Abstract
Quantitative assays that measure immune response to pneumococcal vaccines are not only important for the evaluation of vaccine immunogenicity and efficacy, but are also utilized in the clinical diagnosis of immune deficiency syndromes. Analytical methods have progressed in order to meet changing demands in both of these areas, from early methods to ELISA, and most recently multiplex bead array assays and opsonophagocytosis assays (OPA). It is necessary to understand the evolution of such techniques and the criteria for their interpretation in order to better inform the application of currently available methods, and to guide future investigation into assay development.
Keywords: Streptococcus pneumoniae, pneumococcal, vaccine, immune response, immune deficiency
1. Introduction
Streptococcus pneumoniae (the pneumococcus) is a gram positive bacterium that represents an important human pathogen. Pneumococcal infections can cause significant morbidity and mortality, particularly among young children, elderly adults, and individuals with immune deficiencies [1, 2]. Its virulence is primarily mediated by a polysaccharide capsule, which is the basis for classification of pneumococci into over 90 known serotypes and constitutes an antigenic target for pneumococcal vaccines [3].
The 23-valent pneumococcal polysaccharide vaccine (PPV23) contains capsular polysaccharide from 23 serotypes of Streptococcus pneumoniae and has been licensed for use in adults since 1983 (See Table 1)[4]. Pneumococcal conjugate vaccines (PCVs) consist of pneumococcal capsular polysaccharide conjugated to a protein carrier and result in a T-cell dependent immune response. PCVs, which have been in use since 2000, were initially developed and implemented for use in young children, since PPV23 is not immunogenic in this population [1, 2]. In recent years, there has been growing evidence to support use of PCV in adults as well, particularly among the elderly and those with immune compromising conditions. 2014 US Advisory Committee on Immunization Practices (ACIP) guidelines recommend the administration of PCV13 and PPV23 in series to adults ≥65 years of age (Table 1)[5]. Among European countries, guidelines concerning routine administration of pneumococcal vaccines vary widely, particularly for healthy older adults [6].
Table 1.
Pneumococcal vaccines, with summaries of current ACIP recommendations for use.
| Vaccine | Year licensed | Serotypes included | Indications |
|---|---|---|---|
| PPV23 (Pneumovax 23, Merck) | 1983 | 1, 2, 3, 4, 5, 6B, 7F, 8, 9V, 9N, 10A, 11 A, 12F, 14, 15B, 17F, 18C, 19F, 19A, 20, 22F, 23F, 33F |
|
| PCV7 (Prevnar, Pfizer) | 2000 | 4, 6B, 9V, 14, 18C, 19F, 23F | N/A+ |
| PCV10 (Synflorix, GlaxoSmithKline) | 2008 | PCV7 serotypes + 1, 5,7F | N/A+ |
| PCV13 (Prevnar 13, Pfizer) | 2009 | PCV10 serotypes + 3, 6A, 19A |
|
Chronic medical conditions include chronic heart/lung/liver disease, diabetes mellitus, alcoholism, cigarette smoking
While used in other countries, PCV10 was not implemented in US immunization guidelines and PCV7 was directly replaced by PCV13
As well as adults with asplenia, CSF leaks, cochlear implants
Assays that measure responses to pneumococcal vaccines are important in evaluation of vaccine efficacy as well as in the diagnosis of certain immune deficiency syndromes. Such assays can be broadly divided into two categories: Assays which measure the amount of pneumococcal antibody present in serum, or functional assays that measure serum antibodies’ capacity to kill pneumococci. Initial development and licensure of pneumococcal vaccines were based on quantitative assessment of serologic response (i.e., increase in pneumococcal antibody levels) following vaccine administration, which was correlated with incidence of pneumococcal infections [4, 7]. Vaccine evaluation served as the impetus for development of standardized, reproducible assays to measure serologic response. Various pneumococcal assays that were designed to address this need are described below.
2. Methodologies
2.1. Radioimmunoassay/hemagglutination
Radioimmunoassay (RIA) and hemagglutination assays were several of the early methods developed to measure responses to pneumococcal vaccines. RIA utilized radiolabeled capsular polysaccharide antigens, with precipitation of antigen-antibody complexes via the Farr technique [8, 9]. Expense, as well as potential safety issues related to use of radiolabeled pneumococcal polysaccharide, and lack of isotype specificity represented important limitations of RIA. Furthermore, pneumococcal antibody response as measured by RIA did not correlate with the frequency or severity of infections in a study of diagnostic vaccination in evaluating immune deficiency syndromes [10].
Hemagglutination involved mixing human red blood cells coated with pneumococcal polysaccharide with a serum sample, and documenting the highest serum dilution that still produced agglutination [11]. This technique measured both IgM and IgG antibodies and was difficult to standardize[8], and therefore was not widely adopted.
2.2. ELISA
Largely based on the aforementioned limitations, RIA and hemagglutination were rapidly supplanted by enzyme-linked immunosorbent assay (ELISA) [12]. ELISA for detection of pneumococcal IgG antibodies was developed and refined during the 1980s and 1990s, and was eventually standardized [13], remaining the gold standard to the present day. As compared to RIA, ELISA was less expensive, logistically simpler, required smaller quantities of serum, and did not require use of radiolabeled materials.
Capsule-specific antibodies against pneumococcal polysaccharide are the primary mediators of opsonization and killing of pneumococci. However, antibodies against pneumococcal cell wall polysaccharide (C-PS) as well as other antibodies that do not opsonize (i.e., protect/function) in the immune response can also be found in serum [13]. First-generation ELISA to detect pneumococcal antibodies tended to overestimate antibody levels due to the presence of these non-protective antibodies [13]. Second-generation ELISA removed some of these non-functioning antibodies using preabsorption of test sera with pneumococcal cell wall polysaccharide, however was still not specific. Third-generation ELISA improved specificity via preabsorption with both C-PS and 22F polysaccharide to remove nonfunctioning antibodies, and was ultimately adopted as the WHO ELISA (Figure 1) [13].
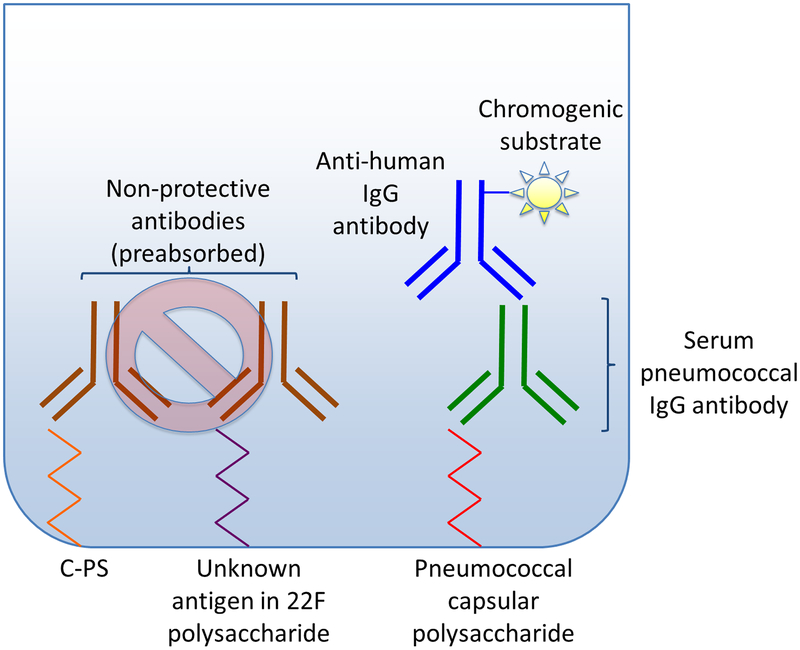
Figure 1. WHO ELISA for measurement of pneumococcal IgG antibodies.
The figure represents a typical plate well in WHO ELISA. The left side of the figure depicts the initial pre-absorption of sera with cell wall polysaccharide (C-PS) and 22F polysaccharide to neutralize non-protective antibodies (shown in brown).
The right side of the figure illustrates binding of serum pneumococcal IgG antibodies (shown in green) to serotype-specific pneumococcal capsular polysaccharide. Anti-human IgG antibodies (shown in blue) are bound to serum IgG antibodies. Using a chromogenic substrate, optical density is measured, and then converted to antibody concentration.
WHO ELISA utilizes micro wells coated with serotype-specific pneumococcal polysaccharide, to which serum antibodies bind. Anti-human IgG antibodies recognize bound serum antibody, and subsequently react with chromogenic substrate (Figure 1). Optical density is then converted to antibody concentration [14]. WHO ELISA was initially standardized against reference serum 89SF, and was later validated using reference serum 007sp which is utilized today [15]. Pneumococcal reference serum 007sp has been assigned reference values for 13 pneumococcal serotypes (1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F, and 23F) through an international collaboration [15]. Reference values for additional serotypes were assigned outside of the international collaboration responsible for these 13 original serotypes [16]. WHO recommendations also suggested criteria for evaluating a new quantitative method for pneumococcal antibody testing, establishing that IgG concentrations for at least 75% of serum samples should fall within 40% error of standardized mean values via ELISA [17].
2.3. Multiplex bead array methods
Since ELISA requires performance of a separate assay for each serotype tested, inclusion of additional serotypes in pneumococcal conjugate vaccines resulted in increased cost and decreased throughput [18]. In contrast, multiplex bead array assays allow for measurement of antibodies against multiple serotypes within a single serum sample. In addition to reducing costs and increasing throughput, they utilize smaller sample volumes and offer increased dynamic range (i.e., require fewer dilutions to obtain result) as compared to ELISA [18, 19]. These assays, which use color coded beads, are commonly referred to as Luminex assays after the developer of the bead assay method. Luminex assays were first developed by commercial laboratories, national public health agencies, and pharmaceutical corporations beginning in the early 2000s [18–20]. While assay developers evaluated their methods based on WHO ELISA performance criteria, laboratories that currently perform these assays may utilize their own proprietary techniques as well as different reference sera (personal communication, September 2017).
Generally, the bead array method uses sets of color coded microspheres, each of which is coated with pneumococcal polysaccharide of a given serotype [18–20]. A set of microspheres is mixed with serum, then exposed to anti-human IgG antibody conjugated to a fluorescent marker, usually phycoerythrin. Mean fluorescence intensity is measured and converted to antibody concentration in μg/mL [18–20]. Serum is preabsorbed with C-PS and/or 22F polysaccharide in concordance with prior methods for ELISA [18, 20].
Multiplex bead array assays performed by individual labs have been demonstrated to produce different results from those performed by other labs as well as from WHO ELISA. Validation studies for various multiplex assays demonstrate variability in specificity and correlation with ELISA based on serotype [18, 21]. A 2010 study evaluated inter-assay variability between three multiplex assays that were available at that time, and their correlation with ELISA. The study analyzed the same 11 or 12 sera using the xMAP Pneumo 13 Luminex assay and two other in-house multiplex bead array assays utilized by the US Centers for Disease Control and UK Health Protection Agency, respectively. Overall, the multiplex assays yielded higher antibody concentrations as compared to ELISA. Multiplex assays demonstrated greater inter-assay variability for certain serotypes as well as for certain sera across all three assays. Overall, none of the assays were able to satisfy the WHO stipulations for qualification of new pneumococcal antibody assays [22]. Epitope modification during conjugation of pneumococcal polysaccharide to microspheres [20] or nonspecific binding of certain sera to microspheres [23, 24] have been proposed as potential explanations for the observed issues with variability and specificity. Multiple studies have demonstrated nonspecific binding of sera to Luminex microspheres, resulting in high nonspecific background [23, 24]. In certain cases, treatment with blocking agents could reduce or eliminate this nonspecific binding [23, 24]. However, the exact causes of these findings have not been identified despite extensive investigation.
Multiplex electrochemiluminescence (ECL) assays obviated the requirement for chemical modification of polysaccharide in conjugation to microspheres. While there were considerably fewer studies of ECL assays as compared to Luminex-based techniques, they were also found to yield variable specificity between different serotypes [25] and overestimation of antibody concentrations as compared to ELISA [26].
2.4. Opsonophagocytosis assays
Killing-type OPAs are functional assays that measure the ability of serum antibodies to promote phagocytosis and killing of bacteria. Classical killing-type OPA involved adding serum to a standardized mixture of phagocytes, complement, and pneumococcus of a given serotype. Serial dilutions of this mixture were plated and incubated, and bacterial colonies were counted to generate a curve which reflected bacterial killing (see Figure 2). Each assay measured killing capacity of a single serotype and required manual counting of bacterial colonies, and therefore was tedious to perform and required large quantities of serum for evaluation of pneumococcal vaccine efficacy [27].
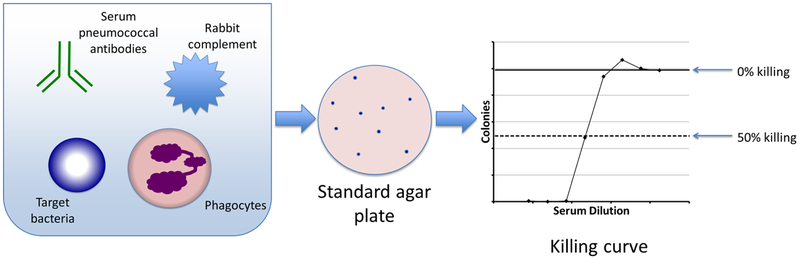
Figure 2. Classical opsonophagocytosis assay to measure killing of pneumococci.
The left side of the figure depicts a plate well used in classical OPA, in which serum is added to a mixture of phagocytes, complement, and pneumococcus of a given serotype. Dilutions of this mixture are plated on a standard agar plate (center). Bacterial colonies are counted to generate a killing curve.
On the far right a representative example of a killing curve is shown. For each of the serum dilutions indicated (X axis), the average surviving colony-forming units (CFU) is shown on the y axis. The bold, solid horizontal line indicates the maximum CFU (i.e., 0% killing), and the dashed horizontal line indicates the CFU with 50% killing.
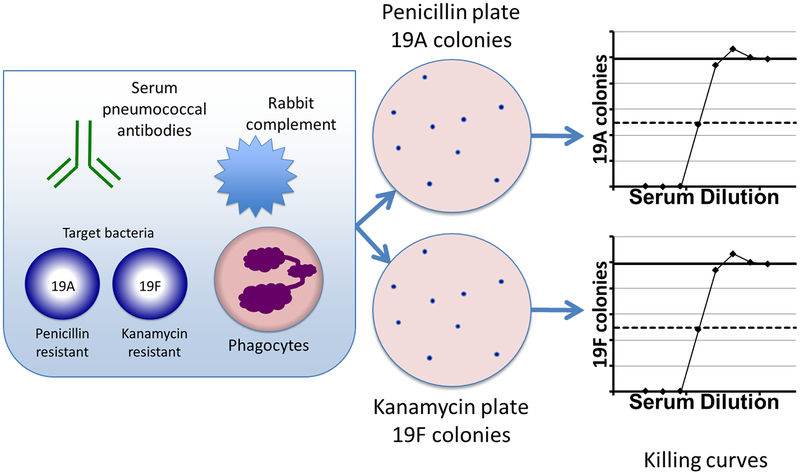
Development of the multiplex opsonophagocytic killing assay (MOPA) addressed the limitations of classical OPA. MOPA utilizes multiple serotypes of pneumococci which each carry resistance to a different antibiotic [28, 29]. By plating on antibiotic-containing media, one can select for the growth of a single serotype, measuring killing of multiple serotypes in a single assay (see Figure 3). Colony counting has also been automated, leading to improvements in efficiency [27, 28]. A detailed procedure is available at www.vaccine.uab.edu, and has facilitated worldwide adoption of MOPA.
Figure 3. Multiplex opsonophagocytosis assay (MOPA) to measure killing of multiple pneumococcal serotypes.
On the left a typical MOPA plate well is shown, in which serum is added to a mixture of phagocytes (differentiated HL-60 cells), rabbit complement, and multiple serotypes of pneumococci, each carrying resistance to a different antibiotic. Dilutions of this mixture are plated on antibiotic-containing media (center) to select for growth of a single serotype. Bacterial colonies are enumerated using an automated counter to generate a killing curve as shown on the right (see Figure 2 for a detailed description).
3. Immunoassays for evaluation of vaccine efficacy
3.1. ELISA
The WHO ELISA was used extensively in evaluation and licensure of pneumococcal vaccines, particularly pneumococcal conjugate vaccines in children. IgG response as measured by WHO ELISA was set forth as the primary efficacy endpoint for initial licensure of PCVs for use in infants, with approval of subsequent formulations to be based on non-inferiority of serologic response [7, 17]. Based on ELISA results from three clinical efficacy trials of PCV7, WHO established a threshold of 0.35 μg/mL for licensing vaccines against invasive pneumococcal disease in children [30]. This threshold was not intended to imply protective status in an individual, nor could it be extrapolated to imply efficacy in preventing other pneumococcal infections such as pneumonia or otitis media, which may require higher antibody levels [30, 31]. While application of this threshold may be appropriate for large-scale studies of vaccine efficacy, it may not be sufficient to predict individuals at risk for infections. Furthermore, actual ‘protective’ thresholds likely vary across different serotypes, patient populations, and sites of infection [31, 32].
3.2. Opsonophagocytosis assays
In some circumstances, IgG antibodies may not reflect protective immunity following administration of a vaccine. Infants who received PCV7, which contains serotype 19F polysaccharide, also produced IgG antibodies cross-reacting with serotype 19A, which is not included in the vaccine but is structurally similar to 19F. However, opsonophagocytosis assay (OPA) data indicated that these antibodies were non-functional, with “19A antibodies” failing to opsonize and kill bacteria in 81% of cases. Clinical experience with PCV7 demonstrated lack of cross-protection against 19A [33]. Furthermore, studies have shown that anti-capsular antibodies provide protection in vivo by opsonizing pneumococci [34]. As a result, OPAs have come to represent important tools for the evaluation of functional immune response produced by pneumococcal vaccines. Since a separate assay run was performed for each serotype, classical OPA was not practical for large-scale vaccine studies involving increasing numbers of serotypes. Such studies are now feasible using MOPA with automated colony counting.
Immunogenicity data generated by OPA was used in licensure and formulation of current recommendations for the administration of PCV13 in infants and children [35]. In this population, assessments of serologic response to vaccination as measured by IgG response via ELISA generally correspond with immunogenicity as measured by OPA [34]. In studies of PCV7 in infants, serotype-specific IgG of ≥0.2 μg/mL and OPA titer ˃1:8 were correlated with protection from invasive pneumococcal infection [7]. However, adults (particularly the elderly) may be susceptible to pneumococcal infections despite ‘adequate’ or normal IgG antibody levels [34]. For this reason, OPA has become an important method for evaluating immunogenicity in vaccine trials for older adults. The specific reasons for this discrepancy between antibody levels and susceptibility to infection are still being elucidated. Immunogenicity as measured by OPA has been proposed as the principal basis for comparing adult vaccines [36], and was a primary endpoint in clinical trials for PCV13 in adults that contributed to its approval and current recommendations for use [5].
4. Immunoassays for diagnosis of immune deficiency syndromes
Evaluating response to pneumococcal vaccines is also an important component of the diagnostic evaluation of suspected primary immunodeficiency disease (PIDD). Normal or deficient responses to pneumococcal vaccines can be integrated with other clinical data to determine intact versus impaired host immunity, respectively [37].
Assessment of vaccine response is utilized as a component in the diagnosis of common variable immunodeficiency (CVID), which is one of the most common primary immunodeficiency diseases in adults [37]. For certain other immunodeficiency syndromes such as IgG2 subclass deficiency and selective antibody deficiency (SAD), diagnosis can depend entirely on response to polysaccharide vaccines [38]. Diagnosis of PIDD may represent an indication for administration of intravenous immune globulin (IVIg) replacement therapy, which is associated with significant cost as well as certain complications. Consequently, American Academy of Allergy, Asthma and Immunology (AAAAI) recommendations also emphasize the importance of integrating other clinical information in the diagnosis of PIDD, particularly a compatible history of infections, prior to initiation of IVIg replacement therapy [37]. To evaluate polysaccharide vaccine response, 2012 AAAAI guidelines recommend measurement of baseline pneumococcal antibody levels via a “reliable quantitative technique” followed by repeat measurement 4–8 weeks following administration of PPV23 [38]. While antibody concentrations that correspond with protection from pneumococcal disease may vary by age, serotype, and site of infection [32], these guidelines define a ‘protective’ antibody level for each serotype as ≥1.3 μg/mL in adults [38]. A normal response to diagnostic vaccination is defined as conversion to a protected concentration (1.3 μg/mL) with a 2-fold rise in antibody concentration in at least 70% of serotypes tested [38]. It should be noted that other thresholds for protection are utilized elsewhere. In the UK, a protective threshold of 0.35 μg/mL has also been applied to diagnostic evaluation of immune deficiency in adults [39].
Initial estimates of pneumococcal antibody levels that corresponded with protection from infection were established using RIA, which reported results in nitrogen-based units (ng antibody nitrogen/mL). However, at that time, precise antibody concentrations corresponding with protection from pneumococcal infection had not been determined. In 1998 a conversion factor of 1 μg/mL per 160 ng antibody nitrogen/mL, which was based on personal communication to the author, was used to establish 1.3 μg/mL as an arbitrary threshold for protection for use with ELISA [40]. This threshold is currently applied to results from multiplex bead array assays as well [38].
4.1. ELISA
WHO ELISA was refined and standardized primarily as a method for evaluation of vaccine immunogenicity. However, use of ELISA for diagnosis of immune deficiency syndromes was impractical, as the assay had to be performed many times in order to measure responses to each of the individual serotypes included in PPV23. For these reasons, direct application of WHO ELISA for clinical diagnosis of PIDD remained problematic.
To address the logistical challenges of performing separate WHO ELISA assays for each serotype, a technique for ELISA measuring combined (as opposed to serotype-specific) pneumococcal antibodies was evaluated for diagnosis of suspected immune deficiency [41]. Individual plate wells were coated with a mixture of pneumococcal polysaccharides of multiple serotypes, and overall IgG antibody binding was measured. However, this assay was found to generate false negative results in patients with primary immune deficiency syndromes [41, 42]. For this purpose, clinicians began utilizing multiplex bead-based assays, which offered less expensive, more efficient measurement of serotype-specific pneumococcal antibodies.
4.2. Multiplex bead array methods
Multiplex bead array assays have come to be widely utilized for evaluation of suspected immune deficiency. However, there is mounting evidence to suggest that issues of specificity and correlation with prior methods may limit their application for this purpose.
A study by Zhang et al. in 2013 investigated inter-assay variation by comparing assay results for 57 serum samples between the xMAP Pneumo14 assay and two proprietary multiplex bead array assays (referred to as ‘lab derived tests,’ or LDTs). While there was 82% agreement by all three assays in terms of meeting a ‘protected’ threshold (˃1.3 μg/mL for 70% of serotypes) for diagnostic purposes, the study found significant variation between results generated by the three assays, with correlation varying by serotype. One LDT systematically yielded lower antibody concentrations than the other two assays [43].
A 2014 study conducted by some of the same investigators examined the effects of inter-lab variability on application of different algorithms for interpretation of diagnostic vaccination with PPV23. The study confirmed the prior findings of significant variability between assays and serotypes. Threshold-based algorithms (using cutoff of >1.3 μg/mL for 70% of serotypes) were less affected by inter-lab variability than were fold-change algorithms (2 or 4-fold rise in antibody concentration in 50% or 70% of serotypes). There was greater disparity between assays with more stringent definitions of fold-change response (i.e., 4-fold as opposed to 2-fold rise in antibody levels) [44].
4.3. Opsonophagocytosis assays
In light of the limitations of current methods as described above, OPA has become attractive as a potential diagnostic test for PIDD. This potential was enhanced following recent efforts to standardize MOPA using pneumococcal reference serum 007sp, which led to improved inter-laboratory comparability and reduced variability [45]. Nevertheless, OPA has not yet been validated for use in diagnosis of suspected immune deficiency syndromes, and correlates between MOPA results and protection against pneumococcal infections for the purposes of diagnostic vaccination with pneumococcal vaccines have not yet been established. Also, since MOPA measures bacterial killing in the presence of serum (which also contains IgM), it does not specifically reflect IgG response in diagnostic evaluation of certain types of immune deficiency syndromes. Additional work is needed prior to use of MOPA for diagnosis of PIDD.
5. Conclusions
Current trends in demographics and use of pneumococcal vaccines predict continued growth in the demand for reliable, high-throughput methods for evaluating vaccine efficacy. Use of PCV in older adults is increasing as a result of changes to US immunization guidelines as well as a dramatic proportional increase in the elderly adult population. However, while current guidelines recommend administration of PPV23 alone for adults ages 19–64 with chronic medical conditions (see Table 1), there is evidence to suggest that PCV may be more immunogenic than PPV23 in individuals with certain conditions such as Chronic Obstructive Pulmonary Disease (COPD) [46]. With the examination of best practices for vaccination of adults with chronic conditions as well as the investigation of new PCVs with expanded coverage to include additional serotypes [47], use of MOPA to evaluate vaccine efficacy will likely continue to expand.Use of pneumococcal vaccines (particularly PPV23) in the diagnostic evaluation of suspected immune deficiency syndromes presents a unique set of challenges. Increasing use of PCV among adults can confound subsequent use of polysaccharide vaccines to evaluate T-independent immune response among serotypes shared between the two vaccines [12]. Furthermore, expanding PCV coverage through the inclusion of additional serotypes would effectively reduce the number of serotypes unique to PPV23. For these reasons it may be necessary to consider alternative polysaccharide vaccines for the evaluation of humoral immune deficiency.
There are significant methodologic issues that may limit the generalizability of the results of multiplex bead array assays for diagnostic purposes. Due to the analytical complexity of these assays, there are relatively few commercial laboratories that perform this assay for clinical diagnostic use. Prior studies have demonstrated issues with specificity, significant variability in results between assays performed by different laboratories using non-uniform techniques, as well as inconsistent correlation with ELISA, which still represents the gold standard for measurement of pneumococcal IgG levels. The ideal strategy for interpreting the results of diagnostic vaccination with PPV23 is even less clear when considering the current criteria for defining an adequate response, which were not initially correlated with susceptibility to infection and have been extrapolated across multiple types of assay.
Clinicians and laboratorians should be aware of the limitations of currently available assays and response criteria, particularly in terms of their potential impacts on treatment decisions. With ongoing improvements in precision and reproducibility, MOPA may eventually be used for diagnosis of PIDD as well as for its current application in vaccine efficacy studies. In addition to the population of individuals who are suspected of having PIDD, increasing use of immune suppressing medications and increased survivorship in HIV/AIDS have led to an expansion of a heterogeneous population of individuals with secondary immune deficiency. While MOPA does not specifically measure IgG response to a polysaccharide vaccine, functional assessment of immune response may be particularly useful in these heterogeneous populations. Studies to establish correlates between vaccine response and susceptibility to infection in these populations would inform interpretation of results for diagnostic purposes.
Regardless of the methods used, it is clear that there is an ongoing need for reliable, reproducible, and efficient techniques for measuring responses to pneumococcal vaccines. Future investigation into these techniques should account for current trends in vaccination practices, demographics, and clinical medicine.
Highlights.
Various methods can be used to evaluate vaccines and diagnose immune deficiency.
Enzyme-linked immunosorbent assays and multiplex bead assays measure serum IgG.
Opsonophagocytosis assays measure serum antibodies’ capacity to kill bacteria.
Multiplex bead array assays have issues that may limit their application.
Opsonophagocytosis assays may eventually become useful for diagnosing immune deficiency.
Acknowledgments
This work was supported by NIH Contract HHSN272201200005C.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The University of Alabama at Birmingham (UAB) has intellectual property rights on some reagents developed in my laboratory, and all study authors are UAB employees.
References
- 1.World Health Organization, Pneumococcal conjugate vaccine for childhood immunization--WHO position paper. Wkly Epidemiol Rec, 2007. 82(12): p. 93–104. [PubMed] [Google Scholar]
- 2.World Health Organization, Pneumococcal vaccines WHO position paper - 2012 - recommendations. Vaccine, 2012. 30(32): p. 4717–8. [DOI] [PubMed] [Google Scholar]
- 3.Geno KA, et al. , Pneumococcal capsules and their types: Past, present, and future. Clin Microbiol Rev, 2015. 28(3): p. 871–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.(CDC), C.f.D.C.a.P., Prevention of pneumococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR, 1997. 46(RR-8): p. 1–24. [PubMed] [Google Scholar]
- 5.Tomczyk S, et al. , Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged >65 years: Recommendations of the advisory committee on immunization practices (ACIP). Morbidity and Mortality Weekly Report, 2014. 63(37): p. 822–5. [PMC free article] [PubMed] [Google Scholar]
- 6.Castiglia P, Recommendations for pneumococcal immunization outside routine childhood immunization programs in Western Europe. Adv Ther, 2014. 31(10): p. 1011–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jodar L, et al. , Serological criteria for evaluation and licensure of pneumococcal conjugate vaccine formultions for use in infants. Vaccine, 2003. 21: p. 3265–3272. [DOI] [PubMed] [Google Scholar]
- 8.Schiffman G, et al. , A Radioimmunoassay for Immunologic Phenomena in Pneumococcal Disease and for the Antibody Response to Pneumococcal Vaccines. I. Method for the Radioimmunoassay of Anticapsular Antibodies and Comparison with Other Techniques. Journal of Immunological Methods, 1980. 33: p. 133–144. [DOI] [PubMed] [Google Scholar]
- 9.Farr RS, A quantitative immunochemical measure of the primary interaction between I*BSA and antibody. J.Inf.Dis, 1958. 103: p. 239–262. [DOI] [PubMed] [Google Scholar]
- 10.Webster AD, et al. , Evaluation of test immunisation in the assessment of antibody deficiency syndromes. Br Med J (Clin Res Ed), 1984. 288(6434): p. 1864–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ammann AJ and Pelger RJ, Determination of antibody to pneumococcal polysaccharides with chromic chloride-treated human red blood cells and indirect hemagglutination. Appl Microbiol, 1972. 24(5): p. 679–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balmer P, Cant AJ, and Borrow R, Anti-pneumococcal antibody titre measurement: what useful information does it yield? J Clin Pathol, 2007. 60(4): p. 345–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wernette CM, et al. , Enzyme-linked immunosorbent assay for quantitation of human antibodies to pneumococcal polysaccharides. Clin Diagn Lab Immunol, 2003. 10(4): p. 514–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Training manual for Enzyme linked immunosorbent assay for the quantitation of Streptococcus pneumoniae serotype specific IgG (Pn PS ELISA).
- 15.Goldblatt D, et al. , Establishment of a new human pneumococcal standard reference serum, 007sp. Clin Vaccine Immunol, 2011. 18(10): p. 1728–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldblatt D, et al. , Assignment of Weight-Based Antibody Units for Seven Additional Serotypes to a Human Pneumococcal Standard Reference Serum, 007sp. Clin Vaccine Immunol, 2015. 22(11): p. 1154–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Plikaytis BD, et al. , An analytical model applied to a multicenter pneumococcal enzyme-linked immunosorbent assay study. J Clin Microbiol, 2000. 38(6): p. 2043–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pickering JW, et al. , A multiplexed fluorescent microsphere immunoassay for antibodies to pneumococcal capsular polysaccharides. Am. J. Clin. Pathol, 2002. 117(4): p. 589–596. [DOI] [PubMed] [Google Scholar]
- 19.Lal G, et al. , Development and validation of a nonaplex assay for the simultaneous quantitation of antibodies to nine Streptococcus pneumoniae serotypes. J Immunol Methods, 2005. 296(1–2): p. 135–47. [DOI] [PubMed] [Google Scholar]
- 20.Biagini RE, et al. , Method for simultaneous measurement of antibodies to 23 pneumococcal capsular polysaccharides. Clin Diagn Lab Immunol, 2003. 10(5): p. 744–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borgers H, et al. , Laboratory diagnosis of specific antibody deficiency to pneumococcal capsular polysaccharide antigens by multiplexed bead assay. Clin Immunol, 2010. 134(2): p. 198–205. [DOI] [PubMed] [Google Scholar]
- 22.Whaley MJ, et al. , Interlaboratory comparison of three multiplexed bead-based immunoassays for measuring serum antibodies to pneumococcal polysaccharides. Clin Vaccine Immunol, 2010. 17(5): p. 862–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pickering JW, et al. , Elimination of false-positive results in a luminex assay for pneumococcal antibodies. Clin Vaccine Immunol, 2010. 17(1): p. 185–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Waterboer T, Sehr P, and Pawlita M, Suppression of non-specific binding in serological Luminex assays. J Immunol Methods, 2006. 309(1–2): p. 200–4. [DOI] [PubMed] [Google Scholar]
- 25.Marchese RD, et al. , Optimization and validation of a multiplex, electrochemiluminescence-based detection assay for the quantitation of immunoglobulin G serotype-specific antipneumococcal antibodies in human serum. Clin Vaccine Immunol, 2009. 16(3): p. 387–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goldblatt D, et al. , Comparison of a new multiplex binding assay versus the enzyme-linked immunosorbent assay for measurement of serotype-specific pneumococcal capsular polysaccharide IgG. Clin Vaccine Immunol, 2011. 18(10): p. 1744–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Romero-Steiner S, et al. , Use of opsonophagocytosis for serological evaluation of pneumococcal vaccines. Clin Vaccine Immunol, 2006. 13(2): p. 165–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burton RL and Nahm MH, Development and validation of a fourfold multiplexed opsonization assay (MOPA4) for pneumococcal antibodies. Clin Vaccine Immunol, 2006. 13(9): p. 1004–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nahm MH, Briles DE, and Yu X, Development of a multi-specificity opsonophagocytic killing assay. Vaccine, 2000. 18(24): p. 2768–71. [DOI] [PubMed] [Google Scholar]
- 30.World Health Organization, Recommendations for the production & control of pneumococcal conjugate vaccines. 2003.
- 31.Siber GR, et al. , Estimating the protective concentration of anti-pneumococcal capsular polysaccharide antibodies. Vaccine, 2007. 25(19): p. 3816–26. [DOI] [PubMed] [Google Scholar]
- 32.Balmer P, et al. , Age-stratified prevalences of pneumococcal-serotype-specific immunoglobulin G in England and their relationship to the serotype-specific incidence of invasive pneumococcal disease prior to the introduction of the pneumococcal 7-valent conjugate vaccine. Clin Vaccine Immunol, 2007. 14(11): p. 1442–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee H, et al. , Immune response in infants to the heptavalent pneumococcal conjugate vaccine against vaccine-related serotypes 6A and 19A. Clin Vaccine Immunol, 2009. 16(3): p. 376–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Song JY, et al. , Pneumococcal vaccine and opsonic pneumococcal antibody. Journal of Infection and Chemotherapy, 2013. 19(3): p. 412–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nuorti JP and Whitney CG, Prevention of pneumococcal disease among infants and children - use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine - recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep, 2010. 59(Rr-11): p. 1–18. [PubMed] [Google Scholar]
- 36.Siber G, Basis for Developing a Pneumococcal Conjugate Vaccine for Adults. 2005.
- 37.Bonilla FA, et al. , Practice parameter for the diagnosis and management of primary immunodeficiency. J Allergy Clin Immunol, 2015. 136(5): p. 1186–205 e1–78. [DOI] [PubMed] [Google Scholar]
- 38.Orange JS, et al. , Use and interpretation of diagnostic vaccination in primary immunodeficiency: a working group report of the Basic and Clinical Immunology Interest Section of the American Academy of Allergy, Asthma & Immunology. J Allergy Clin Immunol, 2012. 130(3 Suppl): p. S1–24. [DOI] [PubMed] [Google Scholar]
- 39.Beck SC, Making sense of serotype-specific pneumococcal antibody measurements. Ann Clin Biochem, 2013. 50(Pt 6): p. 517–9. [DOI] [PubMed] [Google Scholar]
- 40.Sorensen RU, et al. , Influence of age on the response to Streptococcus pneumoniae vaccine in patients with recurrent infections and normal immunoglobulin concentrations. J Allergy Clin Immunol, 1998. 102(2): p. 215–21. [DOI] [PubMed] [Google Scholar]
- 41.Janssen WJ, et al. , Measurement of pneumococcal polysaccharide vaccine responses for immunodeficiency diagnostics: combined IgG responses compared to serotype specific IgG responses. J Clin Immunol, 2014. 34(1): p. 3–6. [DOI] [PubMed] [Google Scholar]
- 42.Sorensen RU and Leiva LE, Measurement of pneumococcal polysaccharide antibodies. J Clin Immunol, 2014. 34(2): p. 127–8. [DOI] [PubMed] [Google Scholar]
- 43.Zhang X, et al. , Impact of analytical variability on clinical interpretation of multiplex pneumococcal serology assays. Clin Vaccine Immunol, 2013. 20(7): p. 957–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Daly TM, et al. , Multilaboratory assessment of threshold versus fold-change algorithms for minimizing analytical variability in multiplexed pneumococcal IgG measurements. Clin Vaccine Immunol, 2014. 21(7): p. 982–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Burton RL, et al. , Assignment of Opsonic Values to Pneumococcal Reference Serum 007sp for Use in Opsonophagocytic Assays for 13 Serotypes. Clin Vaccine Immunol, 2017. 24(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dransfield MT, et al. , Long-term comparative immunogenicity of protein conjugate and free polysaccharide pneumococcal vaccines in chronic obstructive pulmonary disease. Clin Infect Dis, 2012. 55(5): p. e35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Watson WJ, et al. , Immunogenic compositions comprising conjugated capsular saccharide antigens, kits comprising the same and uses thereof. 2017, Google Patents.