Abstract
Little is known about the impact of sleep disturbances (SD) or sleep-related impairment (SRI) in adults with atopic dermatitis (AD), or their relationship with severity of AD and itch and other predictors. We conducted a prospective online questionnaire-based study of 287 adults with AD, including assessment of AD severity by patient-oriented eczema measure (POEM), self-reported global AD severity, self-assessed eczema area and severity index (SA-EASI) and visual analog scale (VAS-) itch; Patient Reported Outcome Measurement Information System (PROMIS) SD and SRI individual items and T-scores. Adults with AD commonly endorsed all SD and SRI symptoms examined; only 58 (21.8%) reported having good or very good sleep quality in the past week. However, only a minority of adults with AD endorsed more profound impact from these individual aspects of SD and SRI in the past week or PROMIS T-scores >55. In particular, SD and SRI were associated with severe or very severe AD (POEM, self-reported severity, VAS-itch and/or SA-EASI). SRI were also associated with comorbid hay fever and/or anxiety. This study suggests that SD and SRI are common in adults with AD, particularly those with severe disease. SD and SRI should be considered when assessing burden of AD and making therapeutic decisions.
Introduction
Atopic dermatitis (AD) is a highly symptomatic, inflammatory skin condition that is associated with intense itch and skin pain(1). A previous study found that fatigue, regular insomnia, and regular daytime sleepiness were reported by 26–34% of US adults with AD and associated with poorer quality of life(2). Another study found that US adults with AD have significantly impaired sleep and fatigue affecting instrumental activities of daily living(3). While sleep disturbances (SD) are a well-established concern in the management of AD, little is known about the relationship of the severity of AD and itch with SD and sleep-related impacts (SRI). We hypothesized that most AD patients have at least some symptoms of SD and SRI, but only a small subset have profound SD and SRI. However, little is known about other predictors of profound SD and SRI among adults with AD. We hypothesized that SD and SRI have a complex relationship with severity of AD and itch. In the present study, we studied the impact of SD and SRI in adults with AD.
Methods
Subjects
This questionnaire-based study was approved by the Institutional Review Board at Northwestern University for adults (age≥18 years, male or female) with AD. The questionnaire was uploaded to Research Data Capture Software (REDCap) and members of non-profit support groups (National Eczema Association and National Eczema Society) for AD were invited to participate by responding to the survey. All patients provided electronic informed consent. Neither the initial invitation to participate in the study nor the informed consent statement explicitly mentioned that the study was about sleep disturbances. Responses from initiation of the study in June 2014–January 2015 were reviewed. The completion rate for the survey was 95.1% among those who began the survey; partial responses were excluded. The questionnaire took an average of 18 minutes (range 15–23) to complete. Data were de-identified, confidential and posed minimal risk to participants.
Questionnaire
The questionnaire was developed to determine the burden of AD in adults (Supplemental Table 1). The questionnaire included questions about socio-demographics, health behaviors, employment, history of atopic and mental health disorders, and previously validated patient-reported outcome (PRO) assessments of eczema, including patient-oriented eczema measure (POEM)(4, 5), self-reported global AD severity, self-administered eczema area and severity index (SA-EASI) as a measure of lesional extent and severity(6), and Visual Analog Scale (VAS) for itch(7).
Sleep was assessed using questions about self-reported sleep duration and the Patient Reported Outcome Measurement Information System (PROMIS) questionnaires for SD and SRI(8). PROMIS was developed by a National Institutes of Health funded consortium that aims to develop questionnaires that measure key health-outcome domains(9). PROMIS SD assesses perceptions of sleep quality, sleep depth, and restoration associated with sleep; perceived difficulties and concerns with getting to sleep or staying asleep; and perceptions of the adequacy of and satisfaction with sleep. PROMIS SRI assesses perceptions of alertness, sleepiness, and tiredness during usual waking hours, and the perceived functional impairments during wakefulness associated with sleep problems or impaired alertness. These instruments were previously validated and found to strongly correlate with both objective and other PRO measures of sleep quality(8). Using lookup tables(8), the total score was converted to a T-score that is referenced to the United States (US) general population.
Data processing and statistical analysis
POEM, SA-EASI, VAS-itch, PROMIS SD and SRI T-scores were analyzed as both interval and ordinal variables. None of the scores were normally distributed; therefore, non-parametric assessments were used. POEM was analyzed as an ordinal variable using previously developed bands (clear/almost clear: 0–2; mild: 3–7; moderate: 8–16; severe: 17–24; very severe: 25–28) (10). VAS-itch was analyzed using previously developed bands (none: 0; mild: >0 – <40; moderate: ≥40 – <70; severe: ≥70 – <90; very severe: ≥90)(11). SA-EASI was analyzed as an ordinal variable by dividing into tertiles.
To determine the relationship of AD severity with sleep outcomes, ordinal logistic regression models were constructed with individual questions from PROMIS SD and SRI as the outcome variables. All questions used a 5-point Likert-scale. This approach was used because it allowed for estimation of a single odds ratios (OR) and 95% CI across all levels of the dependent variable simultaneously, rather than arbitrarily setting a cut-point and analyzing with binary logistic regression. The proportional odds assumption was met for all outcomes (Score test, P>0.05). POEM, self-reported global AD severity VAS-itch and SA-EASI were the ordinal explanatory variables. Multivariable models included comorbid asthma, hay fever, depression and anxiety (binary), body mass index (continuous), insurance status (binary), alcohol consumption (binary), history of smoking cigarettes (binary) or using snuff (binary), race/ethnicity (white, black, Hispanic, Asian, multiracial/other), age (continuous) and gender (male, female). Adjusted OR and 95% CI were estimated.
To test the relationship of AD severity with PROMIS SD and SRI, two different approaches were used. First, linear regression models were constructed with PROMIS SD or SRI as the continuous dependent variable. The continuous independent variables were AD severity, i.e. POEM, VAS-itch or SA-EASI. Based on visual inspection of scatter plots, a nonlinear relationship was examined. Linear and multiple orders of spline functions were tested and retained based on the best statistical fit. A penalized-spline term with one knot was the best fitting model. Inclusion of the penalized-spline in the regression models allowed for a non-linear relationship between variables. Second, the proportion of patients with no-slight (T-score<55), mild (55–59), moderate (60–69) and severe (≥70) SD and SRI were determined across different strata for POEM, self-reported global AD severity, VAS-itch and SA-EASI scores.
Since factors other than itch and AD severity impact sleep, we constructed multivariable logistic regression models with SD and SRI T-scores ≥55 as the binary outcome variables and stepwise selection from 20 independent covariates, including socio-demographics, comorbid allergic and mental health disorders (alpha=0.15).
All data analyses and statistical processes were performed using SAS version 9.4 (SAS Institute, Cary, NC). A two-sided value of p < 0.05 was used to indicate significance for all estimates.
Results
Participant characteristics
Two hundred eighty-seven adults were enrolled in the study. Participants were distributed across ages 18–69 years and a range of household income levels, and were predominantly female (79.1%), Caucasian (80.7%), non-Hispanic (89.9%), and had post-high school education (83.3%) (Table 1).
Table 1.
Baseline demographics and clinical parameters of study cohort (n = 287).
| Parameter | Frequency–no. (%) |
|---|---|
| Age (years) | |
| 18–29 | 111 (39.0%) |
| 30–39 | 71 (24.9%) |
| 40–49 | 48 (16.8%) |
| 50–59 | 39 (13.7%) |
| 60–69 | 16 (5.6%) |
| Sex | |
| Female | 220 (79.1%) |
| Male | 58 (20.9%) |
| Race | |
| Caucasian | 218 (80.7%) |
| African-American | 8 (3.0%) |
| Asian/Pacific-Islander | 24 (8.9%) |
| Other/Multiracial | 20 (7.4%) |
| Hispanic origin | |
| Yes | 26 (10.1%) |
| No | 231 (89.9%) |
| Household income | |
| < $25,000 | 66 (25.9%) |
| $25,000–$74,999 | 102 (40.0%) |
| $75,000–$199,999 | 70 (27.5%) |
| ≥ $200,000 | 17 (6.7%) |
| Highest level of household education | |
| < High school | 2 (0.7%) |
| High school or GED | 43 (15.9%) |
| > High school | 225 (83.3%) |
| Health insurance coverage | |
| No insurance | 47 (17.9%) |
| Government insurance | 90 (34.2%) |
| Private insurance | 126 (47.9%) |
| History of asthma | |
| No | 136 (49.3%) |
| Yes | 140 (50.7%) |
| History of hay fever | |
| No | 97 (35.3%) |
| Yes | 178 (64.7%) |
| History of depression | |
| No | 179 (66.1%) |
| Yes | 92 (34.0%) |
| History of anxiety | |
| No | 197 (73.8%) |
| Yes | 70 (26.2%) |
| Body mass index (median [IQR]) | 24.6 [8.7] |
| Alcohol consumption (mean ± std. dev.) | |
| Number of days per week | 1.4 ± 1.5 |
| Number of drinks per day | 1.8 ± 2.2 |
| Current smoking history | |
| No | 177 (65.1%) |
| Yes | 95 (34.9%) |
| Current snuff/tobacco chewing history | |
| No | 269 (99.3%) |
| Yes | 2 (0.7%) |
The majority of subjects reported their AD to be moderate to very severe over the past week based on the POEM score (92.3%) and self-reported global AD severity (90.5%), with median active itch of 65 on a 100 point scale (range: 5–100) and a median total SA-EASI score of 16.8 (range: 1.1–81).
Sleep disturbance and related impairment
Most patients reported ≥1 night of sleep disturbance in the past week due to their eczema (224 [79.1%]). One hundred and thirty four (47.3%) subjects reported an average of 6 or less hours of sleep (short duration), while 130 (45.9%) and 19 (6.7%) reported 7–8 (normal duration) or 9–10 hours of sleep (prolonged duration).
Only 58 (21.8%) adults with AD endorsed having good or very good sleep quality in the past week. In particular, adults with AD commonly endorsed having restless sleep (41.1% reporting quite a bit or very much), were not satisfied with their sleep (54.8% reporting not at all or a little bit), did not have refreshing sleep (58.5% reporting not at all or a little bit), had difficulty sleeping (36.4% reporting often or always), including falling asleep (31.4% reporting often or always) and staying asleep (31.4% reporting often or always), did not get enough sleep (24.7% reporting often or always) (Table 2). In addition, most adults with AD reported some sleep-related impairment, including difficulty getting things done because of being sleepy, not feeling alert when waking up, feeling tired, problems during the day because of poor sleep, having a hard time concentrating because of poor sleep, being irritable because of poor sleep, feeling sleepy during the day time and having trouble staying awake during the day.
Table 2.
Sleep disturbance and related impairment.
| Variable | Not at all | A little bit | Somewhat | Quite a bit | Very much |
|---|---|---|---|---|---|
| Sleep disturbance | |||||
| Restless sleep | 20 (7.2%) | 73 (26.3%) | 71 (25.5%) | 51 (18.4%) | 63 (22.7%) |
| Satisfied with sleep | 89 (32.3%) | 62 (22.5%) | 77 (27.9%) | 36 (13.0%) | 12 (4.4%) |
| Refreshing sleep | 90 (32.7%) | 71 (25.8%) | 74 (26.9%) | 33 (12.0%) | 7 (2.6%) |
| Difficulty falling asleep | 54 (19.4%) | 62 (22.3%) | 51 (18.4%) | 56 (20.1%) | 55 (19.8%) |
|
|
|||||
| Never | Rarely | Sometimes | Often | Always | |
|
|
|||||
| Trouble staying asleep | 28 (10.1%) | 65 (23.5%) | 97 (35.0%) | 49 (17.7%) | 38 (13.7%) |
| Trouble sleeping | 22 (8.0%) | 61 (22.2%) | 92 (33.5%) | 63 (22.9%) | 37 (13.5%) |
| Got enough sleep | 37 (13.4%) | 76 (27.5%) | 95 (34.4%) | 57 (20.7%) | 11 (4.0%) |
|
|
|||||
| Very poor | Poor | Fair | Good | Very good | |
|
|
|||||
| Sleep quality | 22 (8.2%) | 65 (24.3%) | 122 (45.7%) | 53 (19.9%) | 5 (1.9%) |
|
| |||||
| Sleep-related impairment | Not at all | A little bit | Somewhat | Quite a bit | Very much |
|
| |||||
| Hard to get things done because sleepy | 66 (23.8%) | 89 (32.1%) | 59 (21.3%) | 38 (13.7%) | 25 (9.0%) |
| Felt alert when woke up | 110 (39.6%) | 79 (28.4%) | 53 (19.1%) | 25 (9.0%) | 11 (4.0%) |
| Felt tired | 14 (5.1%) | 64 (23.2%) | 64 (23.2%) | 77 (27.9%) | 57 (20.7%) |
| Problems during day because of poor sleep | 88 (31.7%) | 73 (26.3%) | 62 (22.3%) | 28 (10.1%) | 27 (9.7%) |
| Hard time concentrating because of poor sleep | 73 (26.4%) | 91 (32.9%) | 38 (17.3%) | 35 (12.6%) | 30 (10.8%) |
| Irritable because of poor sleep | 59 (21.2%) | 81 (29.1%) | 56 (20.1%) | 43 (15.5%) | 39 (14.0%) |
| Sleepy during the day time | 42 (15.2%) | 93 (33.7%) | 57 (20.7%) | 48 (17.4%) | 36 (13.0%) |
| Trouble staying awake during the day | 96 (34.8%) | 84 (30.4%) | 47 (17.0%) | 22 (8.0%) | 27 (9.8%) |
However, a subset of subjects with AD endorsed more profound impact from SD than SRI, with less than half of subjects having profound impact from SRI in the past week (quite a bit/very much or often/always) (Table 2). Moreover, most adults with AD had no to slight (PROMIS T-scores <55) SD (208 [74.8%]) or SRI (148 [53.2%]) in the past week compared with the general population. Sixty-five [23.4%] and 5 [1.8%] reported mild and moderate-severe SD, whereas 49 [17.6%] and 81 [29.1%] reported mild and moderate-severe SRI.
Similarly, a subset of adults with AD reported unintentionally falling asleep during the daytime ≥3 times a month (121 [42.1%]), having some or very much difficulty at school or work because of sleepiness (124 [44.6%] and 32 [11.9%]), and falling asleep while driving (22 [7.8%]), respectively.
AD severity, sleep disturbance and related impairment
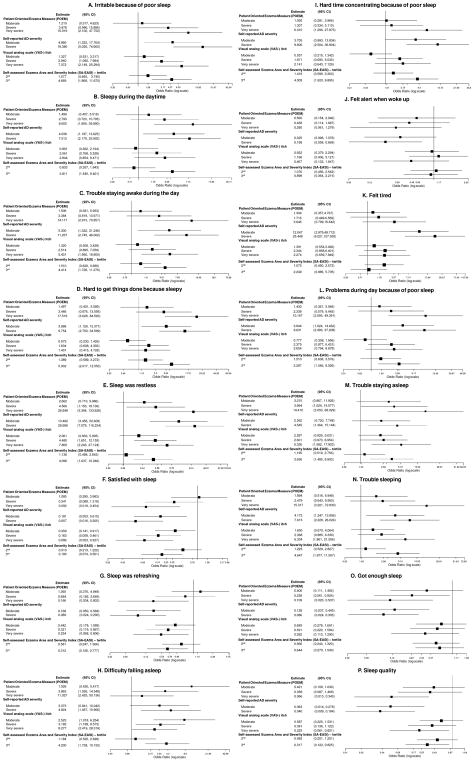
Multivariable ordinal logistic regression models were constructed to determine the relationship between AD severity and individual aspects of SD and SRI. Increasing AD severity was associated with numerically higher odds for all of the abovementioned individual symptoms of SD and SRI. However, only a subset of subjects with severe or very severe POEM, moderate and severe self-reported global assessment of AD, severe or very severe VAS-itch, and highest tertile SA-EASI scores had significantly higher odds for all of aspects of SD and SRI (Figure 1).
Figure 1.
Forest plots of adjusted odds ratios and 95% confidence intervals for the associations between atopic dermatitis severity and individual aspects from the PROMIS sleep disturbance and sleep-related impairment questionnaires.
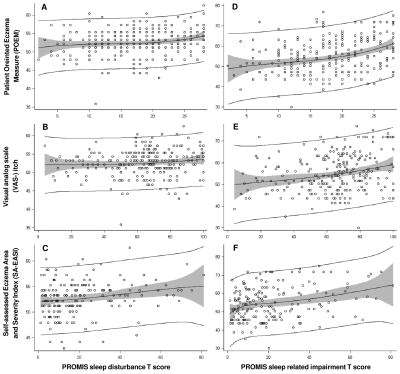
PROMIS SD and SRI T-scores rescale the raw composite SD and SRI scores into standardized scores with a mean of 50 and a standard deviation of 10, where a score of 50 is the average score for people in the US general population. POEM, VAS-itch and SA-EASI were all only weakly or moderately correlated with PROMIS SD (Spearman correlation; rhoPOEM=0.18; rhoVAS-itch=0.13; rhoSA-EASI=0.22) and SRI T-scores (rhoPOEM=0.34; rhoVAS-itch=0.24; rhoSA-EASI=0.28). There were non-linear relationships of PROMIS SD and SRI T-scores with POEM, VAS-itch, and SA-EASI, which were significantly better depicted using higher order polynomial functions (P<0.001) and improvement of model fit (Figure 2). That is, SD and SRI occurred particularly among a subset of adults with severe or very severe POEM, VAS-itch and SA-EASI scores.
Figure 2.
Relationship of POEM, VAS-itch and SA-EASI with PROMIS sleep disturbance (SD) and sleep-related impairment (SRI) T-scores. Scatterplots and penalized-splines are plotted for PROMIS SD T-scores vs. (A) POEM, (B) VAS-itch, and (C) SA-EASI, as well as PROMIS SRI T-scores vs. (D) POEM, (E) VAS-itch, and (F) SA-EASI.
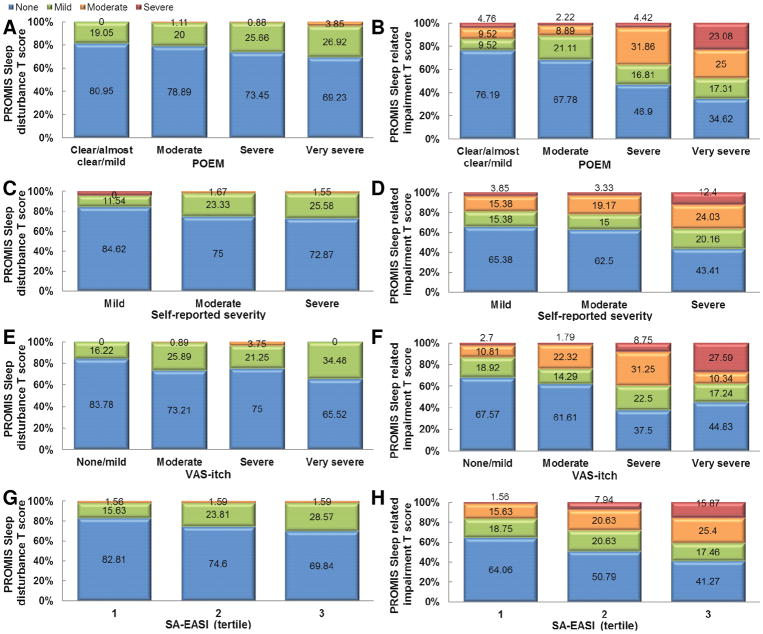
In order to interpret PROMIS SD and SRI T-scores, the proportions of subjects falling within previously established severity strata (none, mild, moderate, severe) were determined across different AD severities (Figure 3). Virtually no AD subjects had moderate or severe SD compared with the US population. In contrast, substantial proportions of AD subjects had moderate and severe SRI. The proportion of patients with mild, moderate and severe SD and SRI generally increased with more severe POEM, self-reported global AD severity, VAS-itch and SA-EASI scores. Though, there was poor concordance between severity of AD and SD and SRI. Even among patients with severe or very severe AD, the majority did not perceive SD. In addition, there were substantial proportions of patients with clear or mild AD that perceived SRI, and patients with severe or very severe AD that did not perceive SRI.
Figure 3.
Proportion of patients with no-slight (T-score<55 [blue]), mild (55–59 [green]), moderate (60–69 [orange]) and severe (≥70 [red]) PROMIS sleep disturbance (A,C,E,G) and sleep-related impairment (B,D,F,H) T-scores stratified by severity of atopic dermatitis severity (POEM [A,B], self-reported global AD severity [C,D], VAS-itch [E,F] and SA-EASI [G,H]).
Other predictors of sleep disturbances and related impairment
To identify other predictors of SD and SRI, multivariable logistic regression models were constructed with stepwise selection from 20 covariates, comorbid allergic and mental health disorders. SD T-scores >55 were significantly associated with VAS-itch scores (OR [95% CI]: 1.025 [1.002–1.049]). In contrast, SRI was associated with self-reported severe AD (aOR [95% CI]: 13.198 [2.041–85.344]), history of hay fever (2.983 [1.148–7.747] and anxiety (7.926 [2.602–24.146]).
Discussion
The present study found that symptoms of SD and SRI were common among adults with AD, including multiple nights of disturbed sleep, difficulty falling asleep, short sleep duration, poor sleep quality, impaired alertness, increased sleepiness, and impaired function secondary to poor sleep, e.g. school or work and falling asleep while driving. However, elevated PROMIS SD and SRI T-scores were only observed in a minority of AD patients, particularly those with severe or very severe AD, indicating that most AD patients do not have more profound SD and SRI than the rest of the US population. Nevertheless, SD and SRI are clinically relevant in a subset of AD patients and may indirectly impact the health of adults with AD. Previous studies found that insomnia, daytime sleepiness and fatigue in US adults with AD were associated with poor overall health, increased healthcare utilization and sick days(2), increased fractures and other injuries(12, 13), headaches(14), and cardiovascular disease(15). Given that potential impact of SD and SRI on overall health and their poor correlation with AD severity, it appears that SD and SRI should specifically be assessed in AD patients aside from AD severity. Further, SD and SRI should be considered together with other AD severity assessments when assessing the burden of and making therapeutic decisions in AD(16).
Multiple previous studies using objective measures, such as actigraphy and polysomnography, demonstrated poor sleep outcomes in children with AD(17, 18). However, few studies have evaluated the impact of AD on sleep in adults. A case-control study of 14 adults with moderate-severe AD and 14 controls found significantly worse sleep outcomes, including lower sleep efficiency and more awakenings, using actigraphy and self-reported measures(19). However, that study neither examined mild AD nor stratified sleep disturbances by AD severity. A previous study of 112 Japanese adults with AD found weak to moderate correlations between self-reported sleep outcomes using the Japanese version of the Pittsburgh Sleep Quality Index (PSQI-J) and AD severity as judged by Scoring AD (SCORAD)(20). The present study demonstrated considerable sleep disturbances and sleep-related impairment using the respective PROMIS SD and SRI short-forms, which were previously validated and found to strongly correlate with actigraphy(8). Together, these studies indicate that severe to very severe AD is particularly associated with poor sleep outcomes.
A substantial proportion of subjects with milder AD still reported SD and SRI. These results are consistent with a previous study of 14 children with AD in remission had a significantly higher number of arousals and awakenings than non-AD controls, most of which were not related to itching or scratching(21). To address this, we constructed regression models with stepwise selection to identify additional associations with poor sleep outcomes in AD. Comorbid hay fever and anxiety were associated with increased SRI; these were previously found to be associated with poor sleep outcomes(22, 23). Future studies are needed to identify the ideal interventions to improve SD and SRI in adult AD.
Given their significant burden, routine screening for sleep disturbances appears warranted in adults with AD. However, the ideal screening instruments have yet to be established. An ideal screening instrument should be low-cost, reliable, quickly and easily completed, and able to incorporate in the busy clinical practice setting. Polysomnography is considered the gold-standard objective measures of sleep, but is fairly expensive and typically performed over an 8 hour period in an inpatient setting. Actigraphy can be used to record nighttime movement over a 7–30 day period and has been pilot and feasibility tested in the ambulatory clinical setting. However, actigraphy watches are expensive and can be easily lost by patients, especially since most are not water proof and must be taken off before showering, swimming, etc. We suggest using a previously validated PRO assessments, such as PSQI or PROMIS SD or SRI short-forms. PSQI is a validated, free, extensively used, self-reported instrument but can take 5–10 minutes to complete and has a complex scoring algorithm(24), which may be a little cumbersome for incorporation into primary care, dermatology and allergy settings. PROMIS SD and SRI short-forms can be completed within 2–3 minutes and are easy to score, with lookup tables to convert to T-scores allowing for comparison with the general population(8). An alternative, perhaps enabled by further study, is the use of 1–2 highly-predictive PROMIS sleep questions, response to which produces adequate sensitivity and specificity for clinical use. Clinical validation studies are underway to validate the use of these instruments in the clinical setting for AD.
This study has several strengths, including being prospective, inclusion of a large number of subjects at all levels of AD severity and use of validated PRO for AD severity (POEM(4, 5), self-reported AD severity(25), SA-EASI(6), and VAS-itch(7)) and SD and SRI (PROMIS). The PROMIS SD and SRI T-scores allowed for comparison of adults with AD from this study with those of the general population. All of the analyses found highly consistent results across multiple measures of AD severity. However, this study has potential limitations. We were not able to determine the impact of sedating anti-histamines and other medications on sleep. While sedating anti-histamines are commonly used to treat SD in AD, they have been shown to cause residual daytime sedation, decreased vigilance and cognitive functioning (26, 27). The study was cross-sectional and was not able to capture the impact of waxing and waning AD on sleep disturbances. Objective assessment of AD extent and severity by a clinician was not assessed. Future longitudinal, clinical studies would further address these points.
In conclusion, SD and SRI occurred even in mild AD, but were most common in severe AD. We recommend routine screening of adults with AD for sleep disturbances in clinical practice. It may be that a multidisciplinary approach which aims to improve itch and AD severity, as well as symptoms of allergic disease and mental health is needed to achieve good sleep outcomes.
Acknowledgments
Funding Support: This publication was made possible with support from the Agency for Healthcare Research and Quality (AHRQ), grant number K12HS023011, and the Dermatology Foundation.
Footnotes
Financial disclosures: None
Conflicts of interest: None
Funding/Sponsor was involved? No
Design and conduct of the study: Yes__ No_X_
Collection, management, analysis and interpretation of data: Yes__ No_X_
Preparation, review, or approval of the manuscript: Yes__ No_X_
Decision to submit the manuscript for publication: Yes__ No_X_
References
- 1.Vakharia PP, Chopra R, Sacotte R, Patel KR, Singam V, Patel N, et al. Burden of skin pain in atopic dermatitis. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology. 2017;119(6):548–52. e3. doi: 10.1016/j.anai.2017.09.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Silverberg JI, Garg NK, Paller AS, Fishbein AB, Zee PC. Sleep disturbances in adults with eczema are associated with impaired overall health: a US population-based study. The Journal of investigative dermatology. 2015;135(1):56–66. doi: 10.1038/jid.2014.325. [DOI] [PubMed] [Google Scholar]
- 3.Yu SH, Attarian H, Zee P, Silverberg JI. Burden of Sleep and Fatigue in US Adults With Atopic Dermatitis. Dermatitis : contact, atopic, occupational, drug : official journal of the American Contact Dermatitis Society, North American Contact Dermatitis Group. 2016;27(2):50–8. doi: 10.1097/DER.0000000000000161. [DOI] [PubMed] [Google Scholar]
- 4.Charman CR, Venn AJ, Williams H. Measuring atopic eczema severity visually: which variables are most important to patients? Archives of dermatology. 2005;141(9):1146–51. doi: 10.1001/archderm.141.9.1146. discussion 51. [DOI] [PubMed] [Google Scholar]
- 5.Schram ME, Spuls PI, Leeflang MM, Lindeboom R, Bos JD, Schmitt J. EASI, (objective) SCORAD and POEM for atopic eczema: responsiveness and minimal clinically important difference. Allergy. 2012;67(1):99–106. doi: 10.1111/j.1398-9995.2011.02719.x. [DOI] [PubMed] [Google Scholar]
- 6.Housman TS, Patel MJ, Camacho F, Feldman SR, Fleischer AB, Jr, Balkrishnan R. Use of the Self-Administered Eczema Area and Severity Index by parent caregivers: results of a validation study. The British journal of dermatology. 2002;147(6):1192–8. doi: 10.1046/j.1365-2133.2002.05031.x. [DOI] [PubMed] [Google Scholar]
- 7.Phan NQ, Blome C, Fritz F, Gerss J, Reich A, Ebata T, et al. Assessment of pruritus intensity: prospective study on validity and reliability of the visual analogue scale, numerical rating scale and verbal rating scale in 471 patients with chronic pruritus. Acta dermato-venereologica. 2012;92(5):502–7. doi: 10.2340/00015555-1246. [DOI] [PubMed] [Google Scholar]
- 8.Yu L, Buysse DJ, Germain A, Moul DE, Stover A, Dodds NE, et al. Development of short forms from the PROMIS sleep disturbance and Sleep-Related Impairment item banks. Behav Sleep Med. 2011;10(1):6–24. doi: 10.1080/15402002.2012.636266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buysse DJ, Yu L, Moul DE, Germain A, Stover A, Dodds NE, et al. Development and validation of patient-reported outcome measures for sleep disturbance and sleep-related impairments. Sleep. 2010;33(6):781–92. doi: 10.1093/sleep/33.6.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Charman CR, Venn AJ, Ravenscroft JC, Williams HC. Translating Patient-Oriented Eczema Measure (POEM) scores into clinical practice by suggesting severity strata derived using anchor-based methods. The British journal of dermatology. 2013;169(6):1326–32. doi: 10.1111/bjd.12590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reich A, Heisig M, Phan NQ, Taneda K, Takamori K, Takeuchi S, et al. Visual analogue scale: evaluation of the instrument for the assessment of pruritus. Acta dermato-venereologica. 2012;92(5):497–501. doi: 10.2340/00015555-1265. [DOI] [PubMed] [Google Scholar]
- 12.Garg N, Silverberg JI. Association between eczema and increased fracture and bone or joint injury in adults: a US population-based study. JAMA dermatology. 2015;151(1):33–41. doi: 10.1001/jamadermatol.2014.2098. [DOI] [PubMed] [Google Scholar]
- 13.Garg NK, Silverberg JI. Eczema is associated with osteoporosis and fractures in adults: a US population-based study. The Journal of allergy and clinical immunology. 2015;135(4):1085–7. e2. doi: 10.1016/j.jaci.2014.10.043. [DOI] [PubMed] [Google Scholar]
- 14.Silverberg JI. Association between childhood eczema and headaches: An analysis of 19 US population-based studies. The Journal of allergy and clinical immunology. 2016;137(2):492–9. e5. doi: 10.1016/j.jaci.2015.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silverberg JI, Greenland P. Eczema and cardiovascular risk factors in 2 US adult population studies. The Journal of allergy and clinical immunology. 2015;135(3):721–8. e6. doi: 10.1016/j.jaci.2014.11.023. [DOI] [PubMed] [Google Scholar]
- 16.Simpson EL, Bruin-Weller M, Flohr C, Ardern-Jones MR, Barbarot S, Deleuran M, et al. When does atopic dermatitis warrant systemic therapy? Recommendations from an expert panel of the International Eczema Council. Journal of the American Academy of Dermatology. 2017;77(4):623–33. doi: 10.1016/j.jaad.2017.06.042. [DOI] [PubMed] [Google Scholar]
- 17.Stores G, Burrows A, Crawford C. Physiological sleep disturbance in children with atopic dermatitis: a case control study. Pediatric dermatology. 1998;15(4):264–8. doi: 10.1046/j.1525-1470.1998.1998015264.x. [DOI] [PubMed] [Google Scholar]
- 18.Dahl RE, Bernhisel-Broadbent J, Scanlon-Holdford S, Sampson HA, Lupo M. Sleep disturbances in children with atopic dermatitis. Archives of pediatrics & adolescent medicine. 1995;149(8):856–60. doi: 10.1001/archpedi.1995.02170210030005. [DOI] [PubMed] [Google Scholar]
- 19.Bender BG, Leung SB, Leung DY. Actigraphy assessment of sleep disturbance in patients with atopic dermatitis: an objective life quality measure. The Journal of allergy and clinical immunology. 2003;111(3):598–602. doi: 10.1067/mai.2003.174. [DOI] [PubMed] [Google Scholar]
- 20.Yano C, Saeki H, Ishiji T, Ishiuji Y, Sato J, Tofuku Y, et al. Impact of disease severity on sleep quality in Japanese patients with atopic dermatitis. Journal of Dermatological Science. 2013;72(2):195–7. doi: 10.1016/j.jdermsci.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 21.Reuveni H, Chapnick G, Tal A, Tarasiuk A. Sleep fragmentation in children with atopic dermatitis. Archives of pediatrics & adolescent medicine. 1999;153(3):249–53. doi: 10.1001/archpedi.153.3.249. [DOI] [PubMed] [Google Scholar]
- 22.Pratt EL, Craig TJ. Assessing outcomes from the sleep disturbance associated with rhinitis. Current opinion in allergy and clinical immunology. 2007;7(3):249–56. doi: 10.1097/ACI.0b013e3280f3c09f. [DOI] [PubMed] [Google Scholar]
- 23.Pires GN, Bezerra AG, Tufik S, Andersen ML. Effects of acute sleep deprivation on state anxiety levels: a systematic review and meta-analysis. Sleep medicine. 2016;24:109–18. doi: 10.1016/j.sleep.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 24.Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 25.Vakharia PP, Chopra R, Sacotte R, Patel N, Immaneni S, White T, et al. Validation of patient-reported global severity of atopic dermatitis in adults. Allergy. 2018;73(2):451–8. doi: 10.1111/all.13309. [DOI] [PubMed] [Google Scholar]
- 26.Zhang D, Tashiro M, Shibuya K, Okamura N, Funaki Y, Yoshikawa T, et al. Next-day residual sedative effect after nighttime administration of an over-the-counter antihistamine sleep aid, diphenhydramine, measured by positron emission tomography. J Clin Psychopharmacol. 2010;30(6):694–701. doi: 10.1097/jcp.0b013e3181fa8526. [DOI] [PubMed] [Google Scholar]
- 27.Wilken JA, Kane RL, Ellis AK, Rafeiro E, Briscoe MP, Sullivan CL, et al. A comparison of the effect of diphenhydramine and desloratadine on vigilance and cognitive function during treatment of ragweed-induced allergic rhinitis. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology. 2003;91(4):375–85. doi: 10.1016/S1081-1206(10)61685-7. [DOI] [PubMed] [Google Scholar]