Abstract
Preeclampsia, a serious multisystem disorder specific to human pregnancy, remains a considerable burden of disease worldwide. Reduced nitric oxide bioavailability is proved to be crucial in the maternal and fetal pathophysiology of preeclampsia. G-protein-coupled Receptor Kinase Interactor-1 (GIT1) is a novel endothelial nitric oxide synthases (eNOS) interactor mediator. The aim of this paper is to investigate the effect of GIT1 on preeclampsia. Blood pressure (BP) was measured using a carotid catheter-calibrated eight-chamber tail-cuff system (CODA) at the same time daily. Urinary albumin excretion (UAE) was determined using Albuwell-M kits (Exocell Inc) and creatinine clearance (CCr) was determined by measuring urinary creatinine concentration with tandem liquid chromatography–mass spectrometry. The release of nitrite was analyzed to detect nitric oxide (NO) production using a Sievers Chemiluminescence NO Analyzer. NOS activity was examined by measuring the conversion of 3H-labeled l-arginine to 3H-labeled l-citrulline. BP was significantly increased in GIT1−/− mice with or without sFIT-1 treatment. In addition, GIT1−/− mice possessed higher UAE and lower CCr. Depletion of GIT1 impedes the NO production and placenta eNOS activity. Additional GIT1 attenuates sFlt-1-induced preeclampsia phenotypes. Our findings suggest that GIT1 significantly extenuates the sFlt-1-induced preeclampsia phenotypes by inhibiting eNOS activity, indicating a crucial role of GIT1 in the progression of preeclampsia.
Keywords: preeclampsia, endothelial nitric oxide synthases (eNOS), G-protein-coupled Receptor Kinase Interactor-1 (GIT1), NO production, urinary albumin excretion (UAE), blood pressure (BP)
Preeclampsia is a pregnancy-specific disorder traditionally diagnosed by increased blood pressure (greater than 90 mmHg diastolic or 140 mmHg systolic) and proteinuria, commonly affects approximately 3–5% of pregnancies (Mol et al. 2016). Especially in less developed countries, preeclampsia accounts for the second leading direct cause of maternal death, and remains one of the main causes of fetal and neonatal mortality (Gidlof and Nisell 2010). In severe diseases, kidney dysfunction, red blood cell breakdown, impaired liver function, visual disturbances, swelling, shortness of breath due to fluid in the lungs, or a low blood platelet count may occur (American College of Obstetricians and Gynecologists and Task Force on Hypertension in Pregnancy 2013). However, the pathogenetic mechanisms of preeclampsia are still not yet fully elucidated, putting limits to efficacious treatments.
Nitric oxide (NO), initially recognized as the endothelium-derived relaxing factor, is the chief vasodilator substance generated by the endothelium in response to various chemical and mechanical stimuli (Johal et al. 2014). By activating soluble guanylate cyclase (sGC), NO causes the relaxation of vascular smooth muscle cells, which in turn results in the activation of cGMP-dependent protein kinases and an increase in intracellular cyclic guanosine 3′,5′-monophosphate (cGMP) (Buhimschi et al. 1998). As a paracrine and autocrine signaling molecule, NO is synthesized from l-arginine by nitric oxide synthases (NOS), which is a family of calcium–calmodulin-dependent enzymes (Conrad and Davis 1995). In mammals, NO is mediated by endothelial NOS (eNOS) and neuronal NOS (nNOS) (Shaamash et al. 2001). It has been found that reduction in the bioavailability of NO is a key feature of endothelial dysfunction in preeclampsia (Brennecke et al. 1997). Moreover, the depletion of eNOS in mice result in high BP, decreased production of NO, hyperlipidemia, and insulin resistance (Orange et al. 2003). Previous studies also show that lack of eNOS exacerbates the preeclampsia–like phenotype induced by overexpression of sFlt-1 in nonpregnant female mice (Li et al. 2012).
G-protein-coupled Receptor Kinase Interactor-1 (GIT1) is a GTPase-activating protein for the ADP-ribosylation factor family of small GTP-binding proteins, which connects the signaling proteins to distinct cellular locations (Premont et al. 2004). Recent studies have shown that GIT1 not only functions as a scaffolding protein, but also possess intrinsic signaling abilities (Schmalzigaug et al. 2007). In addition, it has been demonstrated that GIT1 serves as a novel eNOS interactor modulating after liver injury, suggesting that it plays an important role in regulating the biological function of eNOS (Liu et al. 2012). These findings indicate that GIT1 might be a crucial mediator in preeclampsia progression.
In this study, we aimed to understand the role of GIT1 in sFlt-1–induced preeclampsia phenotype in pregnant mice and to elucidate the underlying mechanisms.
Materials and Methods
Animals
Animals were purchased from Nanjing Animal Model Institute (Nanjing, China). All animal experiments in this study were conducted in accordance with the International Animal Care and Use Committee guidelines of Heze Municipal Hospital. Pregnant C57BL/6 mice (WT and GIT1−/−, embryonic day 13) were injected into the tail veins with 3×109 PFU of adenovirus to overexpress sFlt-1 (sFlt-1) or adenovirus encoding murine Fc protein (control) at equivalent doses. To rule out nonspecific effects of adenovirus, Ad Fc was used as a control.
Measurement of urinary albumin excretion (UAE)
Urinary albumin was determined using Albuwell-M kits (Exocell Inc, Philadelphia, PA) according to the manufacturer’s instructions.
Measurement of creatinine clearance (CCr)
CCr was determined by measuring plasma and urinary creatinine concentration with the method developed before using tandem liquid chromatography–mass spectrometry (Takahashi et al. 2007).
Measurement of BP
A computerized tail-cuff system was used for measuring BPs on unanesthetized, restrained mice (Krege et al. 1995). Continuous recording of the BPs of was performed by radio telemetry.
Nitric Oxide Measurement
The release of nitrite (the stable breakdown product of NO) was detected to assess NO production using a Sievers Chemiluminescence NO Analyzer (Sievers Instruments, Inc., Boulder, CO) according to the manufacturer’s instructions.
NOS Activity Assays
NOS activity was assessed by measuring the conversion of 3H-labeled l-arginine to 3H-labeled l-citrulline as previously described (García-Cardeña et al. 1998) according to the manufacturer’s instructions (Cayman Chemical Co., Ann Arbor, MI).
Statistical analysis
All data were shown as the mean ± SD. Differences between samples were analyzed using the one or two-way ANOVA analysis followed by a post hoc test. Statistical significance was accepted at P < 0.05.
Data availability
The authors state that all data necessary for confirming the conclusions presented in the article are represented fully within the article.
Results
BP was significantly increased in GIT1−/− mice with or without sFIT-1 treatment
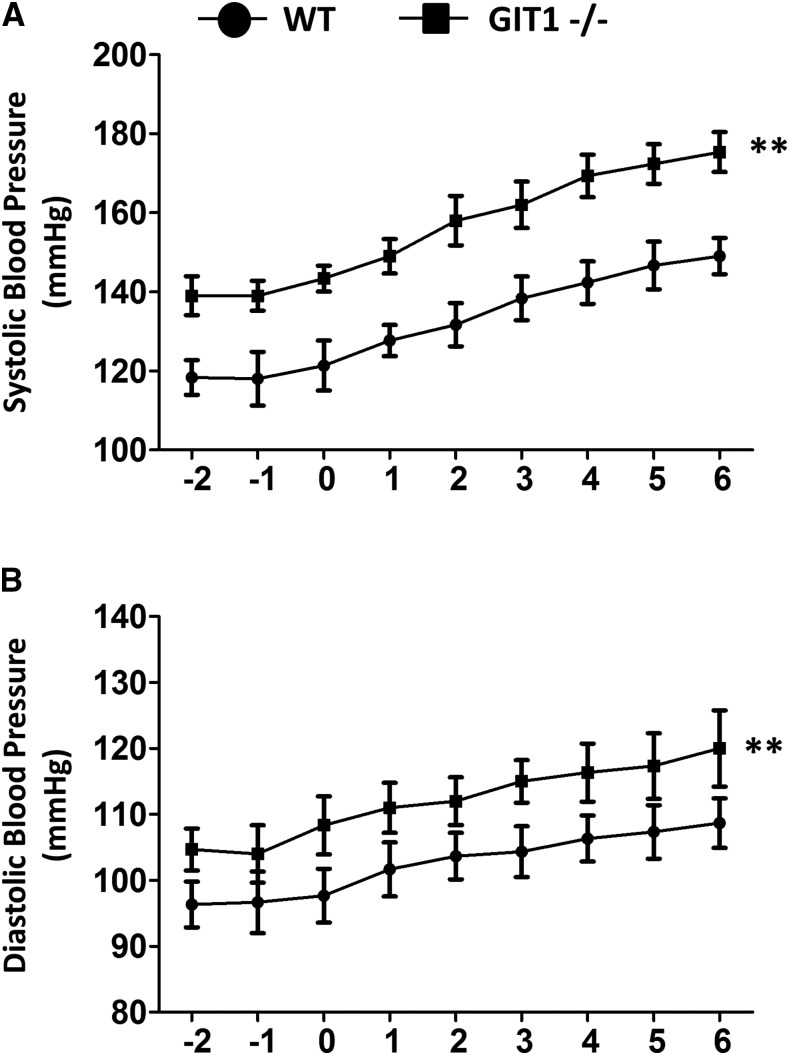
Adenovirus (Ad sFlt-1, 3×109 PFU) was injected into pregnant C57BL/6 mice (GIT1−/− and WT, embryonic day 13) to induce preeclampsia. Since hypertension is a major criterion for diagnose of preeclampsia, we applied telemetry to measure BP at the aortic arch. BP were monitored at the same time daily started at day 2 (-2) before administration of sFlt-1 and finished at day 6 (6) after administration of sFlt-1. As shown in Figure 1A and B, sFlt-1 virus increased SBP and DBP of both GIT1−/− and WT mice. Notably, both SBP and DBP were significantly increased in GIT1−/− mice with or without treated with sFIT-1, indicating that the influence of GIT1 on BP was independent of sFlt-1 treatment.
Figure 1.
Blood pressures were increased in GIT1−/− mice with or without treated with sFIT-1. (A) Systolic blood pressure (B) Diastolic blood pressure. The tests were started at day 2 (-2) before administration of sFlt-1 and finished at day 6 (6) after administration of sFlt-1, day 0(0) was the day administrated with sFlt-1. N = 6 mice in each experimental group. **P < 0.01 vs. WT mice.
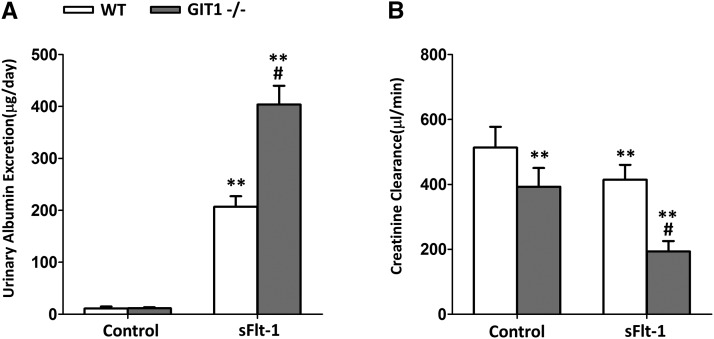
GIT1−/− mice possess higher urinary albumin excretion and lower creatinine clearance
We next examined the UAE and CCr in GIT1−/− and WT mice. As indicated in Figure 2A, daily UAE was not changed in mice of each genotype when treated with control virus (Ad Fc). SFlt-1 virus significantly increased the UAE in both WT and GIT1−/− mice. In addition, the increased sFlt-1 and lack of GIT1 synergistically enhanced the level of UAE (P < 0.01). Besides, GIT1 depletion remarkably decreased CCr in WT and GIT1−/− mice (Figure 2B). Again, there was synergistic interaction between sFlt-1 and lack of GIT1, since a greater extent of CCr reduction was observed in GIT1−/− mice when treated with sFlt-1. Thus, the lack of GIT1 and increased sFlt-1 synergistically exacerbates the increase in UAE and the decrease in CCr.
Figure 2.
Renal function was impacted in GIT1−/− mice. (A) Urinary Albumin Excretion (B) Creatinine Clearance. The tests were done at day 6 after administration of sFlt-1/Fc(control). N = 6 mice in each experimental group. **P < 0.01 vs. WT/control mice, #P < 0.01 vs. WT/ sFlt-1 mice.
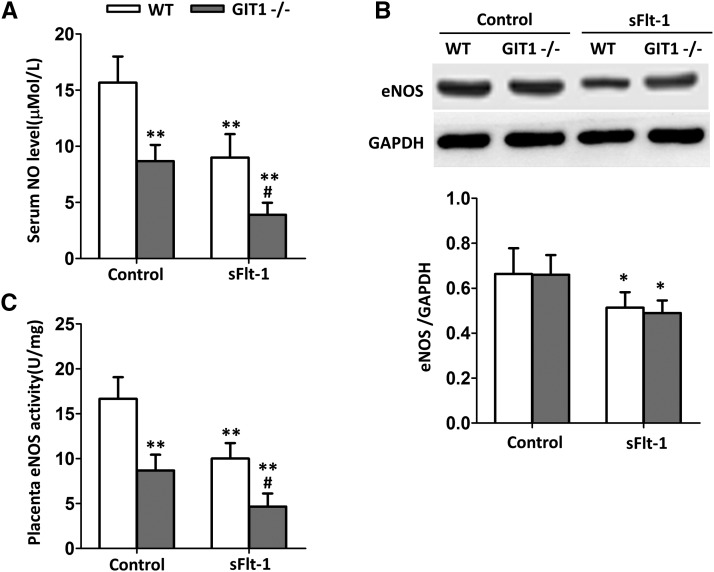
Depletion of GIT1 impedes the NO production and placenta eNOS activity
Reduction in the bioavailability of NO has proved to be a critical feature of endothelial dysfunction in preeclampsia. As shown in Figure 3A, compared with the WT mice, serum NO level was decreased in GIT1−/− mice when treated with Fc, and a more significant decline was detected in GIT1−/− mice when treated with sFlt-1 (P < 0.01). Since eNOS is a key factor monitoring the NO synthesis, we next examined the expression level of eNOS in WT and GIT1−/− mice with or without sFlt-1 treatment by western blot. As shown in Figure 3B, sFlt-1 significantly decreased the eNOS level both in the WT and GIT1−/− mice. However, depletion of GIT1 did not impact the expression level of eNOS (Figure 3B). To further clarify the roles of GIT1 and sFlt-1 in regulating the NO production, placenta eNOS activities were evaluated. Consistent with the results shown in Figure 3A, depletion of GIT1 significantly decreased the eNOS activities with or without sFlt-1 treatment (Figure 3C). Moreover, there was a synergistic effect between sFlt-1 and lack of GIT1 on suppressing the placenta eNOS activities (Figure 3C). Thus, we concluded that GIT1 depletion inhibits the NO synthesis by suppressing the activity, instead of suppressing the protein level of eNOS.
Figure 3.
Serum NO level and placenta eNOS activity were impacted in GIT1−/− mice. (A) Serum NO level (B) Placenta eNOS expression (C) Placenta eNOS activity. The tests were done at day 6 after administration of sFlt-1/Fc (control). N = 6 mice in each experimental group. *P < 0.05, **P < 0.01 vs. WT/control mice, #P < 0.01 vs. WT/ sFlt-1 mice.
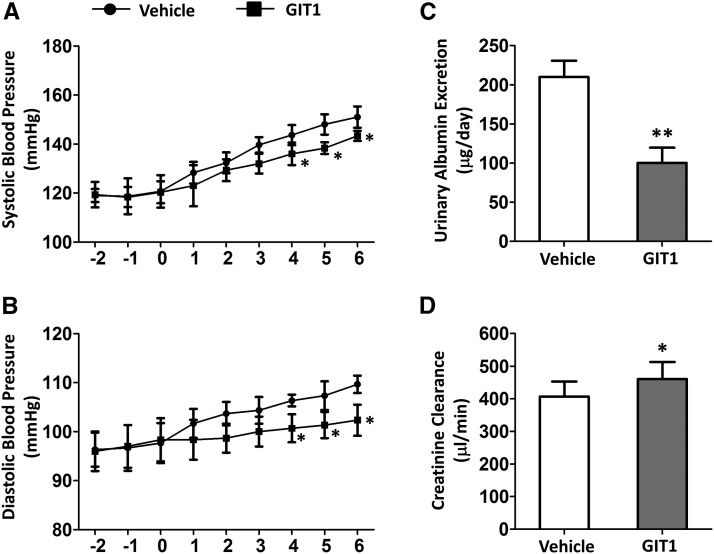
Additional GIT1 attenuates sFlt-1-induced preeclampsia phenotypes
We overexpressed GIT1 in pregnant C57BL/6 mice by injection of adenovirus to further identify the effects of GIT1 on preeclampsia. As shown in Figure 4A and B, the SBP and DBP were decreased in a time-dependent manner when treatment with additional GIT1, reversing the enhancement in BP induce by sFlt-1. Significant differences were observed on the fourth day after the stimulation of sFlt-1 (P < 0.05). In addition, UAE was significantly decreased with additional expression of GIT1 (Figure 4C). Besides, CCr was increased when treated with GIT1 (Figure 4D). Altogether, these data suggest that overexpression of GIT1 attenuates sFlt-1-induced preeclampsia symptoms.
Figure 4.
Additional GIT1 attenuated sFlt-1-induced preeclampsia phenotype in WT mice. (A) Systolic blood pressure (B) Diastolic blood pressure. The tests were started at day 2(-2) before administration of sFlt-1 and finished at day 6(6) after administration of sFlt-1, day 0(0) was the day administrated with sFlt-1, day 1 was the day administrated with GIT1. (C) Urinary Albumin Excretion (D) Creatinine Clearance. The tests were done at day 6 after administration of sFlt-1. Vehicle group was WT mice treated with sFlt-1, GIT1 group was WT mice treated with sFlt-1 and GIT1. N = 6 mice in each experimental group. P < 0.05,**P < 0.01 vs. Vehicle mice.
Additional GIT1 upregulates serum NO level and placenta eNOS activity
To further elucidate the specific mechanism of GIT1 in preeclampsia, serum NO levels and placenta eNOS activities were detected in WT and GIT1 overexpression mice induced by sFlt-1. Serum NO levels (Figure 5A) and eNOS activities (Figure 5B) were significantly increased when treated with additional GIT1 (P < 0.05 and P < 0.01, respectively). Our findings might molecularly explain the mechanism that GIT1 alleviates sFlt-1-induced preeclampsia symptoms by regulating NO production and eNOS activity.
Figure 5.
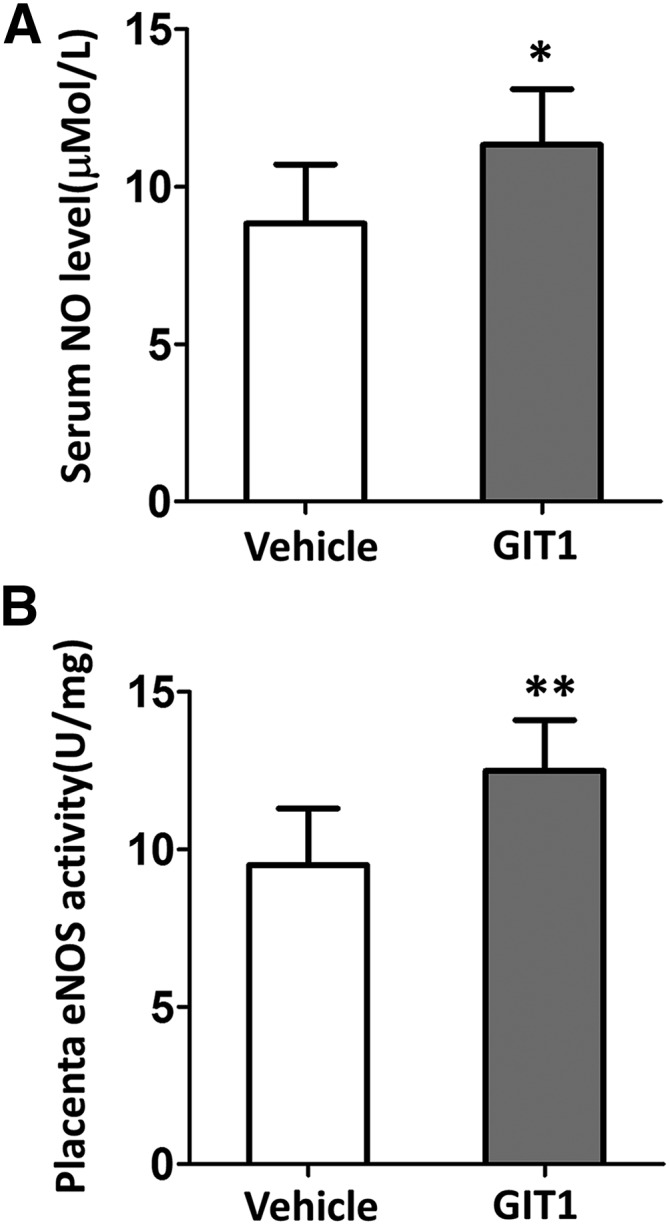
Additional GIT1 upregulated serum NO level and placenta eNOS activity. (A) Serum NO level (B) Placenta eNOS activity. The tests were done at day 6 after administration of sFlt-1/Fc(control). N = 6 mice in each experimental group. *P < 0.05, **P < 0.01 vs. Vehicle mice.
Discussion
Preeclampsia is a multisystem disorder of pregnancy and affects approximately 2–8% of overall pregnancies worldwide (US Preventive Services Task Force et al. 2017). Preeclampsia can be normally characterized by hypertension (the onset of high blood pressure) and proteinuria (a significant amount of protein in the urine) fter 20 weeks of gestation (de Groot and Taylor 1996). Accounting for approximately 16% of the direct maternal deaths, preeclampsia is a major cause of maternal mortality in developed countries, which might lead to a wide spectrum of serious complications, such as hemorrhagic stroke, elevated liver enzymes and low platelets (HELLP) syndrome, eclampsia, and renal failure and pulmonary edema (Lambert et al. 2014; Arulkumaran and Lightstone 2013). Although the etiology of preeclampsia stills remains to be further elucidated, it is widely accepted that the increased production of soluble fms-like tyrosine kinase 1 (sFlt-1), soluble endoglin, and likely other factors in placenta by placental hypoxia/ischemia accounts for the occurrence of preeclampsia (De Vivo et al. 2008). Previous studies have demonstrated that the increased expression of sFlt-1 is considered as one of the most critical factors of preeclampsia maternal symptoms (Carney 2015). Generally, pregnant women with preeclampsia possess higher level of plasma concentration of sFlt-1 compared with normal pregnant women, and this expression is expanded in severe preeclampsia women (Youssef et al. 2011). Thus, in our study, we used adenovirus to overexpress sFlt-1 for inducing preeclampsia in mice and explored the function of GIT1 in monitoring sFlt-1-induced preeclampsia phenotype.
Previous studies have proved that eNOS plays a crucial role in vascular homeostasis (Sandrim et al. 2010). A complex set of signaling and post-translational structural biological processes are involved in the regulation of eNOS function (Singh et al. 2010). For instance, eNOS has been found to be interact with various proteins, such as HSP-90, G-protein-coupled receptor kinase 2 (GRK2), caveolin-1, and notably, GIT1 (Niu and Qi 2011). These protein interactions generally result in changes in the activity and function of eNOS by modifying the phosphorylation of several key serine residues (Fatini et al. 2006). It has been reported that GIT1 significantly enhances the eNOS activity by direct interaction with eNOS protein in sinusoidal endothelial cells (Li et al. 2012). A simple model has been proposed that activated Akt could phosphorylate eNOS at Ser1177 to stimulate NO production (Dimmeler et al. 1999). However, recent studies have shown that eNOS could be phosphorylated by a relatively complex system. It has been found that the interaction between GIT1 and eNOS depends on the phosphorylation of specific residues in GIT1 (Tyr293 and Tyr554) (Watts and Motley 2009). A tightly linked system of regulatory events centered on the GIT1 scaffold was identified to regulate the activation of eNOS (Tanaka et al. 2015). Upon endothelin stimulation, GIT1 was phosphorylated on tyrosine residues, predominantly Tyr293 and Tyr554 in sinusoidal endothelial cells (Wu et al. 2014). Previous findings have demonstrated that Src kinase family are responsible for the phosphorylation of these two sites (Tanaka et al. 2015). Here, we extend these findings by exploring the function of GIT1 in regulating eNOS activity in sFlt-1–induced preeclampsia mice. In this paper, we demonstrate that depletion of GIT1 significantly impedes the NO production and placenta eNOS activity in mice (Figure 3). However, more detailed molecular mechanisms of how GIT1 regulates eNOS activity in preeclampsia mice still need to be further elucidated. In view of the fact described above, here we hypothesize that GIT1 might directly bind to eNOS in placenta, and phosphorylation of GIT1 is crucial in the GIT1-eNOS interaction and in stimulation of eNOS phosphorylation. Moreover, Src and Akt might both act upstream and downstream of GIT1 in the GIT1-eNOS pathway, leading to eNOS activation that we observed mediated through the GIT1 scaffold.
Previous studies have found that overexpression of sFlt-1 induces a preeclampsia–like phenotype, which includes increased BP, proteinuria, and endothelial dysfunction (Caillon et al. 2018). Also, it has been demonstrated that increased sFlt-1 will lead to a more severe preeclampsia-like phenotype in eNOS−/− mice (Navaratnam et al. 2017). Symptoms including the increase of UAE, the decrease of CCr, and more severe endotheliosis are aggravated by a synergistic effect combining the overexpression of sFlt-1 and the depletion of eNOS.(Reddy et al. 2009) In addition, when overexpressed sFlt-1, eNOS−/− mice has a higher renal expression of the ET system compared with the WT mice (Zhang et al. 2013). Preproendothelin-1 (ET-1) has been demonstrated to exacerbates the pathologic changes generated from the additional expression of sFlt-1 (Kamoi et al. 1990). Pregnant women with preeclampsia normally exhibit higher levels of plasma ET-1 (Boulanger and Luscher 1990). Since NO suppresses the expression level of ET-1, previous studies have proved that the decreased production of NO and the suppressed eNOS activity might exacerbate preeclampsia-like phenotypes by upregulating ET-1 (Liang et al. 1996). In addition, depletion of eNOS and increased sFlt-1 enhance the expression level of preproET-1 and ETAR in the kidney (Li et al. 2012). In this paper, we demonstrate that depletion of GIT1 significantly impedes the NO production and placenta eNOS activity (Figure 3). Thus, we can assume that GIT1 might be influential on the regulation of ET-1.
In conclusion, our data demonstrate that absence of GIT1 exacerbates the preeclampsia-like phenotypes induced by the overexpression of sFlt-1 in pregnant female mice. Depletion of GIT1 decreases BP and UAE, and increases CCr both in WT and sFlt-1 mice. In addition, GIT−/− sFlt-1 mice exhibit reduced expression of NO production and decreased activity of eNOS. Overexpression of GIT1 demonstrates a reversed effect. Although future studies could be made to further reveal the specific molecular mechanisms of how GIT1 aggravates the sFlt-1–induced preeclampsia-like phenotype by regulating NO/eNOS pathway, our research provides reliable clues and basis for the follow-up study on preeclampsia.
Acknowledgments
The authors declare that they have no conflict of interest.
Footnotes
Communicating editor: A. McCallion
Literature Cited
- American College of Obstetricians and Gynecologists, and Task Force on Hypertension in Pregnancy , 2013. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet. Gynecol. 122: 1122–1131. [DOI] [PubMed] [Google Scholar]
- Arulkumaran N., Lightstone L., 2013. Severe pre-eclampsia and hypertensive crises. Best Pract. Res. Clin. Obstet. Gynaecol. 27: 877–884. 10.1016/j.bpobgyn.2013.07.003 [DOI] [PubMed] [Google Scholar]
- Boulanger C., Luscher T. F., 1990. Release of endothelin from the porcine aorta. Inhibition by endothelium-derived nitric oxide. J. Clin. Invest. 85: 587–590. 10.1172/JCI114477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennecke S. P., Gude N. M., Di Iulio J. L., King R. G., 1997. Reduction of placental nitric oxide synthase activity in pre-eclampsia. Clin. Sci. (Lond.) 93: 51–55. 10.1042/cs0930051 [DOI] [PubMed] [Google Scholar]
- Buhimschi I. A., Saade G. R., Chwalisz K., Garfield R. E., 1998. The nitric oxide pathway in pre-eclampsia: pathophysiological implications. Hum. Reprod. Update 4: 25–42. 10.1093/humupd/4.1.25 [DOI] [PubMed] [Google Scholar]
- Caillon H., Tardif C., Dumontet E., Winer N., Masson D., 2018. Evaluation of sFlt-1/PlGF Ratio for Predicting and Improving Clinical Management of Pre-eclampsia: Experience in a Specialized Perinatal Care Center. Ann. Lab. Med. 38: 95–101. 10.3343/alm.2018.38.2.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney E. F., 2015. Hypertension: sFlt-1 removal seems to be beneficial in women with pre-eclampsia. Nat. Rev. Nephrol. 11: 690. [DOI] [PubMed] [Google Scholar]
- Conrad K. P., Davis A. K., 1995. Nitric oxide synthase activity in placentae from women with pre-eclampsia. Placenta 16: 691–699. 10.1016/0143-4004(95)90013-6 [DOI] [PubMed] [Google Scholar]
- de Groot C. J., Taylor R. N., 1996. Preeclampsia: an update. Eur. J. Obstet. Gynecol. Reprod. Biol. 69: 59–60. 10.1016/0301-2115(95)02546-4 [DOI] [PubMed] [Google Scholar]
- De Vivo A., Baviera G., Giordano D., Todarello G., Corrado F., et al. , 2008. Endoglin, PlGF and sFlt-1 as markers for predicting pre-eclampsia. Acta Obstet. Gynecol. Scand. 87: 837–842. 10.1080/00016340802253759 [DOI] [PubMed] [Google Scholar]
- Dimmeler S., Fleming I., Fisslthaler B., Hermann C., Busse R., et al. , 1999. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature 399: 601–605. 10.1038/21224 [DOI] [PubMed] [Google Scholar]
- Fatini C., Sticchi E., Gensini F., Genuardi M., Tondi F., et al. , 2006. Endothelial nitric oxide synthase gene influences the risk of pre-eclampsia, the recurrence of negative pregnancy events, and the maternal-fetal flow. J. Hypertens. 24: 1823–1829. 10.1097/01.hjh.0000242407.58159.87 [DOI] [PubMed] [Google Scholar]
- García-Cardeña G., Fan R., Shah V., Sorrentino R., Cirino G., et al. , 1998. Dynamic activation of endothelial nitric oxide synthase by Hsp90. Nature 392: 821–824. 10.1038/33934 [DOI] [PubMed] [Google Scholar]
- Gidlof S., Nisell H., 2010. [Pre-eclampsia] Lakartidningen 107: 3288–3292. [PubMed] [Google Scholar]
- Johal T., Lees C. C., Everett T. R., Wilkinson I. B., 2014. The nitric oxide pathway and possible therapeutic options in pre-eclampsia. Br. J. Clin. Pharmacol. 78: 244–257. 10.1111/bcp.12301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamoi K., Sudo N., Ishibashi M., Yamaji T., 1990. Plasma endothelin-1 levels in patients with pregnancy-induced hypertension. N. Engl. J. Med. 323: 1486–1487. 10.1056/NEJM199011223232113 [DOI] [PubMed] [Google Scholar]
- Krege J. H., Hodgin J. B., Hagaman J. R., Smithies O., 1995. A noninvasive computerized tail-cuff system for measuring blood pressure in mice. Hypertension 25: 1111–1115. 10.1161/01.HYP.25.5.1111 [DOI] [PubMed] [Google Scholar]
- Lambert G., Brichant J. F., Hartstein G., Bonhomme V., Dewandre P. Y., 2014. Preeclampsia: an update. Acta Anaesthesiol. Belg. 65: 137–149. [PubMed] [Google Scholar]
- Li F., Hagaman J. R., Kim H. S., Maeda N., Jennette J. C., et al. , 2012. eNOS deficiency acts through endothelin to aggravate sFlt-1-induced pre-eclampsia-like phenotype. J. Am. Soc. Nephrol. 23: 652–660. 10.1681/ASN.2011040369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X., Lin Y., Cheng Y., 1996. [Changes in plasma endothelin-1 and lipid peroxidate levels and amount of superoxidate dismutase in red blood cell in patients with pregnancy-induced hypertension] Zhonghua Fu Chan Ke Za Zhi 31: 220–222. [PubMed] [Google Scholar]
- Liu S., Premont R. T., Rockey D. C., 2012. G-protein-coupled receptor kinase interactor-1 (GIT1) is a new endothelial nitric-oxide synthase (eNOS) interactor with functional effects on vascular homeostasis. J. Biol. Chem. 287: 12309–12320. 10.1074/jbc.M111.320465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mol B. W. J., Roberts C. T., Thangaratinam S., Magee L. A., de Groot C. J. M., et al. , 2016. Pre-eclampsia. Lancet 387: 999–1011. 10.1016/S0140-6736(15)00070-7 [DOI] [PubMed] [Google Scholar]
- Navaratnam K., Abreu P., Clarke H., Jorgensen A., Alfirevic A., et al. , 2017. Evaluation of agreement of placental growth factor (PlGF) tests and the soluble FMS-like tyrosine kinase 1 (sFlt-1)/PlGF ratio, comparison of predictive accuracy for pre-eclampsia, and relation to uterine artery Doppler and response to aspirin. J. Matern. Fetal Neonatal Med. 1–9. 10.1080/14767058.2017.1373760 [DOI] [PubMed] [Google Scholar]
- Niu W., Qi Y., 2011. An updated meta-analysis of endothelial nitric oxide synthase gene: three well-characterized polymorphisms with hypertension. PLoS One 6: e24266 10.1371/journal.pone.0024266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orange S. J., Painter D., Horvath J., Yu B., Trent R., et al. , 2003. Placental endothelial nitric oxide synthase localization and expression in normal human pregnancy and pre-eclampsia. Clin. Exp. Pharmacol. Physiol. 30: 376–381. 10.1046/j.1440-1681.2003.03844.x [DOI] [PubMed] [Google Scholar]
- Premont R. T., Perry S. J., Schmalzigaug R., Roseman J. T., Xing Y., et al. , 2004. The GIT/PIX complex: an oligomeric assembly of GIT family ARF GTPase-activating proteins and PIX family Rac1/Cdc42 guanine nucleotide exchange factors. Cell. Signal. 16: 1001–1011. 10.1016/S0898-6568(04)00023-3 [DOI] [PubMed] [Google Scholar]
- Reddy A., Suri S., Sargent I. L., Redman C. W., Muttukrishna S., 2009. Maternal circulating levels of activin A, inhibin A, sFlt-1 and endoglin at parturition in normal pregnancy and pre-eclampsia. PLoS One 4: e4453 10.1371/journal.pone.0004453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandrim V. C., Palei A. C., Sertorio J. T., Cavalli R. C., Duarte G., et al. , 2010. Effects of eNOS polymorphisms on nitric oxide formation in healthy pregnancy and in pre-eclampsia. Mol. Hum. Reprod. 16: 506–510. 10.1093/molehr/gaq030 [DOI] [PubMed] [Google Scholar]
- Schmalzigaug R., Phee H., Davidson C. E., Weiss A., Premont R. T., 2007. Differential expression of the ARF GAP genes GIT1 and GIT2 in mouse tissues. J. Histochem. Cytochem. 55: 1039–1048. 10.1369/jhc.7A7207.2007 [DOI] [PubMed] [Google Scholar]
- Shaamash A. H., Elsonosy E. D., Zakhari M. M., Radwan S. H., El-Dien H. M., 2001. Placental nitric oxide synthase (NOS) activity and nitric oxide (NO) production in normal pregnancy, pre-eclampsia and eclampsia. Int. J. Gynaecol. Obstet. 72: 127–133. 10.1016/S0020-7292(00)00314-3 [DOI] [PubMed] [Google Scholar]
- Singh A., Sharma D., Raghunandan C., Bhattacharjee J., 2010. Role of inflammatory cytokines and eNOS gene polymorphism in pathophysiology of pre-eclampsia. Am. J. Reprod. Immunol. 63: 244–251. 10.1111/j.1600-0897.2009.00781.x [DOI] [PubMed] [Google Scholar]
- Takahashi N., Boysen G., Li F., Li Y., Swenberg J. A., 2007. Tandem mass spectrometry measurements of creatinine in mouse plasma and urine for determining glomerular filtration rate. Kidney Int. 71: 266–271. 10.1038/sj.ki.5002033 [DOI] [PubMed] [Google Scholar]
- Tanaka M., Miyajima M., Hishioka N., Nishimura R., Kihara Y., et al. , 2015. Humic acid induces the endothelial nitric oxide synthase phosphorylation at Ser1177 and Thr495 Via Hsp90alpha and Hsp90beta upregulation in human umbilical vein endothelial cells. Environ. Toxicol. 30: 223–231. 10.1002/tox.21888 [DOI] [PubMed] [Google Scholar]
- US Preventive Services Task Force. Bibbins-Domingo K., Grossman D. C., Curry S. J., Barry M. J., et al. , 2017. Screening for Preeclampsia: US Preventive Services Task Force Recommendation Statement. JAMA 317: 1661–1667. 10.1001/jama.2017.3439 [DOI] [PubMed] [Google Scholar]
- Watts V. L., Motley E. D., 2009. Role of protease-activated receptor-1 in endothelial nitric oxide synthase-Thr495 phosphorylation. Exp. Biol. Med. (Maywood) 234: 132–139. 10.3181/0807-RM-233 [DOI] [PubMed] [Google Scholar]
- Wu P. R., Chen B. R., Hsieh C. C., Lin W. C., Wu K. K., et al. , 2014. The N-terminal portion of autoinhibitory element modulates human endothelial nitric-oxide synthase activity through coordinated controls of phosphorylation at Thr495 and Ser1177. Biosci. Rep. 34: e00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youssef A., Righetti F., Morano D., Rizzo N., Farina A., 2011. Uterine artery Doppler and biochemical markers (PAPP-A, PIGF, sFlt-1, P-selectin, NGAL) at 11 + 0 to 13 + 6 weeks in the prediction of late (> 34 weeks) pre-eclampsia. Prenat. Diagn. 31: 1141–1146. [DOI] [PubMed] [Google Scholar]
- Zhang Z. F., Li B., Chen D. J., 2013 [Effects of danshen on NO and ET-1 secreted by endothelial cells induced by the serum of pre-eclampsia patients.] Zhongguo Zhong Xi Yi Jie He Za Zhi 33: 538–540. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors state that all data necessary for confirming the conclusions presented in the article are represented fully within the article.