Abstract
Presbyopia results from loss or insufficiency of the eye's accommodative ability, and clinically manifests as the inability to focus near objects on the retina. It is one of the most common causes of visual impairment worldwide especially in adults of productive or working age. Various means of compensating for the loss of accommodative ability have been devised from optical tools such as spectacles and contact lenses, to topical medications and to surgical procedures. A comprehensive search on journal articles about topical and surgical correction of presbyopia was undertaken. The various techniques for presbyopia correction, as enumerated in these articles, are discussed in this paper with the addition of our personal experience and perspective on the future of these techniques.
Keywords: Accomodating intraocular lenses, electrostimulation, intracorneal inlays, laser sclerectomy, monovision, multifocal intraocular lenses, presbyopic eyedrops, PresbyLASIK, scleral implants
Introduction
Accommodation is the process by which the eye increases the power of the crystalline lens for it to focus near objects on the retina. The accommodative triad consists of an increase in the anterior and posterior curvatures of the lens, along with convergence and miosis.[1,2,3]
Presbyopia is the loss or insufficiency of the accommodative ability of the eye. It is an irreversible, normal physiologic process that affects all primates. There are four types of presbyopia. Incipient presbyopia is the earliest stage, at which reading small print at near requires extra effort. Functional presbyopia is the stage at which visual difficulty occurs with clinical findings – there is gradually declining accommodative amplitude and continued near tasks demand assistance. Absolute presbyopia is the endpoint of continuous gradual accommodation decline, wherein no accommodative ability remains. Premature presbyopia is when accommodative ability becomes insufficient for near vision tasks at an earlier age than expected – usually due to factors such as environment, disease, medication use, or nutrition.
It has long been of interest to ophthalmologists and visual science experts. Thomas Young, in 1804, clarified the nature of the processes underlying accommodation. Sixty years later Donders charted the loss of subjective amplitude of accommodation with age as well as the demand for reading correction.[1] Since then, several theories have been postulated to explain this phenomenon. The theory of Heimholtz proposes that accommodation occurs as a result of the elastic properties of the lens and the vitreous, which allow the lens to become more round when zonular muscle tension is relieved during ciliary muscle contraction. When age-related sclerosis occurs in the lens, this ability is lost.[4] Schachar, in contrast, suggests that the longitudinal fibers of the ciliary muscle contract during accommodation. This places more tension on the equatorial zonules while relaxing the anterior and posterior zonules, and the force distribution causes an increase in the equatorial diameter of the lens. Under this theory, presbyopia occurs because of the increased equatorial diameter of the aging lens, which causes a reduction in the resting tension of the zonules.[5] Dysfunctional lens syndrome (DLS) is a term proposed and described by several ophthalmologists, as a deterministic model for characterizing the aging lens according to several stages. In DLS Stage I, the lens becomes more rigid and less flexible, corresponding with presbyopia. DLS Stage II is characterized by contrast sensitivity loss, increased higher-order aberrations (HOAs) and light scatter; while DLS Stage III corresponds with significant lens clouding and cataracts.[6,7]
Presbyopia is one of the most common causes of vision impairment, affecting an estimated 1.09 billion people worldwide in 2015, regardless of race or income.[8,9,10] Presbyopia has been associated with negative impacts on quality of life in people aged 40 and above from developing and developed nations alike – because it causes difficulties with reading and with accomplishing near vision tasks. It affects individuals in the prime of professional and creative activity, and the conservative estimated burden of presbyopia is 11.023 billion USD or 0.016% of the global gross domestic product regarding potential productivity lost.[2]
Optical correction of presbyopia may be accomplished through the use of spectacles and contact lenses. Advances have been made in the refinement of spectacle and contact lens design and manufacture. Moreover, adjustment for any change in refraction is straightforward. Conventional spectacle correction involves single-vision passive spectacles for reading or near work. Bifocal, trifocal, and progressive spectacles give the benefit of vision for multiple distances without changing spectacles. However, the nonzero vertex distance from the eye means that there is no direct coupling between the lens and movements of the eye. Clear vision at particular distances requires an appropriate relationship between the visual axis and the lens. Very careful glazing of the lenses and spectacle frame fitting are also essential in achieving good vision. Another limitation to using spectacles is image jump, as the fixation axis crosses the top edge of the bifocal, and peripheral vision distortion-which make spectacle wearers more prone to falls or accidents. Presbyopes have also been recognized as a large potential market for contact lens wear, especially for those who might not be amenable to spectacle use. Some options for contact lens wearers include contact lenses for distance correction with single vision spectacles for near addition, monovision in which one eye is corrected for distance and the other for near, and bi- or multi-focal contact lenses.[11] However, contact lens use is limited by the need to observe proper care and hygiene. People with certain lifestyles find the use of contact lenses or spectacles inconvenient – whether due to cosmesis or due to the limitation of daily or athletic activities.
The advent of refractive surgery has led to an interest in the development of techniques to correct presbyopia while eliminating the need for spectacle or contact lens use. The dynamic approach to presbyopia correction uses the residual accommodative capacity of the eye and attempts to reverse the steps associated with the pathophysiology of presbyopia. Static or passive approaches attempt to enhance the depth of focus of the optical system, bringing about pseudoaccommodation because they provide functional near vision from non-accommodative factors. Monovision has been employed, with one eye treated for distance vision and another for near or intermediate vision. On the other hand, eyes can be rendered optically multifocal to increase the range of distances at which objects can be perceived.
This paper offers to the interested readers an integrated perspective on the different procedures, both surgical and non-surgical, currently available for presbyopia correction. It aims to discuss the various advantages and disadvantages associated with each technique and technology; and include our experiences and personal opinions about the immediate future perspectives of these techniques.
Methods
A comprehensive search was conducted on PubMed. Keywords used include Accommodation, refractive surgery, presbyopia correction, monovision, corneal inlays, multifocal intraocular lenses (IOLs), and multifocal corneal ablation. No date restrictions were used. Papers included were meta-analyses, systematic reviews, experimental papers, and clinical trial reports.
Discussion
Nonsurgical treatment of presbyopia
Pharmacologic treatment
While several surgical techniques for correcting presbyopia have already been developed or are being explored, various groups are interested in finding noninvasive measures. As such, several classes of eyedrops that address presbyopia are being developed or are currently under clinical evaluation.[12]
Parasympathomimetics in various combinations either with an nonsteroidal anti-inflammatory drug or with tropicamide have been tested. These are said to stimulate parasympathetic innervation and induce ciliary body stimulation and miosis causing an increased depth of focus.[13] Pilot studies have reported improvement of both uncorrected distance visual acuity (UDVA) and uncorrected near visual acuity (UNVA), and some are currently in phase IIb trials. Most of the effect diminished after several hours, however, adverse effects include headache, ocular stinging, and nausea.[14,15,16]
Another set of eyedrops uses a different approach – targeting the crystalline lens to treat presbyopia. Pirenoxine eyedrops have been shown to suppress crystalline lens hardening in rats. The same effect was also observed in a small randomized controlled study performed on 18 Japanese males with early presbyopia, clinically evidenced by improvement in accommodative amplitude – but not in males with an accommodative amplitude nearing zero. In spite of the small sample size, these results give encouraging evidence that pyridoxine eyedrops may prevent progression of presbyopia.[17] A 1.5% lipoic acid choline ester eyedrop is also being developed. It has been shown to reduce crystalline protein disulfide bonds, softening the lens, and preserving its shape-changing ability during accommodation. Phase I and II studies have reported good outcomes.[18]
Authors' comments
Pharmacologic treatment offers one of the most attractive options for the future of presbyopic treatment, especially in cases with early manifestations of subjective near vision problems. The regulations involved in approving these medications are complex, and it would take years for them to be available, but we anticipate an important success in the treatment of presbyopia with these medications.
Electrostimulation
The use of pulsed electrostimulation is being studied with the Ocufit machine (SOOFT italia, Fermo, Italy) as a minimally invasive means of restoring accommodation. It aims to revitalize ciliary muscle contraction to overcome higher resistance in the accommodative complex – the lens, ciliary muscles, zonules, and choroid-which is brought about by aging. UNVA improved after the second treatment, and ultrasound biomicroscopy performed after the procedure showed a decreased anterior and posterior ray of lens curvature. The only adverse effect was dry eye sensation. Further studies are underway, especially with regard to optimizing electrostimulation parameters and developing customized protocols or programs to retain the positive effect of the treatment. This procedure is believed to be effective for patients with early presbyopia.[19]
Authors' comment
Electrostimulation is a complex treatment that will need special equipment. Its results are anticipated to be marginal and probably very limited over time, requiring continuous and therapeutic application. Our opinion is negative due to the low practicality and the limitations involved in the use of this technique.
Surgical treatment of presbyopia
Presbyopia correction at the corneal level
Corneal surface excimer procedures
PresbyLASIK is an encompassing term for the surgical techniques that use the principles of Laser-Assisted In situ Keratomileusis (LASIK) to create a multifocal corneal surface. There are three main types of multifocal corneal excimer laser profiles: (1) multifocal transition profile – which is no longer in use because it induced significant levels of vertical coma, (2) Central PresbyLASIK, and (3) peripheral PresbyLASIK. The principles of each algorithm are based on the dioptric power of refractive error and presbyopia correction calculation, corneal asphericity quotient (Q-value), and higher-order spherical aberrations changes or optical and transition zone manipulation.[20,21,22]
In Central PresbyLASIK a hyperpositive area for near vision is created at the center, and the periphery is left for far vision.[23,24] It is suitable for both hyperopes and myopes as only minimal corneal excision is required. This technique is pupil dependent. Its main limitation is the lack of adequate alignment among the line of sight, the central pupil, and the corneal vertex, which may induce coma aberrations.
Some available variations of central presbyLASIK include:
AMO/Visx hyperopia-presbyopia multifocal approach® (AMO Development LLC, Milpitas, California, USA) steepens the central zone to improve near vision and the peripheral zone for distance vision. It is for hyperopes with up to +4.0 diopters (D) and −2.00 D of astigmatism
-Supracor® (Technolas Perfect Vision GmbH, Munich, Germany) is an aberration-optimized presbyopic algorithm that uses the Technolas® 217P excimer laser (Bausch and Lomb, Rochester, NY). This technique creates a hyperpositive area in the central 3.0 mm zone, and targets 0.50 D of myopia in both eyes.[25] It treats hyperopic presbyopia while minimizing the aberrations normally induced during treatment
-PresbyMAX® is performed using the Esiris Laser® (SCHWIND eye-tech-solutions, Kleinostheim, Germany). It is based on the creation of a biaspheric multifocal corneal surface with a central hyper-positive area to achieve +0.75 to +2.50 D of near vision correction. The central treatment zone is surrounded by an area in which the ablation is calculated to correct the distance refractive error.[20,21] It can be performed in hyperopes and myopes.
In peripheral PresbyLASIK, the center of the cornea is left for distance, and the periphery is ablated such that a negative peripheral asphericity is created to increase the depth of field for near vision.[24] For myopes, a significant amount of corneal tissue needs to be removed when performing peripheral PresbyLASIK. This is why this technique is only recommended for presbyopic hyperopes or presbyopic low myopes. Peripheral PresbyLASIK also requires an efficient excimer laser beam profile that can compensate for the loss of energy that happens while ablating the peripheral cornea – this is one of the main difficulties in targeting specifically high negative asphericity values with this technique. Figure 1 gives a schematic illustration of central and peripheral PresbyLASIK.[24]
Figure 1.
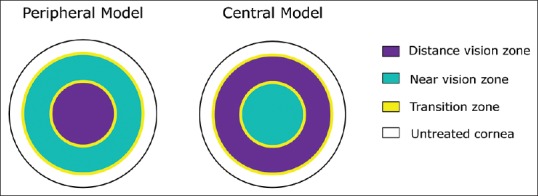
Ablation differences between central and peripheral PresbyLASIK
Laser blended vision combines a low degree of asphericity and micro-monovision in the near eye to achieve good near and distance vision.[26] A sphericity between −0.58 and −0.70 is created to increase the depth of field. Reinstein et al.[26] reported binocular visual acuity of 20/20 at distance and J3 at near in 99% of patients.
Reports on spectacle independence with central presbyLASIK range from 72%[27] to 93%.[28] The loss of corrected distance visual acuity (CDVA) of at least 1 line has been reported with central[22,25,28,29,30] and peripheral[31,32,33] presbyLASIK. Laser-blended vision combines the best features of the multifocal cornea and monovision, achieving good visual outcomes. The main disadvantage of presbyLASIK is the lack of long-term results, and the presence of a multifocal cornea is a limitation for further multifocal IOL implantation.
Author comments
PresbyLASIK is a successful procedure, when properly indicated, especially in high mesopic conditions or when the photopic pupil is <4 mm-which is a critical condition for patient selection. PresbyMAX is more effective than the procedures based on modifications of peripheral corneal asphericity, which are more limited and similar to outcomes of monovision. In the future, issues will be more general as far as studies consolidate the confidence of the refractive surgeons in these corneal procedures.
Intracorneal inlays
In 1964, keratophakia was developed for the treatment of hyperopia and presbyopia. Here, an alloplastic lenticule is placed at the interface of the free corneal cap and the stromal bed. While this procedure has been abandoned for now due to the technical difficulty of implantation as well as unpredictable refractive results, this led to the development of corneal inlays.[34]
Early synthetic corneal implants were made of polymethylmethacrylate (PMMA) or polysulfone. Although they corrected the refractive error, these implants were also associated with corneal necrosis and implant extrusion.[35] Nowadays, the material used in corneal inlays allows for sufficient nutrient flow into the stroma – avoiding nutrient flow interruption, which can cause loss of transparency, corneal thinning, epithelial and stromal decompensation, and melting.[36] The permeability of hydrogel material used in the inlays is similar to that of the corneal stroma, permitting the exchange of nutrients[37,38] such as glucose and oxygen.
Corneal inlays are advantageous in that there is no need to remove corneal tissue, implantation is relatively easy, they are minimally invasive, and they are all removable hence their effects are reversible.[39,40] The inlays are implanted in the nondominant eye, under a stromal flap or within a corneal pocket made by femtosecond laser – which is preferred as it might decrease the occurrence of dry eye.[35] Implantation depth depends on the inlay: inlays that alter the curvature of the cornea are implanted more superficially whereas inlays with small aperture or those that have a different index of refraction are implanted deeper to avoid changes in the cornea curvature and to allow a proper diffusion of nutrients in the corneal stroma.[35,39] Up to now, new implant designs are being proposed such as a diffractive corneal inlay design concept. Another alternative being proposed is the use of lenticules excised from Small Incision Lenticule Extraction (SMILE) procedures as implants for presbyopia treatment.[41] Table 1 enumerates the advantages and disadvantages of corneal inlay implantation for presbyopia treatment.
Table 1.
Advantages and disadvantages of corneal inlays
| Advantages | Disadvantages |
|---|---|
| Minimally invasive Reversible No need to remove corneal tissue Quick recovery Does not affect visual field testing Can be combined with other refractive procedures Enables normal visualization of central and peripheral fundus |
Requires patients who can tolerate monovision Decreased distance visual acuity Decreased contrast sensitivity Perception of halos Corneal topography changes (long-term) Induces HOAs Corneal haze (with long-term implantation) Dependent on inlay centration Dry eye |
HOAs=Higher order aberrations
There are three types of corneal inlays[39]
Corneal reshaping inlays enhance near and intermediate vision through a multifocal effect, changing the shape of the anterior curvature of the cornea and making it hyper-prolate to increase power[39,40]
Refractive inlays alter the refractive index with a bifocal optic[39]
Small aperture inlays improve depth of focus.[39]
Until recently, four corneal inlays were available in the market:
Corneal reshaping inlay – the Raindrop™
The Raindrop™ (ReVision Optics, Lake Forest, California, USA) is a reshaping inlay made of biocompatible hydrogel material and water, making it permeable to the passage of nutrients and oxygen.[39,40,42] The implant is 10 μm thick at the periphery, and 32 μm at the center, with a diameter of 2 mm.[35] By itself, the implant has no refractive power.[39,40] It changes the anterior corneal surface and creates a hyper prolate region, resulting in a multifocal cornea.[42] The central corneal thickness of the eye should be 500 μm or thicker, with a residual stromal bed thickness of 300 μm.
It is placed in the nondominant eye at a minimum depth of 150 μm and has to be aligned over the center of the light-constricted pupil.[40,42,43] After the inlay is placed over the center of the pupil, it has to dry for 30 s before the flap is repositioned.[43]
The results of a 1-year follow-up using the Raindrop inlay in emmetropic presbyopes were reported by Garza et al.[35] 100% achieved a UNVA of 0.2 logMAR or better in the operative eye; binocularly 100% of patients achieved a UNVA of 0.18 logMAR or better. No eye lost more than two lines of CDVA or corrected near visual acuity (CNVA). Yoo et al.,[44] meanwhile measured the corneal and optical aberrations in 22 emmetropic presbyopes with a mean addition power of +1.97 ± 0.30 D. They found that all patients gained monocular and binocular UNVA. For a 4 mm pupil size, there were significant increases in total root mean square (RMS), coma-like RMS, and spherical-like RMS. A total of 82% of the patients were satisfied or very satisfied with their near vision, and 13.6% reported that they needed near glasses more often after surgery than before surgery. Thirty-seven percent of patients reported glare. The group concluded that the procedure can induce HOA's but had moderate effects on the entire optical system. Alió et al.[38] reported an increase in spherical, coma, and total HOAs with the implantation of hydrogel inlays.
Whitman et al. followed clinical outcomes of the raindrop inlay in patients with emmetropic presbyopia.[45] A total of 340 patients completed 1 year of follow-up with an average improvement of five lines in UNVA, and 2.5 line improvement in Uncorrected Intermediate Visual Acuity (UIVA); UDVA decreased by 1.2 lines. Contrast sensitivity loss occurred at the highest spatial frequencies with no loss binocularly. Eighteen inlays were replaced because of decentration, and 11 were explanted-five patients were dissatisfied with their vision, two had inlay misalignment, two had epithelial ingrowth, one had visual symptoms associated with decreased visual acuity, one had recurrent central corneal haze that failed to respond to topical treatment. The Raindrop implant is no longer available as of January 2018.
Refractive inlay-Flexivue Microlens™
The Flexivue Microlens™ (Presbia Cooperatief U. A., Amsterdam, The Netherlands) is a transparent hydrophilic concave-convex disc that is made from a clear copolymer of hydroxyethylmethacrylate and methylmethacrylate with an ultraviolet (UV) blocker.[36,42,46] It has a diameter of 3.2 mm, and a thickness of 15–20 μm, depending on the additional power. The central 1.8 mm diameter of the disc is plano, and the peripheral zone has an add power which ranges from +1.25D to +3.0 D in +0.25 D increments. At the center, there is a 0.15 mm opening that facilitates the transfer of nutrients and oxygen through the cornea.[36,39,40,42,46] [Figure 2]-Picture courtesy of Dr. JL Alió. The inlay has a refractive power of 1.4583 and a light transmission of 95% at a wavelength above 410 nm.[42,46]
Figure 2.
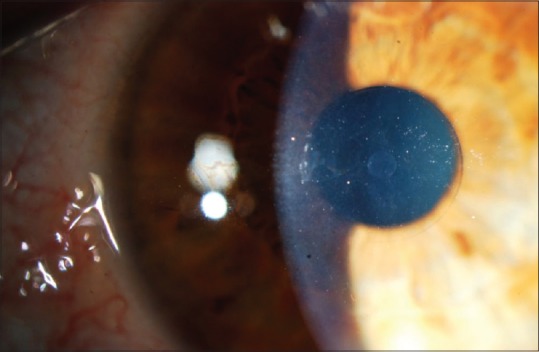
Flexivue® inlay
During distance vision, light rays pass through the plano central zone of the inlay, so that they are sharply focused on the retina. Light rays that pass through the refractive peripheral zone will focus in front of the retina. During near vision, rays passing through the central zone of the inlay will focus behind the retina, and those passing through the peripheral refractive zone of the inlay will be focused on the retina.[36,46] The rays passing through the peripheral clear cornea will be blocked by the pupil.[46]
The Flexivue microlens is implanted in the nondominant eye, in a corneal pocket that is 280–300 μm deep,[39,42,46] and it is centered over the patient's visual axis based on the first Purkinje reflex. Corneal inlay power is calculated by decreasing the preoperative CNVA manifest refraction spherical equivalent by 0.25 D.[36]
Limnopoulou et al.[46] reported in their 1-year follow-up study an UNVA of 20/32 or better in 75% of operated eyes; UDVA decreased significantly in the operated eye from 20/20 to 20/50, but binocular UDVA was not significantly altered. HOA increased and contrast sensitivity decreased in the operated eye. The said study included 47 emmetropic presbyopes between 45 and 60 years old. No intra or postoperative complications were noted, and none of the patients required inlay explantation. In a 36-month follow-up study, Malandrini et al.[36] reported that mean preoperative UNVA and UDVA were 0.76 logMAR and 0.00 logMAR, respectively, in 26 eyes, compared with 0.10 logMAR and 0.15 logMAR postoperative. Sixty-two percent of the eyes lost more than one line of UDVA, and 19% lost more than two lines of UDVA, and 8% of the eyes lost more than one line of CDVA at 36 months. Mean spherical aberration increased after surgery. Explantation was performed in six eyes because of reduced UDVA, halos and glare, 6 months after explantation, the CDVA in all cases returned to preoperative levels.
Refractive inlay-Icolens™
The Icolens™ (Neoptics AG, Huenenberg, Switzerland) is a refractive inlay made of a copolymer of hydroxyethyl methacrylate and methyl methacrylate. It has a diameter of 3 mm, a peripheral thickness of 15 μm and a central 0.15 mm hole for nutrient flow [Figure 3].[42,47] This inlay has a bifocal design with a peripheral positive refractive zone for near and a central zone for distance vision.[47]
Figure 3.
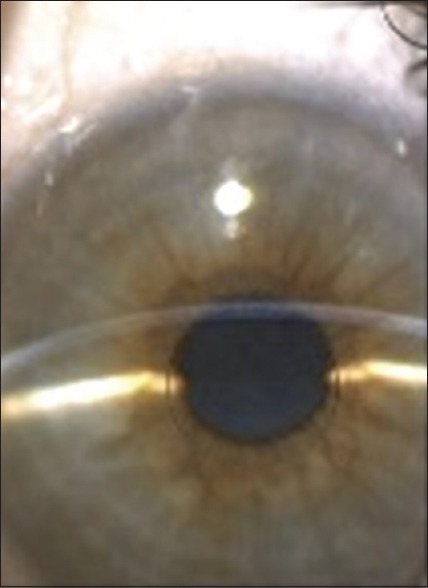
Icolens® inlay
Baily et al.[47] reported the results of the Icolens 12 months after implantation in 52 patients. The inlay was implanted in the nondominant eye of emmetropic patients through a corneal pocket created by femtosecond laser at a depth of 290 μm. UNVA improved from N18/N24 preoperatively to N8 postoperatively, with 100% of patients having N16 or better, and nine patients having N5 or better. The mean UDVA in the surgical eye worsened significantly from 0.05 ± 0.12 logMAR preoperatively to 0.22 ± 0.15 logMAR postoperatively. There was a loss of CDVA, with 77% of the patients losing more than one line – which was attributed to a neuro-optical phenomenon related to the implant. Eleven inlays were removed in total – seven because of inadequate centration, three due to ambiguous ocular dominance, and one because the patient had unrealistic expectations.
Small aperture inlays-Kamra™
The Kamra Inlay™ (Acufocus Inc., Irvine, CA, USA) is the most widely used corneal inlay[39,40] with nearly 20,000 inlays implanted worldwide. It is a small aperture inlay and is made of polyvinylidene fluoride. It is 5 μm thick and has a 3.8 mm diameter with a central 1.6 mm aperture. There are 8400 microperforations ranging in diameter from 5 to 11 mm to allow nutritional flow through the cornea.[40,42,48] The inlay contains carbon nanoparticles,[49,50] which have a light transmission of 5%.[51] Since this inlay is opaque, it may be visible in light-colored eyes [Figure 4].[50]
Figure 4.
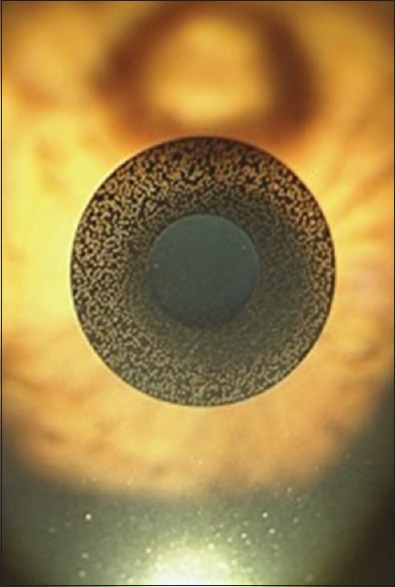
Kamra® inlay
This inlay improves near vision by increasing the depth of focus through the principle of small aperture optics.[39,40,42] It is implanted in the nondominant eye, in a lamellar pocket 200-220 μm deep. Its implantation does not cause scotomas in the visual field[40] and allows a normal visualization of the central and peripheral fundus with a good quality of central and peripheral imaging scans.[49]
Inlay design has evolved over the years, although the artificial aperture size of 3.8 mm or outer diameter and 1.6 mm inner diameter has been maintained. Tomita et al.[51] evaluated the outcomes of Kamra inlay implantation and simultaneous LASIK in hyperopic, myopic and emmetropic patients. After 6 months, they concluded that the procedure was safe and improved distance and NVA; although postoperative symptoms such as halos, glare, and night-vision disturbances had been observed.[52] Meanwhile, a 1-year follow-up of combined LASIK and Kamra inlay implantation was done by Igras et al. They concluded that there was a significant improvement in NVA with a slight compromise in uncorrected monocular DVA in the implanted eye without binocular effect on the UDVA.[53] 132 patients were evaluated: 85% were hypermetropic, 11% emmetropic and 4% myopic. By 12 months, 97% of patients had J3 or better UNVA. While 6.3% of patients lost one line of CDVA in the implanted eye, none lost more than two lines. Two inlays were explanted-one due to poor night vision, and one secondary to persistent hyperopic shift and corneal haze. On a 3-year follow-up of 32 emmetropic presbyopic patients, Seyeddain et al.[54] reported a significant gain in UNVA and UIVA, although 28.3% of patients lost one line of CDVA.
Iron corneal deposits have been noted after implantation of the Kamra inlay in 18 eyes.[55] These deposits did not influence the distance and NVA– corrected and uncorrected.
Alió et al.[56] conducted a follow-up on 10 eyes that had received the Kamra inlay, up to 6 months after inlay removal. After removal of the Kamra inlay, corneal topography and aberrometry were not permanently affected and that more than 60% of the patients had a CNVA, CDVA, UNVA, and UDVA similar to their preoperative value. The reason for the removal in eight eyes was subjective dissatisfaction with visual symptoms (glare, starburst, blurry vision, and halos), one case was related to inadvertent thin flap, and another was related to insufficient near vision.
Corneal tissue appearance was analyzed by Abbouda et al.,[57] using confocal microscopy, 6 months after KAMRA Inlay implantation. Twelve eyes that had been implanted with three models of the Kamra inlay were included in this study. The epithelium layers appeared normal in all patients, but a low grade of keratocyte activation was found in all. A few patients had an elevated number of activated keratocytes, with reduced CNVA, CDVA, and UNVA requiring the use of reading glasses. UDVA was not affected. The subbasal nerve plexus was detected in 10 patients, and the branch pattern was found in eight patients. Four patients had the inlay explanted – the main reason being subjective dissatisfaction with visual symptoms and poor vision. None of these patients had refractive postoperative changes. These findings support the role of keratocyte activation in refractive outcomes after Kamra inlay implantation. Flap thickness depth, low-laser energy cut, and topical steroid treatment help prevent it.
Lin et al.[58] compared contrast sensitivity in 507 patients before and after the implantation of the KAMRA Inlay. They reported that postoperatively contrast sensitivity was mildly reduced monocularly, but not binocularly, and that it remained within the normative ranges.
The Kamra inlay can be implanted in patients with previous cataract surgery and monofocal IOL implantation, as reported by Huseynova et al.[59] In 13 pseudophakic patients with monofocal IOL and subsequent Kamra implantation, there was no change in mean UDVA, mean UNVA improved by five lines. Two eyes lost two lines and one eye lost one line of CDVA.
Author comments
Intracorneal inlays have been the subject of clinical investigation for more than 15 years and have never gained full popularity among refractive surgeons due to the frequent problems of centration, biological tolerance, and optical performance. At present, they should be considered with marginal indications. Declining use is foreseen due to high-explantation rates over time secondary to late complications such as corneal stromal opacity, late hyperopic shift, and inadequate visual performance caused by corneal irregularity.
Corneal monovision
Monovision is a concept that was initially developed to correct presbyopia for contact lens users and later implemented for use in refractive surgery. It is an induced anisometropia, wherein the dominant eye is corrected for emmetropia and the nondominant eye is corrected with a certain degree of myopia. Crossed monovision is also possible, wherein distance correction is given to the nondominant eye. Neuroadaptation occurs, after which the brain uses the distance image from the dominant eye as well as the near image from the nondominant eye to achieve a wider range of functional vision.[60,61,62]
In general, most patients do well with laser-induced monovision; studies report ranges of 86% to 92.5% regarding optical results and patient satisfaction. Jain et al. did a retrospective observational case series on 144 presbyopic patients who underwent laser refractive surgery, and whose surgical outcome was monovision (n = 42). Of these patients, 88% were satisfied with their vision.[62] In 37 presbyopic myopes, monovision with LASIK was found to produce an acceptable binocular UNVA of 0.74, and a mean binocular CDVA of 1.08, in spite of a slight decrease in contrast sensitivity and stereopsis.[63] In a retrospective study, SMILE monovision was found to produce highly satisfactory binocular distance and NVA – VA 20/20 or better for distance and J2 for near – for 84% of 49 myopics with early presbyopia.[64]
Successful monovision treatment is said to depend on the power given to the non-dominant eye – near vision improves with increased power but diminishes distance vision. The degree of anisometropia is also a concern. There is no guide as yet for the target level of anisometropia although the maximum advised by Jain et al. would be −2.00D as anything higher than that would compromise patient acceptance.[65] Intolerable anisometropia may be reversed by performing an enhancement to correct residual myopia. Trial contract lenses may be beneficial while planning for surgery, especially with hyperopes, as it prevents unintentional overcorrection.[66]
Author's comments
Monovision, as will be mentioned later on, is one of the most successful methods for the compensation of presbyopia. Corneal monovision is usually performed in initial or intermediate presbyopes in whom full presbyopia has not yet developed. Its simplicity, good clinical outcomes, and potential reversibility-by a touch up with the excimer laser-makes it highly efficient and frequently used now and in the future by refractive surgeons.
Procedures that target the lens
Several groups have started looking into the possibility of targeting the crystalline lens to reverse presbyopia. One such procedure is phaco-ersatz, where the natural lens nucleus and cortex are replaced with a man-made substitute. Its proponents say that presbyopia is brought about by stiffness of the lens material and changes in ciliary muscle function – which the procedure would overcome. Early work on animals has been said to be promising, but this is as yet an unproven method for restoring human accommodation.
Another alternative that has been suggested would be to disrupt the lens material, making sure to maintain its clarity, with the femtosecond laser. As the laser is capable of delivering tightly focused energy pulses into the lens, it can in theory avoid causing damage to the surrounding lens material.[67] With this in mind, some groups have proposed scanning the point of focus in three dimensions to generate cleavage patterns within the lens-creating so-called gliding planes that exert an effect similar to elasticity. Various patterns have been tried– all avoiding the axial region of the lens, to avoid image-degrading opacities. Initial in vitro and in vivo experimental studies have been performed on cadaver lenses and animal lenses, all demonstrating improvement in effective elasticity. The first human experiments, on patients aged 45–60 who were to undergo later refractive surgery for later cataract, had disappointing results however.[68] While none of the eyes involved developed cataracts, the results for accommodation were disappointing – with mean and SD of objective amplitude improvement only at 0.20 ± 0.29 D. This technique is still in development, and further investigation on long-term effects and procedure refinement is needed.
Anterior and posterior chamber phakic lenses were originally designed to correct high levels of ametropia in younger patients. This concept was extended to presbyopia correction, using simultaneous-image refractive bifocal lenses with a central 1.5 mm in the optic to provide distance correction. Unfortunately, endothelial cell loss was found to be a problem, occurring even years after implantation.[60] This and the rising popularity of refractive lens exchange led to diminished interest in phakic IOLs for presbyopia.
Refractive lens exchange, which involves removing the lens and replacing it with premium IOL implants – which provide good visual outcomes for distance and near, and promise spectacle independence has so far been deemed the most popular option for managing presbyopia. Premium IOL's can be divided into two main groups: multifocal IOL's and accommodative IOL's.
Multifocal intraocular lenses
Multifocal IOL's aim to provide spectacle independence for both near and distance vision by dividing light into two or more foci,[69,70,71,72] independently of capsular mechanics and ciliary body function.[73] The perfect multifocal IOL should provide excellent vision for near, intermediate, and distance activities. Its design has to be aspheric, and the material should be malleable enough as to be implanted through incisions <2 mm – allowing for the performance of microincision cataract surgery. Ideally, these IOL's should not produce photic phenomena and should be pupil independent as well.
There are various types of multifocal IOL's available in the market today. These can be grouped based on their design – the main IOL classifications are either rotationally symmetrical or rotationally asymmetrical (or varifocal). Rotationally symmetrical IOL's can be further divided into diffractive, refractive, or combined IOL designs.[70,71,72,73,74]
Diffractive IOLs were designed based on the principle of diffraction, wherein light changes direction or slows down when it encounters an edge of discontinuity. These lenses have rings on the surface, which form a discontinued optical density. When light particles hit these rings, these light particles are directed toward two focal points[70,71,75] (as in the case of bifocal IOLs) or three focal points (as in the case of trifocal IOLS).[70] Diffractive IOLs may either be apodized or nonapodized. Apodized IOL's – to apodize means to remove the foot[71]-have a gradual decrease in diffractive step heights from the center to the periphery.[71,75] The steps on the non-apodized IOLs have a uniform height from the center to the periphery, such that light is equally distributed in both focal points independently of the pupil size.[71] This creates a smooth transition of light between the focal points. These are the most commonly implanted multifocal IOL.[76] Extended depth of focus (EDOF) IOLs are also diffractive lenses that apply positive and negative spherical aberrations in the lens center to create a continuous range of focus.[77]
Refractive IOLs use concentric zones of different dioptric power to achieve multifocality. They are pupil dependent and may be affected by decentration.[71]
Rotationally asymmetric or varifocal IOLs are characterized by an inferior segmental near add.[74] The IOL has a larger section for distance vision, and a smaller reading segment with only one transition zone. The near add varies from +1.5 to +3.0 D, depending on the patient's visual needs.[78]
Considerations prior to multifocal intraocular lens implantation
Careful patient selection is the most important part in implanting a multifocal IOL. One has to know the patient's visual expectations, and make sure that this can be fulfilled by the chosen IOL. It is important to give the patients realistic expectations about surgical outcomes and to give them advanced warning of possible visual side effects, such as glares or haloes that may be experienced with multifocal IOLs. Patients also have to be counseled as to the process of neuroadaptation, which may require several months for them to grow accustomed to vision with the multifocal lens. It is important to carefully check pupillary function before and after surgery, as the majority of multifocal IOLs are pupil dependent. Intraoperatively, in a patient with a very small pupil size which might require surgical manipulation, extra care should be taken to avoid causing damage to the pupillary sphincter. Patients with larger pupils may experience glare and halos after multifocal IOL implantation.[78,79,80]
Existing ophthalmologic conditions should be taken into account while planning for multifocal IOL implantation. Patients with corneal abnormalities such as central scars and Fuchs dystrophy are not suitable candidates for multifocal IOL implantation.[80] Careful zonular examination is needed in the preoperative evaluation. Identification of zonular weakness during surgery is very important as decentration or tilt of the multifocal IOL can have a detrimental effect on the visual acuity. This can be prevented by the implantation of a capsular tension ring.[80,81] A macular condition is a relative contraindication for the implantation of a multifocal IOL[82] as it causes reduced contrast sensibility that can be worsened by implantation of a multifocal IOL. The macula should be carefully evaluated, with a macular function test or a posterior segment optical coherence tomography (OCT) scan – especially in patients with risk factors (males, with a significant smoking history, and a history of heart disease).[82] Retinal dystrophies such as Stargardt and retinitis pigmentosa are absolute contraindications for multifocal IOL implantation. Other diseases such as diabetic retinopathy and macular degeneration cause decreased contrast sensitivity that may be worsened by the multifocal IOL.[80] Glaucoma is a relative contraindication to multifocal IOL implantation. If the patient has early glaucoma or controlled ocular hypertension, a multifocal IOL may be considered; however, it should be avoided in patients with uncontrolled progressive and advanced glaucoma.[83]
Correct IOL power calculation and selection is crucial, and emmetropia should be the target. It has been reported that 20% of the causes of multifocal IOL explantation is due to incorrect IOL power.[79] Astigmatic correction is mandatory to ensure good multifocal IOL results. As such, patients with irregular astigmatism are not good candidates for a multifocal IOL[78,80] since its correction is neither easy nor predictable. Further studies are needed to determine the visual effects of multifocal IOLs in patients with a history of previous refractive surgery.[84]
A variety of multifocal IOLs are already commercially available – these will be briefly discussed below.
The AcrySof® Restor® SN6AD3 (Alcon Laboratories, Inc., Fort Worth, Texas) is a one-piece multifocal IOL that uses both apodized diffractive and refractive technology. It is made of hydrophobic acrylic with UV and blue light filter and has a refractive index of 1.47. This lens is not pupil dependent. It has an optic diameter of 6 mm, and an overall diameter of 13 mm, and implantation requires an incision size more than 2.2 mm [Figure 5].[85] Available powers range from +6.00 to +34.00 D, and near addition at the lens plane is +4.00 D. The central 3.5 mm of the optic zone has 12 concentric steps with gradually decreasing step height. Surrounding this apodized region is the refractive area that directs light to a distance focal point for large pupil diameters.[85,86] In bright light with constricted pupils, the lens sends light energy to near and distant focal points; in low light with dilated pupils, the apodized diffractive lens sends a greater amount of energy to distance vision to minimize visual disturbances.[87]
Figure 5.
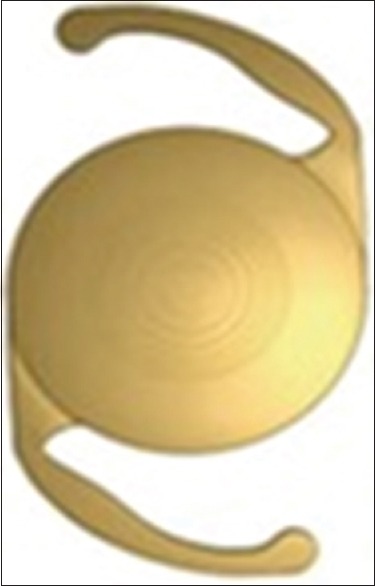
AcrySof® Restor SN6AD3
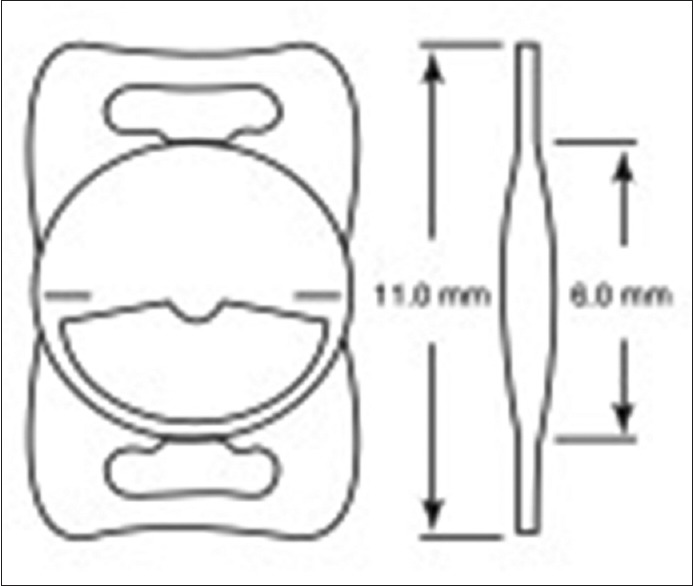
The Lentis® Mplus LS-313 (Oculentis GmbH, Berlin, Germany) is a one piece, refractive rotationally asymmetric (varifocal) multifocal IOL [Figure 6].[85] It is made of HydroSmart acrylate copolymer with a hydrophobic surface and a refractive index of 1.46. It has a 360° continuous square optic and haptic edge. The optic diameter is 6.0 mm, and the overall diameter is 11.0 mm-implantation of this lens requires an incision size of 2.6 mm. This is a pupil-dependent IOL. Available powers range from +15.0 to +25.0 D in 0.5 D steps for the Mplus; and −10.0 to +1.0 D in 1.0 D steps and from +0.00 to +36.0 D in 0.5 D steps for the Mplus X. Additional power at the IOL plane is +3.0D, and additional power at the spectacle plane is +2.5 D.
Figure 6.
Lentis® Mplus LS-313 intraocular lens
The Lentis has an inferior surface-embedded segment with the optical power required for near vision and seamless transitions between the near and far zones. Light in the near vision zone is refracted to the near focus, the rest of it is refracted to the far focus.[76,85] Light hitting the transition area of the embedded sector is reflected away from the optical axis to prevent superposition of interference or diffraction.[74,76] It is said to cause the highest HOA's when compared with the Symphony and Miniwell.[88]
The Tecnis® Symfony (Abbot Medical Optics, Inc., Santa Ana California, USA) is a diffractive nonapodized achromatic IOL. It is made of UV blocking hydrophobic acrylic and has a refractive index of 1.47. Its optic diameter is 6 mm, and its overall diameter is 13 mm. Available powers range from +5.00 to +34.00 D in 0.50 D increments. This IOL has a biconvex wavefront-designed anterior aspheric surface and a posterior achromatic diffractive surface [Figure 7].[85,89]
Figure 7.
The Tecnis® Symfony
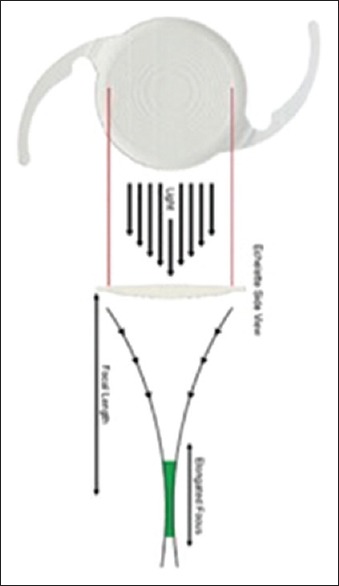
The Symfony elongates the focus and corrects the corneal chromatic and spherical aberration using an achromatic technology, also known as “extended range of vision.”[89] Chromatic aberrations have a detrimental effect on vision since they reduce contrast vision and induce blur.[90] As such, the improvement of both aberrations increases the retinal image quality with a better tolerance to decentration and without sacrificing the depth of field.[91]
This lens has been said to provide better near, intermediate, and distance vision than aspheric monofocal IOLs. Contrary to other multifocal IOLs, it does not induce aberrations to the same extent.[88] Because it provides an elongated focal area rather than various focal points, halos are not as common as with the multifocal IOLs. In fact, a study[89] reported that 90% of the patients implanted with the Symfony IOL reported no or mild halos or photic phenomena, and the visual results were better than those obtained with rotationally asymmetric multifocal IOL or apodized diffractive IOLs.
The AT LISA® tri 839 MP (Carl Zeiss Meditec, Jena, Germany) is a one-piece trifocal diffractive aspheric IOL, made of hydrophilic acrylic 25% with hydrophobic surface. Its optic diameter is 6 mm, and its overall diameter is 11 mm. Implantation requires an incision size of 1.8 mm. Available powers range from plano to +32.00 in 0.5 D increments. Additional powers are +3.33 for near vision, and +1.66 for intermediate vision. The central 4.34 mm of the IOL optic is the trifocal zone; the peripheral bifocal zone is from 4.34–6 mm with diffractive rings covering the entire optic diameter [Figure 8].[85]
Figure 8.

AT LISA® tri 839 mp intraocular lens
The FineVision® Micro F (PhysIOL, Liège, Belgium) is a one-piece trifocal IOL made of 25% hydrophilic acrylic. It is pupil dependent; its optic has a diffractive anterior surface and an aspheric posterior surface. The optic diameter is 6.15 mm, and the overall diameter is 10.75 mm; implantation requires an incision size >1.8 mm. Available powers range from +10 to +35 D in 0.5 D increments. Near addition at spectacle plane is +1.75 D intermediate vision, and +3.5D near vision [Figure 9].[85,92]
Figure 9.

FineVision® Micro F intraocular lens
The anterior surface of this IOL is convoluted. By varying the height of the diffractive step, the amount of light distributed to the near, intermediate, and distant foci are adjusted according to the pupil aperture. The IOL distributes 43% of light energy to far vision, 28% to near vision, and 15% to intermediate vision.[93]
The Panoptix® (Alcon Laboratories, Fort Worth, Texas, USA) is a single-piece, aspheric IOL made of hydrophobic UV and blue light filtering acrylate/methacrylate copolymer. Its optic diameter is 6.0 mm, and its overall diameter is 13.0 mm. This IOL has a 4.5 mm diffractive area in the center with 15 diffractive zones and an outer refractive rim. Light distribution is 25% for near (40 cm), 25% for intermediate (60 cm), and 50% for far vision.[85]
There is a more physiological transition from different distances because of an ENLIGHTEN™ optical technology, such that when the light from the first focal point is diffracted to the distance focus, a fourth focal point is created at 1.20 m. Thus, this IOL is quadrafocal although it acts as a trifocal.[93] Based on laboratory simulations, the performance in image quality, photic phenomena, and resolution are equivalent between the Panoptix IOL and the AT LISA Tri 839MP and FineVision Micro F trifocal IOL.[94]
Clinical outcomes and quality of life
Patient satisfaction and visual function with multifocal and toric multifocal IOLs are very good.[70,93,94,95,96] Carballo-Alvarez et al.[93] evaluated visual outcomes 3 months after bilateral implantation of FineVision® trifocal IOL. They reported good near, intermediate, and far vision, with satisfactory contrast sensitivity and no significant photic phenomena. When a comparison was done between the FineVision® trifocal and the Restor® IOL, similar refractive outcomes were noted, with similar reading speed and patient satisfaction. Intermediate distance in the defocus curve was better, however, in the trifocal IOL group. Spectacle independence was achieved in 80% of patients with trifocal IOLs and in 50% of patients with bifocal IOLs.[97] Similar outcomes were reported in studies that compared the Restor® IOL with the AT LISA® tri. The latter had better intermediate visual acuity, while near and DVA was good with both IOLs. The Quality of Vision (QoV) questionnaire reported similar visual disturbances between the two groups.[98,99] Comparison of the visual outcomes and intraocular optical quality between the Lentis® Mplus and the Restor® IOL has shown that Uncorrected Near Visual Acuity (UNVA) and Distance-Corrected Near Visual Acuity (DCNVA) was better with the Lentis® Mplus, although it significantly induced HOAs. Intermediate VA and photopic contrast sensitivity were better with the Restor® IOL.[76]
Complications
The most common visual symptoms associated with multifocal IOLs are glares and halos. These phenomena are secondary to the effect produced by multifocal IOL's on light-light in the out of focus image reduces the contrast of the in-focus image.[70] Other symptoms include starbursts, shadows, negative, and positive dysphotopsia.[70] Decreased contrast sensitivity has also been reported.[69,70] Aspheric multifocal IOL's were developed to avoid these visual phenomena. A meta-analysis compared the visual outcomes between aspheric and spherical multifocal IOLs; aspheric multifocal IOLs achieved better image quality than spherical multifocal IOLs, and also had less spherical aberrations.[69]
Uncorrected blurry vision has been identified as the main cause of dissatisfaction after multifocal IOL implantation – it is mainly caused by residual refractive error and dry eye. Careful patient screening for pre-existing pathology helps avoid patient dissatisfaction due to these symptoms.[100]
Posterior capsule opacification is the most common complication after cataract surgery. A study[92] compared the ND:YAG capsulotomy rates after the implantation of the FineVision® Micro F, and AT LISA® tri 839MP trifocal IOLs. It found that posterior capsule opacification occurrence is significantly higher in the AT LISA® tri 839MP IOL-although both IOLs had the same incidence of posterior cystoid macular edema.
There is no IOL implant so far that does not induce night vision disturbances. Patients may, however, adapt over a period of around 6 months through a process known as neuroadaptation.[80] Neuroadaptation occurs faster with fully diffractive IOLs because the pupil does not affect the visual outcome.[93]
Every patient should ideally eventually adapt to multifocality and the visual effects of the multifocal IOL's. Unfortunately, some never do – which warrants an IOL exchange. Kamiya et al.[79] reported the reasons for multifocal IOL explantation. The most common reasons for IOL exchange were as follows: decreased contrast sensitivity (36%), photopic phenomena (34%), incorrect IOL power (20%), preoperative excessive expectation (14%), IOL decentration (4%), anisometropia (4%), and unknown causes including neuroadaptation failure (32%). It is important to note that 70% of the eyes that had the multifocal IOL explanted had an UDVA of 20/20 or better-which means that that the visual side effects rather than the postoperative visual acuity were significant enough to warrant IOL exchange.
Author comments
Multifocal IOLs, with their different optical designs, are to be considered today as very successful. They provide very good results, with high patient and doctor satisfaction. The art of choosing the best lens and optical design for a particular case is the key to success. The main problem for multifocal IOLs is that they depend on the neuroadaptation process, which is unpredictable and may lead to a long postoperative recovery and even to explantation. This level of failure is rare today, if the surgeon follows the correct process for selecting the right patient. Refractive precision is an essential target to accomplish during the postoperative period.
EDOF lenses are very weak, with no benefits over standard trifocal designs. Most patients with EDOF lenses require near vision glasses, which is a cause of visual dissatisfaction. The best results today are accomplished with trifocal designs and refractive varifocal lenses, frequently implanted in asymmetrical powers to improve patient neuroadaptation.
Once accommodative lenses are fully developed, however, we foresee that multifocal lenses will decline in use.
Accommodating intraocular lenses
True accommodating IOLs are supposed to be capable of undergoing a progressive power change related to active ciliary body contraction. Majority of accommodating IOL's today mimic physiologic accommodation by enabling a forward movement of the optic component during an accommodative effort.[101] These IOLs are monofocal, thus avoiding the optical side effects associated with multifocal IOLs.
There are three basic design strategies for accommodating IOL's, all of which are at various stages of development and commercialization. These will be discussed below, along with some examples.
Position-changing intraocular lenses
Single optic IOL's provide near and intermediate vision by anterior axial movement of the lens optic and the accommodative effect is dependent on IOL power.[102]
The CrystaLens® HD (Bausch and Lomb, Rochester, NY, USA) was the first accommodative IOL approved by the FDA.[101] It is a biconvex hinged single-optic IOL made of biocompatible 3rd generation silicone (Biosil), with a refractive index of 1.428. There are two sizes available depending on the required power: 12.0 mm (HD520) for 10–16.50 D and the 11.5 mm (HD500) for 17–33 D. The center of the IOL is biaspheric to increase the depth of focus so it provides better near and intermediate vision. The CrystaLens® uses a double mechanism to improve near vision – first through axial movement of the optic, and through the variation of the radius of curvature of the anterior surface [Figure 10].[103,104] This lens is commercially available.
Figure 10.

Crystalens® intraocular lens
Alió et al.[103] compared the visual acuity outcomes and ocular optical quality between the Crystalens® HD and a monofocal IOL (Acri. Smart® 48S). The UNVA was significantly better in the accommodating IOL group; however, there was no significant difference in CNVA between the two groups. No difference was also noted between the intraocular aberrometric coefficient.
Visual outcomes between the Crystalens® HD and the Lentis® M-Plus were also compared.[105] The latter had better DCNVA, and there were no significant differences in postoperative UNVA or CNVA between groups. The near add was reduced significantly after surgery in both groups, with a lower near-add power in the Lentis® M-Plus group. Regarding optical quality, there was a significantly larger amount of IOL tilt in the Lentis® M-Plus group; however, the difference in mean ocular HOAs was not statistically significant. The Crystalens® HD had significantly better contrast sensitivity results under photopic conditions at all spatial frequencies. Both IOLs had limitations in providing complete near-vision outcomes. Ray tracing aberrometry showed that the Crystalens® accommodative power was lower than 0.4D.[106]
Capsular contraction syndrome, also known as Z-syndrome, is uniquely associated with the Crystalens® because of its hinged plate haptic design. An asymmetric capsular contraction causes the plate haptics to vault in opposite directions [Figure 11][107] inducing astigmatism.[107,108,109,110] Intraoperative measures to mitigate the risk of Z-syndrome include central capsulorrhexis with adequate coverage of the plate haptics and meticulous cortical material removal. Mild fibrosis may be treated with a Nd YAG laser capsulotomy; more pronounced Z-syndromes may need IOL repositioning or an IOL exchange.[109,110]
Figure 11.

Z-syndrome induced by capsular contraction
The 1CU® (Human Optics, Erlangen, Germany) is a single piece biconvex IOL made of hydrophilic acrylic. It has an optic diameter of 5.5 mm and a total diameter of 9.8 mm. This IOL has four flexible haptics that bend when constricted, allowing anterior movement of the optic during the accommodative effort.[111]
Saiki et al.[112] did a 4-year follow-up in patients with the 1CU IOL and reported that amplitude of accommodation was not enough to provide a good near vision. One possible explanation for the lack of accommodation is the contraction of the capsule. This IOL has been discontinued and is no longer commercially available.
The TetraFlex® (Lenstec Inc, St. Petersburg, Fla, USA) is a single piece IOL made of hydroxyethylmethacrylate with a refractive index of 1.46. It has an optic diameter of 5.75 mm, an overall diameter of 11.5 mm, and its insertion requires an incision size of 2.5–3.0 mm. It has flexible angulated closed-loop haptics that also allow movement within the capsular bag [Figure 12].[111]
Figure 12.
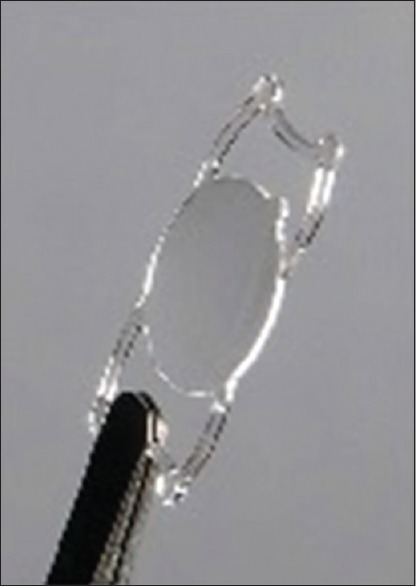
Tetraflex® intraocular lens
While the original primary mechanism of action for this IOL was its ability to move forward within the capsular bag, it has been found to increase HOAs with accommodative effort.[113]
This lens is still commercially available although it has demonstrated contradictory results.
Dual optic IOL's consist of a mobile front optic and a stationary rear optic, which are interconnected with spring-type haptics.[111]
The Synchrony® (Visiogen Inc, Abbott Medical Optics, Santa Ana, California, USA) is a single piece, dual optic IOL made of silicone. Available powers range from +16.0 to +28.0 D in 0.50 D steps. The anterior biconvex optic has a high plus power (around +32 D), while the posterior concave optic has a low minus power to return the eye to emmetropia. A bridge with a spring function connects the two components [Figure 13].[114] Once in the capsular bag, the tension of the bag compresses the optics, leading to strain energy in the haptics that is released when there is an attempt to accommodate.[115,116] This IOL has been discontinued and is no longer commercially available.
Figure 13.
Synchrony® intraocular lens
A comparison of the visual and ocular performance between the Crystalens® HD and the Synchrony® IOL was made by Alió et al.[115] There were no statistically significant differences in UDVA, CDVA, near, or intermediate visual outcomes between the 2 IOLs. Reading acuity or reading speed was similar in both groups. Contrast sensitivity was significantly better in patients with the Synchrony®. HOAs, however, were higher in the Crystalens® HD group. Both IOLs were found to have limitations in providing adequate near visual outcomes.
Bohórquezand Alarcon[116] evaluated reading ability 1 and 2 years after the implantation of the Synchrony IOL. Reading speed, mean reading acuity, and mean critical print size were significantly better 2 years postoperatively. They concluded that these results were a consequence of true accommodation, although the reasons for the improved reading skills 2 years as opposed to 1 year postoperatively were not fully clear.
Shape-changing intraocular lenses are capable of changing lens curvature to bring about changes in dioptric power
The FluidVision® (Powervision, Belmont, California, USA) has an overall diameter of 10.0 mm and an optic diameter of 6.0 mm. It is made of acrylic material while the haptics and interior of the optic are filled with silicone oil. During accommodation, the silicone oil is pushed into the optic through fluid channels that connect the haptics to the optic. This inflates the lens, which increases the dioptric power for near vision.[117] When the eye focuses at far, fluid flows from the optic body back into the haptics, flattening the lens and decreasing the[118] dioptric power. The fifth generation of this particular IOL is already in clinical trials.
Another, the NuLens® (DynaCurve, Herzliya Pituah, Israel) consists of PMMA haptics, a PMMA anterior reference plane that provides distance vision correction, a small chamber that contains a solid silicone gel, and a posterior piston with an aperture in the center [Figure 14].[118] It exerts its mechanism of action when the piston is pressed, making the flexible gel bulge and resulting in an increase or decrease in IOL optical power. This lens is implanted at the ciliary sulcus and has to be inserted through a limbal incision of 9 mm. It can provide up to 10.00 of accommodation, improving NVA without compromising DVA.[118] It is not yet commercially available.
Figure 14.
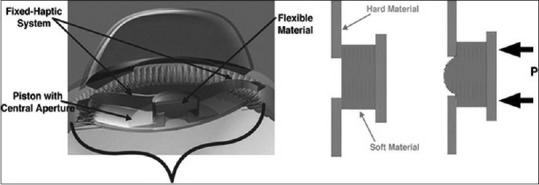
Nulens® intraocular lens
Power-changing intraocular lenses dynamically change in refractive power
The Lumina® (Akkolens International, Breda, The Netherlands) consists of two optical elements, each having an elastic U-shaped loop with a spring function, and non-elastic connections to the main body of the lens [Figure 15]. The optics are aspherical – the anterior optic has a power of 5.0D, and the power of the posterior optic depends on the required correction of the eye (available powers range from 10 to 25 D). This lens has to be implanted at the sulcus, and its size is customized based on the sulcus to sulcus diameter, measured by an OCT at the 12 o'clock meridian.[119] This lens is not yet commercially available.
Figure 15.

Lumina® intraocular lens
During accommodation, the IOL is compressed by the contraction of the ciliary muscle. The optics move in opposite directions to increase the optical power of the lens. When the ciliary muscle relaxes, the springs force the elements back to their original state, decreasing the optical power. It has been proven through subjective and objective methods that the Lumina IOL improves near, intermediate and far vision without affecting contrast sensitivity, with an accommodative power between 1.5 and 6.0D.[119]
Authors' comments
In spite of the failures and disappointments of the past, accommodative lenses do have a future in refractive surgery. Sulcus-based lenses will prevail once the confirmation of their performance is more consolidated. The obvious advantages of accommodative lenses-such as no neuroadaptation needed, lack of optical photic phenomenon, and physiological behavior-will make accommodative lenses, once they are fully available, the option of choice for pseudophakic presbyopia.
Pseudophakic monovision
The same principles used for corneal monovision and contact lenses apply to monovision with IOL implants – with the dominant eye corrected for emmetropia, and the nondominant eye with a certain degree of myopia up to −2.00D.[120,121,122,123,124,125,126,127,128,129] The range of vision is determined by the targeted refraction in the non-dominant eye. However, neuroadaptation, contrast sensitivity, and stereopsis are compromised with increased anisometropia. This is why mini-monovision has also been developed– with myopia of −0.75 to −1.25 D targeted in the nondominant eye.
Monovision with phakic IOLs is not routinely done because it has been associated with a higher rate of cataract formation in presbyopic patients.[130] A report has been published, however, of using a posterior chamber phakic IOL with a central hole (Hole ICL) – which is said to be associated with less risk for cataract formation-for moderate-to-high ametropía in early presbyopic patients. Binocular visual acuity was good, 20/25 or better for distance and J2 for near in 94% of patients, with no adverse events noted.[121]
Pseudophakic monovision is a reasonable alternative for treating presbyopia. The IOL implants used in monovision definitely cost less than premium multifocal IOLs. No significant difference was reported between monovision and multifocal IOL implantation regarding patient satisfaction and distance vision. Patients implanted with multifocal IOL's are more probable to be independent of spectacles; however, they are also more prone to have glares and halos. Patients with monovision have better intermediate vision, but might likely require spectacles for certain near activities.[122,127]
High-quality randomized control trials with harmonized methodologies for outcome assessment are still needed before accurate estimates of the comparative efficacy of both techniques become available. At present, the choice of which to use depends on the patient's needs and should require an in-depth discussion by the surgeon as to the pros and cons of each.[122]
Author's comments
Without a doubt, monovision is the most popular refractive procedure in implantology. It is successful in most of the cases, and even then neuroadaptation is required, most of the patients quickly develop a good tolerance to the asymmetrical refractive condition when it is obvious for the patient the advantages that this refractive approach for the correction of presbyopia has. Monovision is now and will be in the future, as it has been so far, one of the best options for the compensation of presbyopia in all ages.
Scleral procedures
The premise for scleral procedures is based on Schachar's theory of accommodation. Historically, scleral procedures for the treatment of presbyopia started with the anterior ciliary sclerotomy (ACS) – wherein radial scleral incisions were made overlying the ciliary muscle to tighten the zonules by increasing the space between the lens and ciliary muscle. Only slight and short-term improvement in accommodation was noted, prompting the use of silicone implants to reduce regression.[5] Both techniques are no longer in use today.
Scleral implants, called scleral expansion bands, were first used by Schachar. These PMMA rods had mixed results, and low patient satisfaction – they were retired because of anterior ischemia reports.[5]
Scleral implant development remains an active area of research at present. The procedure known as PresVIEW® (Refocus group, Dallas, Texas) is on the phase III study for the microinsert implant-four PMMA injection molded segments are placed in scleral tunnels at a depth of 400 μm, 4 mm from the limbus. These aim to lift the sclera and ciliary muscle, tightening the zonular fibers holding the lens. Preliminary reports indicate a good uncorrected near and intermediate vision without compromising the distance vision; while primary outcomes are still pending, some substantial risks involved include anterior segment ischemia and conjunctival erosion.[131] Another group describes the development of four supraciliary contraction segments placed in scleral tunnels, with the goal of relaxing the zonula and facilitating ciliary body shift toward the center. Five patients (10 eyes) were included in the pilot study. At 3 months of follow-up, four patients were able to read books without glasses. This gradually decreased until only one patient (20%) could read at near without spectacles. At 6 months, 15 of 40 implants placed had become superficial. Five additional implants became superficial by the 12th month.[132]
Scleral laser excision or radial sclerectomy first came about because ACS was believed to be unsuccessful due to rapid scleral healing. The idea was to ablate almost the full scleral thickness up to 500–600 μm, with an Erbium-doped yttrium aluminum garnet (Er: YAG) laser. Up to 2D of subjective accommodation was noted after 12 months, nevertheless, this treatment is no longer available.[133] Scleral laser anterior ciliary excision or LaserACE® (Ace Vision Group Inc., Silver Lake, Ohio, USA) is the only scleral laser micro-excision procedure currently available. It is based on the VisioDynamics theory which argues that the aging connective tissues in the eye impacts ocular biomechanical efficiency. A matrix array of microincisions is made up to 85%–90% scleral thickness, in four oblique quadrants over 3 zones that are 0.5–6.0 mm from the anatomical limbus. LaserACE® aims to create differential stiffness, increasing the plasticity and compliance of scleral tissue during ciliary muscle contraction, and improving the efficiency of the accommodation apparatus. Preliminary results were promising – with statistically significant UNVA and DCNVA changes sustained even at 24 months postoperatively. The primary risk is accidental micro perforation of the sclera, however, the advantage of this procedure is that the cornea and the crystalline remains untouched and allows LaserACE® to be performed after or even in combination with other procedures.[134]
Author's comments
In spite of the many years of experimental and clinical studies, scleral procedures have so far uniformly failed and are not in use. Most probably, these techniques will disappear as an option in the near future due to the inconsistent outcomes that they provide.
Conclusion
A myriad of treatment options have been devised to correct presbyopia, yet its complex multifactorial nature and the conflicting reports on treatment outcomes make it arguably the last frontier in refractive surgery. The arrival of new technology, which increases understanding of its pathophysiology, will eventually lead clinicians and scientists to develop the most effective method to recover accommodative function and provide good vision at all distances. For now, the task at hand is to make use of the available methods-which are adequate and effective for the most part. These can be used individually or in various combinations, to individualize treatment to meet patients' needs, and to maximize visual function. Careful patient selection and screening and thorough explanation of risks and benefits associated with each treatment option cannot be emphasized enough during the preoperative planning period.
Financial support and sponsorship
This publication has been carried out in the framework of the Red Temática de Investigación Cooperativa en Salud (RETICS), reference number RD16/0008/0012, financed by the Instituto Carlos III – General Subdirection of Networks and Cooperative Investigation Centers (R&D&I National Plan 2008-2011) and the European Regional Development Fund (Fondo Europeo de Desarrollo Regional FEDER).
Conflicts of interest
The authors declare that there are no conflicts of interests of this paper.
References
- 1.Charman WN. The eye in focus: Accommodation and presbyopia. Clin Exp Optom. 2008;91:207–25. doi: 10.1111/j.1444-0938.2008.00256.x. [DOI] [PubMed] [Google Scholar]
- 2.Goertz AD, Stewart WC, Burns WR, Stewart JA, Nelson LA. Review of the impact of presbyopia on quality of life in the developing and developed world. Acta Ophthalmol. 2014;92:497–500. doi: 10.1111/aos.12308. [DOI] [PubMed] [Google Scholar]
- 3.Glasser A. Accommodation: Mechanism and measurement. Ophthalmol Clin North Am. 2006;19:1–12. doi: 10.1016/j.ohc.2005.09.004. v. [DOI] [PubMed] [Google Scholar]
- 4.Gilmartin B. The aetiology of presbyopia: A summary of the role of lenticular and extralenticular structures. Ophthalmic Physiol Opt. 1995;15:431–7. [PubMed] [Google Scholar]
- 5.Schachar RA. Theoretical basis for the scleral expansion band procedure for surgical reversal of presbyopia [SRP] Compr Ther. 2001;27:39–46. doi: 10.1007/s12019-001-0006-4. [DOI] [PubMed] [Google Scholar]
- 6.Durrie DS, Moshifar M. Section II: Intraocular Refractive Surgery Topics. Chicago, IL: USA; 2016. “Dysfunctional lens syndrome,” in Proceedings of the Annual Meeting of ISRS. Pursuit of Perfection. [Google Scholar]
- 7.Durrie D. Dysfunctional lens syndrome, a New Way to Educate Patients. [Last accessed 2018 Mar 10]. Available at https://www.aao.org/eyenet/academylive/detail/dysfunctional-lens-syndrome-educate-patients .
- 8.Bourne RR, Jonas JB, Bron AM, Cicinelli MV, Das A, Flaxman SR, et al. Prevalence and causes of vision loss in high-income countries and in Eastern and central Europe in 2015: Magnitude, temporal trends and projections. Br J Ophthalmol. 2018;102:575–85. doi: 10.1136/bjophthalmol-2017-311258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bourne RR, Flaxman SR, Braithwaite T, Cicinelli MV, Das A, Jonas JB, et al. Magnitude, temporal trends, and projections of the global prevalence of blindness and distance and near vision impairment: A systematic review and meta-analysis. Lancet Glob Health. 2017;5:e888–97. doi: 10.1016/S2214-109X(17)30293-0. [DOI] [PubMed] [Google Scholar]
- 10.Ajibode HA, Fakolujo VO, Onabolu OO, Jagun O, Ogunlesi TA, Abiodun OA, et al. A community-based prevalence of presbyopia and spectacle coverage in Southwest Nigeria. J West Afr Coll Surg. 2016;6:66–82. [PMC free article] [PubMed] [Google Scholar]
- 11.Charman WN. Developments in the correction of presbyopia I: Spectacle and contact lenses. Ophthalmic Physiol Opt. 2014;34:8–29. doi: 10.1111/opo.12091. [DOI] [PubMed] [Google Scholar]
- 12.Renna A, Alió JL, Vejarano LF. Pharmacological treatments of presbyopia: A review of modern perspectives. Eye Vis (Lond) 2017;4:3. doi: 10.1186/s40662-017-0068-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krader C, Feinbaum C. Ophthalmology Times; Published; 2015. [Last accessed on 2018 Mar 10]. Simple solution for presbyopia: Topical agent acts by reducing pupil size to increase depth of focus. Available from: http://www.ophthalmologytimes.com/modern-medicine-feature-articles/simple-solution-presbyopia . [Google Scholar]
- 14.Patel S, Salamun F, Matovic K. Pharmacological correction of presbyopia. Poster Presentation European Society of Cataract and Refractive Surgery Congress XXXI; Published. 2013. [Last accessed on 2018 Mar 28]. Available from: http://www.escrs.org/amsterdam2013/programme/posters-details.asp?id=19804 .
- 15.Renna A, Vejarano LF, De la Cruz E, Alió JL. Pharmacological treatment of presbyopia by novel binocularly instilled eye drops: A pilot study. Ophthalmol Ther. 2016;5:63–73. doi: 10.1007/s40123-016-0050-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cole J. Can an eye drop eliminate presbyopia? Review of Optometry, Newtown Square, PA, USA. 2017. [Last accessed on 2018 Mar 10]. Available from: https://www.reviewofoptometry.com/article/ro0617-can-aneye-drop-eliminate-presbyopia .
- 17.Tsuneyoshi Y, Higuchi A, Negishi K, Tsubota K. Suppression of presbyopia progression with pirenoxine eye drops: Experiments on rats and non-blinded, randomized clinical trial of efficacy. Sci Rep. 2017;7:6819. doi: 10.1038/s41598-017-07208-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krader C. Topical drops show promise as treatment for presbyopia. Ophthalmol Times Eur. 2016;12:18–20. [Google Scholar]
- 19.Gualdi L, Gualdi F, Rusciano D, Ambrósio R, Jr, Salomão MQ, Lopes B, et al. Ciliary muscle electrostimulation to restore accommodation in patients with early presbyopia: Preliminary results. J Refract Surg. 2017;33:578–83. doi: 10.3928/1081597X-20170621-05. [DOI] [PubMed] [Google Scholar]
- 20.Luger MH, McAlinden C, Buckhurst PJ, Wolffsohn JS, Verma S, Arba Mosquera S, et al. Presbyopic LASIK using hybrid bi-aspheric micro-monovision ablation profile for presbyopic corneal treatments. Am J Ophthalmol. 2015;160:493–505. doi: 10.1016/j.ajo.2015.05.021. [DOI] [PubMed] [Google Scholar]
- 21.Baudu P, Penin F, Arba Mosquera S. Uncorrected binocular performance after biaspheric ablation profile for presbyopic corneal treatment using AMARIS with the presbyMAX module. Am J Ophthalmol. 2013;155:636–647. doi: 10.1016/j.ajo.2012.10.023. 647.e1. [DOI] [PubMed] [Google Scholar]
- 22.Alió JL, Amparo F, Ortiz D, Moreno L. Corneal multifocality with excimer laser for presbyopia correction. Curr Opin Ophthalmol. 2009;20:264–71. doi: 10.1097/icu.0b013e32832a7ded. [DOI] [PubMed] [Google Scholar]
- 23.Jackson WB, Tuan KM, Mintsioulis G. Aspheric wavefront-guided LASIK to treat hyperopic presbyopia: 12-month results with the VISX platform. J Refract Surg. 2011;27:519–29. doi: 10.3928/1081597X-20101110-02. [DOI] [PubMed] [Google Scholar]
- 24.Vargas-Fragoso V, Alió JL. Corneal compensation of presbyopia: PresbyLASIK: An updated review. Eye Vis (Lond) 2017;4:11. doi: 10.1186/s40662-017-0075-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luger MH, Ewering T, Arba-Mosquera S. One-year experience in presbyopia correction with biaspheric multifocal central presbyopia laser in situ keratomileusis. Cornea. 2013;32:644–52. doi: 10.1097/ICO.0b013e31825f02f5. [DOI] [PubMed] [Google Scholar]
- 26.Reinstein DZ, Carp GI, Archer TJ, Gobbe M. LASIK for presbyopia correction in emmetropic patients using aspheric ablation profiles and a micro-monovision protocol with the carl zeiss meditec MEL 80 and VisuMax. J Refract Surg. 2012;28:531–41. doi: 10.3928/1081597X-20120723-01. [DOI] [PubMed] [Google Scholar]
- 27.Saib N, Abrieu-Lacaille M, Berguiga M, Rambaud C, Froussart-Maille F, Rigal-Sastourne JC, et al. Central presbyLASIK for hyperopia and presbyopia using micro-monovision with the technolas 217P platform and SUPRACOR algorithm. J Refract Surg. 2015;31:540–6. doi: 10.3928/1081597X-20150727-04. [DOI] [PubMed] [Google Scholar]
- 28.Ryan A, O'Keefe M. Corneal approach to hyperopic presbyopia treatment: Six-month outcomes of a new multifocal excimer laser in situ keratomileusis procedure. J Cataract Refract Surg. 2013;39:1226–33. doi: 10.1016/j.jcrs.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 29.Uthoff D, Pölzl M, Hepper D, Holland D. A new method of cornea modulation with excimer laser for simultaneous correction of presbyopia and ametropia. Graefes Arch Clin Exp Ophthalmol. 2012;250:1649–61. doi: 10.1007/s00417-012-1948-1. [DOI] [PubMed] [Google Scholar]
- 30.Alió JL, Chaubard JJ, Caliz A, Sala E, Patel S. Correction of presbyopia by technovision central multifocal LASIK (presbyLASIK) J Refract Surg. 2006;22:453–60. doi: 10.3928/1081-597X-20060501-06. [DOI] [PubMed] [Google Scholar]
- 31.Pinelli R, Ortiz D, Simonetto A, Bacchi C, Sala E, Alió JL, et al. Correction of presbyopia in hyperopia with a center-distance, paracentral-near technique using the technolas 217z platform. J Refract Surg. 2008;24:494–500. doi: 10.3928/1081597X-20080501-07. [DOI] [PubMed] [Google Scholar]
- 32.Epstein RL, Gurgos MA. Presbyopia treatment by monocular peripheral presbyLASIK. J Refract Surg. 2009;25:516–23. doi: 10.3928/1081597X-20090512-05. [DOI] [PubMed] [Google Scholar]
- 33.El Danasoury AM, Gamaly TO, Hantera M. Multizone LASIK with peripheral near zone for correction of presbyopia in myopic and hyperopic eyes: 1-year results. J Refract Surg. 2009;25:296–305. doi: 10.3928/1081597X-20090301-10. [DOI] [PubMed] [Google Scholar]
- 34.Yilmaz OF, Bayraktar S, Agca A, Yilmaz B, McDonald MB, van de Pol C, et al. Intracorneal inlay for the surgical correction of presbyopia. J Cataract Refract Surg. 2008;34:1921–7. doi: 10.1016/j.jcrs.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 35.Garza EB, Gomez S, Chayet A, Dishler J. One-year safety and efficacy results of a hydrogel inlay to improve near vision in patients with emmetropic presbyopia. J Refract Surg. 2013;29:166–72. doi: 10.3928/1081597X-20130129-01. [DOI] [PubMed] [Google Scholar]
- 36.Malandrini A, Martone G, Menabuoni L, Catanese AM, Tosi GM, Balestrazzi A, et al. Bifocal refractive corneal inlay implantation to improve near vision in emmetropic presbyopic patients. J Cataract Refract Surg. 2015;41:1962–72. doi: 10.1016/j.jcrs.2015.01.018. [DOI] [PubMed] [Google Scholar]
- 37.Mulet ME, Alio JL, Knorz MC. Hydrogel intracorneal inlays for the correction of hyperopia: Outcomes and complications after 5 years of follow-up. Ophthalmology. 2009;116:1455–60. doi: 10.1016/j.ophtha.2009.05.019. 1460.e1. [DOI] [PubMed] [Google Scholar]
- 38.Alió JL, Shabayek MH, Montes-Mico R, Múlet ME, Ahmed AG, Merayo J, et al. Intracorneal hydrogel lenses and corneal aberrations. J Refract Surg. 2005;21:247–52. doi: 10.3928/1081-597X-20050501-07. [DOI] [PubMed] [Google Scholar]
- 39.Lindstrom RL, Macrae SM, Pepose JS, Hoopes PC., Sr Corneal inlays for presbyopia correction. Curr Opin Ophthalmol. 2013;24:281–7. doi: 10.1097/ICU.0b013e328362293e. [DOI] [PubMed] [Google Scholar]
- 40.Konstantopoulos A, Mehta JS. Surgical compensation of presbyopia with corneal inlays. Expert Rev Med Devices. 2015;12:341–52. doi: 10.1586/17434440.2015.1007124. [DOI] [PubMed] [Google Scholar]
- 41.Lim CH, Riau AK, Lwin NC, Chaurasia SS, Tan DT, Mehta JS, et al. LASIK following small incision lenticule extraction (SMILE) lenticule re-implantation: A feasibility study of a novel method for treatment of presbyopia. PLoS One. 2013;8:e83046. doi: 10.1371/journal.pone.0083046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Arlt E, Krall E, Moussa S, Grabner G, Dexl A. Implantable inlay devices for presbyopia: The evidence to date. Clin Ophthalmol. 2015;9:129–37. doi: 10.2147/OPTH.S57056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Steinert RF, Schwiegerling J, Lang A, Roy A, Holliday K, Barragán Garza E, et al. Range of refractive independence and mechanism of action of a corneal shape-changing hydrogel inlay: Results and theory. J Cataract Refract Surg. 2015;41:1568–79. doi: 10.1016/j.jcrs.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 44.Yoo A, Kim JY, Kim MJ, Tchah H. Hydrogel inlay for presbyopia: Objective and subjective visual outcomes. J Refract Surg. 2015;31:454–60. doi: 10.3928/1081597X-20150623-03. [DOI] [PubMed] [Google Scholar]
- 45.Whitman J, Dougherty PJ, Parkhurst GD, Olkowski J, Slade SG, Hovanesian J, et al. Treatment of presbyopia in emmetropes using a shape-changing corneal inlay: One-year clinical outcomes. Ophthalmology. 2016;123:466–75. doi: 10.1016/j.ophtha.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 46.Limnopoulou AN, Bouzoukis DI, Kymionis GD, Panagopoulou SI, Plainis S, Pallikaris AI, et al. Visual outcomes and safety of a refractive corneal inlay for presbyopia using femtosecond laser. J Refract Surg. 2013;29:12–8. doi: 10.3928/1081597X-20121210-01. [DOI] [PubMed] [Google Scholar]
- 47.Baily C, Kohnen T, O'Keefe M. Preloaded refractive-addition corneal inlay to compensate for presbyopia implanted using a femtosecond laser: One-year visual outcomes and safety. J Cataract Refract Surg. 2014;40:1341–8. doi: 10.1016/j.jcrs.2013.11.047. [DOI] [PubMed] [Google Scholar]
- 48.Naroo SA, Bilkhu PS. Clinical utility of the KAMRA corneal inlay. Clin Ophthalmol. 2016;10:913–9. doi: 10.2147/OPTH.S89132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Casas-Llera P, Ruiz-Moreno JM, Alió JL. Retinal imaging after corneal inlay implantation. J Cataract Refract Surg. 2011;37:1729–31. doi: 10.1016/j.jcrs.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 50.Dexl AK, Seyeddain O, Riha W, Hohensinn M, Hitzl W, Grabner G, et al. Reading performance after implantation of a small-aperture corneal inlay for the surgical correction of presbyopia: Two-year follow-up. J Cataract Refract Surg. 2011;37:525–31. doi: 10.1016/j.jcrs.2010.10.044. [DOI] [PubMed] [Google Scholar]
- 51.Tomita M, Kanamori T, Waring GO, 4th, Nakamura T, Yukawa S. Small-aperture corneal inlay implantation to treat presbyopia after laser in situ keratomileusis. J Cataract Refract Surg. 2013;39:898–905. doi: 10.1016/j.jcrs.2013.01.034. [DOI] [PubMed] [Google Scholar]
- 52.Tomita M, Kanamori T, Waring GO, 4th, Yukawa S, Yamamoto T, Sekiya K, et al. Simultaneous corneal inlay implantation and laser in situ keratomileusis for presbyopia in patients with hyperopia, myopia, or emmetropia: Six-month results. J Cataract Refract Surg. 2012;38:495–506. doi: 10.1016/j.jcrs.2011.10.033. [DOI] [PubMed] [Google Scholar]
- 53.Igras E, O'Caoimh R, O'Brien P, Power W. Long-term results of combined LASIK and monocular small-aperture corneal inlay implantation. J Refract Surg. 2016;32:379–84. doi: 10.3928/1081597X-20160317-01. [DOI] [PubMed] [Google Scholar]
- 54.Seyeddain O, Hohensinn M, Riha W, Nix G, Rückl T, Grabner G, et al. Small-aperture corneal inlay for the correction of presbyopia: 3-year follow-up. J Cataract Refract Surg. 2012;38:35–45. doi: 10.1016/j.jcrs.2011.07.027. [DOI] [PubMed] [Google Scholar]
- 55.Dexl AK, Ruckhofer J, Riha W, Hohensinn M, Rueckl T, Messmer EM, et al. Central and peripheral corneal iron deposits after implantation of a small-aperture corneal inlay for correction of presbyopia. J Refract Surg. 2011;27:876–80. doi: 10.3928/1081597X-20110802-02. [DOI] [PubMed] [Google Scholar]
- 56.Alió JL, Abbouda A, Huseynli S, Knorz MC, Homs ME, Durrie DS, et al. Removability of a small aperture intracorneal inlay for presbyopia correction. J Refract Surg. 2013;29:550–6. doi: 10.3928/1081597X-20130719-05. [DOI] [PubMed] [Google Scholar]
- 57.Abbouda A, Javaloy J, Alió JL. Confocal microscopy evaluation of the corneal response following acuFocus KAMRA inlay implantation. J Refract Surg. 2014;30:172–8. doi: 10.3928/1081597X-20140217-04. [DOI] [PubMed] [Google Scholar]
- 58.Lin L, van de Pol C, Vilupuru S, Pepose JS. Contrast sensitivity in patients with emmetropic presbyopia before and after small-aperture inlay implantation. J Refract Surg. 2016;32:386–93. doi: 10.3928/1081597X-20160217-04. [DOI] [PubMed] [Google Scholar]
- 59.Huseynova T, Kanamori T, Waring GO, 4th, Tomita M. Outcomes of small aperture corneal inlay implantation in patients with pseudophakia. J Refract Surg. 2014;30:110–6. doi: 10.3928/1081597X-20140120-06. [DOI] [PubMed] [Google Scholar]
- 60.Davidson RS, Dhaliwal D, Hamilton DR, Jackson M, Patterson L, Stonecipher K, et al. Surgical correction of presbyopia. J Cataract Refract Surg. 2016;42:920–30. doi: 10.1016/j.jcrs.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 61.Farid M, Steinert RF. Patient selection for monovision laser refractive surgery. Curr Opin Ophthalmol. 2009;20:251–4. doi: 10.1097/ICU.0b013e32832a0cdb. [DOI] [PubMed] [Google Scholar]
- 62.Jain S, Ou R, Azar DT. Monovision outcomes in presbyopic individuals after refractive surgery. Ophthalmology. 2001;108:1430–3. doi: 10.1016/s0161-6420(01)00647-9. [DOI] [PubMed] [Google Scholar]
- 63.Garcia-Gonzalez M, Teus MA, Hernandez-Verdejo JL. Visual outcomes of LASIK-induced monovision in myopic patients with presbyopia. Am J Ophthalmol. 2010;150:381–6. doi: 10.1016/j.ajo.2010.03.022. [DOI] [PubMed] [Google Scholar]
- 64.Luft N, Siedlecki J, Sekundo W, Wertheimer C, Kreutzer TC, Mayer WJ, et al. Small incision lenticule extraction (SMILE) monovision for presbyopia correction. Eur J Ophthalmol. 2018;28:287–93. doi: 10.5301/ejo.5001069. [DOI] [PubMed] [Google Scholar]
- 65.Jain S, Arora I, Azar DT. Success of monovision in presbyopes: Review of the literature and potential applications to refractive surgery. Surv Ophthalmol. 1996;40:491–9. doi: 10.1016/s0039-6257(96)82015-7. [DOI] [PubMed] [Google Scholar]
- 66.Cox CA, Krueger RR. Monovision with laser vision correction. Ophthalmol Clin North Am. 2006;19:71–5. doi: 10.1016/j.ohc.2005.10.002. vi. [DOI] [PubMed] [Google Scholar]
- 67.Schumacher S, Oberheide U, Fromm M, Ripken T, Ertmer W, Gerten G, et al. Femtosecond laser induced flexibility change of human donor lenses. Vision Res. 2009;49:1853–9. doi: 10.1016/j.visres.2009.04.028. [DOI] [PubMed] [Google Scholar]
- 68.Krueger RR, Uy H, McDonald J, Edwards K. Ultrashort-pulse lasers treating the crystalline lens: Will they cause vision-threatening cataract.(An American Ophthalmological Society Thesis)? Trans Am Ophthalmol Soc. 2012;110:130–65. [PMC free article] [PubMed] [Google Scholar]
- 69.Liu JP, Zhang F, Zhao JY, Ma LW, Zhang JS. Visual function and higher order aberration after implantation of aspheric and spherical multifocal intraocular lenses: A meta-analysis. Int J Ophthalmol. 2013;6:690–5. doi: 10.3980/j.issn.2222-3959.2013.05.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rosen E, Alió JL, Dick HB, Dell S, Slade S. Efficacy and safety of multifocal intraocular lenses following cataract and refractive lens exchange: Metaanalysis of peer-reviewed publications. J Cataract Refract Surg. 2016;42:310–28. doi: 10.1016/j.jcrs.2016.01.014. [DOI] [PubMed] [Google Scholar]
- 71.Gooi P, Ahmed I. Review of presbyopic IOLs: Multifocal and accommodating IOLs. Int Ophthalmol Clin. 2012;52:41–50. doi: 10.1097/IIO.0b013e31824b87be. [DOI] [PubMed] [Google Scholar]
- 72.Alió JL, Grzybowski A, Romaniuk D. Refractive lens exchange in modern practice: When and when not to do it? Eye Vis (Lond) 2014;1:10. doi: 10.1186/s40662-014-0010-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lane SS, Morris M, Nordan L, Packer M, Tarantino N, Wallace RB, 3rd, et al. Multifocal intraocular lenses. Ophthalmol Clin North Am. 2006;19:89–105. doi: 10.1016/j.ohc.2005.09.002. vi. [DOI] [PubMed] [Google Scholar]
- 74.Alio JL, Plaza-Puche AB, Javaloy J, Ayala MJ, Moreno LJ, Piñero DP, et al. Comparison of a new refractive multifocal intraocular lens with an inferior segmental near add and a diffractive multifocal intraocular lens. Ophthalmology. 2012;119:555–63. doi: 10.1016/j.ophtha.2011.08.036. [DOI] [PubMed] [Google Scholar]
- 75.Davison JA, Simpson MJ. History and development of the apodized diffractive intraocular lens. J Cataract Refract Surg. 2006;32:849–58. doi: 10.1016/j.jcrs.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 76.Alió JL, Plaza-Puche AB, Javaloy J, Ayala MJ. Comparison of the visual and intraocular optical performance of a refractive multifocal IOL with rotational asymmetry and an apodized diffractive multifocal IOL. J Refract Surg. 2012;28:100–5. doi: 10.3928/1081597X-20120110-01. [DOI] [PubMed] [Google Scholar]
- 77.Pedrotti E, Bruni E, Bonacci E, Badalamenti R, Mastropasqua R, Marchini G, et al. Comparative analysis of the clinical outcomes with a monofocal and an extended range of vision intraocular lens. J Refract Surg. 2016;32:436–42. doi: 10.3928/1081597X-20160428-06. [DOI] [PubMed] [Google Scholar]
- 78.McNeely RN, Pazo E, Spence A, Richoz O, Nesbit MA, Moore TC, et al. Comparison of the visual performance and quality of vision with combined symmetrical inferonasal near addition versus inferonasal and superotemporal placement of rotationally asymmetric refractive multifocal intraocular lenses. J Cataract Refract Surg. 2016;42:1721–9. doi: 10.1016/j.jcrs.2016.10.016. [DOI] [PubMed] [Google Scholar]
- 79.Kamiya K, Hayashi K, Shimizu K, Negishi K, Sato M, Bissen-Miyajima H, et al. Multifocal intraocular lens explantation: A case series of 50 eyes. Am J Ophthalmol. 2014;158:215–220.e1. doi: 10.1016/j.ajo.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 80.Braga-Mele R, Chang D, Dewey S, Foster G, Henderson BA, Hill W, et al. Multifocal intraocular lenses: Relative indications and contraindications for implantation. J Cataract Refract Surg. 2014;40:313–22. doi: 10.1016/j.jcrs.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 81.Alió JL, Plaza-Puche AB, Piñero DP. Rotationally asymmetric multifocal IOL implantation with and without capsular tension ring: Refractive and visual outcomes and intraocular optical performance. J Refract Surg. 2012;28:253–8. doi: 10.3928/1081597X-20120314-01. [DOI] [PubMed] [Google Scholar]
- 82.Klein BR, Brown EN, Casden RS. Preoperative macular spectral-domain optical coherence tomography in patients considering advanced-technology intraocular lenses for cataract surgery. J Cataract Refract Surg. 2016;42:537–41. doi: 10.1016/j.jcrs.2016.01.036. [DOI] [PubMed] [Google Scholar]
- 83.Teichman JC, Vold SD, Ahmed II. Top 5 pearls for implanting premium IOLs in patients with glaucoma. Int Ophthalmol Clin. 2012;52:65–71. doi: 10.1097/IIO.0b013e31824b3ec0. [DOI] [PubMed] [Google Scholar]
- 84.Sachdev GS, Sachdev M. Optimizing outcomes with multifocal intraocular lenses. Indian J Ophthalmol. 2017;65:1294–300. doi: 10.4103/ijo.IJO_1072_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Duran-Garcia M, Alió J. Multifocal intraocular lenses: Types and models. In: Alió J, Pikkel J, editors. Multifocal Intraocular Lenses. The Art and the Practice. 1st ed. London: Springer Healthcare; 2014. [Google Scholar]
- 86.Alió JL, Grabner G, Plaza-Puche AB, Rasp M, Piñero DP, Seyeddain O, et al. Postoperative bilateral reading performance with 4 intraocular lens models: Six-month results. J Cataract Refract Surg. 2011;37:842–52. doi: 10.1016/j.jcrs.2010.11.039. [DOI] [PubMed] [Google Scholar]
- 87.Ortiz D, Alió JL, Bernabéu G, Pongo V. Optical performance of monofocal and multifocal intraocular lenses in the human eye. J Cataract Refract Surg. 2008;34:755–62. doi: 10.1016/j.jcrs.2007.12.038. [DOI] [PubMed] [Google Scholar]
- 88.Camps VJ, Tolosa A, Piñero DP, de Fez D, Caballero MT, Miret JJ, et al. In vitro aberrometric assessment of a multifocal intraocular lens and two extended depth of focus IOLs. J Ophthalmol 2017. 2017 doi: 10.1155/2017/7095734. 7095734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cochener B Concerto Study Group. Clinical outcomes of a new extended range of vision intraocular lens: International Multicenter Concerto Study. J Cataract Refract Surg. 2016;42:1268–75. doi: 10.1016/j.jcrs.2016.06.033. [DOI] [PubMed] [Google Scholar]
- 90.Pérez-Merino P, Dorronsoro C, Llorente L, Durán S, Jiménez-Alfaro I, Marcos S, et al. In vivo chromatic aberration in eyes implanted with intraocular lenses. Invest Ophthalmol Vis Sci. 2013;54:2654–61. doi: 10.1167/iovs.13-11912. [DOI] [PubMed] [Google Scholar]
- 91.Weeber HA, Piers PA. Theoretical performance of intraocular lenses correcting both spherical and chromatic aberration. J Refract Surg. 2012;28:48–52. doi: 10.3928/1081597X-20111103-01. [DOI] [PubMed] [Google Scholar]
- 92.Bilbao-Calabuig R, Llovet-Osuna F, González-López F, Beltrán J. Nd:YAG capsulotomy rates with two trifocal intraocular lenses. J Refract Surg. 2016;32:748–52. doi: 10.3928/1081597X-20160803-02. [DOI] [PubMed] [Google Scholar]
- 93.Carballo-Alvarez J, Vazquez-Molini JM, Sanz-Fernandez JC, Garcia-Bella J, Polo V, García-Feijoo J, et al. Visual outcomes after bilateral trifocal diffractive intraocular lens implantation. BMC Ophthalmol. 2015;15:26. doi: 10.1186/s12886-015-0012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kohnen T. First implantation of a diffractive quadrafocal (trifocal) intraocular lens. J Cataract Refract Surg. 2015;41:2330–2. doi: 10.1016/j.jcrs.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 95.Carson D, Xu Z, Alexander E, Choi M, Zhao Z, Hong X, et al. Optical bench performance of 3 trifocal intraocular lenses. J Cataract Refract Surg. 2016;42:1361–7. doi: 10.1016/j.jcrs.2016.06.036. [DOI] [PubMed] [Google Scholar]
- 96.Mencucci R, Favuzza E, Caporossi O, Rizzo S. Visual performance, reading ability and patient satisfaction after implantation of a diffractive trifocal intraocular lens. Clin Ophthalmol. 2017;11:1987–93. doi: 10.2147/OPTH.S142860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jonker SM, Bauer NJ, Makhotkina NY, Berendschot TT, van den Biggelaar FJ, Nuijts RM, et al. Comparison of a trifocal intraocular lens with a+3.0 D bifocal IOL: Results of a prospective randomized clinical trial. J Cataract Refract Surg. 2015;41:1631–40. doi: 10.1016/j.jcrs.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 98.Kretz FT, Choi CY, Müller M, Gerl M, Gerl RH, Auffarth GU, et al. Visual outcomes, patient satisfaction and spectacle independence with a trifocal diffractive intraocular lens. Korean J Ophthalmol. 2016;30:180–91. doi: 10.3341/kjo.2016.30.3.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gundersen KG, Potvin R. Comparison of visual outcomes and subjective visual quality after bilateral implantation of a diffractive trifocal intraocular lens and blended implantation of apodized diffractive bifocal intraocular lenses. Clin Ophthalmol. 2016;10:805–11. doi: 10.2147/OPTH.S107162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gibbons A, Ali TK, Waren DP, Donaldson KE. Causes and correction of dissatisfaction after implantation of presbyopia-correcting intraocular lenses. Clin Ophthalmol. 2016;10:1965–70. doi: 10.2147/OPTH.S114890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Werner L, Olson RJ, Mamalis N. New technology IOL optics. Ophthalmol Clin North Am. 2006;19:469–83. doi: 10.1016/j.ohc.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 102.Beiko G. Status of accommodative intraocular lenses. Curr Opin Ophthalmol. 2007;18:74–9. doi: 10.1097/ICU.0b013e328011fbab. [DOI] [PubMed] [Google Scholar]
- 103.Alió JL, Piñero DP, Plaza-Puche AB. Visual outcomes and optical performance with a monofocal intraocular lens and a new-generation single-optic accommodating intraocular lens. J Cataract Refract Surg. 2010;36:1656–64. doi: 10.1016/j.jcrs.2010.04.040. [DOI] [PubMed] [Google Scholar]
- 104.Jardim D, Soloway B, Starr C. Asymmetric vault of an accommodating intraocular lens. J Cataract Refract Surg. 2006;32:347–50. doi: 10.1016/j.jcrs.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 105.Alió JL, Plaza-Puche AB, Montalban R, Javaloy J. Visual outcomes with a single-optic accommodating intraocular lens and a low-addition-power rotational asymmetric multifocal intraocular lens. J Cataract Refract Surg. 2012;38:978–85. doi: 10.1016/j.jcrs.2011.12.033. [DOI] [PubMed] [Google Scholar]
- 106.Pérez-Merino P, Birkenfeld J, Dorronsoro C, Ortiz S, Durán S, Jiménez-Alfaro I, et al. Aberrometry in patients implanted with accommodative intraocular lenses. Am J Ophthalmol. 2014;157:1077–89. doi: 10.1016/j.ajo.2014.02.013. [DOI] [PubMed] [Google Scholar]
- 107.Yuen L, Trattler W, Boxer Wachler BS. Two cases of Z syndrome with the crystalens after uneventful cataract surgery. J Cataract Refract Surg. 2008;34:1986–9. doi: 10.1016/j.jcrs.2008.05.061. [DOI] [PubMed] [Google Scholar]
- 108.Pepose JS, Burke J, Qazi MA. Benefits and barriers of accommodating intraocular lenses. Curr Opin Ophthalmol. 2017;28:3–8. doi: 10.1097/ICU.0000000000000323. [DOI] [PubMed] [Google Scholar]
- 109.Page TP, Whitman J. A stepwise approach for the management of capsular contraction syndrome in hinge-based accommodative intraocular lenses. Clin Ophthalmol. 2016;10:1039–46. doi: 10.2147/OPTH.S101325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kramer GD, Werner L, Neuhann T, Tetz M, Mamalis N. Anterior haptic flexing and in-the-bag subluxation of an accommodating intraocular lens due to excessive capsular bag contraction. J Cataract Refract Surg. 2015;41:2010–3. doi: 10.1016/j.jcrs.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 111.Menapace R, Findl O, Kriechbaum K, Leydolt-Koeppl Ch. Accommodating intraocular lenses: A critical review of present and future concepts. Graefes Arch Clin Exp Ophthalmol. 2007;245:473–89. doi: 10.1007/s00417-006-0391-6. [DOI] [PubMed] [Google Scholar]
- 112.Saiki M, Negishi K, Dogru M, Yamaguchi T, Tsubota K. Biconvex posterior chamber accommodating intraocular lens implantation after cataract surgery: Long-term outcomes. J Cataract Refract Surg. 2010;36:603–8. doi: 10.1016/j.jcrs.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 113.Wolffsohn JS, Davies LN, Gupta N, Naroo SA, Gibson GA, Mihashi T, et al. Mechanism of action of the tetraflex accommodative intraocular lens. J Refract Surg. 2010;26:858–62. doi: 10.3928/1081597X-20100114-04. [DOI] [PubMed] [Google Scholar]
- 114.McLeod S, Vargas L, Portney V, Ting A. Synchrony dual optic accommodating lens. J Cataract Refract Surg. 2007;33:37–46. doi: 10.1016/j.jcrs.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 115.Alió JL, Plaza-Puche AB, Montalban R, Ortega P. Near visual outcomes with single-optic and dual-optic accommodating intraocular lenses. J Cataract Refract Surg. 2012;38:1568–75. doi: 10.1016/j.jcrs.2012.05.027. [DOI] [PubMed] [Google Scholar]
- 116.Bohórquez V, Alarcon R. Long-term reading performance in patients with bilateral dual-optic accommodating intraocular lenses. J Cataract Refract Surg. 2010;36:1880–6. doi: 10.1016/j.jcrs.2010.06.061. [DOI] [PubMed] [Google Scholar]
- 117.Kohl JC, Werner L, Ford JR, Cole SC, Vasavada SA, Gardiner GL, et al. Long-term uveal and capsular biocompatibility of a new accommodating intraocular lens. J Cataract Refract Surg. 2014;40:2113–9. doi: 10.1016/j.jcrs.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 118.Alió JL, Ben-nun J, Rodríguez-Prats JL, Plaza-Puche A. Visual and accommodative outcomes 1 year after implantation of an accommodating intraocular lens based on a new concept. J Cataract Refract Surg. 2009;35:1671–8. doi: 10.1016/j.jcrs.2009.04.043. [DOI] [PubMed] [Google Scholar]
- 119.Alio JL, Simonov A, Plaza-Puche AB, Angelov A, Angelov Y, van Lawick W, et al. Visual outcomes and accommodative response of the lumina accommodative intraocular lens. Am J Ophthalmol. 2016;164:37–48. doi: 10.1016/j.ajo.2016.01.006. [DOI] [PubMed] [Google Scholar]
- 120.Gil-Cazorla R, Shah S, Naroo SA. A review of the surgical options for the correction of presbyopia. Br J Ophthalmol. 2016;100:62–70. doi: 10.1136/bjophthalmol-2015-306663. [DOI] [PubMed] [Google Scholar]
- 121.Kamiya K, Takahashi M, Takahashi N, Shoji N, Shimizu K. Monovision by implantation of posterior chamber phakic intraocular lens with a central hole (Hole ICL) for early presbyopia. Sci Rep. 2017;7:11302. doi: 10.1038/s41598-017-11539-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kelava L, Barić H, Bušić M, Čima I, Trkulja V. Monovision versus multifocality for presbyopia: Systematic review and meta-analysis of randomized controlled trials. Adv Ther. 2017;34:1815–39. doi: 10.1007/s12325-017-0579-7. [DOI] [PubMed] [Google Scholar]
- 123.Ito M, Shimizu K, Iida Y, Amano R. Five-year clinical study of patients with pseudophakic monovision. J Cataract Refract Surg. 2012;38:1440–5. doi: 10.1016/j.jcrs.2012.03.031. [DOI] [PubMed] [Google Scholar]
- 124.Finkelman YM, Ng JQ, Barrett GD. Patient satisfaction and visual function after pseudophakic monovision. J Cataract Refract Surg. 2009;35:998–1002. doi: 10.1016/j.jcrs.2009.01.035. [DOI] [PubMed] [Google Scholar]
- 125.Wilkins MR, Allan BD, Rubin GS, Findl O, Hollick EJ, Bunce C, et al. Randomized trial of multifocal intraocular lenses versus monovision after bilateral cataract surgery. Ophthalmology. 2013;120:2449–550. doi: 10.1016/j.ophtha.2013.07.048. [DOI] [PubMed] [Google Scholar]
- 126.Zhang F, Sugar A, Arbisser L, Jacobsen G, Artico J. Crossed versus conventional pseudophakic monovision: Patient satisfaction, visual function, and spectacle independence. J Cataract Refract Surg. 2015;41:1845–54. doi: 10.1016/j.jcrs.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 127.Greenstein S, Pineda R., 2nd The quest for spectacle independence: A comparison of multifocal intraocular lens implants and pseudophakic monovision for patients with presbyopia. Semin Ophthalmol. 2017;32:111–5. doi: 10.1080/08820538.2016.1228400. [DOI] [PubMed] [Google Scholar]
- 128.Kim J, Shin HJ, Kim HC, Shin KC. Comparison of conventional versus crossed monovision in pseudophakia. Br J Ophthalmol. 2015;99:391–5. doi: 10.1136/bjophthalmol-2014-305449. [DOI] [PubMed] [Google Scholar]
- 129.Hayashi K, Ogawa S, Manabe S, Yoshimura K. Binocular visual function of modified pseudophakic monovision. Am J Ophthalmol. 2015;159:232–40. doi: 10.1016/j.ajo.2014.10.023. [DOI] [PubMed] [Google Scholar]
- 130.Lackner B, Pieh S, Schmidinger G, Simader C, Franz C, Dejaco-Ruhswurm I, et al. Long-term results of implantation of phakic posterior chamber intraocular lenses. J Cataract Refract Surg. 2004;30:2269–76. doi: 10.1016/j.jcrs.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 131.Beckman K. What a Novel Scleral Implant may Mean for Presbyopia. Ophthalmology Times; 2016. [Last accessed on 2018 Mar 10]. Available from: http://www.ophthalmologytimes.com/modern-medicine-feature-articles/what-novel-scleral-implant-may-mean-presbyopia . [Google Scholar]
- 132.Tunc Z, Helvacioglu F, Ercalik Y, Baikoff G, Sencan S. Supraciliary contraction segments: A new method for the treatment of presbyopia. Indian J Ophthalmol. 2014;62:116–23. doi: 10.4103/0301-4738.97554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hipsley A, Hall B, Rocha KM. Scleral surgery for the treatment of presbyopia: Where are we today? Eye Vis (Lond) 2018;5:4. doi: 10.1186/s40662-018-0098-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hipsley A, Ma DH, Sun CC, Jackson MA, Goldberg D, Hall B, et al. Visual outcomes 24 months after laserACE. Eye Vis (Lond) 2017;4:15. doi: 10.1186/s40662-017-0081-y. [DOI] [PMC free article] [PubMed] [Google Scholar]


