AMH levels were measured at 3, 6, and 9 months in virgin females to assess the effect of perinatal BPA exposure on the ovarian reserve over time. Production of AMH is highest in preantral and small antral follicles and stops as antral follicles continue to grow. AMH levels decreased significantly with age, but did not differ significantly by treatment group at any time point (Fig. 4A). Of interest, a significant decline in AMH levels from 3 to 9 months of age was noted in all treatment groups except 25 μg BPA. Additionally, when comparing AMH levels in 9 month-old single-bred females with the levels in their forced-bred siblings (Fig. 4B), there was no significant difference in control females; however, there were significant differences between single-bred and forced-bred animals in DES and all BPA treatment groups.
Fig. 4.
Effect of perinatal DES and BPA treatment on serum anti-Müllerian (AMH) hormone levels. [A] There was a significant decline of serum AMH levels (ANOVA, p < 0.05) over time within each treatment group. There were no significant differences among treatments at any single timepoint. [B] Comparison of AMH levels between single bred and their forced bred siblings at 9 months. Student t-tests revealed significant differences between AMH levels in the single bred and their forced bred siblings in all but the control group (* p < 0.05, ** p < 0.01), n = 12–22 dams/treatment.
3.4. Fertility and fecundity
The percent fertility across all BPA treatment groups decreased as the age of the dam increased (Table 2). There was a pronounced decline in fertility between 3 and 9 months of age in the 250 ng BPA animals (100% and 25%, respectively), but this decline did not reach statistical significance. Among the siblings bred only once at 9 months of age, only those exposed perinatally to DES exhibited a significant decrease in fertility compared to controls (20% and 57%, respectively). It is interesting to note that when the littermates of these females were subjected to a forced-breeding protocol as previously described [24], the 25 μg BPA group was significantly less fertile, producing an average of 4.7 litters over the 32-week period of breeding while the control mice produced an average of 6.4 litters (Table 3).
Table 2.
Comparison of fertility of exposed females after a single mating with an unexposed male. Fertility at 3, 6 or 9 months of age is expressed as the % of animals producing litters. Increased age of dam resulted in decreased fertility across all treatment groups. There was a pronounced decline in fertility between 3 and 9 months in the BPA 250 ng animals, but this decline did not reach significance. Only females exposed perinatally to DES exhibited a significant decrease in fertility at 9 months compared to age-matched controls (*p < 0.05; χ2 analysis).
| Treatment | 3 month |
6 month |
9 month |
|||
|---|---|---|---|---|---|---|
| n | % fertility | N | % fertility | N | % fertility | |
| Vehicle | 37 | 100 | 29 | 79 | 21 | 57 |
| 25 ng BPA | 25 | 90 | 26 | 77 | 16 | 44 |
| 250 ng BPA | 33 | 100 | 27 | 81 | 16 | 25 |
| 25 μg BPA | 30 | 100 | 26 | 69 | 18 | 39 |
| 10 ng DES | 29 | 100 | 27 | 74 | 20 | 20* |
Table 3.
Measurements of fertility and fecundity in a forced-bred regimen at timepoints comparable to those of single bred siblings in the current study. Data in this table is re-expressed from Figs. 2 and 3 in Cabaton et al. [24]. Fertility after forced-breeding was assessed as the cumulative number of litters/dam over 32 weeks. Fecundity is expressed as the total number of pups per dam at 6 and 9 months of age.
| n | Forced-bred for 32 weeks Cumulative number of litters |
18 Weeks of breeding – 26 weeks of age (6 mo) Cumulative number of pups |
31 weeks of breeding – 39 weeks of age (9 mo) Cumulative number of pups |
|
|---|---|---|---|---|
| Vehicle | 21 | 6.37 ± 0.39 | 71.67 ± 4.63 | 81.57 ± 5.44 |
| 25 ng BPA | 19 | 5.74 ± 0.48 | 52.32 ± 4.56a | 66.16 ± 5.51a |
| 250 ng BPA | 18 | 6.89 ± 0.61 | 69.72 ± 5.73 | 88.22 ± 7.95 |
| 25 μg BPA | 20 | 4.7 ± 0.53a | 53.35 ± 3.58a | 58.25 ± 4.77a |
| DES | 20 | 5.5 ± 0.44 | 58.15 ± 5.47a | 65.70 ± 6.66 |
Denotes signficance from respective controls, p < 0.05.
While the number of live pups decreased in relation to increased age of the dam, there was no significant difference in fecundity across exposure groups at each time point (data not shown). Thus regarding fecundity, no differences were significant in single-bred dams. However, there were significant decreases in the cumulative number of pups at 6 and 9 months in their forced-bred siblings exposed to 25 ng or 25 μg BPA (Table 3). In addition, there was a significant increase in the number of days to delivery from male pairing between virgin dams exposed to either 250 ng or 25 μg BPA (28.75 and 27.14 days respectively) compared to controls (23.30 days) at 9 months (a difference of 5.5 and 3.8 days, respectively) (Table 4). There was also a significant increase in the number of days to delivery from male pairing between 250 ng BPA or DES-exposed dams at 9 months compared to their 3-month counterparts (a difference of 6.28 and 3.16 days, respectively).
Table 4.
Perinatal BPA and DES exposure significantly increased the number of days to delivery from male pairing in females at 9 months of age in a dose-dependent manner.
| Treatment | 3 month |
6 month |
9 month |
|||
|---|---|---|---|---|---|---|
| n | Average # (days) | n | Average # (days) | n | Average # (days) | |
| Vehicle | 37 | 22.02 ± 0.22 | 29 | 23.85 ± 0.71 | 21 | 23.30 ± 0.80 |
| 25 ng BPA | 25 | 22.49 ± 0.35 | 26 | 21.96 ± 0.22 | 16 | 23.14 ± 0.70 |
| 250 ng BPA | 33 | 22.47 ± 0.22 | 27 | 23.61 ± 0.57 | 16 | 28.75 ± 1.31a,b |
| 25 μg BPA | 30 | 21.74 ± 0.18 | 26 | 22.70 ± 0.40 | 18 | 27.14 ± 2.33a |
| 10 ng DES | 29 | 22.34 ± 0.27 | 27 | 23.63 ± 1.33 | 20 | 25.50 ± 1.19b |
Different from controls at 9 months (p < 0.05).
Different from females exposed to same treatment at 3 months of age (p < 0.05; ANOVA).
4. Discussion
Over the last 15 years, our laboratory has generated substantial data on the effects of perinatal exposure to environmentally-relevant doses of BPA on the reproductive axis and reproductive tissues in prepubertal and adult rodents. The perinatal BPA exposure regime used in the current study has been shown to alter sexual differentiation of the AVPV, a brain region essential for the regulation of the preovulatory LH surge [17,34]. We have previously shown that perinatal exposure to 250 ng BPA results in altered differentiation of the sexually dimorphic population of TH neurons in the AVPV as well as AVPV size [17].
In the current study, we followed up on this observation by investigating the potential contribution of the hypothalamus to impaired cyclicity in BPA-exposed females. Mice were ovariectomized and provided with a regimen of ovarian hormones to induce an LH surge. This protocol allowed us to examine the response of the population of GnRH neurons without the confounding issue of differences in circulating levels of endogenous hormones or differences in surge timing due to lengthened or shortened estrous cycles. This model also allowed us to minimize the impact of pituitary changes. Alterations in GnRH signaling at the pituitary level can still be reflected in circulating LH levels as observed in animals that have been exposed to much higher BPA doses than those in this study [35].
Evidence of a significant decrease in activation of GnRH neurons occurred in 3–6 month-old females perinatally-exposed to either BPA or DES. This finding reveals that early BPA and DES exposure can exert lasting effects on the hypothalamic regulation of GnRH release in adulthood. The decrease in the percent of activated GnRH neurons in BPA-exposed females is consistent with an accelerated decline in the functionality of the HPG axis in response to estrogen positive feedback that could in turn impair spontaneous ovulation. This finding is in agreement with known effects of estrogen exposure on the hypothalamus during the critical period of sexual differentiation in rodents [36]. These data suggest that perinatal BPA exposure may result in persistent changes in components of the neuronal circuitry involved in cyclical LH release or in the sensitivity of hypothalamic neurons to estradiol. With regard to potential changes in estrogen sensitivity, an altered ratio of estrogen receptor alpha and beta has been reported in the medial preoptic area of sheep exposed to BPA during gestation [37]. In addition, perturbed estrogen receptor expression has been reported in the AVPV and preoptic area of prepubertal and young adult female rats following early exposure to BPA [38]. These studies suggest lasting effects of early BPA exposure that could have a long term impact on neuroendocrine regulation.
Other studies have examined the effects of BPA on GnRH activation or release. Adewale et al. [39] examined GnRH/fos double labeled neurons in female rats directly exposed to daily subcutaneous injections of higher doses of BPA (50 μg/kg BW or 50 mg/kg BW) on PND 0–3 and unlike the current study, no decline in double-labeled GnRH neurons was observed in BPA-exposed females. Differences in these outcomes may result from differences in species, or BPA dose, and most likely from large differences in exposure period in the two studies. Several in vitro studies examined the effects of BPA on GnRH neurons. Increased GnRH pulsatility was reported in ex vivo hypothalamic explants from adult rats that had received daily subcutaneous injections of much higher levels of BPA (ranging from 2.5–62.5 mg/kg/day) from PND1–10 [40]. More recently, a study exposed rat pups to low levels of BPA analogous to the low dose used in the current study (25 ng/kg BW/day) from PND 1–15 and reported a decrease in GnRH pulsatility when examined prepubertally (PND 20) in ex vivo explants [41]. In addition, when primary GnRH neurons in cultured nasal explants from embryonic day 11.5 Swiss mice were examined with calcium imaging, BPA exposure was found to directly decrease GnRH neuronal activity [42]. These three in vitro studies reveal the ability of BPA to affect GnRH secretion and GnRH neuronal activity.
In our model, perinatal exposure to BPA significantly altered estrous cyclicity in 6 month-old females. Specifically, females exposed to 25 μg BPA experienced periods of persistent proestrus and revealed an increased number of anovulatory cycles. Therefore, the results of this current study suggest that alterations in the events critical for the generation of the preovulatory LH surge may manifest by 6 months of age in BPA-exposed females. It is interesting to note that middle-aged female rats (10–12 months of age) also exhibited a decline in the number of activated GnRH neurons, and this decline was postulated to be responsible for the attenuated amplitude of the LH surge that eventually leads to the age-related onset of acyclicity [30,43].
While there are reports of a decline in the follicular reserve in animals exposed to much higher concentrations of BPA than those used here [21,44–46], we did not see evidence of a reduction in our BPA-exposed females relative to controls. Levels of serum AMH were not significantly lower in BPA-exposed animals as would be expected if the follicular reserve was severely compromised. The AMH data do suggest a slowing of the decline of the follicular reserve in the 9 month-old 25 μg BPA females which might have resulted from the decreased number of ovulatory cycles observed in this group. In addition, the comparison of AMH levels between the 9 month-old animals in this study and those measured in their sisters assessed in the forced-breeding study [24] suggests an increased preservation of the follicular reserve in the forced-bred BPA and the DES-exposed females, which may be due to a decline in the number of ovulations experienced in that regimen.
Of interest, reduced GnRH neuronal activation in females exposed perinatally to 250 ng BPA in the current study contrast with the apparent lack of effect of that same exposure on fertility and fecundity in the forced-breeding regime [24]. These data stress the potential differences between the mechanisms involved in postpartum estrus and/or the forced breeding regime and spontaneous estrus in cycling females. There was a pronounced decline in fertility by 9 months in the single-bred animals exposed perinatally to 250 ng in the current study, but this decline was not statistically significant. However, there was a significant increase in the number of days from male pairing to delivery at 9 months of age in females exposed to 250 ng BPA and 25 μg BPA compared to their age-matched controls. This outcome could indicate altered ovulatory cycles, reduced capacity for reflex ovulation, decreased receptiveness to mating and/or reduction in attractiveness of the exposed females to the males.
When the results of the prior forced-breeding study [24] and the prior AVPV study [17] are viewed along with the current data, it becomes apparent that the same dose of BPA may affect one path to ovulation without impairing another. This is exemplified by the marked effect of the 250 ng dose on the AVPV [17] and on the activation of GnRH neurons and its lack of effect in the forced-bred regime. In spontaneous estrous cycles, ovulation occurs through temporally mediated estrogen-induced positive feedback; in the forced-bred regimen, ovulation during postpartum estrus may be triggered by hormone changes at the time of parturition, [47] and in the early postpartum period [1] as well as cervical vaginal stimulation associated with labor [2]. In addition, prior parity or reproductive experience in rodents can result in altered circulating hormone levels and changes in sensitivity to neurotransmitters [48,49]. Therefore, if multiple pregnancies result in similar permanent changes, the hormonal milieu and regulation of a multiparous mouse may be very different from an agematched nulliparous female. The higher AMH levels in the forced-bred relative to the once-bred females at 9 months of age suggest more follicular reserve available in the forced-bred animals.
In summary, when we review the datasets obtained in our lab under identical experimental conditions, the results suggest that early BPA exposure interferes with at least 2 of the 3 potential mechanisms of ovulation: spontaneous ovulation and ovulation stimulated during postpartum estrus. Additionally, the increased time to delivery in 9 month-old BPA-exposed females in persistent estrus or persistent proestrus, suggests that a third possible mechanism for pregnancy induction, mating-stimulated ovulation may also be affected. Although rodents are spontaneous ovulators, they are capable of reflex ovulation in certain circumstances [3,4]. For example, this has been reported to be the case in aging where animals that are anovulatory or in persistent estrus are capable of ovulating in response to the mating stimulus of a male [50]. These reports suggest that although the hormonal regulation of GnRH secretion may be disrupted, the ability of mating to induce an LH surge may be enhanced in these conditions [4]. Of interest, 5 of the 6 control females that exhibited persistent proestrus delivered healthy litters following breeding at 9 months of age whereas only 1 out of 4 of the 25 μg BPA females did so. In contrast, none of the 250 ng BPA females exhibiting persistent proestrus delivered litters following male pairings. Although these data are derived from a small number of animals, they are suggestive of an impaired ability of the BPA-exposed females to exhibit reflex ovulation in conditions that would be favorable for mating induced LH surge induction.
Finally, the studies described herein show that two known triggers of GnRH secretion necessary to stimulate an LH surge and ovulation can be affected by perinatal BPA exposure, albeit at different doses. While the number of pregnancies and the number of pups born were decreased in the 25 ng and 25 μg BPA females in the forced-breeding regimen described previously [24], the 250 ng BPA dose did not affect pup number or fertility. On the contrary, the siblings of the 250 ng females examined in the current study were negatively affected in a single breeding experiment. In addition, ovariectomy and steroidpriming to induce an LH surge in virgin females by positive feedback resulted in decreased activation of GnRH neurons in both 25 ng and 250 ng females (the 25 μg dose was not evaluated). Although we cannot prove it with our limited current data, we have hypothesized that the increase in the time from pairing to delivery in the BPA or DES-exposed females, particularly those in persistent estrus or persistent proestrus could be consistent with impaired ability to ovulate in response to the mating stimulus of a male (reflex ovulation) as has been reported in rodents. When viewed together, these findings suggest that developmental BPA exposure may have the ability to interfere with reproduction by affecting three potential pathways that lead to the activation of GnRH neurons required for stimulating an LH surge and ovulation.
Acknowledgements
A special thank you to Lucia Speroni and Nafis Hasan for critically reading the manuscript.
We would like to acknowledge Paul Ronsheim for his technical contributions. Perinaaz Wadia and Nicolas Cabaton participated in the generation of animals for study, the measurement of AMH and the monitoring of estrous cyclicity. This work was supported by National Institute of Environmental Health Sciences grant ES08314. The content does not necessarily represent the official views of the funding agencies including the National Institute of Environmental Health Sciences or the National Institutes of Health.
Footnotes
Competing financial interest declaration
The authors have nothing to declare.
References
- [1].Carrillo-Martínez GE, Gómora-Arrati P, González-Arenas A, Morimoto S, Camacho-Arroyo I, González-Flores O, Role of progesterone receptors during postpartum estrus in rats, Horm. Behav 59 (2011) 37–43. [DOI] [PubMed] [Google Scholar]
- [2].Fox SR, Smith MS, Postpartum preovulatory surge of gonadotropin secretion in the rat may be initiated by the labor process, Biol. Reprod 31 (1984) 619–626. [DOI] [PubMed] [Google Scholar]
- [3].Bakker J, Baum MJ, Neuroendocrine regulation of GnRH release in induced ovulators, Front. Neuroendocrinol 21 (2000) 220–262. [DOI] [PubMed] [Google Scholar]
- [4].Kauffman AS, Rissman EF, Neuroendocrine control of mating-induced ovulation, in: Neill JD (Ed.), Knobil and Neill's Physiology of Reproduction, 3rd ed., Elsevier, 2006, pp. 2283–2326. [Google Scholar]
- [5].Cooke B, Hegstrom CD, Villeneuve LS, Breedlove SM, Sexual differentiation of the vertebrate brain: principles and mechanisms, Front. Neuroendocrinol 19 (1998) 323–362. [DOI] [PubMed] [Google Scholar]
- [6].Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, et al. , Endocrine-disrupting chemicals: an Endocrine Society scientific statement, Endocr. Rev 30 (June) (2009) 293–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Gore AC, Chappell VA, Fenton SE, Flaws JA, Nadal A, Prins GS, et al. , EDC-2: the endocrine society's second scientific statement on endocrine-disrupting chemicals, Endocr. Rev 36 (2015) E1–E150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL, Exposure of the U.S. population to bisphenol A and 4-tertiary-Octylphenol: 2003–2004, Environ. Health Perspect 116 (2008) 39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Vandenberg LN, Chahoud I, Padmanabhan V, Paumgartten FJ, Schoenfelder G, Biomonitoring studies should be used by regulatory agencies to assess human exposure levels and safety of bisphenol a, Environ. Health Perspect 118 (2010) 1051–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Balakrishnan B, Henare K, Thorstensen EB, Ponnampalam AP, Mitchell MD, Transfer of bisphenol A across the human placenta, Am. J. Obstet. Gynecol 202 (April) (2010) 393–397. [DOI] [PubMed] [Google Scholar]
- [11].Schonfelder G, Wittfoht W, Hopp H, Talsness CE, Paul M, Chahoud I, Parent bisphenol A accumulation in the human maternal-fetal-placental unit, Environ. Health Perspect 110 (2002) A703–A707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Rubin BS, Paranjpe M, DaFonte T, Schaeberle C, Soto AM, Obin M, et al. , Perinatal BPA exposure alters body weight and composition in a dose specific and sex specific manner: the addition of peripubertal exposure exacerbates adverse effects in female mice, Reprod. Toxicol 68 (2016) 130–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wang W, Hafner KS, Flaws JA, In utero bisphenol A exposure disrupts germ cell nest breakdown and reduces fertility with age in the mouse, Toxicol. Appl. Pharmacol 276 (2014) 157–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Nah WH, Park MJ, Gye MC, Effects of early prepubertal exposure to bisphenol A on the onset of puberty ovarian weights, and estrous cycle in female mice, Clin. Exp. Reprod. Med 38 (2011) 75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Rubin BS, Murray MK, Damassa DA, King JC, Soto AM, Perinatal exposure to low doses of bisphenol-A affects body weight: patterns of estrous cyclicity and plasma LH levels, Environ. Health Perspect 109 (2001) 675–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Markey CM, Coombs MA, Sonnenschein C, Soto AM, Mammalian development in a changing environment: exposure to endocrine disruptors reveals the developmental plasticity of steroid-hormone target organs, Evol. Dev 5 (2003) 1–9. [DOI] [PubMed] [Google Scholar]
- [17].Rubin BS, Lenkowski JR, Schaeberle CM, Vandenberg LN, Ronsheim PM, Soto AM, Evidence of altered brain sexual differentiation in mice exposed perinatally to low environmentally relevant levels of bisphenol A, Endocrinology 147 (2006) 3681–3691. [DOI] [PubMed] [Google Scholar]
- [18].Susiarjo M, Hassold TJ, Freeman E, Hunt PA, Bisphenol A, exposure in utero disrupts early oogenesis in the mouse, PLoS Genet. 3 (2007) e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Berger A, Ziv-Gal A, Cudiamat J, Wang W, Zhou C, Flaws JA, The effects of in utero bisphenol A exposure on the ovaries in multiple generations of mice, Reprod. Toxicol 60 (2016) 39–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lawson C, Gieske M, Murdoch B, Ye P, Li Y, Hassold T, et al. , Gene expression in the fetal mouse ovary is altered by exposure to low doses of bisphenol A, Biol. Reprod 84 (2011) 79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Zhang HQ, Zhang XF, Zhang LI, Chao HH, Pan B, Feng YM, et al. , Fetal exposure to bisphenol A affects the primordial follicle formation by inhibiting the meiotic progression of oocytes, Biol. Reprod 39 (2012) 5651–5657. [DOI] [PubMed] [Google Scholar]
- [22].Newbold RR, Jefferson WN, Padilla-Banks E, Prenatal exposure to bisphenol a at environmentally relevant doses adversely affects the murine female reproductive tract later in life, Environ. Health Perspect 117 (June) (2009) 879–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Markey CM, Wadia PR, Rubin BS, Sonnenschein C, Soto AM, Long-term effects of fetal exposure to low doses of the xenoestrogen bisphenol-A in the female mouse genital tract, Biol. Reprod 72 (2005) 1344–1351. [DOI] [PubMed] [Google Scholar]
- [24].Cabaton NJ, Wadia PR, Rubin BS, Zalko D, Schaeberle CM, Askenase MH, et al. , Perinatal exposure to environmentally relevant levels of Bisphenol-A decreases fertility and fecundity in CD-1 mice, Environ. Health Perspect 119 (2011) 547–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Soto AM, Lin T-M, Justicia H, Silvia RM, Sonnenschein C, An in culture bioassay to assess the estrogenicity of xenobiotics, in: Colborn T, Clement C (Eds.), Chemically Induced Alterations in Sexual Development: the Wildlife/human Connection, Princeton Scientific Publishing, Princeton, 1992, pp. 295–309. [Google Scholar]
- [26].McLachlan JA, Newbold RR, Shah HC, Hogan MD, Dixon RL, Reduced fertility in female mice exposed transplacentally to diethylstilbestrol (DES), Fertil. Steril 38 (1982) 364–371. [DOI] [PubMed] [Google Scholar]
- [27].Vandenbergh JG, Male odor accelerates female sexual maturation in mice, Endocrinology 84 (1969) 658–660. [DOI] [PubMed] [Google Scholar]
- [28].Whitten WK, Modification of the oestrous cycle of the mouse by external stimuli associated with the male: changes in the oestrous cycle determined by vaginal smears, J. Endocrinol 17 (1958) 307–313. [DOI] [PubMed] [Google Scholar]
- [29].Bronson FH, vom Saal FS, Control of the preovulatory release of luteinizing hormone by steroids in the mouse, Endocrinology 104 (1979) 1247–1255. [DOI] [PubMed] [Google Scholar]
- [30].Rubin BS, Lee CE, King JC, A reduced proportion of luteinizing hormone (LH) releasing hormone neurons express fos protein during the preovulatory or steroid induced LH surge in middle-aged rats. Biol. Reprod 51 (1994) 1264–1272. [DOI] [PubMed] [Google Scholar]
- [31].Paxinos G, Franklin KBJ, The Mouse Brain in Stereotaxic Coordinates, 2nd ed., Academic Press, San Diego, 2001. [Google Scholar]
- [32].Kevenaar ME, Meerasahib MF, Kraner P, van de Lang-Born BM, de Jong FH, Groome NP, et al. , Serum anti-mullerian hormone levels reflect the size of the primordial follicle pool in mice, Endocrinology 147 (2006) 3228–3234. [DOI] [PubMed] [Google Scholar]
- [33].Kalaiselvi VS, Saikumar P, Prabhu K, Prashanth Krishna G, The anti mullerian hormone-a novel marker for assessing the ovarian reserve in women with regular menstrual cycles, J. Clin. Diagn. Res 6 (2012) 1636–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Patisaul HB, Fortino AE, Polston EK, Neonatal genistein or bisphenol-A exposure alters sexual differentiation of the AVPV, Neurotoxicol. Teratol 28 (Jane) (2006) 111–118. [DOI] [PubMed] [Google Scholar]
- [35].Fernandez M, Bianchi M, Lux-Lantos V, Libertun C, Neonatal exposure to bisphenol an alters reproductive parameters and gonadotropin releasing hormone signaling in female rats, Environ. Health Perspect 117 (May) (2009) 757–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Pinilla L, Barreiro ML, Gonzalez LC, Tena-Sempere M, Aquilar E, Comparative effects of testosterone propionate, oestradiol benzoate ICI 182,780, tamoxifen and raloxifene on hypothalamic differentiation in the female rat, J. Endocrinol 172 (2002) 441–448. [DOI] [PubMed] [Google Scholar]
- [37].Mahoney MM, Padmanabhan V, Developmental programming: impact of fetal exposure to endocrine-disrupting chemicals on gonadotropin-releasing hormone and estrogen receptor mRNA in sheep hypothalamus, Toxicol. Appl. Pharmacol 247 (2010) 98–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Rebuli ME, Cao J, Sluzas E, Delclos KB, Camacho L, Lewis SM, et al. , Investigation of the effects of subchronic low dose oral exposure to bisphenol A (BPA) and ethinyl estradiol (EE) on estrogen receptor expression in the juvenile and adult female rat hypothalamus, Toxicol. Sci 140 (2014) 190–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Adewale HB, Jefferson WN, Newbold RR, Patisaul HB, Neonatal bisphenol-a exposure alters rat reproductive development and ovarian morphology without impairing activation of gonadotropin-releasing hormone neurons, Biol. Reprod 81 (2009) 690–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Fernandez M, Bourguignon N, Lux-Lantos V, Libertun C, Neonatal exposure to Bisphenol A and reproductive and endocrine alterations resembling the polycystic ovarian syndrome in adult rats, Environ. Health Perspect 118 (2010) 1217–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Franssen D, Gérard A, Hennuy B, Donneau AF, Bourguignon JP, Parent AS, Delayed neuroendocrine sexual maturation in female rats after a very low dose of Bisphenol A through altered GABAergic neurotransmission and opposing effects of a high dose, Endocrinology 157 (2016) 1740–1750. [DOI] [PubMed] [Google Scholar]
- [42].Klenke U, Constantin S, Wray S, BPA directly decreases GnRH neuronal activity via noncanonical pathway, Endocrinology 157 (2016) 1980–1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Lloyd JM, Hoffman GE, Wise PM, Decline in immediate early gene expression in gonadotropin-releasing hormone neurons during proestrus in regularly cycling middle-aged rats, Endocrinology 134 (1994) 1800–1805. [DOI] [PubMed] [Google Scholar]
- [44].Rodriguez HA, Santambrosio N, Santamaria CG, Munoz-de-Toro M, Luque EH, Neonatal exposure to bisphenol A reduces the pool of primordial follicles in the rat ovary, Reprod. Toxicol 30 (2010) 550–557. [DOI] [PubMed] [Google Scholar]
- [45].Li Y, Zhang W, Liu J, Wang W, Lin H, Zhu J, et al. , Prepubertal bisphenol A exposure interferes with ovarian follicle development and its relevant gene expression, Reprod. Toxicol 44 (2014) 33–40. [DOI] [PubMed] [Google Scholar]
- [46].Delclos KB, Camacho L, Lewis SM, Vanlandingham MM, Latendresse JR, Olson GR, et al. , Toxicity evaluation of bisphenol A administered by gavage to Sprague Dawley rats from gestation day 6 through postnatal day 90, Toxicol. Sci 139 (2014) 174–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Morishige WK, Pepe GJ, Rothchild I, Serum luteinizing hormone: prolactin and progesterone levels during pregnancy in the rat, Endocrinology 92 (1973) 1527–1530. [DOI] [PubMed] [Google Scholar]
- [48].Bridges RS, Long-term alterations in neural and endocrine processes induced by motherhood in mammals, Horm. Behav 77 (2015) 193–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Bridges RS, Byrnes EM, Reproductive experience reduces circulating 17beta-estradiol and prolactin levels during proestrus and alters estrogen sensitivity in female rats, Endocrinology 147 (2006) 2575–2582. [DOI] [PubMed] [Google Scholar]
- [50].Matt DW, Coquelin A, Lu JK, Neuroendocrine control of luteinizing hormone secretionand reproductive function in spontaneously persistent estrous aging rats, Biol. Reprod 37 (1987) 1198–1206. [DOI] [PubMed] [Google Scholar]