Abstract
Oral administration of berberine chloride to mice induced an obvious enhancement in jejunal health status as expressed by the significant reduction of apoptotic cells within the intestinal villi from 15.5 to 8.3 apoptotic cell/10 VCU. In addition, jejunal antioxidant biomarkers were significantly improved as revealed by the increase in the activities of catalase and glutathione peroxidase enzymes with a concurrent increase in reduced glutathione levels and total antioxidant capacity. Also, it was associated with a significant decrease in oxidative damage biomarkers of hydrogen peroxides, malondialdehyde, nitrite/nitrate, inducible nitric oxide synthase and protein carbonyl content. Moreover, BBR treatment induced a reduction in the pro-inflammatory cytokine, TNF-α by about 40%. It is highly recommended to use berberine as food supplements or as natural drug therapy to enhance the antioxidant status within the intestinal tissue.
Keywords: Mice, Berberine, Oxidative damage, Antioxidants, Apoptosis
1. Introduction
Normal human and animal diet contains a wide range of various pro-oxidants formed during food preparation for cooking. It includes oxidized polyunsaturated fatty acids, oxysterols, nitrites, nitrates, heavy metals, mycotoxins and many other organic pollutants. These harmful substances are found in food in low levels; however, various combinations of these pro-oxidants in food are considered to be the source of free radical production in the gastrointestinal tract (GIT). Therefore effective antioxidant protection in the GIT is needed to maintain gut health and this protection is based on food derived or supplemented antioxidants (Surai et al., 2003, Surai et al., 2004).
Berberine is an isoquinoline alkaloid, present in root and shoot systems of clinically important medicinal plants (Timothy et al., 1997). Berberine based products are widely applied in traditional medicine (Wongbutdee, 2008). It has been known to have a wide range of pharmacological activities due to its non-toxic effect on humans (Vuddanda et al., 2010).
Many studies were carried out to use berberine against several diseases for the in vivo mammalian system (Dkhil, 2014, Malik et al., 2014, Dkhil et al., 2015). Moreover, Previous studies showed that, berberine has a wide range of antioxidant activities including activation of the antioxidant enzyme (Siow et al., 2011).
This study aims to test the effect of berberine treatment in enhancing the antioxidant status within mouse jejunum tissue.
2. Materials and methods
2.1. Animals
Twelve male Swiss albino mice, Mus musculus (9–11 weeks) were allocated randomly into two groups with 6 mice per group. The first group is considered as the control group which received saline. The second group was daily treated with 10 mg/kg berberine chloride (Sigma Company, St. Louis, MO, USA) for five successive days via oral gavaging using an epigastric tube. The experiment was approved by state authorities of Zoology Department at King Saud University. The used dose is in agreement with previous studies on cytotoxicity measurements (Timothy et al., 1997, Jahnke et al., 2006).
2.2. Sample collection
Animals were killed by cervical dislocation at day 5 p.i. Jejunum tissue was separated and washed several times with ice cold sterile phosphate buffer saline and divided into different portions.
2.3. Immunohistochemical staining of apoptosis and apoptotic score
Jejunum was isolated and cut into small pieces, then fixed in 10% neutral buffered formalin. After fixation, specimens were dehydrated in ethanol then, embedded in paraffin wax. Jejunum was sectioned to 5 μ thickness. Jejunum sections were tested for apoptotic changes in their villi using the TUNEL Apoptosis detection kit (GenScript, USA). The test is based on detection of fragmented DNA in the nucleus during apoptosis. Biotinylated nucleotide is labeled at the DNA 3′-OH ends using terminal deoxynucleotidyl transferase (TDT). Then, horseradish peroxidase labeled streptavidin (Streptavidin-HRP) is bound to these biotinylated nucleotides, which detect the peroxidase substrate, H2O2, and 3,3′-diaminobenzidine (DAB) solution staining the apoptotic nuclei dark brown color. Sections were then counterstained with hematoxylin solution for 1 min before the final processing of slides (Dkhil et al., 2013). Apoptotic score was determined via counting the average number of apoptotic cells for ten better-orientated villous crypt units (VCU). Results were presented as the mean number of apoptotic cells per ten VCU.
2.4. Preparation of tissue homogenate
Pieces of jejunum were weighed then immediately homogenized in ice-cold phosphate buffered saline then centrifuged at 2000g × 15 min at 4 °C to give a final yield of (10% w/v) jejunal homogenates that were kept at −20 °C until use.
2.5. Oxidative damage biomarkers in jejunal homogenates
Oxidized proteins were determined via the estimation of protein carbonyl content through the formation of labeled protein hydrazone derivatives using 2,4-dinitro phenyl hydrazine (Levine et al., 1990), while, malondialdehyde was determined via the reaction with thiobarbituric acid in an acidic medium and high temperature yielding a colored product with a color intensity directly proportional to the amount of MDA at 534 nm (Ohkawa et al., 1979). In addition, hydrogen peroxide level was measured after the reaction with peroxidase enzyme, and with 3,5-dichloro-2-hydroxybenzensulfonic acid and 4-aminophenazone to form a chromophore with color intensity directly proportional to the amount of hydrogen peroxides in the test samples at 540 nm (Aebi, 1984). Moreover, nitrite/nitrate levels were determined via the formation of nitrous acid diazotise sulfanilamide in an acidic medium and the products were coupled with N-(1-naphthyl) ethylenediamine. The formed azo dye appeared bright reddish-purple in color that can be measured at 540 nm (Berkels et al., 2004).
TNF-α level and iNOs activity were determined using antigen antibody interaction in 96-microplate wells coated with specific monoclonal antibodies. Tumor necrosis factor-α was detected via solid phase sandwich enzyme linked immunosorbent assay, while inducible nitric oxide synthase activity was determined via competitive enzyme immunoassay (ALPCO Diagnostics, USA).
2.6. Antioxidant biomarkers in jejunal homogenates
Jejunal reduced glutathione level was determined colorimetrically via precipitation of proteins using tungstate sulfuric acid solution and the formation of a yellow color after reaction with DNTB (Prins and Loose, 1969), while, the activity of glutathione peroxidase was determined kinetically depending on the catalyzing potential of GPX to reduce H2O2 and to oxidize reduced glutathione forming oxidized glutathione (GSSG) which in turn is reduced by glutathione reductase and NADPH forming NADP+ resulting in a decreased absorbance of solution at 340 nm. This decrease in absorption is directly proportional to GPX activity (Paglia and Valentine, 1967). Also, total antioxidant capacity of jejunum tissue was determined colorimetrically via its activity to eliminate certain amounts of exogenously added H2O2. Residual amounts of H2O2 are enzymatically determined yielding colored products (Koracevic et al., 2001). To determine the catalase activity, we used the method of Aebi (1984) which is based on the catalytic activities of catalase on a known amount of H2O2 for a certain time, and then reaction is stopped using a catalase inhibitor. The remaining amounts of H2O2 are determined colorimetrically in the presence of peroxidase enzyme and DHBS and aminophenazone to form a chromophore with the color intensity inversely proportional to the amount of catalase in the original sample.
2.7. Statistical analysis
Statistical analysis was carried out by using an unpaired Student’s t-test. The obtained data were analyzed by MS Excel 2007 (Microsoft, Rochester, NY, USA) and SigmaPlot 2011 (Systat Software, Inc., Chicago, IL, USA).
3. Results
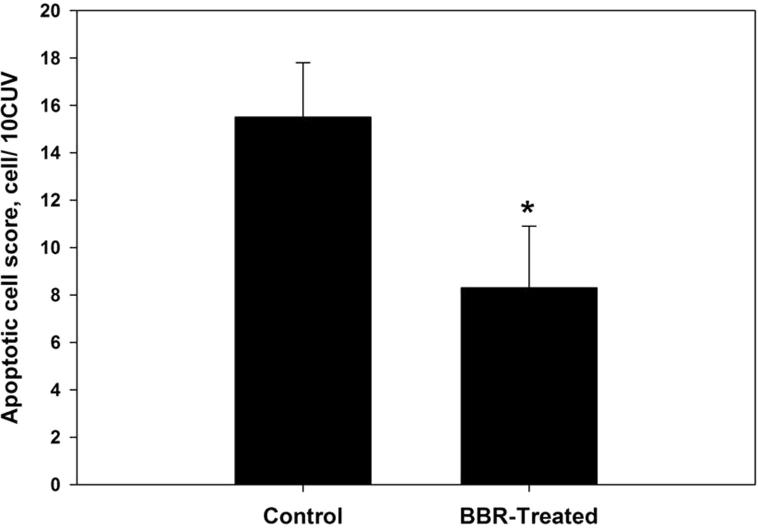
Berberine treatment caused a significant reduction in the number of apoptotic cells (P ⩽ 0.05) within the jejunum tissue from 15.5 ± 2.3 to 8.3 ± 2.6 apoptotic cell/10 VCU (Figs. 1 and S1).
Figure 1.
Berberine induced reduction of apoptotic cell number within jejunum villous tissue in laboratory mice, Mus musculus. All values are mean ± SD. *Significant against control non-treated group at P ⩽ 0.05.
Upon oral administration of berberine to mice, a great enhancement of the antioxidant system within the jejunum tissue occurred. The total antioxidant capacity of jejunum tissues was raised by more than two fold (Table 1). Also, reduced glutathione level was increased to 1.8 μmol/g (Table 1). In addition, the antioxidant enzyme GPX was duplicated (Table 1) and that of CAT activity was increased by more than two fold (Table 1).
Table 1.
Effect of berberine administration on jejunal glutathione peroxidase activity, catalase activity and reduced glutathione and total antioxidant capacity in laboratory mice.
| Glutathione peroxidase (mU/g) | Reduced glutathione (μmol/g) | Catalase (U/g) | Total antioxidant capacity (mM/g) | |
|---|---|---|---|---|
| Control | 86.5 ± 12.1 | 1.3 ± 0.15 | 0.3 ± 0.07 | 1.2 ± 0.16 |
| BBR-treated | 147.8 ± 10.9⁎ | 1.8 ± 0.11⁎ | 0.79 ± 0.25⁎ | 2.6 ± 0.3⁎ |
All values are mean ± SD.
Significant against non-treated control group at P ⩽ 0.05.
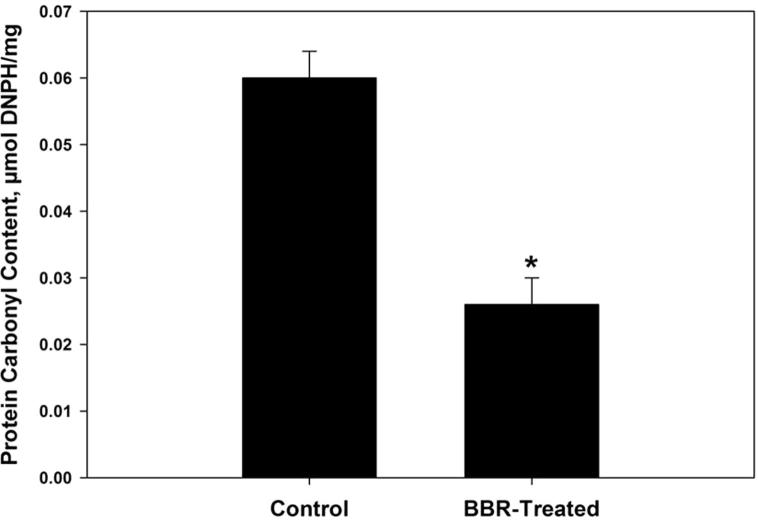
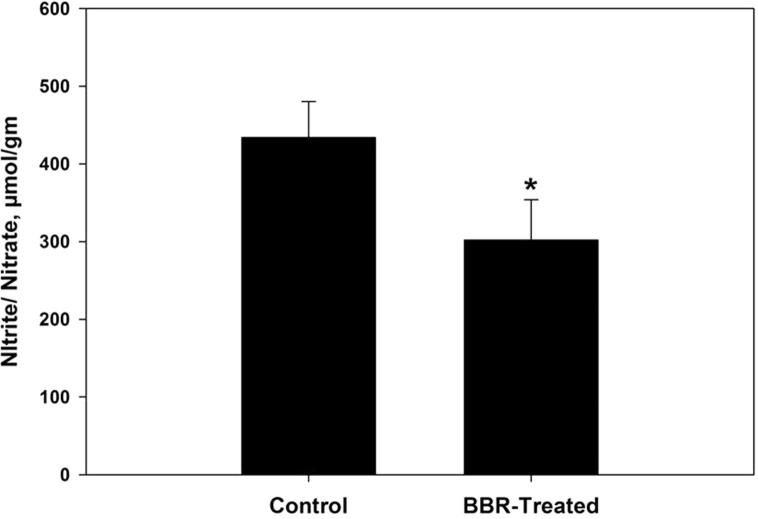
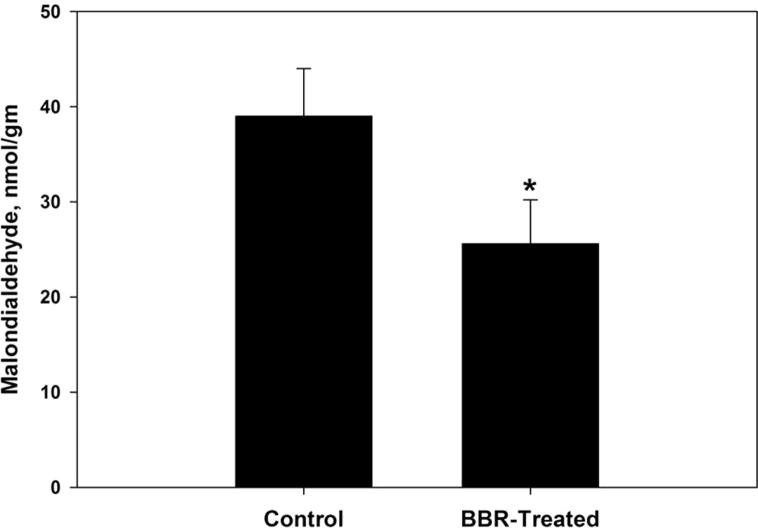
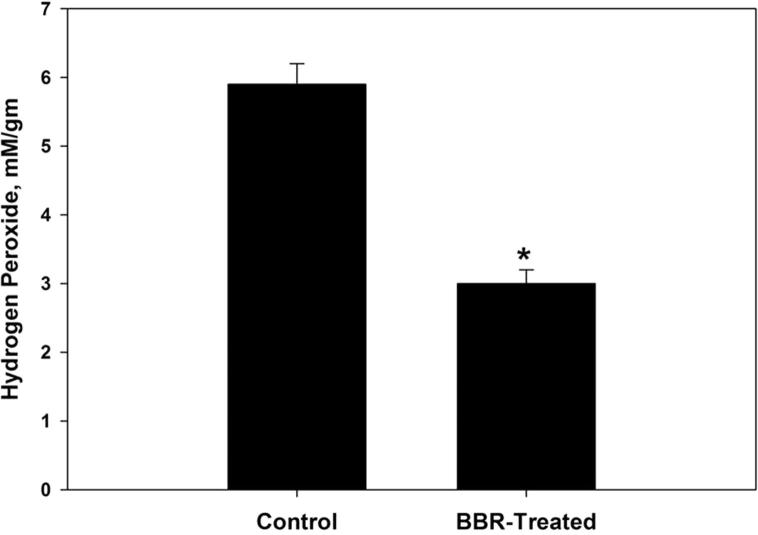
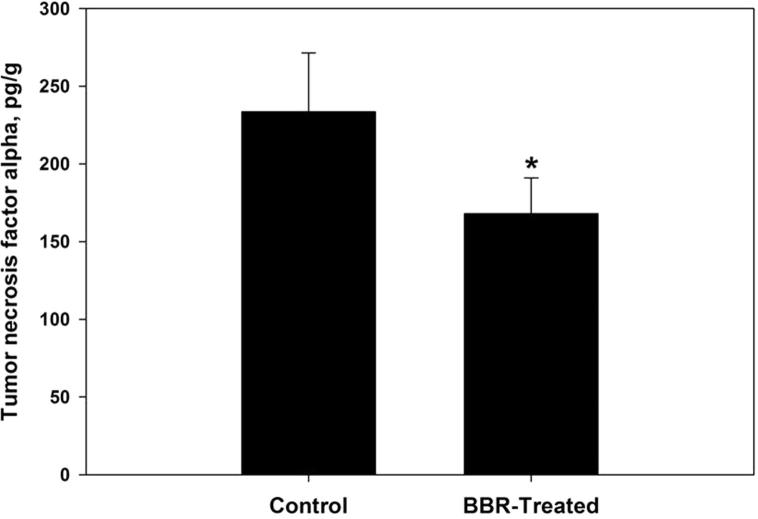
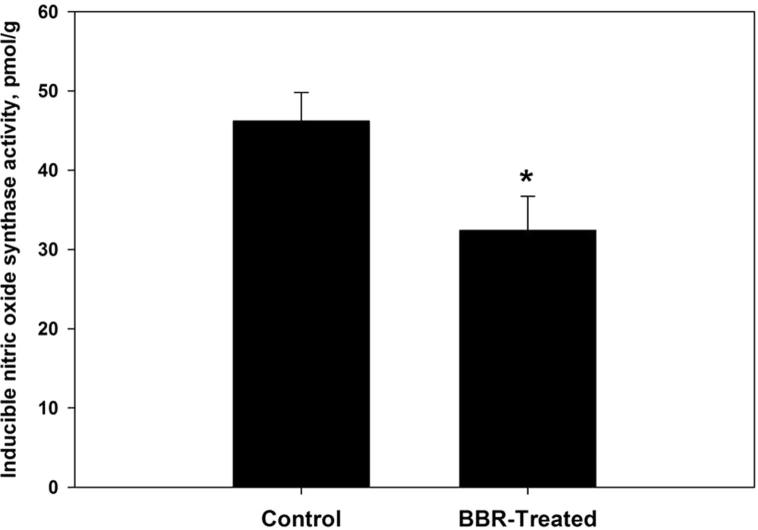
Oxidative damage biomarkers in the infected jejunum tissue were significantly decreased after berberine treatment. Oxidized protein level was decreased by more than 50% (Fig. 2). In addition, nitrite/nitrate level was reduced to 302 ± 52 μmol/g (Fig. 3). Malondialdehyde and hydrogen peroxide contents were decreased to 25.6 nmol/g and 3.0 mM/g, respectively after berberine treatment (Figure 4, Figure 5). Also, the activity of the pro-inflammatory cytokine (TNF-α) and inducible nitric oxide synthase (iNOs) was decreased from 233.6 pg/g and 46.2 pmol/g to 168 pg/g and 32.4 pmol/g, respectively (Figure 6, Figure 7).
Figure 2.
Berberine induced reduction of oxidized protein level within jejunum tissue of laboratory mice, Mus musculus. All values are mean ± SD. *Significant against control non-treated group at P ⩽ 0.05.
Figure 3.
Berberine induced reduction of nitrite/nitrate level within jejunum tissue of laboratory mice, Mus musculus. All values are mean ± SD. *Significant against control non-treated group at P ⩽ 0.05.
Figure 4.
Berberine induced reduction of lipid peroxidation products within jejunum tissue of laboratory mice, Mus musculus. All values are mean ± SD. *Significant against control non-treated group at P ⩽ 0.05.
Figure 5.
Berberine induced reduction of hydrogen peroxide level within jejunum tissue of laboratory mice, Mus musculus. All values are mean ± SD. *Significant against control non-treated group at P ⩽ 0.05.
Figure 6.
Berberine induced reduction of the pro-inflammatory cytokine (TNF-α) level within jejunum tissue of laboratory mice, Mus musculus. All values are mean ± SD. *Significant against control non-treated group at P ⩽ 0.05.
Figure 7.
Berberine induced reduction of inducible nitric oxide synthase activity within jejunum tissue of laboratory mice, Mus musculus. All values are mean ± SD. *Significant against control non-treated group at P ⩽ 0.05.
4. Discussion
Pro-oxidants cannot be avoided in our food but we need to make sure that they are made by consumption of naturally formed antioxidants (Surai et al., 2004). Antioxidants are essential for the gastrointestinal tract (GIT) to prevent lipid peroxidation. Also, antioxidant enzymes are needed in the intestinal mucosa to fight the induced oxidative damage (Srigiridhar and Nair, 1997). Disturbance of the antioxidant system of the GIT could lead to various diseases as cells become more susceptible to infections by various microorganisms or the loss of their function in food absorption.
Experimental mice treated with berberine showed a great enhancement in the antioxidant status of their intestinal tissue. The activity of glutathione peroxidase and catalase enzymes was elevated, the glutathione level reduced and the total antioxidant capacity was also increased (Table 1). Also, lipid peroxidation and protein oxidation processes were significantly diminished as revealed by the decreased amounts of malondialdehyde and protein carbonyl contents after berberine treatment (Figure 2, Figure 4. Moreover, hydrogen peroxide level was significantly reduced by about half of its value in control non-treated mice (Fig. 5). Finally, berberine effectively down-regulated nitric oxide pathway of inflammation as revealed by the decreased amounts of the pro-inflammatory cytokine (TNF-α) (Fig. 6), decreased activity of inducible nitric oxide synthase enzyme (Fig. 7) and reduced nitrite/nitrate levels within the jejunum tissue (Fig. 3).
Berberine was found to have a great capacity for quenching different free radicals such as DPPH, O2−, NO− and OH− (Hwang et al., 2006, Siow et al., 2011). It could decrease the formation of malondialdehyde in cultured rat hepatocytes with prevention capacity on the depletion of glutathione (Altman et al., 1994, Hwang et al., 2006, Thirupurasundari et al., 2009). It also caused downregulation of the gene expression ratio of inflammatory biomarkers such as TNF-α and iNOs (Choi et al., 2006, Kim et al., 2007, Kim et al., 2008, Lou et al., 2011, Domitrović et al., 2013). In addition, berberine has a wide range of antioxidant activities including activation of the antioxidant enzyme systems of SOD, CAT, GST and GPX, with conservation activities of non-enzymatic antioxidant levels (Siow et al., 2011, Domitrović et al., 2013).
BBR protective activities against oxidative and cytotoxic damage occur via activation and/or suppression of different cellular pathways. It could inhibit inducible cyclooxygenase-2 (COX-2) over production through the activation of Peroxisome proliferator-activated receptor-gamma (PPARγ) pathway (Feng et al., 2012), and suppress β-phosphorylation processes leading to diminished production of IL-1β and TNF-α (Lee et al., 2007). In addition, its inhibitory effect of nitric oxide production and iNOs expression occurs via AMP-activated protein kinase sensitive pathway (Siow et al., 2011). It was also found to inhibit ROS production through the inhibition of NADPH-oxidase mediated O2− release (Siow et al., 2011). Moreover, it caused the activation of redox sensing transcription factor Nrf2 and inhibition of NF-κβ dependent proinflammatory mediator’s production of iNOs, COX-2 and TNF-α (Chitra et al., 2013).
Apoptosis in the gastro intestinal system is vitally essential, where constant and rapid renewal of intestinal cells is in progress providing normal function holding tissue homeostasis. In addition, many factors contribute to apoptosis including the elevated reactive oxygen and nitrogen intermediates and inflammatory cytokines within cells (Ramachandran et al., 2000, Major et al., 2011).
Results of the current study show that berberine treatment of normal mice induced protection of villous epithelial cells from apoptotic changes as it caused a decrease in the number of apoptotic cells by about 50% (Figs. 1 and S1). This prevention of apoptosis may be due to the downregulatory effects of berberine on Bax/Bcl-2 gene expression ratio (Chueh and Lin, 2012a, Chueh and Lin, 2012b, Domitrović et al., 2013, Malik et al., 2014).
Collectively, our data show that berberine treatment induced a significant enhancement of intestinal health in laboratory mice as revealed by decreasing apoptotic changes and reducing oxidative damage biomarkers with great activation of antioxidant biomarkers. So, it is recommended to use berberine in food or drug mixtures to enhance the intestinal health status.
Acknowledgement
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for its funding this research group No (RG-198).
Footnotes
Peer review under responsibility of King Saud University.
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.sjbs.2015.10.012.
Appendix A. Supplementary data
Supplementary Fig. S1.
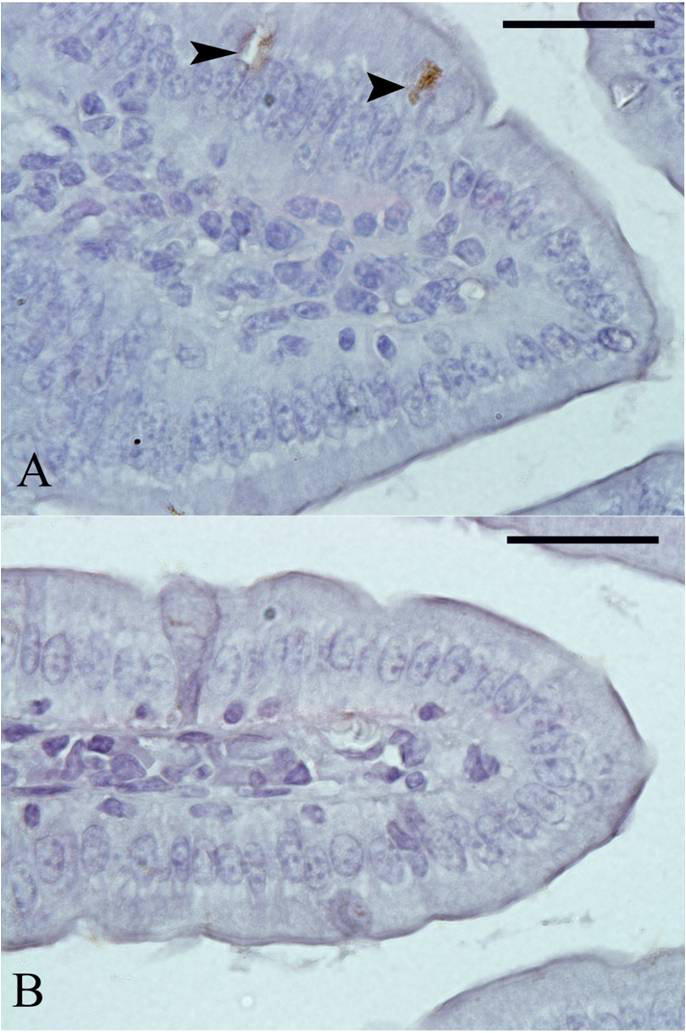
Berberine induced reduction of apoptosis within jejunum tissue of laboratory mice, Mus musculus. (A) Control untreated jejunum. (B) Berberine treated mice jejunum. The arrow head refers to apoptotic cells. Sections were stained with TUNEL Apoptosis method. Scale bar = 50 μm
References
- Aebi H.U. Academic press; New York: 1984. Methods in Enzymatic Analysis; pp. 121–126. [Google Scholar]
- Altman S.A., Zastawny T.H., Randers L., Lin Z., Lumpkin J.A., Remacle J., Dizdaroglu M., Rao G. tert-Butyl hydroperoxide-mediated DNA base damage in cultured mammalian cells. Mutat. Res. 1994;306:35–44. doi: 10.1016/0027-5107(94)90165-1. [DOI] [PubMed] [Google Scholar]
- Berkels R., Purol-Schnabel S., Roesen R. Measurement of nitric oxide by reconversion of nitrate/nitrite to NO. Methods Mol. Biol. 2004;279:1–8. doi: 10.1385/1-59259-807-2:001. [DOI] [PubMed] [Google Scholar]
- Chitra P., Saiprasad G., Manikandan R., Sudhandiran G. Berberine attenuates bleomycin induced pulmonary toxicity and fibrosis via suppressing NF-B dependant TGF-B activation: a biphasic experimental study. Toxicol. Lett. 2013;219:178–193. doi: 10.1016/j.toxlet.2013.03.009. [DOI] [PubMed] [Google Scholar]
- Choi B.H., Ahn I.S., Kim Y.H., Park J.W., Lee S.Y., Hyun C.K., Do M.S. Berberine reduces the expression of adipogenic enzymes and inflammatory molecules of 3T3-L1 adipocyte. Exp. Mol. Med. 2006;38:599–605. doi: 10.1038/emm.2006.71. [DOI] [PubMed] [Google Scholar]
- Chueh W.H., Lin J.Y. Protective effect of isoquinoline alkaloid berberine on spontaneous inflammation in the spleen, liver and kidney of non-obese diabetic mice through downregulating gene expression ratios of pro-/anti-inflammatory and Th1/Th2 cytokines. Food Chem. 2012;131:1263–1271. [Google Scholar]
- Chueh W.H., Lin J.Y. Berberine, an isoquinoline alkaloid, inhibits streptozotocin-induced apoptosis in mouse pancreatic islets through down-regulating Bax/Bcl2 gene expression ratio. Food Chem. 2012;132:252–260. doi: 10.1016/j.foodchem.2011.10.065. [DOI] [PubMed] [Google Scholar]
- Dkhil M.A. Role of berberine in ameliorating Schistosoma mansoni-induced hepatic injury in mice. Biol. Res. 2014;47(1):8. doi: 10.1186/0717-6287-47-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dkhil M.A., Delic D., Al-Quraishy S. Goblet cells and mucin related gene expression in mice infected with Eimeria papillata. Sci. World J. 2013;2013 doi: 10.1155/2013/439865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dkhil M.A., Metwaly M.S., Al-Quraishy S., Sherif N.E., Delic D., Al Omar S.Y., Wunderlich F. Anti-Eimeria activity of berberine and identification of associated gene expression changes in the mouse jejunum infected with Eimeria papillata. Parasitol Res. 2015;114:1581–1593. doi: 10.1007/s00436-015-4344-z. [DOI] [PubMed] [Google Scholar]
- Domitrović R., Cvijanović O., Pernjak-Pugel E., Skoda M., Mikelić L., Crnčević-Orlić Z. Berberine exerts nephroprotective effect against cisplatin-induced kidney damage through inhibition of oxidative/nitrosative stress, inflammation, autophagy and apoptosis. Food Chem. Toxicol. 2013;62:397–406. doi: 10.1016/j.fct.2013.09.003. [DOI] [PubMed] [Google Scholar]
- Feng A.W., Gao W., Zhou G.R., Yu R., Li N., Huang X.L., Li Q.R., Li J.S. Berberine ameliorates COX-2 expression in rat small intestinal mucosa partially through PPARγ pathway during acute endotoxemia. Int. Immunopharmacol. 2012;12:182–188. doi: 10.1016/j.intimp.2011.11.009. [DOI] [PubMed] [Google Scholar]
- Hwang J.M., Kuo H.C., Tseng T.H., Liu J.Y., Chu C.Y. Berberine induces apoptosis through a mitochondria/caspases pathway in human hepatoma cells. Arch. Toxicol. 2006;80:62–73. doi: 10.1007/s00204-005-0014-8. [DOI] [PubMed] [Google Scholar]
- Jahnke G.D., Price C.J., Marr M.C., Myers C.B., George J.D. Developmental toxicity evaluation of berberine in rats and mice. Birth Defects Res. B Dev. Reprod. Toxicol. 2006;77:195–206. doi: 10.1002/bdrb.20075. [DOI] [PubMed] [Google Scholar]
- Kim S., Kim Y., Kim J.E., Cho K.H., Chung J.H. Berberine inhibits TPA-induced MMP-9 and IL-6 expression in normal human keratinocytes. Phytomedicine. 2007;15:340–347. doi: 10.1016/j.phymed.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Kim S., Choi J.H., Kim J.B., Nam S.J., Yang J.H., Kim J.H., Lee J.E. Berberine suppresses TNF alpha-induced MMP-9 and cell invasion through inhibition of AP-1 activity in MDA-MB-231 human breast cancer cells. Molecules. 2008;13:2975–2985. doi: 10.3390/molecules13122975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koracevic D., Koracevic G., Djordjevic V., Andrejevic S., Cosic V. Method for the measurement of antioxidant activity in human fluids. J. Clin. Pathol. 2001;54:356–361. doi: 10.1136/jcp.54.5.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C.H., Chen J.C., Hsiang C.Y., Wu S.L., Wu H.C., Ho T.Y. Berberine suppresses inflammatory agents-induced interleukin-1beta and tumor necrosis factor-alpha productions via the inhibition of I-kappaβ degradation in human lung cells. Pharmacol. Res. 2007;56:193–201. doi: 10.1016/j.phrs.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Levine R.L., Garland D., Oliver C.N., Amici A., Climent I., Lenz A.G., Ahn B.W., Shaltiel S., Stadtman E.R. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990;186:464–478. doi: 10.1016/0076-6879(90)86141-h. [DOI] [PubMed] [Google Scholar]
- Lou T., Zhang Z., Xi Z., Liu K., Li L., Liu B.A.N.D., Huang F. Berberine inhibits inflammatory response and ameliorates insulin resistance in hepatocytes. Inflammation. 2011;34:659–667. doi: 10.1007/s10753-010-9276-2. [DOI] [PubMed] [Google Scholar]
- Major P., Revajová V., Levkut M., Ševčíková Z., Spišáková V., Faixová Z., Levkutová M., Kožárová I., Goldová M.A.N.D., Levkut M. Intestinal mucin dynamic and leukocytic responses of chickens infected with Eimeria acervulina and fed oregano supplemented diet. Acta Vet. Brno. 2011;80:147–156. [Google Scholar]
- Malik T.A., Kamili A.N., Chishti M.Z., Tanveer S., Ahad S., Johri R.K. In vivo anticoccidial activity of berberine [18, 5,6-dihydro-9,10-dimethoxybenzo(g)-1,3-benzodioxolo(5,6-a) quinolizinium] – an isoquinoline alkaloid present in the root bark of Berberis lycium. Phytomedicine. 2014;21(5):663–669. doi: 10.1016/j.phymed.2013.11.005. [DOI] [PubMed] [Google Scholar]
- Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Paglia D.E.A.N.D., Valentine W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967;70:158–169. [PubMed] [Google Scholar]
- Prins G.K., Loose J.A. Biochemical methods in red blood cell genetics. Academic Press; N.Y.D, London: 1969. Glutathione; pp. 126–129. (Chapter 4) [Google Scholar]
- Ramachandran A., Muniswamy M., Balasubramanian K. Apoptosis in the intestinal epithelium: its relevance in normal and pathophysiological conditions. J. Gastroenterol. Hepatol. 2000;15:109–120. doi: 10.1046/j.1440-1746.2000.02059.x. [DOI] [PubMed] [Google Scholar]
- Siow Y.L., Sarna L., Karmin O. Redox regulation in health and disease, therapeutic potential of berberine, a review. Food Res. Int. 2011;44:2409–2417. [Google Scholar]
- Srigiridhar K.A.N.D., Nair K.M. Protective effects of antioxidant enzymes and GSH in vivo on iron mediated lipid peroxidation in gastrointestinal tract of rat. Indian J. Biochem. Biophys. 1997;34:402–405. [PubMed] [Google Scholar]
- Surai K.P., Surai P.F., Speake B.K.A.N.D., Sparks N.H.C. Antioxidant-prooxidant balance in the intestine: food for through 1. Prooxidants. Nutr. Genomics Funct. Foods. 2003;1:51–70. [Google Scholar]
- Surai K.P., Surai P.F., Speake B.K.A.N.D., Sparks N.H.C. Antioxidant-prooxidant balance in the intestine: food for through 2. Antioxidants. Curr. Top. Nutraceutical Res. 2004;2:27–46. [Google Scholar]
- Thirupurasundari C.J., Padmini R., Devaraj S.N. Effect of berberine on the antioxidant status, ultrastructural modifications and protein bound carbohydrates in azoxymethane-induced colon cancer in rats. Chem. Biol. Interact. 2009;177:190–195. doi: 10.1016/j.cbi.2008.09.027. [DOI] [PubMed] [Google Scholar]
- Timothy C., Birdsall N.D., Gregory S., Kelly N.D. Berberine: therapeutic potential of an alkaloid found in several medicinal plants. Altern. Med. Rev. 1997;2:94–103. [Google Scholar]
- Vuddanda P.R., Chakraborty S., Singh S. Berberine: a potential phytochemical with multispectrum therapeutic activities. Expert Opin. Invest. Drugs. 2010;19:1297–1307. doi: 10.1517/13543784.2010.517745. [DOI] [PubMed] [Google Scholar]
- Wongbutdee J. Physiological effects of berberine. Review article. Thai Pharm. Health Sci. J. 2008;4:78–83. [Google Scholar]