Abstract
Dracaena cinnabari Balf. fil. is an endangered endemic species growing on the Yemeni island of Soqotra. Dracaena woodlands are considered as one of the oldest forest communities on Earth. Uncontrolled grazing unfortunately caused a lack of naturally occurring regeneration. Our two-year research was focused on the growth dynamics of Dracaena seedlings from two separate populations. One hundred of germinated seeds from two different altitudes from the island were sown and planted under the same conditions. Average increment and difference between the growth dynamics of plants from the two localities were investigated. The observed data on this plant species revealed very interesting, hitherto unknown results. (1) The seedlings germinated within a time period from four to ten weeks. Germination rate was 90% on the Firmihin highland plateau and 78% on the Scand Mountain. (2) Average plant length from both localities was almost the same (24.9 cm) at the end of measurement. Differences in values between the two populations proved as non-significant. (3) A significant difference was found in the number of leaves and in the sum of lengths of all leaves on one plant. While the seedlings from Firmihin featured a wide spreading above-ground part with a large number of leaves, the plants from Scand invested more energy into faster leaves elongation rate. (4) Growth dynamics reflected seasonal changes. Increments were slower or ceased during the period of vegetative rest from autumn to spring. (5) Average mortality rate was 13%. Most of the plants died during the period of vegetative rest. Further study on germination and regeneration under artificial conditions seems like the only way to prevent species extinction.
Keywords: Dragon’s Blood Tree, Germination, Growth rate, Height increment, Mortality
1. Introduction
The genus Dracaena comprises between 60 and 100 species (Adolt and Pavliš, 2004). It belongs in the family of Asparagaceae or Dracaenaceae (Brown and Mies, 2012). Dracaena species are exceptional among monocotyledonous plants because of their ability of the secondary growth of stems, branches and roots (Habrová et al., 2009). Most of Dracaena species grow as shrubs or geophytes. There are only seven species featuring arborescent growth and the xeromorphic Dracaena cinnabari belongs to them (Bekele, 2007).
D. cinnabari is a large, single-trunked tree with height up to 10 m and smooth grey bark. Branches with sausage-shaped sections form an umbrella-shaped crown. The crown shape is adapted to arid climates and is affected by the availability of atmospheric moisture. Tough leaves are densely tufted, dark green and elongated, up to 60 cm long and up to 4 cm wide. The leaves are scleromorphic as a specialised feature to prevent excessive loss of water (Brown and Mies, 2012). Small creamy flowers grow in large terminal panicles. Globose fruits have about 1 cm in diameter and contain from 1 to 3 spherical and brownish-red seeds, which are very hard. Their stage of ripening goes from black to red (Miller and Morris, 2004). The seeds are spread by birds. Since ancient times, the plant has been used for harvesting blood-red medicinal resin and as fuel wood. Moreover, flowers, fruits and leaves have been used as a source of dry season feed for livestock.
D. cinnabari (Dragon’s Blood Tree) is an endemic species and the most iconic plant of the Soqotra Island. It is a highly conspicuous element in the landscape of Soqotra, specifically at altitudes between ca. 300 and 1480 m (Brown and Mies, 2012). Soqotra is an isolated island lying in the Indian Ocean between the Horn of Africa and the Arabian Peninsula. It belongs to the Republic of Yemen. Separated from the continent during the Tertiary period, the island’s floral endemism rate makes it one of the most biodiverse islands in the world (Grant, 2005). Today, it hosts more than 950 plant species, including some 825 terrestrial plants (430 genera, 114 families) and about 130 algae and seagrasses (Miller and Morris, 2004, Cheung and DeVantier, 2006, Brown and Mies, 2012). Of the total number of flowering plants and ferns, 37% are endemic, when compared with other archipelagos. In 2003, upon the international recognition of these outstanding attributes, the island became a UNESCO Man and Biosphere Reserve. In 2005, the islands were nominated for World Heritage listing (Cheung and DeVantier, 2006, Scholte et al., 2011). According to Cheung and DeVantier (2006), during the long period of isolation, evolution of the island’s flora and fauna has proceeded in various adaptations to cope with the arid, wind-swept environment. The umbrella-shaped shrubs and trees have adopted eco-morphological strategy. The unique vegetation formation adapted to semi-arid environment is an evergreen woodland dominated by the Dragon’s Blood Tree (D. cinnabari) (Miller et al., 2006). The Dracaena woodland covers 3658 ha, i. e. 1.1% of the island (Král and Pavliš, 2006). The general distribution of Dracaena on Soqotra reflects the size of areas that are affected by the monsoon mists (Brown and Mies, 2012).
The Soqotra’s flagship species D. cinnabari suffers from the lack of regeneration due to intensified goat grazing (Miller and Morris, 2004). Therefore, the seedlings and young trees grow mainly on rock ledges and other sites that are inaccessible to goats. There are only mature and overmature trees in the accessible terrain. Plant density is not homogenous. The Dracaena woodland on Firmihin is considered as one of the oldest forest communities on Earth (Miller and Morris, 2004). However, further development of this community is not optimistic. Prediction of tree density development was made for Firmihin, the locality with the highest density of Dracaena trees (Hubálková, 2011). It showed that the Dracaena tree density would decrease by 36% until 2110, if the actual grazing intensity remains unchanged. A similar situation occurs with the Frankincense tree Boswellia papyrifera growing in Eritrea and in the Horn of Africa. Its populations are declining due to human pressure and environmental degradation, the trees are found mainly in hilly areas on steep slopes as an adaption to harsh growing conditions (Ogbazghi et al. 2006). To assess the perspectives of woodland restoration, Negussie et al. (2008) examined Boswellia seedling densities in grazed woodland and a grazing exclosure in the lower Geba river catchment in northern Ethiopia. According to the results of their experiment, the number of Boswellia seedlings varied throughout the year, showing higher values in the rainy season. There were more seedlings in the exclosure than in grazed woodland. The authors also mention dry season as a serious cause of seedling mortality which limits the potential of native woodland recovery. Ogbazghi et al. (2006) devoted to the role of environment and land use factors determining the distribution limits of B. papyrifera in Eritrea. Their field survey was conducted in 113 village areas. Species occurrence was related to rainfall, air temperature, length of growing period, physical and chemical soil factors, topography and land-use types. The results show decreasing distribution as a result of several interrelated human factors such as the conversion of woodlands into agricultural fields and increasing livestock pressure.
The vulnerable Dragon’s Blood Tree has been therefore one of the main concerns for conservation efforts and research activities on Soqotra in recent years (Attore et al., 2007, Habrová et al., 2009). Several detailed studies have been conducted to assess the potential impacts of various environmental factors on the plant development (Brown and Mies, 2012, Van Damme and Banfield, 2011, Scholte et al., 2011). However, there is little information about phenology and growth of D. cinnabari. There are few current studies related to the growth dynamics of D. cinnabari (Adolt et al., 2012, Attore et al., 2007, Habrová et al., 2009). Germination of Dracaena seeds under greenhouse conditions appears to be unproblematic (Brown and Mies, 2012). Adolt and Pavliš (2004) in Brown and Mies (2012) reported that germination rates as high as 77% could be achieved under greenhouse conditions, and that the mortality of seedlings amounted to only 10%. Beyhl (1996) mentioned comparably low rates of 35% germinability, which would appear to be adequate to maintain populations. In his thesis, Adolt (2001) carried out a germination experiment using 50 seeds of D. cinnabari from the Diksam area and 100 seeds of Dracaena draco from Tenerife. The experiment was conducted in the greenhouse of Mendel University in Brno, Czech Republic, at an average temperature of 22 °C. The germination was boosted by variously diluted solutions of hydrogen peroxide. The germination rate of D. draco was significantly higher (34%) than that of D. cinnabari (5%). One percent hydrogen peroxide solution increased the germination of Dragon’s Blood Tree to 22%. Petroncini et al. (2003) studied anatomy and genetic variability of the species using 45 randomly collected seeds from a highland plateau on Soqotra (14 seeds sown in summer 2001, 31 seeds sown in February 2002). The plants were cultivated in a greenhouse of the Botanical Garden of Florence. The authors observed the development of a thick layer of cuticular waxes on the leaves. Unfortunately, they didn’t devote their interest to growth dynamics of the seedlings. Under natural conditions, germination and successful establishment are going to be substantially lower (Brown and Mies, 2012). According to Beyhl (1998), during their germination, the seeds of D. cinnabari form a little root and a cotyledon, that remains stuck to the seed like in other monocotyledons. Directly after the germination with the outlet of the little root and of the little leaf, the seeds develop a cylindrical swelling or a tubular epicotyl, which gives rise to a tuber. Beyhl (1998) also claims that at the first stage of germination the little leaves remain etiolated for some days after exposure to light. After a short time, they start to produce chlorophyll and assimilate. Other leaves are green since the beginning. The first three or five leaves are distich disposed, just the next leaves have a pent – spiral collocation. Koopowitz and Kay (1990) suppose that Dracaena trees grow easily from the seed but their seedlings are very vulnerable to grazing animals. They also point out the fact, that the information about the growth dynamics of D. cinnabari is scarce. Beyhl (1996) asserts that the growth of the branches of Dracaena trees happens after the development of the terminal bud, after flourishing, or after a traumatic event. Earlier botanists (e.g. Symon, 1974, Wright, 1901) in their studies of palms and other arborescent monocotyledons devoted considerable attention to few forms with secondary vascular tissues. Tomlinson and Zimmermann (1969) created and described sketches of the habitus of monocotyledons with the secondary growth. According to these authors, the development of Beaucarnea recurvata, for example, begins with a rapidly growing main axis, which remains unbranched for several years. Tomlinson (1970) studied the peculiarities of branching and crown shape changes in dependence on the plant age. According to Banfield et al. (2011), widely spread branches and tightly packed leaves may increase the surface area available for the condensation of water from the surrounding fogs and mists. The canopy also creates a cooling shade, which reduces solar radiation and evaporation in the area around the trunk or stem, benefiting both the tree and other plants growing thereunder (Banfield et al., 2011). However, the age at which Dracaena reaches a particular height or begins to branch, remains unexplored.
The aim of this study is to describe the growth dynamics of D. cinnabari seedlings in the first two years after germination. We included also the germination phase as we considered the germination rates reported by Adolt (2001) very low. Moreover, we focused on two populations (one from the highland plateau of Firmihin and the other one from the Scand Mountain) from different elevations as we had a hypothesis about a certain genetic adaptation to the different conditions. Another intention was to compare seed germination and seedling mortality between the two groups and to find a rational explanation to the obtained results. We also wanted to know if the total plant biomass could be estimated from the plant height. Within the growth dynamics, we hypothesised as follows: (1) the average germination rate would be around 80% and thus the results published by Adolt and Pavliš (2004) would be corroborated. (2) The length of the highest leaf would be in favour of seedlings from Scand. Let us assume that this was due to genetic adaptation and higher plant resistance in the Scand area (e.g. Banfield et al., 2011, Brown and Mies, 2012). (3) The number of leaves would be higher in the plants from Firmihin as an adaptation to the drier and warmer climate and more effective interception of horizontal precipitation (e.g. Banfield et al., 2011, Brown and Mies, 2012). (4) The leaf length increment would reflect seasonal changes. (5) The average mortality rate of seedlings would be about 10% as well as in the study written by Adolt and Pavliš (2004).
2. Materials and methods
2.1. Study area
The Soqotra Archipelago, situated in the northern part of the Indian Ocean between 12°06′–12°42′N and 52°03′–54°32′E, comprises the name-giving Soqotra Island, the islets of Abd-al Kuri, Samha and Darsa and a few cliffs (Kürschner et al., 2006). The major island, Soqotra, the furthest east of the group, is approximately 3600 km2 in area, spanning 133 km west to east and 43 km north to south (Cheung and DeVantier, 2006). According to Mies and Beyhl (1996), the islands are situated in the arid tropical zone where evapotranspiration generally surpasses precipitation by far. The climate of ecoregions is influenced by the south-west (summer) and north-east (winter) monsoons. The south-west monsoon (from May to September) brings only humidity, the north-east monsoon (from November to March) is milder but brings expected winter rain (Scholte and De Geest, 2010, Culek et al., 2006). The remaining part of the year is characterised by dry weather conditions. Soqotra can be divided into three physiographic zones: the coastal plains, the limestone plateau and the igneous Haggeher Mountains (Miller and Morris, 2004). According to Attorre et al. (2007), D. cinnabari, absent in the west, has a fragmented distribution in the central and eastern part of the island. It is common and often abundant on the granites of the Haggeher Mountains (Scand) and the adjacent limestone plateaus (Firmihin) where it is frequently dominant in the evergreen and semi-deciduous woodland (Miller and Morris, 2004). The species occurrence dominates above 600 m a.s.l.
The seeds used in our experiment originated from the two last closed populations of D. cinnabari: from the Firmihin plateau at an altitude of approx. 580 m a.s.l. and from Scand situated at approximately 1450 m above sea level.
Firmihin is a highland plateau in the central part of the island. Altitude of the plateau ranges from 390 to 760 m a.s.l. According to Miller et al. (2000), the area belongs to the third vegetation belt where the plant starts to be dominant. According to Habrová et al. (2007), annual mean temperature on Firmihin is 23.4 °C, daily means range between 20 °C in February and 29 °C in May. The minimum temperature recorded on the plateau was 14.35 °C in January, the maximum temperature recorded was 36.26 °C in May. Annual mean relative air humidity is 71.87%. Horizontal precipitation is relatively low and infrequent compared to Scand; therefore, the crowns are wide. Firmihin is a limestone plateau. Karstification of the landscape is a conspicuous feature and locally, the limestone is interrupted by small areas of sandstone (Brown and Mies, 2012). The soil is characterised by high calcium content and lower content of organic carbon with quick mineralisation. In general, the surface topography is characterised by the eroded limestone bedrock and by soils deficient in organic material (Brown and Mies, 2012). Two deep ravines surround and protect this amazing protected area. Due to this phenomenon, the unique vegetation has been preserved there, headed by the largest closed stand of Dragon’s Blood Tree on the island. In spite of geographic barriers, all natural regeneration is decimated by browsing.
Scand is situated northeast of Firmihin, in the Haggeher Mountains, which are strongly dissected by deep wadis, sheltered gullies and cliffs (Brown and Mies, 2012). With 1526 m a.s.l., Scand is the highest peak of the island. According to Miller et al. (2000), the area belongs to the fifth vegetation belt where the plant is dominant. According to Habrová et al. (2007), annual mean temperature on Scand is 17.85 °C. Minimum temperature recorded on Soqotra was 8.16 °C on Scand in January, the maximum recorded was 31.96 °C in May. Annual mean relative air humidity is 80.03%. Horizontal precipitation is typical of the Scand area (higher air humidity, frequent fogs). Relative humidity is at or close to 100% for most of the period of darkness (Brown and Mies, 2012). Therefore, the crowns are narrower up to conical shape. The Haggeher Mountains represent a massif of granite. Thin and little developed soil is characterised by higher sodium content due to the presence of Riebeckite mineral. Natural regeneration is more successful in contrast with Firmihin due to the broken topography and inaccessible localities with steep slopes, which are protected against grazing and human impact (particularly in sheltered gullies, in cracks and on the peaks).
2.2. Seed collection, planting and measurement of seedlings
In total one hundred and forty seeds were collected at the turn of January and February 2011 on Firmihin (N12°28,867′; E54°0,602′) and on Scand (N12°34,592′; E54°01,642′), 70 seeds per locality. On both localities, sites of less exposed eastern slopes were chosen. Slope was 15° on Firmihin and 45° on Scand. Altitude was 580 m a.s.l. on Firmihin and 1450 m a.s.l. on Scand. The seeds have probably been lying on the ground for no more than seven days. They were collected at the slope bottom under crowns of ca. 10 trees on each locality, stored in laboratory tubes and with the permission of Yemeni nature conservation authorities they were transported to the Czech Republic. The research could not be done on the island because the observation of plants and their periodic measurement were impossible.
The two-year experiment was conducted in Brno (Czech Republic) in the period from March 2011 to June 2013. The Czech Republic has a moderate continental climate with warm, dry summers and fairly cold and snowy winters. Brno belongs among the warmest places in the country with an average annual temperature of 8.8 °C, an average annual precipitation of 493 mm and an average annual hours of sunshine of 1678 h (Climatedata.eu, 2015). One hundred high quality – large, brownish and undamaged – seeds were sown on 9 March 2011 separately into pots of 350 ml and planted under uniform conditions – constant room temperature (20–23 °C) and relative humidity (55–60%), twice-weekly watering, the same soil substrate (universal full-featured cultivation soil substrate with adjusted organic matter content and proper acidity), the same light intensity (S-facing windows, no curtains). We did not use any germinator or solution accelerating the germination.
After germination, measurements of the above-ground part were carried out at one week interval. Every new leaf was numbered and the length of each leaf was measured continuously until the end of the experiment. The seedlings were transplanted into larger pots on 22 December 2011.
2.3. Data analysis
To assess differences in germination rates and seedling mortality between the two localities (Firmihin/Scand), we used a logistic regression approach. Locality (Firmihin/Scand) was used as an explanatory variable. The growth dynamics during the whole experiment was visually explored using graphs for the length of the first leaf, length of the highest leaf, total length of all leaves and for the number of leaves with a smoothed curve added separately for Firmihin and Scand seedlings using the loess method (local polynomial regression, Cleveland et al., 1992). The significance of differences between the localities was tested at the end of the first half of the experiment and at the end of the whole experiment (week 50 and week 122) using the double sided t-test (variant with unequal variance). All statistical analyses were performed in the R statistical environment (R Core Team, 2013), graphs within ggplot2 package (Wickham, 2009).
3. Results
3.1. Germination
The seedlings germinated within a time period from four to ten weeks after sowing the seeds. The highest germination rate was observed in the fifth and sixth week (between 1 and 7 April 2011) in the case of seeds from Firmihin, and in the seventh and eighth week (between 14 and 21 April 2011) in the case of seeds from Scand. The result indicates that 73 out of 100 seeds (Firmihin/Scand) germinated under proper conditions. The germination rate was somewhat higher on Firmihin (90%) than on Scand (78%); however, this difference was not significant (p-value = 0.098). One week after germination, the first leaf started to develop and elongate.
3.2. Growth dynamics
An average increment for the whole period of measurement was 0.211 cm per week. The length of the first leaf (Fig. A.1) could not be compared with the length of the highest leaf (Fig. A.2). While the average length of the highest leaf was almost the same (24.9 cm) in the two groups (Firmihin/Scand), the average length of the first leaf was 23.5 cm for Firmihin/Scand at the end of measurement. Although the length of the first leaf (Fig. A.1) was slightly greater in the seedlings from Scand than from Firmihin in the first half of observation (until week 75 at the beginning of August 2012), the difference was not significant for week 50 (Table 1). Similarly, it was not significant at the end of the experiment when the first leaf on Firmihin was somewhat longer than on Scand. We observed a similar pattern for the height of the above-ground part of the plant expressed by the length of the highest leaf (Fig. A.2). The height of seedlings from Scand was somewhat greater throughout the period of observation, but the difference was not significant (Table 1). Significant differences between the seedlings from Firmihin/Scand were found in the number of leaves and in the sum of lengths of all leaves on one plant. The number of leaves ranged from 8 to 16 in the seedlings from Firmihin and between 8 and 12 in the seedlings from Scand at the end of measurement (Fig. A.3). On average, it was higher on Firmihin than on Scand, 12 versus 10 leaves respectively. The difference in the total number of leaves was 132 in favour of plants from Firmihin at the end of June 2013. In the case of seedlings from Scand, the number of leaves was increasing more or less constantly up to week 75 of measurement (early August 2012), slowed down in the autumn of 2012, and then increased in the spring. Compared with Scand, the process of leaf development in the plants from Firmihin was more dynamic. The seedlings from Firmihin had less leaves in the first half of the experiment, but more leaves from the second half up to the end of the experiment. This trend was very similar for the sum of the lengths of all leaves indicating that the number of leaves mostly influenced this summed leaf length (Fig. A.4). The total length of leaves was greater in the Scand plants until the end of November 2012. Two months later (in week 100), the total length between the two groups was equalled, followed by a greater total leaf length of Firmihin seedlings at the end of the two-year measurement.
Figure A.1.
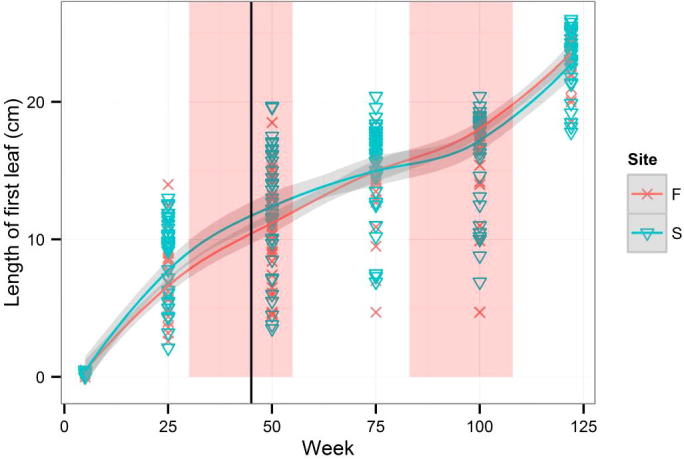
Development of the first leaf length during the whole experiment. Smoothed curves are fitted separately for Firmihin and Scand using the loess method. Grey areas show a period from the autumnal to vernal equinox. The bold black vertical line represents week 43 when the seedlings were transplanted.
Figure A.2.
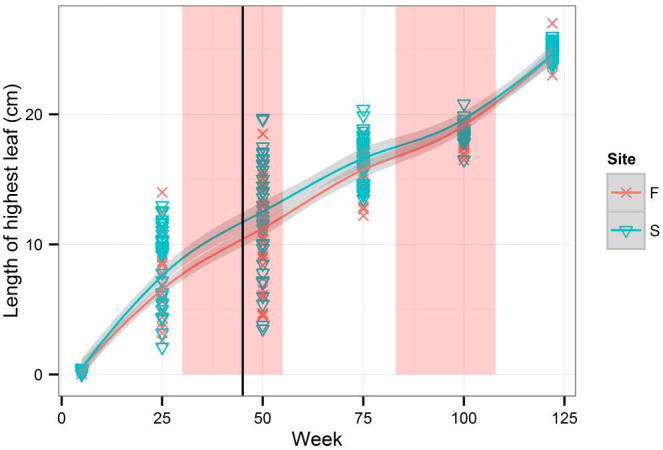
Length of the highest leaf.
Table 1.
t-Test results showing the significance of the effect of locality (Firmihin/Scand) on the measured parameters in week 50 and at the end of the experiment (week 122). The effect of locality was significant only for the length of all leaves and for the number of leaves per plant.
| Parameter | Week 50 |
Week 122 |
||||
|---|---|---|---|---|---|---|
| t | d.f. | p-Value | t | d.f. | p-Value | |
| Length of the first leaf | −1.220 | 60.0 | 0.227 | 1.599 | 58.9 | 0.115 |
| Length of the highest leaf | −1.326 | 60.7 | 0.190 | −0.258 | 70.7 | 0.797 |
| Length of all leaves | −2.942 | 51.7 | 0.005 | 4.116 | 59.0 | <0.001 |
| Number of leaves | −2.683 | 54.9 | 0.010 | 3.963 | 65.0 | <0.001 |
Figure A.3.
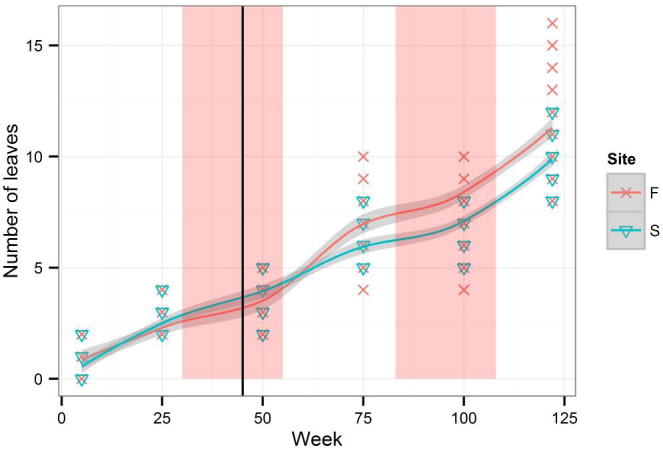
Number of leaves.
Figure A.4.
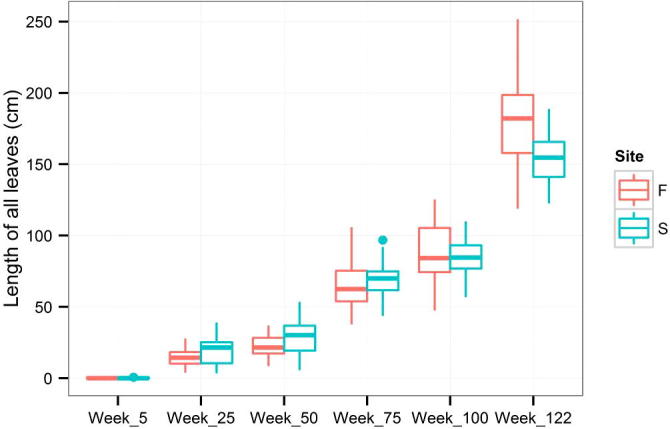
Length of all leaves.
3.3. Mortality
Out of 45 germinated seedlings from Firmihin, five died during the experiment. Out of 39 germinated seedlings from Scand, six died. This shows that the mortality was almost the same (difference was not significant with p-value = 0.568). The first seedling died about 3 months after seeding (source from Firmihin). Most of the seedlings withered during the autumn and winter of 2011, in the period from 13 October 2011 to 5 January 2012. We responded to the increasing mortality rate by transplanting the seedlings into larger pots on 22 December 2011. The last two plants withered in the summer of 2012 (plants from Scand).
4. Discussion
4.1. Germination
The highest germination was observed in the sixth and seventh week after sowing, during April 2011. The seeds from Firmihin germinated about ten days before the seeds from Scand. It could have been given by the lower vitality of seeds from Scand, which were visibly smaller than the seeds from Firmihin. Moreover, the seeds were collected from the ground, because infructescences in the crowns were already dry. The phenology of D. cinnabari is not yet known, but local Soqotrans claim that phenological phases of the species do not repeat in regular time periods. The difference in the germination rate on Firmihin (90%) and on Scand (78%) was non-significant. The slightly higher germination rate on Firmihin could have been also caused by the better seed quality. The results confirm our hypothesis 1 and the statement of Adolt and Pavliš (2004) that germination rates as high as 77% could be achieved under greenhouse conditions. Our results showed even a slightly higher germinability under controlled room conditions than in the greenhouse, particularly for Firmihin. Adolt and Pavliš (2004) used the seeds from the Diksam plateau situated at the same altitude as Firmihin. The air temperature in their greenhouse (22 °C) was similar to our in-room temperature (21 °C). The results also confirm the opinion that the germination of Dracaena seeds under artificial conditions seems to be unproblematic (Brown and Mies, 2012). We agree with Brown and Mies (2012) that germination under natural conditions is going to be significantly lower. The seeds are eaten by birds (on the other hand, scarified seeds in bird droppings may shorten dormancy). Some seeds exposed to sunlight dry out from the lack of moisture, namely those on the bare ground without a litter layer. Higher humidity in the mountain area ensures successful germination of seeds fallen into the cracks of bare rocks.
4.2. Growth dynamics
Weekly measurements of seedlings increments have not been taken before. Beyhl (1998) described the plant morphology of young plants. According to him, at the first stage of germination, little leaves remain etiolated for some days after exposure to light. According to our observations, the first leaf remains etiolated no later than 2 days after exposure to light and then turns green due to chlorophyll production and assimilation. As Beyhl (1998) states, further leaves are green since the beginning. Based on our observations, we can confidently confirm the opinion of Tomlinson and Zimmermann (1969) that the plant growth begins with a rapidly growing main axis, which is typical for monocots with the secondary growth. After germination, the new plant probably puts most of its energy into creating a storage organ in the form of underground tuber, and into terminal leaf development. Other parts of the plant body develop only after the above-described main growth has been completed. Another formation of leaves was observed in May 2011, approximately from the third week at an average length of the above-ground part being 1.7 cm. New leaves started to elongate rapidly, overgrowing the first leaf at the beginning of the growing season in 2012. This trend was more pronounced in the seedlings from Scand where the further leaves overgrew the first leaf in almost a half of the seedlings (data from week 75). In contrast, the further leaves of plants from Firmihin overgrew the first leaf in less than a quarter of plants. Nevertheless, the differences in values between the two populations proved as non-significant. The results were almost equal at the end of measurement in June 2013, when the other leaves in more than a half of the seedlings from both populations overgrew the first leaves, as seen in Fig. A.1. In view of the results, the first developed leaf wasn’t generally the highest leaf on the plant in the end of our experiment. Fig. A.2 shows the height of the above-ground part of the plant expressed by the length of the highest leaf. The seedlings from Scand were somewhat higher throughout the period of observation, but the difference was not significant. An average plant length for both populations started to be similar almost at the end of measurement between March and May 2013. The recorded outcome disproved our hypothesis 2 that the length of the highest leaf would be in favour of seedlings from Scand due to the genetic adaptation and higher plant resistance in the Scand area. The gradual equalisation in the length of the highest leaf might have been caused by the lateral development of roots in the seedlings from Scand. The development of other leaves may increase the surface area available for the condensation of water from the surrounding fogs and mists (Banfield et al., 2011) to compensate for the lacking soil moisture. Thus, the plant ensures a sufficient water supply necessary for the dissolution of substances, moving nutrients throughout the plant body, vital metabolic processes and thermoregulation (Taiz and Zeiger, 2010). Banfield et al. (2011) or Brown and Mies (2012) mentioned that the leaves are narrow, elongated and sometimes curved due to reduced solar radiation and evaporation. As shown in Fig. A.3, the increase in the number of leaves on one plant from Scand was more or less gradual, without significant fluctuations. Compared to plants from Firmihin, the number of leaves in the seedlings from Scand was slightly higher until early May 2012 in response to shorter sunshine during the period of vegetative rest. The difference in the number of leaves in favour of plants from Firmihin started to increase noticeably from the beginning of the vegetation season in the second year of measurement. Our hypothesis 3 about a higher number of leaves in the plants from Firmihin as an adaptation to drier and warmer climate and a more effective interception of horizontal precipitation was confirmed. A significant difference was found also in the sum of the lengths of all leaves on one plant (Fig. A.4). The results show the same trend as in the number of leaves. The results indicate different growth dynamics at the first stage of development. While the seedlings from Firmihin invest more energy into the construction of root system and into the wide spreading above-ground part with a large number of leaves, the plants from Scand invest more energy into faster leaf elongation rate, at the expense of root development and increased number of leaves. It should be emphasised that the plants from Scand were exposed to less favourable growth conditions compared to natural conditions, where much higher air humidity with frequent drizzles and mists is typical. According to Brown and Mies (2012), investing more energy into the construction of tougher, longer-lived leaves with a thick layer of cuticular waxes protects the plants considerably from the mechanical damage and makes them less attractive to small herbivores. Moreover, the shape of the above-ground parts of seedlings confirms the well-known theory published by Valladares and Pearcy (1998) in Brown and Mies (2012), that leaves consistently exposed to the sun are inclined at a much steeper angle to the horizontal plane than shade leaves of the same species. Hypothesis 4 about the dependence of the length increment on seasonal changes was verified. Compared to the growing season, the plants grew slower during the period of vegetative rest due to worsened ambient conditions.
4.3. Mortality
Out of 84 germinated plants, about 13% died during the period of measurement. The recorded number confirms the data published by Adolt and Pavliš (2004) and our hypothesis 5 that an average mortality rate of seedlings ex-situ is about 10%. As Brown and Mies (2012) mentioned, the survival of plants is substantially lower under natural conditions. The significant difference between Firmihin and Scand was not proven. Most of the plants died during the period of vegetative rest in the second year of observation. The higher mortality rate could have been due to a combination of several negative factors (esp. shorter sunshine duration and lower light intensity).
Despite an undeniable importance of the endemic species of Soqotra, the research on its growth dynamics is still in its infancy. Only a few studies have been devoted to the germination and growing patterns of D. cinnabari at the stages of early growth. The reason is a ban on exporting the products of nature out of the island and difficulties with ensuring a long-term stay. This research was carried out thanks to a special permission issued by The Environment Protection Authority, which is an administrative body of the Ministry of Water and Environment in Yemen. Previously published data regarding the observation and measurement of young plants are thin, the seeds originating from a single population and the seedlings being planted artificially, under regulated in-room or greenhouse conditions. Natural conditions differ from the controlled cultivation by specific features of the subtropical monsoon climate together with insular biogeography (absence of vertical precipitation for most of the year, horizontal precipitation, two monsoon seasons accompanied by rains, temperature fluctuations between day and night, much higher sunlight intensity, different soil types and bedrock, very little or no soil layer, overgrazing, etc.). The Czech research team from the Mendel University in Brno established a fenced sample plot with the Dracaena seedlings on the Dixam plateau six years ago. The ongoing measurement of seedlings at two-year intervals is a next step to elucidate the growth dynamics of young Dracaena plants in situ.
The paper demonstrates very little known facts about D. cinnabari and its distinct phases during early stages of development as well as about growth of seedlings. The species is dramatically threatened with extinction, because the natural regeneration is extremely limited by excessive goat grazing and changing environmental conditions. It is very important to study germination and growth dynamics of this highly endangered species under controlled conditions, because the pasture management is deep-rooted in the culture of local people. Artificial regeneration seems to be the only way to protect one of the oldest plant species on Earth.
Acknowledgments
This research is funded by the Internal Grant Agency of the Faculty of Forestry and Wood Technology, Mendel University in Brno, Czech Republic (IGA 37/2012). Special thanks to the Environment Protection Authority of the Republic of Yemen for their kind permission to conduct the research in the Czech Republic.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Irena Hubálková, Email: irena.hubalkova@mendelu.cz.
Petr Maděra, Email: petr.madera@mendelu.cz.
Daniel Volařík, Email: daniel.volarik@mendelu.cz.
References
- Adolt, R., 2001. Návrh zásad ochrany genofondu dračinců v lesích Sokotry a Kanárských ostrovů, in: Diploma thesis, Mendel University in Brno, Czech Republic.
- Adolt R., Pavliš J. Age structure and growth of Dracaena cinnabari populations on Socotra. Trees-Struct. Funct. 2004;18:43–53. [Google Scholar]
- Adolt R., Habrová H., Maděra P. Crown age estimation of a monocotyledonous tree species Dracaena cinnabari using logistic regression. Trees-Struct. Funct. 2012;26:1287–1298. [Google Scholar]
- Attore F., Francesconi F., Taleb N., Scholte P., Saed A., Alfo M., Bruno F. Will dragonblood survive the next period of climate change? Currentand future potential distribution of Dracaena cinnabari (Socotra, Yemen) Biol. Conserv. 2007;138:430–439. [Google Scholar]
- Banfield L., Van Damme K., Miller A.G. University Press, Cambridge; Biology of Island Floras: 2011. Evolution and Biogeography of the Flora of the Socotra Archipelago (Yemen) pp. 197–223. [Google Scholar]
- Bekele T.A. RELMA in ICRAF Project; Nairobi: 2007. Useful Trees of Ethiopia: Identification, Propagation and Management in 17 Agroecological Zones. [Google Scholar]
- Beyhl, F.E., 1996. Attempts at raising the Socotran Dragon tree, Dracaena cinnabari, outside the island, In: Dumont, H.J. (Ed.), Proceedings of the First International Symposium on Socotra Island: Present and Future. United Nations, New York, pp. 125–133.
- Beyhl, F.E., 1998. Attempts in raising the Soqotran Dragon-tree. Dracaena cinnabari Balf. fil., outside the island (Monocotyledones: Liliales: Agavaceae), In: Dumont, H.J. (Ed.), Proceedings of the First International Symposium on Soqotra Island: Present and Future. United Nations Development Programme, Conservation and Sustainable Use of Biodiversity of Soqotra Archipelago, Technical Series, Hong Kong, pp. 125–133.
- Brown G., Mies B. Springer; Netherlands: 2012. Vegetation Ecology of Socotra. [Google Scholar]
- Cheung, C., DeVantier, L., 2006. Socotra – A Natural History of the Island and their People, Odyssey Books and Guides, Airphoto International Ltd.
- Cleveland, W.S., Grosse, E., Shyu, W.M., 1992. Local regression models, in: Chambers, J.M., Hastie, T.J. (Eds.), Chapter 8 of Statistical Models in S. Wadsworth & Brooks/Cole.
- Climatedata.eu, 2015. Temperature – Precipitation – Sunshine. Styleshout & Belsoft. [2014-05-01 online at http://www.climatedata.eu/].
- Culek M., Král K., Habrová H., Adolt R., Pavliš J., Maděra P. Socotra’s annual weather pattern. In: Cheung C., Devantier L., editors. Odyssey Books and Guides, Airphoto International Ltd; 2006. pp. 42–43. (Socotra – A Natural History of the Island and their People). [Google Scholar]
- Grant, G., 2005. Socotra: Hub of the Frankincense Trade. Explorations: An Undergraduate Research Journal. [2014-01-15 online at http://undergraduatestudies.ucdavis.edu/explorations/2005/grant.pdf].
- Habrová H., Král K., Maděra P. The Weather Pattern in one of the Oldest Forest Ecosystems on Earth – Dragon’s Blood Tree Forest (Dracaena cinnabari) on Firmihin – Soqotra Island. In: Rožnovský J., Litschmann T., Vyskot I., editors. Klima lesa. Česká bioklimatologická společnost; Praha: 2007. p. 13. [Google Scholar]
- Habrová H., Čermák Z., Pavliš J. Dragon’s blood tree – threatened by overmaturity, not by extinction: Dynamics of a Dracaena cinnabari woodland in the mountains of Soqotra. Biol. Conserv. 2009;142:772–778. [Google Scholar]
- Hubálková I. Prediction of Dragon’s Blood Tree (Dracaena cinnabari Balf.) stand sample density on Soqotra Island. J. Landsc. Ecol. 2011;4:5–17. [Google Scholar]
- Koopowitz H., Kay H. Christopher Helm; London: 1990. Plant Extinction: A Global Crisis. [Google Scholar]
- Kürschner, H., Hein, P., Kilian, N., Hubaishan, M.A., 2006. Diversity and zonation of the forests and woodlands of the mountains of northern Socotra, Yemen, In: Kilian, N., Hubaishan, M.A. (Eds.), Biodiversity of Socotra: Forests, Woodlands and Bryophytes. Englera, Berlin, pp. 11 –55.
- Král K., Pavliš J. The first detailed land cover map of Socotra Island by Landsat /ETM+ data. Int. J. Remote Sens. 2006;27:3239–3250. [Google Scholar]
- Mies, B.A., Beyhl, F.E., 1996. The vegetation ecology of Soqotra. In: Dumont, H.J. (Ed.), Proceedings of the First International Symposium on Soqotra Island: Present and Future. United Nations, New York, pp. 35–82.
- Miller A.G., Morris M. Edinburgh; Royal Botanic Garden Edinburgh: 2004. Etnoflora of the Soqotra Archipelago. [Google Scholar]
- Miller M.J.S., Macnaughton W.K., Zhang X.J., Thompson J.H., Charbonnet R.M., Bobrowski P., Lao J., Trentacosti A.M., Sandoval M. Treatment of gastric ulcers and diarrhea with the Amazonian herbal medicine sangre de grado. Am. J. Physiol. Gastrointest. Liver Physiol. 2000;279:G192–G200. doi: 10.1152/ajpgi.2000.279.1.G192. [DOI] [PubMed] [Google Scholar]
- Miller A.G., Morris M., Wranik W., Knees S. Edinburgh; Royal Botanic Garden Edinburgh: 2006. Soqotra – Land of the Dragon’s Blood Tree. [Google Scholar]
- Negussie A., Aerts R., Gebrehiwot K., Muys B. Seedling mortality causes recruitment limitation of Boswellia papyrifera in northern Ethiopia. J. Arid Environ. 2008;72:378–383. [Google Scholar]
- Ogbazghi W., Rijkers T., Wessel M., Bongers F. Distribution of the frankincense tree Boswellia papyrifera in Eritrea: the role of environment and land use. J. Biogeogr. 2006;33:524–535. [Google Scholar]
- Petroncini S., Marcheselli M.P., Bruschi P. Preliminary aspects of the anatomy and genetic variability of Dracaena cinnabari Balf. fil.: environment for international development. J.Agric. Environ. Int. Dev. 2003;97:185. [Google Scholar]
- R Core Team, 2013. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. [2014-07-01 online at http://www.R-project.org/].
- Scholte P., De Geest P. The climate of Socotra Island (Yemen): a first-time assessment of the timing of the monsoon wind reversal and its influence on precipitation patterns and vegetation. J. Arid Environ. 2010;74:1507–1515. [Google Scholar]
- Scholte P., Al-okaishi A., Suleyman A.S. When conservation precedes development: a case study of the opening up of the Socotra archipelago, Yemen. Oryx. 2011;45:401–410. [Google Scholar]
- Symon D.E. The Growth of Dracaena draco – Dragon’s Blood Tree. J. Arnold Arboretum. 1974;55:51–58. [Google Scholar]
- Taiz L., Zeiger E. Sinauer Associates; Sunderland: 2010. Plant Physiology. [Google Scholar]
- Tomlinson P.B. Monocotyledons – towards an understanding of their morphology and anatomy. Adv. Bot. Res. 1970;3:207–292. [Google Scholar]
- Tomlinson P.B., Zimmermann M.H. Vascular anatomy of monocotyledons with secondary growth – an introduction. J. Arnold Arboretum. 1969;50:159–179. [Google Scholar]
- Valladares F., Pearcy R.W. The functional ecology of shoot architecture in sun and shade plants of Heteromelesarbutifolia M. Roem., a Californian chaparral shrub. Oecologia, 114, pp. 1–10. In: Brown G., Mies B., editors. Springer; Netherlands: 1998. (Vegetation Ecology of Socotra). [DOI] [PubMed] [Google Scholar]
- Van Damme K., Banfield L. Past and present human impacts on the biodiversity of Socotra Island (Yemen): implications for future conservation. Biodiversity Conservation in the Arabian Peninsula. In: Knight M., Mallon D., Seddon P., editors. Zoology in the Middle East. Kasparek Verlag; Heidelberg: 2011. pp. 31–88. [Google Scholar]
- Wickham H. Springer; New York: 2009. Ggplot2: elegant graphics for data analysis. [Google Scholar]
- Wright H. Royal Botanic Garden Edinburgh; Peradeniya, Edinburgh: 1901. Observations on Dracaena reflexa Lam, Ann. pp. 165–172. [Google Scholar]


