Abstract
Learning to respond optimally under a broad array of environmental conditions is a critical brain function that requires engaging the cognitive systems that are optimal for solving the task at hand. Serotonin is implicated in learning and decision-making, but the specific functions of serotonin in system-level cognitive control remain unclear. Across 3 studies, we examined the influence of a polymorphism within the promoter region of the serotonin transporter gene (5-HTTLPR polymorphism in SLC6A4 ) on participants' ability to engage the task appropriate cognitive system when the reflexive (Experiments 1 and 2) or the reflective (Experiment 3) system was optimal. Critically, we utilized a learning task for which all aspects remain fixed with only the nature of the optimal cognitive processing system varying across experiments. Using large community samples, Experiments 1 and 2 (screened for psychiatric diagnosis) found that 5-HTTLPR S/L G allele homozygotes, with putatively lower serotonin transport functionality, outperformed L A allele homozygotes in a reflexive-optimal learning task. Experiment 3 used a large community sample, also screened for psychiatric diagnosis, and found that 5-HTTLPR L A homozygotes, with putatively higher serotonin transport functionality, outperformed S/L G allele homozygotes in a reflective-optimal learning task.
Keywords: category learning, decision-making, hypothesis-testing, prefrontal cortex, procedural learning, striatum
Introduction
Learning to make the optimal decision under a broad array of environmental conditions is one of the most important of brain functions and an important aspect of navigating our everyday lives. Optimal learning and decision-making requires selecting the most advantageous behavior from a suite of choices and depends upon a number of factors including the rewards and punishments associated with each behavioral choice, as well as one's ability to engage the cognitive systems that are optimal for solving the task. Serotonin has long been implicated in learning and decision-making, but the specific function of serotonin in system-level cognitive control remains unclear ( Cools et al. 2011 ; Seymour et al. 2012 ).
Dissociable systems subserving optimal learning and decision-making have been identified. One highly successful multiple systems approach is the COmpetition between Verbal and Implicit System (COVIS) model ( Ashby et al. 1998 , 2011 ; Ashby and Maddox 2005 , 2010 ). COVIS assumes 2 systems, 1 reflective and 1 reflexive that are dissociable and are thought to be competitive with each generating a preferred output on each trial, and the more confident of the 2 outputs driving the response ( Ashby et al. 1998 ; Paul and Ashby 2014 ). The reflective system uses working memory and executive attention to develop and test explicit rules and is hypothesized to primarily involve the prefrontal cortex, the anterior cingulate cortex, and the medial temporal lobe ( Filoteo et al. 2005 ; Seger and Cincotta 2006 ; Nomura et al. 2007 ). These brain regions interact with high-level sensory processing areas during learning and contribute to the generation, selection, and maintenance of explicit rules ( Seger and Cincotta 2006 ). In contrast, the reflexive learning system is reliant on the striatum (e.g., putamen) and motor areas (e.g., supplementary motor area) and operates by implicitly associating high-level perceptual representations with actions that lead to immediate reward ( Seger and Cincotta 2002 , 2005 ; Nomura et al. 2007 ; Yi et al. 2014 ) (the reflective system is also referred to as the hypothesis-testing system, and the reflexive system is also referred to as the procedural-based learning system [ Ashby et al. 1998 ; Ashby and Maddox 2010 ]).
In addition to the strong neuroscience evidence cited here, a large body of behavioral support for the 2 systems exists. These data come in the form of a series of behavioral dissociations ( Maddox and Ashby 2004 ; Ashby and Maddox 2005 , 2010 ). For example, increasing the working memory load or reducing the time available to process the feedback adversely affects learning in the reflective, but not the reflexive system ( Waldron and Ashby 2001 ; Maddox, Ashby et al. 2004 ; Zeithamova and Maddox 2006 ; Filoteo et al. 2010 ). Analogously, manipulations of the motor mapping between category label and response location adversely affect learning in the reflexive, but not the reflective system ( Ashby et al. 2003 ; Maddox, Bohil et al. 2004 ; Spiering and Ashby 2008 ). Because these systems are interactive, in many cases enhanced performance in one system is associated with attenuated performance in the other ( Ashby et al. 1998 ; DeCaro et al. 2008 ; Maddox et al. 2008 ; Filoteo et al. 2010 ).
A detailed understanding of serotonin's involvement in system-level control is important for understanding day-to-day cognitive function in healthy individuals but is also critical to our understanding of psychiatric disorders, such as depression. Prior comprehensive and integrative reviews of the relationship between serotonin and these 2 modes of information processing indicate that lower serotonin function is associated with a more dominant reflexive processing system that performs well when reflexive processing is optimal, but because of the competitive nature of the 2 systems can impair performance when reflective processing is required ( Clarke et al. 2004 ; Carver et al. 2008 ; Eskenazi and Neumaier 2011 ; Worbe et al. 2015 ). Although there is strong theoretical evidence for this hypothesis, relatively few direct tests have been performed.
One approach is to examine whether genetic variants that putatively influence serotonergic function are associated with performance on reflexive and reflective processing tasks. The serotonin transporter (5-HTT) contributes to the active clearance of extracellular serotonin and thereby influences the duration and intensity of serotonin signaling via pre- and post-synaptic receptors located on target neurons (for a review, see Hariri and Holmes 2006 ). The efficiency with which the 5-HTT returns serotonin to the presynaptic neuron appears to be influenced by a polymorphism in the proximal gene promoter (i.e., the 5-HTT linked polymorphic region, or 5-HTTLPR). The 5-HTTLPR is most commonly represented by 2 variants: a short (S) allele and a long (L) allele. The presence of 1 or 2 S alleles, rather than 2 copies of the L allele, may be associated with reduced transcriptional efficiency that putatively results in significant decreases (∼50%) in serotonin reuptake ( Lesch et al. 1996 ; Heinz et al. 2000 ).
Further, it has recently been suggested that 5-HTTLPR expression may be modulated by an additional single-nucleotide polymorphism, namely rs25531, which is composed of an adenine to guanine change at the sixth nucleotide in the first of 2 extra 20- to 23-basepair repeats of the L allele ( Wendland et al. 2006 ). It is important to note that the L allele with guanine at the sixth nucleotide (L G ) and the S allele are similar in terms of transcriptional activity; therefore, only the L allele with adenine at the sixth nucleotide (L A ) is associated with relatively increased transcriptional activity ( Hu et al. 2005 ). For the sake of brevity, we refer to the L G and S alleles as S′ and the L A allele as L′ throughout this article.
Consistent with theory, there is a growing body of research to suggest that carriers of the S′ 5-HTTLPR allele show performance advantages relative to L′ allele carriers in tasks that are likely to be mediated by reflexive processes ( Homberg and Lesch 2011 ). For example, S′ allele carriers demonstrate advantages when learning to avoid risky stimuli in a passive avoidance task ( Finger et al. 2007 ; Blair et al. 2008 ), as well as versions of the Balloon Analogue Risk task ( Crisan et al. 2009 ) and an investment task for financial risk ( Kuhnen and Chiao 2009 ).
In contrast, there is accumulating evidence that individuals with 2 copies of the L′ 5-HTTLPR allele show enhanced reflective processing relative to individuals with the S′ allele. For instance, adult women who carry at least 1 copy of the L′ 5-HTTLPR allele displayed greater memory monitoring and updating during a cognitive flexibility task than S′ allele homozygotes ( Weiss et al. 2014 ). Similarly, in an aging population, 5-HTTLPR L′ allele homozygotes had better source memory monitoring than S′ allele carriers. Further, this advantage for the L′ allele homozygote group was accompanied by greater neural activity in regions of prefrontal cortex that have been shown to support accurate memory monitoring ( Pacheco et al. 2012 ). Indeed, several studies have found greater gray matter volume in L′ allele homozygotes compared with S′ allele carriers in key brain regions involved in reflective processing ( Pezawas et al. 2005 ; Selvaraj et al. 2011 ).
Optimal system-level control involves identifying and engaging the task appropriate cognitive system—that is, the system that is needed to optimize performance in the task at hand. To date existing paradigms have not probed the distinct cognitive processing systems involved during learning in a selective and controlled manner. In addition, reflective and reflexive processing has not been studied within the framework of a single task domain in which all aspects of the task remain constant across reflective-optimal and reflexive-optimal versions of the task with only the nature of the optimal cognitive processing system varying across conditions. The goal of the present study was to examine the role of serotonin in learning using a single task (categorization) that cleanly dissociated reflective from reflexive processing systems using tasks that only differ in the nature of the cognitive processing system that mediated optimal performance.
Categorization is a form of decision-making that is critical to daily living. Category learning is an ideal paradigm to test dissociable reflective and reflexive systems because the neurobiological underpinnings of category learning are well understood ( Ashby et al. 1998 ; Poldrack et al. 2001 ; Seger and Cincotta 2002 , 2005 , 2006 ; Reber et al. 2003 ; Shohamy et al. 2004 ; Filoteo et al. 2005 ; Nomura et al. 2007 ; Poldrack and Foerde 2008 ; Seger 2008 ; Ashby and Maddox 2010 ). In the category learning literature, researchers have developed paradigms that selectively target reflective and reflexive learning ( Ashby et al. 1998 , 2011 ; Ashby and Maddox 2005 , 2010 ). Importantly, learning performance in reflective- and reflexive-optimal task are dissociable and interactive ( Azzopardi and Cowey 1998 ; Maddox et al. 1998 , 2002 , 2003 , 2007 , 2013 ; Ashby et al. 2002 ; Maddox, Ashby et al. 2004 ; Maddox, Bohil et al. 2004 ; Maddox, Filoteo et al. 2004 ; Zeithamova and Maddox 2006 , 2007 ; Tam et al. 2013 ). Given that L′ allele carriers of the 5-HTTLPR polymorphism in the serotonin transporter gene are thought to show enhanced reflective processing, we predict an increase in reflective-optimal category learning as the number of 5-HTTLPR L′ alleles increases. Given the interactive nature of the 2 systems, we also predict that this enhancement in reflective processing will be associated with a commensurate attenuation in reflexive processing as the number of L′ alleles increases. Put another way, and based on the predictions from COVIS and the small but growing literature to suggest some reflexive processing advantages in S′ allele carriers, we predict enhanced reflexive-optimal category learning and attenuated reflective-optimal category learning as the number of 5-HTTLPR S′ alleles increases.
Across 3 experiments, we examined the relationship between serotonin transporter promoter region polymorphism (5-HTTLPR) status and reflexive- and reflective-optimal categorization. On each trial in all studies, participants were presented with a stimulus and were asked to classify it into 1 of 2 categories. Critically, the stimulus-to-category assignments were manipulated in such a way that optimal responding in the reflexive-optimal condition involves recruiting the reflexive learning system whereas optimal responding in the reflective-optimal condition involves recruiting the reflective learning system. Because all aspects of the task are identical, except the cognitive/neural system that mediates optimal performance, any performance differences that are observed across allele groups are likely due to differential processing within the 2 systems.
Experiment 1 examined reflexive-optimal category learning in a community sample and found a performance advantage for individuals with 2 copies of the S′ allele of the serotonin transporter (5-HTTLPR). Like Experiment 1, Experiment 2 also examined reflexive-optimal category learning but Experiment used a task with a different long-run goal (maximize long-run accuracy vs. attain 10 correct in a row as quickly as possible). Experiment 2 also used a community sample that was screened for current or past psychiatric diagnosis such as drug and alcohol addiction using the Mini International Neuropsychiatric Interview (MINI; Sheehan et al. 1998 ). The results from Experiment 2 complemented those from Experiment 1 and suggested that individuals with 2 copies of the S′ allele of the serotonin transporter (5-HTTLPR) showed a larger performance increase across trials than individuals with 2 copies of the L′ allele. Experiment 3 examined reflective-optimal category learning in a community sample that was screened using the MINI. In line with the predictions from COVIS, we found a performance advantage for individuals with 2 copies of the L′ allele of the serotonin transporter (5-HTTLPR).
Experiment 1
Participants
One hundred and ninety-seven participants (130 females, 67 males; Mage = 22.82) were recruited from the Austin, Texas community, and were compensated $10 per hour for participating in the experiment. Participants were recruited via ads placed online, flyers posted in the surrounding community, and via e-email sent to individuals that are subscribed to listserves that are affiliated with the university. Our sample was composed of 107 Caucasian, 57 Asian, 4 African-American, 16 Pacific Islander, 10 identified as “other,” and 3 declined to state their race. The ratio distribution of tri-allelic 5-HTTLPR genotypes was 34:84:79 (L′L′:L′S′:S′S′). A multiallelic likelihood ratio test indicated that genotype frequencies was just below significance from Hardy Weinberg equilibrium, P (LR) = 0.08. Experiment 1 was undertaken with the understanding and written consent of each participant and was approved by the Institutional Review Board at the University of Texas.
Genotyping
Genomic DNA was isolated from buccal cells and saliva using methods published elsewhere ( Pearson et al. 2015 ). Previous research ( Hu et al. 2005 ; Zalsman et al. 2006 ) suggests that the L G allele and the S allele are similar in terms of transcriptional activity. Therefore, the S and L G alleles were treated as equivalents. For the sake of brevity and ease, L G and S will hereafter be referred to as S′ and L A and will be hereafter referred to as L′. Genotype and allele distributions for all 3 experiments are presented in Table 1 .
Table 1.
Genotype and allele distributions by experiment
| Experiment 1 ( n = 197) | ||||||||
| 5-HTTLPR genotype count (frequency) | 5-HTTLPR allele count (frequency) | |||||||
| L/L | L/S | S/S | L | S | ||||
| 57 (0.29) | 80 (0.41) | 60 (0.30) | 194 (0.49) | 200 (0.51) | ||||
| 5-HTTLPR/rs25531 genotype count (frequency) | 5-HTTLPR/rs25531 allele count (frequency) | |||||||
| L′/L′ | L′/S′ | S′/S′ | L′ | S′ | ||||
| 34 (0.17) | 84 (0.43) | 79 (0.40) | 152 (0.39) | 242 (0.61) | ||||
| L A /L A | L A /L G | L A /S | L G /L G | L G /S | S/S | L A | L G | S |
| 34 (0.17) | 19 (0.10) | 65 (0.33) | 4 (0.02) | 15 (0.08) | 60 (0.30) | 152 (0.39) | 42 (0.11) | 200 (0.51) |
| Experiment 2 ( n = 201) | ||||||||
| 5-HTTLPR genotype count (frequency) | 5-HTTLPR allele count (frequency) | |||||||
| L/L | L/S | S/S | L | S | ||||
| 42 (0.21) | 69 (0.34) | 90 (0.45) | 153 (0.38) | 249 (0.62) | ||||
| 5-HTTLPR/rs25531 genotype count (frequency) | 5-HTTLPR/rs25531 allele count (frequency) | |||||||
| L′/L′ | L′/S′ | S′/S′ | L′ | S′ | ||||
| 37 (0.18) | 59 (0.29) | 105 (0.52) | 133 (0.33) | 269 (0.67) | ||||
| L A /L A | L A /L G | L A /S | L G /L G | L G /S | S/S | L A | L G | S |
| 37 (0.18) | 4 (0.02) | 55 (0.27) | 1 (0.00) | 14 (0.07) | 90 (0.45) | 133 (0.33) | 20 (0.05) | 249 (0.62) |
| Experiment 3 ( n = 194) | ||||||||
| 5-HTTLPR genotype count (frequency) | 5-HTTLPR allele count (frequency) | |||||||
| L/L | L/S | S/S | L | S | ||||
| 42 (0.22) | 65 (0.34) | 87 (0.45) | 149 (0.38) | 239 (0.62) | ||||
| 5-HTTLPR/rs25531 genotype count (frequency) | 5-HTTLPR/rs25531 allele count (frequency) | |||||||
| L′/L′ | L′/S′ | S′/S′ | L′ | S′ | ||||
| 38 (0.20) | 56 (0.29) | 100 (0.52) | 132 (0.34) | 256 (0.66) | ||||
| L A /L A | L A /L G | L A /S | L G /L G | L G /S | S/S | L A | L G | S |
| 38 (0.20) | 3 (0.02) | 53 (0.27) | 1 (0.01) | 12 (0.06) | 87 (0.45) | 132 (0.34) | 17 (0.04) | 239 (0.62) |
Note: L′ = L A , S′ = L G and S, all frequencies rounded to 2 decimal places.
Stimuli
Stimuli consisted of color images of houses, flowers, or food. Each stimulus was 4 dimensional with 1 of the 2 possible values for each dimension being presented (16 stimuli total). For houses, the stimuli varied along the following 4 dimensions: shape of window (circle vs. rectangle), color (pink vs. green), number of clouds (1 vs. 2), and landscape (tree vs. lawn) (see Fig. 1A for examples). For flowers, the stimuli varied along these 4 dimensions: petal shape (long and thin vs. short and fat), shape of center (circular vs. square), number of leaves (3 vs. 6), and color of pot (blue vs. yellow). For food, the stimuli varied along these 4 dimensions: number of strawberries (1 vs. 2), color of plate (blue vs. yellow), utensil (fork vs. knife), and carbohydrate (pancake vs. toast). One of these stimulus types was randomly sampled for each participant.
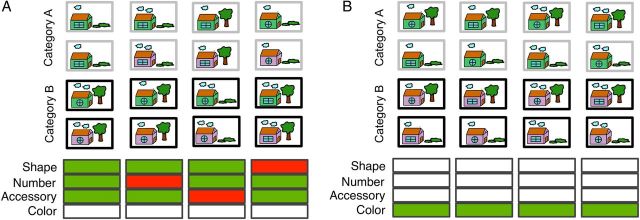
Figure 1.
( A ) A schematic of one possible reflexive-optimal categorization problem in which the shape of window (rectangle vs. circle), number of clouds (1 vs. 2), and landscaping (tree vs. lawn) dimensions were relevant and the color of the house was irrelevant. ( B ) A schematic of one possible reflective-optimal categorization problem in which the dimension of color of wall (green or pink) was relevant, and the 3 other dimensions were irrelevant.
For the reflexive-optimal task, we first made one stimulus dimension irrelevant (e.g., color of the house). Then, for the 3 remaining relevant stimulus dimensions, the possible properties of each stimulus were given a value of 1 or −1 (e.g., for shape of window in houses, rectangle = 1 and circle = −1). Then, each category structure was created by the following mathematical formula (where the 3 relevant stimulus dimensions are X , Y , and Z ):
This yielded 8 unique A and 8 unique B items. A schematic of one possible reflexive-optimal category learning problem is displayed in Figure 1A .
Procedure
After providing demographic information and a saliva sample for genetic analysis, participants then completed the category learning task using Pygame software on a personal computer in a testing room along with some standardized neuropsychological tests. Participants were informed that they would be viewing pictures that vary across trials in 4 dimensions. They were informed that each picture was a member of 1 of 2 categories: A or B, and that their task was to determine the category membership for each picture by using the computer key and pressing either the “1” button which corresponded to Category A or the “2” button which corresponded to Category B. Participants were informed that they would receive feedback following each response that would state whether their response was “correct” or “incorrect.” Finally, they were informed that their goal was to generate 10 correct responses in a row. Once they achieved 10 correct responses in a row, or after 150 trials, whichever came first, the task would end.
Results
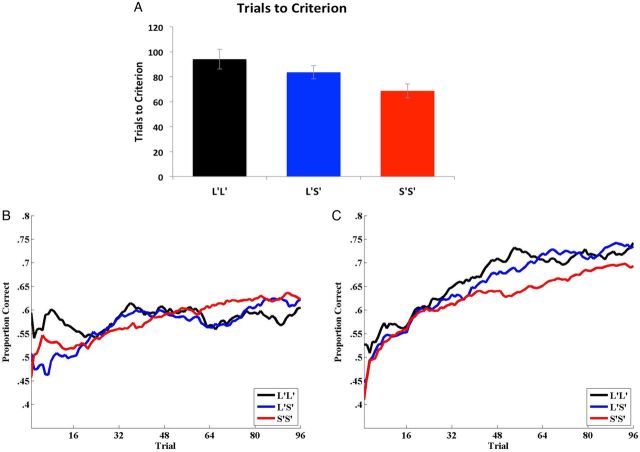
The primary dependent measure was the number of trials needed to reach criteria (i.e., 10 correct responses in a row). If an individual did not reach criterion after 150 trials, we assume a trials-to-criterion of 151. Figure 2A displays the average trials-to-criterion for the L′L′, L′S′, and S′S′ genotype groups. An ANOVA was conducted on the trials-to-criterion measure and yielded a significant effect of allele group ( F2,194 = 3.733, P = 0.026, partial eta squared = 0.037). Post hoc analyses indicated that S′S′ homozygotes (average trials to criterion = 68.63) learned significantly faster than L′L′ homozygotes (average trials to criterion = 94.09) ( P = 0.012), and nearly significantly faster than L′S′ heterozygotes (average trials to criterion = 83.51) ( P = 0.054). The L′L′ and L′S′ groups did not differ significantly in their rate of learning ( P = 0.289). The linear trend was also significant ( F1,194 = 7.380, P = 0.007) suggesting that performance improved linearly with an increase in the number of S′ alleles. Finally, we observed the same pattern of results when the analysis was restricted to Caucasian participants (N: L′L′ = 20, L′S′ = 43, S′S′ = 28; average trials-to-criterion: L′L′ = 96.85, L′S′ = 86.44, S′S′ = 75.18), and importantly the linear trend was still significant ( F1,88 = 4.091, P = 0.002).
Figure 2.
( A ) Trials to criterion for the 3 tri-allelic serotonin transporter genotype groups from the Experiment 1 reflexive-optimal task. Error bars denote standard error of the mean. ( B ) Average learning curves (based on a sliding 16-trial average window) for the 3 tri-allelic serotonin transporter groups from the Experiment 2 reflexive-optimal task. ( C ) Average learning curves (based on a sliding 16 trial average window) for the 3 tri-allelic serotonin transporter genotype groups from the Experiment 3 reflective-optimal task.
Summary
The results from Experiment 1 suggest that S′ allele homozygotes were fastest to attain 10 correct responses in a row in the reflexive-optimal task, and L′ allele homozygotes were slowest to attain 10 correct responses in a row, with L′S′ heterozygotes showing an intermediate learning rate. The results showed a linear trend with performance improving linearly as the number of copies of the S′ allele increased. These results support our hypothesis that the presence of the S′ allele enhances processing in the reflexive system relative to the reflective system. This finding is promising but given the fact that candidate gene studies have been criticized for poor replicability, additional testing of this hypothesis is needed ( Ioannidis et al. 2001 ). Although Experiment 2 does not provide a direct replication of Experiment 1, it does examine learning of the same reflexive-optimal category structure but in a task for which the goal is to maximize long-run accuracy as opposed to attain 10 correct responses in a row. In addition, the participants in Experiment 1 were not screened for neuropsychiatric disorders, which could bias the results, especially given the relationship between the serotonin transporter gene and depression ( Caspi et al. 2003 ; Beevers 2005 ; Carver et al. 2008 , 2009 ). Thus, in Experiment 2, all participants were screened using the MINI ( Sheehan et al. 1998 ) to ensure that they did not met criteria for a current or past psychiatric diagnosis. Finally, to obtain more information regarding the time-course of learning, we had all participants complete a fixed number of trials in the experiment with the goal of maximizing accuracy.
Experiment 2
Participants
Two hundred and one participants (114 females, 87 males; Mage = 25.10) were recruited from the Austin, Texas community, and were compensated $10 per hour for participating in the experiment. Participants were recruited via ads placed online, flyers posted in the surrounding community, and via e-email sent to individuals that are subscribed to listserves that are affiliated with the university. Participants who showed interest in the study were contacted and were administered the Mini International Neuropsychiatric Interview (MINI; Sheehan et al. 1998 ) to rule out individuals who met criteria for serious current psychopathology. Only healthy individuals with no past or current psychopathology were included in the study in an effort to remove third variable explanations, such as the presence of psychopathology. Our sample was composed of 120 Caucasian, 40 Asian, 9 African-American, 2 Pacific Islander, 19 “other,” and 11 participants who declined to state their race. The distribution of tri-allelic 5-HTTLPR genotypes was 37:59:105 (L′L′:L′S′:S′S′). A multiallelic likelihood ratio test indicated that genotype frequencies did significantly differ from Hardy Weinberg equilibrium, P (LR) < 0.001. Experiment 2 was undertaken with the understanding and written consent of each participant and was approved by the Institutional Review Board at the University of Texas.
Genotyping and Stimuli
The genotyping methodology and stimuli were identical to Experiment 1.
Procedure
The procedures were identical to those from Experiment 1 except that each participant completed 6 16-trial blocks with each stimulus being presented once in each block. We took this approach because it is more common in the category learning literature than trials to criterion, and it allows one to compare learning curves across groups. Given that trial number was fixed in this study, participants' were encouraged to maximize accuracy, and they were not given the goal of generating 10 consecutive correct responses.
Results
The participant's goal was to respond accurately on each trial and to maximize overall proportion correct. To determine whether performance differences emerged across genotype groups, we employed a generalized linear mixed effects model to analyze the results statistically, using the statistical computing package R ( Team 2014 ) in conjunction with the package lme4 ( Bates et al. 2012 ). This analysis was designed to estimate the log odds of producing a correct response given each trial and genotype group. The dependent variable was trial-by-trial accuracy for individual participants coded as “correct” or “incorrect,” with the reference level set as “incorrect.” The use of mixed effects modeling is increasing rapidly in the field of learning, in large part because of its focus on trial-by-trial behavior as opposed to more traditional ANOVA analyses that operates at the arbitrary-sized block-by-block level. This trial-by-trial level of analysis is appropriate given the participant goal to maximize performance on each trial. Even so, and for completeness, we include traditional ANOVA analyses in Appendix. Not surprisingly, the statistical findings from ANOVA are weaker, due mainly to the fact that they have less statistical power. Importantly though, the overall pattern of findings converges across the mixed effects and ANOVA analyses.
The fixed effects in our mixed effects model included the genotype group (L′L′, L′S′, and S′S′: with S′S′ serving as the reference level), trial number (1 to 96) and the interaction term between these 2 factors. The model was corrected for the random intercept for each participant. The most complex random effects structure as justified the data was by-subject and by-item random intercepts ( P < 0.05):
The learning curves are displayed in Figure 2B . The L′L′ and L′S′ genotype groups were independently compared against the S′S′ group that served as the reference. For both the S′S′ versus L′S′ and S′S′ versus L′L′ analyses, the effect of trial was significant, b = 0.005, SE = 7.44 × 10 −4 , z = 7.054, 95% CI [0.0038, 0.0067], P < 0.001, indicating that accuracy increased over trials for all allele groups (each successive trial increased the probability of an accurate response for all groups). For the comparison of the L′S′ group against the S′S′ group, neither the condition effect, b = −6.89 × 10 −4 , SE = 0.0876, z = −0.008, 95% CI [−0.1724, 0.1710], P = 0.994, nor the trial by condition interaction was significant, b = −7.60 × 10 −4 , SE = 1.23 × 10 −3 , z = −0.615, 95% CI [−0.0032, 0.0017], P = 0.538. For the comparison of the L′L′ group against the S′S′ group, the condition effect was not significant, b = 0.170, SE = 0.103, z = 1.652, 95% CI [−0.0318, 0.3726], P = 0.099. However, the trial by condition interaction was significant, b = −3.67 × 10 −3 , SE = 1.45 × 10 −3 , z = −2.528, 95% CI [−0.0065, −0.0008], P = 0.011, indicating that the learning rate for the S′S′ group was significantly faster than that for the L′L′ group. Next we compared the L′L′ and L′S′ groups and found no significant difference between the 2 for condition effect or trial by condition interaction ( P = 0.122, P = 0.068, respectively). Therefore, we combined the L′L′ and L′S′ groups and then compared them to the S′S′ group. For this analysis, the condition effect was nonsignificant, b = 0.065, SE = 0.076, z = 0.851, 95% CI = [−0.0843, 0.2139], P = 0.395, and the trial by condition interaction was just below statistical significance, b = −1.88 × 10 −3 , SE = 1.07 × 10 −3 , z = −1.749, 95% CI [−0.0040, 0.0002], P = 0.080. The same pattern of results held when we restricted the analysis to Caucasians only [although participants were not given the goal of generating 10 consecutive correct responses in a row, for completeness we examined several trials-to-criterion measures in these data. We examined the number of trials needed to obtain 10 consecutive correct responses, as well as 3 more liberal measures of learning. Specifically, the trials needed to obtain 90% accuracy over the last 10, 16 or 20 trials. The pattern of results mirrored those from the mixed effects modeling with faster learning for the S′S′ group relative to the L′L′ group, although the effects were smaller and in no case was the effect of genotype group significant].
Summary
The results from Experiment 2 are generally in line with those from Experiment 1 in an MINI ( Sheehan et al. 1998 ) screened sample that ensures that no participants met criteria for a current or past psychiatric diagnosis and utilizes a slight variant of the task that emphasizes accuracy over the goal of generating 10 consecutive correct responses. As predicted, the mixed effects modeling suggests that S′ homozygotes showed a faster learning rate than L′ homozygotes in the reflexive-optimal task. Interestingly, it is worth noting that L′ homozygotes show enhanced performance early relative to S′ homozygotes. This pattern is also predicted since COVIS assumes that there is an initial bias toward reflective processes even when in a reflexive-optimal task. Efficient reflective processing early (as predicted for L′ homozygotes) can lead to an early boost in performance, but in the long-run leads to poor performance in reflexive-optimal tasks. These results provide additional support for our hypothesis that the presence of the S′ allele enhances processing in the reflexive system relative to the reflective system. Taken together, the results from Experiments 1 and 2 provide strong evidence to suggest that the S′ allele of the serotonin transporter leads to enhanced reflexive processing. In 2 variants of a reflexive-optimal category learning task S′ homozygotes learned the task faster than L′ homozygotes.
Experiment 3 complements Experiments 1 and 2 by exploring the relationship between 5-HTTLPR and reflective-optimal category learning. Critically, most aspects of the task were identical to those from Experiment 2 except the stimulus-to-category mappings. We predict that the S′ allele advantage observed in the reflexive-optimal task will reverse in the reflective-optimal task with L′ allele individuals showing faster learning than S′ allele individuals.
Experiment 3
Participants
One hundred and ninety-four participants (108 females, 86 males; Mage = 24.96) were recruited from the Austin, Texas community, and were compensated $10 per hour for participating in the experiment. Participants were recruited via ads placed online, flyers posted in the surrounding community, and via e-email sent to individuals that are subscribed to listserves that are affiliated with the university. Participants who showed interest in the study were contacted and were administered the Mini International Neuropsychiatric Interview (MINI; Sheehan et al. 1998 ) to rule out individuals who met criteria for significant psychopathology. Only healthy individuals with no past or current psychopathology were included in the study in an effort to remove third variable explanations, such as the presence of psychopathology. Our sample was composed of 116 Caucasian, 44 Asian, 5 African-American, 5 Pacific Islander, 16 identified as “other,” and 8 declined to state their race. The ratio distribution of tri-allelic 5-HTTLPR genotypes was 38:56:100 (L′L′:L′S′:S′S′). A multiallelic likelihood ratio test indicated that genotype frequencies did significantly differ from Hardy Weinberg equilibrium, P (LR) < 0.001. Experiment 3 was undertaken with the understanding and written consent of each participant and was approved by the Institutional Review Board at the University of Texas.
Genotyping
The genotyping methodology was identical that used in Experiments 1 and 2.
Stimuli
The house, plant, or food stimuli from Experiments 1 and 2 were also used in Experiment 3 with one stimulus type being randomly sampled and used for each participant. For the reflective-optimal task, we first made one stimulus dimension relevant (e.g., color of wall for houses, Fig. 1B ) with the 3 remaining stimulus dimensions being irrelevant. One value along the relevant dimension was assigned to Category A and the other to Category B. This yielded 8 unique A and 8 unique B items. A schematic of one possible reflective-optimal category learning problem is displayed in Figure 1B .
Procedure
The procedures were identical to those from Experiment 2.
Results
Performance differences across L′L′, L′S′, and S′S′ genotype groups were assessed using the same mixed effects modeling analysis used in Experiment 2. Traditional ANOVA results are also included in Appendix. The learning curves are displayed in Figure 2C . The L′L′ and L′S′ genotype groups were independently compared against the S′S′ group that served as the reference. For both the S′S′ versus L′S′ and S′S′ versus L′L′ analyses, the effect of trial was significant, b = 0.008, SE = 8.4 × 10 −4 , z = 9.605, 95% CI [0.0065, 0.0098], P < 0.0001, indicating that accuracy increased over trials for all genotype groups. For the comparison of the L′S′ group against the S′S′ group, the group effect was not significant, b = 0.027, SE = 0.228, z = 0.118, 95% CI [−0.419, 0.471], P = 0.906. The trial by group interaction was significant, b = 3.20 × 10 −3 , SE = 1.44 × 10 −3 , z = 2.218, 95% CI = [0.0004, 0.0060], P = 0.026, indicating that the learning rate for the L′S′ group was significantly faster than that for the S′S′ group. For the comparison of the L′L′ group against the S′S′ group, neither the group effect, b = 0.128, SE = 0.260, z = 0.491, 95% CI [−0.382, 0.635], P = 0.623, nor the trial × group interaction were significant, b = 2.50 × 10 −3 , SE = 1.68 × 10 −3 , z = 1.50, 95% CI [−0.0008, 0.0058], P = 0.133. Using the same mixed regression analysis, we found no group ( P = 0.73) or group by trial interaction ( P = 0.71) effects for the L′L′ and L′S′ groups. Thus, to increase our power, we combined these into an L′ carrier group and compared directly with the S′S′ group. The group effect was not significant, b = 0.068, SE = 0.195, z = 0.342, 95% CI = [−0.316, 0.450], P = 0.733, but the group by trial interaction was significant, b = 2.94 × 10 −3 , SE = 1.24 × 10 −3 , z = 2.37, 95% CI [0.0005, 0.0054], P = 0.018, indicating that the learning rate for the L′ carrier group was significantly faster than that for the S′S′ group. The same pattern of results held when we restricted the analysis to Caucasians only.
Summary
As predicted, and counter to the results from Experiments 1 and 2, L′ allele carriers of the 5-HTTLPR serotonin transporter learned faster than S′ homozygotes when the task was one that relied on reflective processing. These results add to the large body of research suggesting that the presence of the L′ allele enhances processing in the reflective system. Taken together with the results from Experiment 2—that is identical in all respects except the optimal cognitive system (reflexive vs. reflective) that mediates learning in the task—the findings are clear. S′ homozygotes of the 5-HTTLPR serotonin transporter gene polymorphism are faster to learn in a reflexive-optimal task but are slower to learn in a reflective-optimal task.
General Discussion
To our knowledge, this is the first study to examine the relationship between serotonin transporter allele status and reflexive- and reflective-optimal learning in a highly controlled task for which all aspects were held equivalent except the nature of the cognitive and neural system that mediated optimal learning ( Ashby et al. 1998 ; Poldrack et al. 2001 ; Waldron and Ashby 2001 ; Seger and Cincotta 2002 , 2005 , 2006 ; Reber et al. 2003 ; Maddox and Ashby 2004 ; Shohamy et al. 2004 ; Ashby and Maddox 2005 , 2010 ; Filoteo et al. 2005 ; Zeithamova and Maddox 2006 ; Poldrack and Foerde 2008 ; Seger 2008 ; Smith et al. 2012 , 2013 ). Experiment 1 utilized a large community sample and revealed that individuals with 2 copies of the S′ allele of the serotonin transporter (5-HTTLPR) outperformed individuals with 2 copies of the L′ allele in a reflexive-optimal learning task. Experiment 2 used the same reflexive-optimal category structure as Experiment 1, but extended Experiment 1 by using a large community sample who were screened for current or past psychiatric diagnosis, and whose goal was to maximize long-run accuracy as opposed to getting 10 correct in a row. The results from Experiment 2 converged with those from Experiment 1 in revealing a reflexive-optimal learning advantage for individuals with 2 copies of the S′ allele of the serotonin transporter (5-HTTLPR) relative to individuals with 2 copies of the L′ allele. Specifically, we found that individual with 2 copies of the S′ allele showed a greater increase in performance over blocks than individuals with 2 copies of the L′ allele. Interestingly, the results from Experiment 2 also suggested that individuals with 2 copies of the L′ allele performed better than individuals with 2 copies of the S′ allele early in task, but L′ allele participants showed effectively no performance improvement over trials, whereas the S′ allele participants showed clear learning.
This pattern is predicted from the dual learning systems framework if one assumes that participants approach the task with an initial bias toward reflective processing. Briefly, L′L′ participants are thought to have enhanced reflective processing relative to L′S′ and S′S′ participants. Since reflexive-optimal tasks can be solved to some reasonable level of accuracy with a sophisticated reflective strategy, it makes sense that L′L′ participants might show a performance advantage early in a reflexive-optimal task because they are better able to identify a reasonably accurate reflective strategy quickly. However, with additional training in a reflexive-optimal task, S′ carriers should more quickly abandon reflective strategies in favor of the more optimal reflexive strategy and should learn at a faster rate. The results from Experiment 2 support this prediction.
Experiment 3 used a large community sample of participants who were also screened for current or past psychiatric diagnosis and revealed that individuals with 2 copies of the S′ allele of the serotonin transporter (5-HTTLPR) were outperformed by individuals with the L′ allele in a reflective-optimal learning task. Taken together, the results from these 3 experiments suggest that serotonin transporter (5-HTTLPR) allele status strongly affects reflexive- and reflective-optimal learning with a reflexive-optimal learning advantage holding for individuals with 2 copies of the S′ allele of the serotonin transporter, and a reflective-optimal learning advantage holding for individuals with 2 copies of the L′ allele of the serotonin transporter.
Limitations
The observed association between the tri-allelic serotonin transporter and reflexive- and reflective-optimal processing should be interpreted with several limitations in mind. First, as with any genetics study of this sort, the association may be driven by another genetic variant in linkage disequilibrium with the 5-HTTLPR polymorphism, or another unmeasured third variable (e.g., within-ethnicity population stratification). Second, given the departure from HWE in our samples screened for psychological dysfunction (Experiments 2 and 3), participants recruited for these experiments may not represent a random sample of the population. Hardy Weinberg equilibrium tests examine the genotype frequencies in a sample of individuals to determine whether any genotypes are relatively under- or over-represented. Departures from Hardy Weinberg equilibrium are nonspecific indicators of something driving the relative frequencies of the observed genotypes. The deviation from Hardy Weinberg equilibrium seen in this report can be explained in many ways. Among the many possible threats to Hardy Weinberg equilibrium are nonrandom mating, natural selection, genetic drift, genotyping error, and when the study sample was selected based upon a phenotype that is associated with the tested genotype ( Sham 1998 ; Hosking et al. 2004 ). We are unable to determine which, if any, of these threats to Hardy Weinberg equilibrium occurred. Importantly, we genotyped 20% of the samples twice to explore the possibility of genotyping error and observed no genotyping discrepancies in these samples. The relatively small sample size may have led to a sampling error, and the possibility that SLC6A4 genotypes are associated with the inclusion criteria used to recruit for the study cannot be discounted. We do not believe that the HWE findings were driven by the grouping L G and S participants together as we used a multiallelic exact and Monte-Carlo-based test to avoid such groupings. It is possible that ethnic differences in our sample (i.e., the proportion of Asian participants) may have contributed to these departures from Hardy Weinberg Equilibrium as genotype frequencies are known to vary by ethnicity ( Haberstick et al. 2015 ). Though this may raise concerns about the possibility of population stratification, the consistency of effects when the analyses were run using only Caucasian participants ameliorates this concern. Indeed, the fact that we found similar results in both the full sample and the “Caucasian-only” sample across 2 variants of the reflexive-optimal category learning task is suggestive that this effect is valid.
Third, although we screened participants in Experiments 2 and 3 with the Mini International Neuropsychiatric Interview, we may have failed to screen out various other conditions linked to the reflexive and reflective processing. Fourth, in the present study, we used well-studied category learning paradigms to target reflexive and reflective learning. Future research should determine the extent to which the observed genetic effects generalize to other types of learning and decision-making. Finally, for a genetic association study, the samples in the present study are relatively small. Thus, a larger sample would be needed to further increase our confidence in the findings reported in this study. Even so, the fact that we found similar results across 2 versions of the less well-studied reflexive-optimal condition instills confidence in the validity of our findings.
Conclusions
Three studies examined the relationship between tri-allelic serotonin transporter status and reflexive- and reflective-optimal learning and decision-making in a highly controlled task for which all aspects were held equivalent except the nature of the cognitive system that mediated optimal learning. One study and a subsequent extension found that individuals with 2 copies of the 5-HTTLPR S′ allele outperformed L′ allele homozygotes in a reflexive-optimal learning task, whereas a third study found that individuals with 2 copies of the 5-HTTLPR S′ allele performed worse than L′ allele carriers in a reflective-optimal learning task.
Funding
This study was supported by the National Institute of Drug Abuse (DA032457 to W.T.M. and C.G.B.) and shared equipment grants to J.E.M. from the Department of Veteran Affairs Research Service.
Notes
We would like to thank the MaddoxLab RAs for all data collection with special thanks to Alex Kline. We would also like to thank Kayla Dwyer for genotyping work. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs. Conflict of Interest : None declared.
Appendix
In this Appendix, we present the results from more traditional ANOVA as applied to Experiments 2 and 3. Because the stimuli were presented in 6 16-trial blocks with each stimulus presented once in each block, we estimate performance at these 6 time points.
Experiment 2
The learning curves for each of the 3 genotype groups across the 6 blocks are displayed in Figure A1A . A 3 genotype group (L′L′ vs. L′S′ vs. S′S′) × 6 block mixed design ANOVA was conducted on the average block-by-block accuracy rates. The main effect of genotype group was nonsignificant ( F2,198 = 0.154, P = 0.858, partial η2 = 0.002). The effect of block was significant ( F5,990 = 13.855, P = 0.001, partial η2 = 0.065). The interaction between genotype group and block was just below statistical significance, ( F10,990 = 1.700, P = 0.070, partial η2 = 0.017). Although the interaction did not reach statistical significance, we conducted a number of follow-up analyses in the interest of a deeper understanding of the results. First, we examined the learning curves separately for each genotype group. An examination of Figure A1A suggests that whereas L′S′ and S′S′ participant showed an increase in performance over blocks, the L′L′ participants appear not to show learning. The statistical results support this conclusion. The effect of block was nonsignificant in the L′L′ group ( F5,180 = 1.045, P = 0.393, partial η2 = 0.028), but the effect of block was significant in both the L′S′ ( F5,290 = 8.366, P = 0.001, partial η2 = 0.126) and S′S′ ( F5,520 = 14.187, P = 0.001, partial η2 = 0.120) groups. Second, we wished to determine whether Block 1 performance in the L′L′ group was in fact better than that of the L′S′ and S′S′ groups. T -tests suggest that L′L′ block 1 accuracy was significantly higher than L′S′ block 1 accuracy ( t94 = 2.088, P = 0.039) but was just below significantly higher than S′S′ block 1 accuracy ( t140 = 1.670, P = 0.097). In addition, Block 1 performance across the L′S′ and S′S′ groups did not differ significantly ( t162 = 0.790, P = 0.431). If we compare S′ carriers with L′L′ homozygotes, Block 1 performance is significantly better in the L′L′ group ( t199 = 2.001, P = 0.039). Finally, we wanted to determine whether there were differences in the rate of learning across blocks 1 and 6 as a function of genotype group. As a measure of learning rate, we computed the learning slope defined as block 6 performance minus block 1 performance; the larger the value the greater the increase in performance. These data are included as an inset in Figure A1A . The main effect of learning slope was just below significance ( F2,198 = 2.551, P = 0.080, partial η2 = 0.025). Follow-up analyses suggested that the learning slope for the L′S′ group was larger than that for the L′L′ group ( P = 0.03), the learning slope for the S′S′ group was larger than that for the L′L′ group, although not by the traditional P < 0.05 metric ( P = 0.06), and the learning slopes for the L′S′ and S′S′ groups did not differ ( P = 0.57). When we compared S′ carriers with L′L′ homozygotes the learning slope difference was significant ( P = 0.030).
Figure A1.
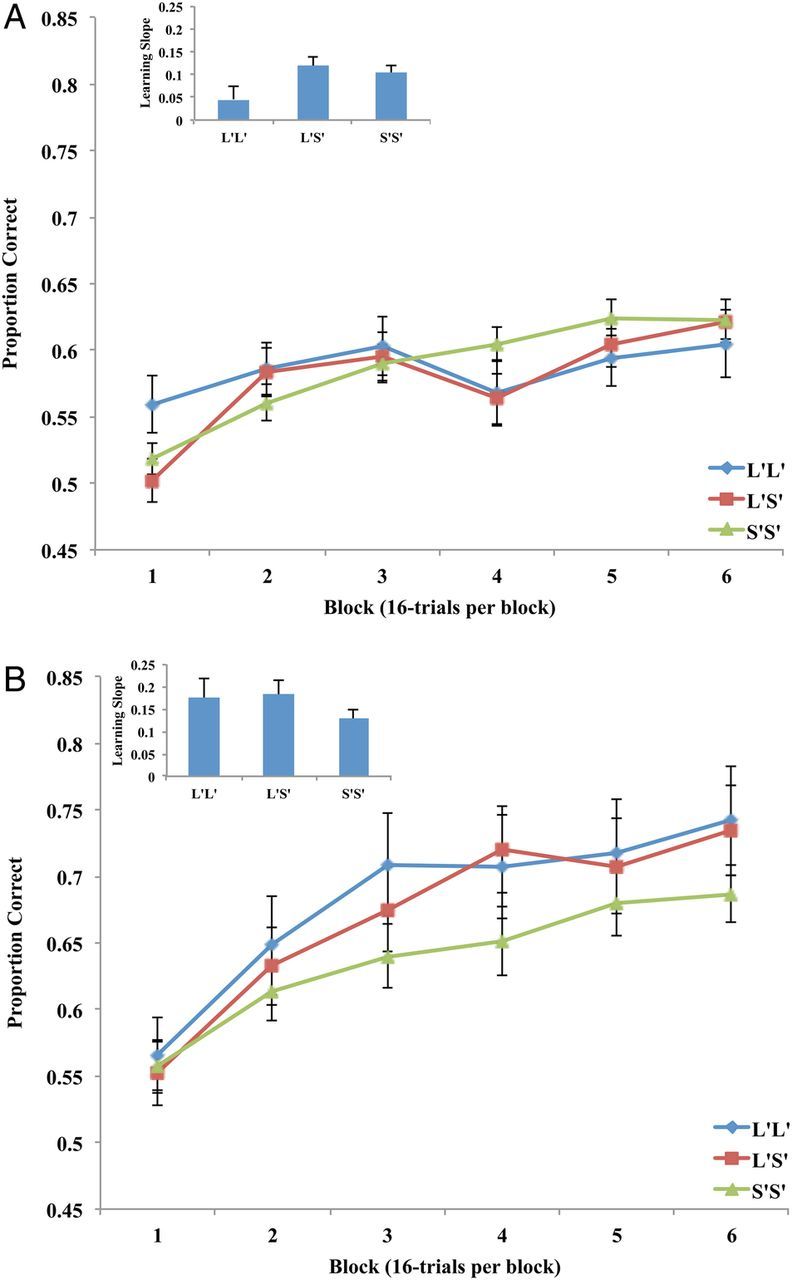
( A ) Block-by-block proportion correct for the 3 tri-allelic serotonin transporter genotype groups from the Experiment 2 reflexive-optimal task. The inset displays the learning slope data where the learning slope is defined as block 6 accuracy minus block 1 accuracy. Error bars denote standard error of the mean. ( B ) Block-by-block proportion correct for the 3 tri-allelic serotonin transporter genotype groups from the Experiment 3 reflective-optimal task. The inset displays the learning slope data. Error bars denote standard error of the mean.
Although these results do not jibe perfectly with those from the mixed effects modeling, they do converge in many important areas. First, both analyses suggest that S′S′ and L′S′ participants show a larger effect of trial (or block) than L′L′ carriers. This follows from the mixed effects trial-by-trial and ANOVA block-by-block analyses, as well as the analyses of the learning slopes. Second, there appears to be little learning across the experimental session in the L′L′ group, but robust learning in the other 2 groups. Finally, both analyses suggest that L′L′ homozygotes show better initial learning than S′ carriers. Although this possibility was not outlined in Introduction, this pattern is predicted from the dual learning systems framework if one assumes that participants approach the task with an initial bias toward reflective processing. Briefly, L′L′ participants are thought to have enhanced reflective processing relative to L′S′ and S′S′ participants. Since reflexive-optimal tasks can be solved to some reasonable level with a sophisticated reflective strategy, it makes sense that L′L′ participants might show a performance advantage early in a reflexive-optimal task because they are better able to identify a reasonably accurate reflective strategy quickly. However, with additional training in a reflexive-optimal task, S′ carriers should more quickly abandon reflective strategies in favor of the more optimal reflexive strategy and should learn at a faster rate.
Experiment 3
The learning curves for each of the 3 genotype groups across the 6 blocks are displayed in Figure A1B along with the learning slope inset. A 3-genotype group (L′L′ vs. L′S′ vs. S′S′) × 6 block mixed design ANOVA was conducted on the average block-by-block accuracy rates. The main effect of genotype group was nonsignificant ( F2,191 = 0.889, P = 0.413, partial η2 = 0.009). The effect of block was significant ( F5,955 = 32.236, P = 0.001, partial η2 = 0.144), and the interaction was nonsignificant ( F10,955 = 0.812, P = 0.617, partial η2 = 0.008). The analysis of the learning slopes was also nonsignificant ( F2,191 = 1.270, P = 0.284, partial η2 = 0.013). Despite the lack of statistical significance in these analyses, these results again jibe (at least ordinally) with those from the mixed effects modeling. The L′ carriers showed enhanced performance relative to the S′S′ homozygotes with the L′L′ and L′S′ groups showing a performance improvement of ∼18% whereas the S′S′ group showed a performance improvement of just over 12%.
References
- Ashby FG , Alfonso-Reese LA , Turken AU , Waldron EM . 1998. . A neuropsychological theory of multiple systems in category learning . Psychol Rev . 105 : 442 – 481 . [DOI] [PubMed] [Google Scholar]
- Ashby FG , Ell SW , Waldron EM . 2003. . Procedural learning in perceptual categorization . Mem Cognit . 31(7) : 1114 – 1125 . [DOI] [PubMed] [Google Scholar]
- Ashby FG , Maddox WT . 2005. . Human category learning . Annu Rev Psychol . 56 : 149 – 178 . [DOI] [PubMed] [Google Scholar]
- Ashby FG , Maddox WT . 2010. . Human category learning 2.0 . Ann N Y Acad Sci . 1224 : 147 – 161 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashby FG , Maddox WT , Bohil CJ . 2002. . Observational versus feedback training in rule-based and information-integration category learning . Mem Cognit . 30(5) : 666 – 677 . [DOI] [PubMed] [Google Scholar]
- Ashby FG , Paul E , Maddox WT . 2011. . COVIS . In: Pothos EM , Wills EPA , editors. Formal Approaches in Categorization . New York: Cambridge University Press . [Google Scholar]
- Azzopardi P , Cowey A . 1998. . Blindsight and visual awareness . Conscious Cogn . 7(3) : 292 – 311 . [DOI] [PubMed] [Google Scholar]
- Bates D , Maechler M , Bolker B . 2012. . lme4: linear mixed-effects models using S4 classes. [Computer software] . [Google Scholar]
- Beevers CG . 2005. . Cognitive vulnerability to depression: a dual process model . Clin Psychol Rev . 25(7) : 975 – 1002 . [DOI] [PubMed] [Google Scholar]
- Blair KS , Finger E , Marsh AA , Morton J , Mondillo K , Buzas B , Goldman D , Drevets WC , Blair RJ . 2008. . The role of 5-HTTLPR in choosing the lesser of two evils, the better of two goods: examining the impact of 5-HTTLPR genotype and tryptophan depletion in object choice . Psychopharmacology (Berl) . 196(1) : 29 – 38 . [DOI] [PubMed] [Google Scholar]
- Carver CS , Johnson SL , Joormann J . 2008. . Serotonergic function, two-mode models of self-regulation, and vulnerability to depression: What depression has in common with impulsive aggression . Psychol Bull . 134(6) : 912 – 943 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver CS , Johnson SL , Joormann J . 2009. . Two-mode models of self-regulation as a tool for conceptualizing effects of the serotonin system in normal behavior and diverse disorders . Curr Dir Psychol Sci . 18(4) : 195 – 199 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A , Sugden K , Moffitt TE , Taylor A , Craig IW , Harrington H , McClay J , Mill J , Martin J , Braithwaite A et al. . 2003. . Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene . Science . 301(5631) : 386 – 389 . [DOI] [PubMed] [Google Scholar]
- Clarke HF , Dalley JW , Crofts HS , Robbins TW , Roberts AC . 2004. . Cognitive inflexibility after prefrontal serotonin depletion . Science . 304(5672) : 878 – 880 . [DOI] [PubMed] [Google Scholar]
- Cools R , Nakamura K , Daw ND . 2011. . Serotonin and dopamine: unifying affective, activational, and decision functions . Neuropsychopharmacology . 36(1) : 98 – 113 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crisan LG , Pana S , Vulturar R , Heilman RM , Szekely R , Druga B , Dragoş N , Miu AC . 2009. . Genetic contributions of the serotonin transporter to social learning of fear and economic decision making . Soc Cogn Affect Neurosci . 4(4) : 399 – 408 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCaro MS , Thomas RD , Beilock SL . 2008. . Individual differences in category learning: sometimes less working memory capacity is better than more . Cognition . 107(1) : 284 – 294 . [DOI] [PubMed] [Google Scholar]
- Eskenazi D , Neumaier JF . 2011. . Increased expression of 5-HT(6) receptors in dorsolateral striatum decreases habitual lever pressing, but does not affect learning acquisition of simple operant tasks in rats . Eur J Neurosci . 34(2) : 343 – 351 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filoteo JV , Lauritzen JS , Maddox WT . 2010. . Removing the frontal lobes: the effects of engaging executive functions on perceptual category learning . Psychol Sci . 21(3) : 415 – 423 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filoteo JV , Maddox WT , Simmons AN , Ing AD , Cagigas XE , Matthews S , Paulus MP . 2005. . Cortical and subcortical brain regions involved in rule-based category learning . Neuroreport . 16(2) : 111 – 115 . [DOI] [PubMed] [Google Scholar]
- Finger EC , Marsh AA , Buzas B , Kamel N , Rhodes R , Vythilingham M , Pine DS , Goldman D , Blair JR . 2007. . The impact of tryptophan depletion and 5-HTTLPR genotype on passive avoidance and response reversal instrumental learning tasks . Neuropsychopharmacology . 32(1) : 206 – 215 . [DOI] [PubMed] [Google Scholar]
- Haberstick BC , Smolen A , Williams RB , Bishop GD , Foshee VA , Thornberry TP , Conger R , Siegler IC , Zhang X , Boardman JD et al. . 2015. . Population frequencies of the Triallelic 5HTTLPR in six ethnicially diverse samples from North America, Southeast Asia, and Africa . Behav Genet . 45(2) : 255 – 261 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR , Holmes A . 2006. . Genetics of emotional regulation: the role of the serotonin transporter in neural function . Trends Cogn Sci . 10(4) : 182 – 191 . [DOI] [PubMed] [Google Scholar]
- Heinz A , Jones DW , Mazzanti C , Goldman D , Ragan P , Hommer D , Linnoila M , Weinberger DR . 2000. . A relationship between serotonin transporter genotype and in vivo protein expression and alcohol neurotoxicity . Biol Psychiatry . 47(7) : 643 – 649 . [DOI] [PubMed] [Google Scholar]
- Homberg JR , Lesch KP . 2011. . Looking on the bright side of serotonin transporter gene variation . Biol Psychiatry . 69(6) : 513 – 519 . [DOI] [PubMed] [Google Scholar]
- Hosking L , Lumsden S , Lewis K , Yeo A , McCarthy L , Bansal A , Riley J , Purvis I , Xu CF . 2004. . Detection of genotyping errors by Hardy-Weinberg equilibrium testing . Eur J Hum Genet . 12(5) : 395 – 399 . [DOI] [PubMed] [Google Scholar]
- Hu X , Oroszi G , Chun J , Smith TL , Goldman D , Schuckit MA . 2005. . An expanded evaluation of the relationship of four alleles to the level of response to alcohol and the alcoholism risk . Alcohol Clin Exp Res . 29(1) : 8 – 16 . [DOI] [PubMed] [Google Scholar]
- Ioannidis JP , Ntzani EE , Trikalinos TA , Contopoulos-Ioannidis DG . 2001. . Replication validity of genetic association studies . Nat Genet . 29(3) : 306 – 309 . [DOI] [PubMed] [Google Scholar]
- Kuhnen CM , Chiao JY . 2009. . Genetic determinants of financial risk taking . PLoS One . 4(2) : e4362 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesch KP , Bengel D , Heils A , Sabol SZ , Greenberg BD , Petri S , Benjamin J , Müller CR , Hamer DH , Murphy DL . 1996. . Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region . Science . 274(5292) : 1527 – 1531 . [DOI] [PubMed] [Google Scholar]
- Maddox WT , Ashby FG . 2004. . Dissociating explicit and procedural-learning based systems of perceptual category learning . Behav Processes . 66(3) : 309 – 332 . [DOI] [PubMed] [Google Scholar]
- Maddox WT , Ashby FG , Bohil CJ . 2003. . Delayed feedback effects on rule-based and information-integration category learning . J Exp Psychol Learn Mem Cogn . 29(4) : 650 – 662 . [DOI] [PubMed] [Google Scholar]
- Maddox WT , Ashby FG , Gottlob LR . 1998. . Response time distributions in multidimensional perceptual categorization . Percept Psychophys . 60 : 620 – 637 . [DOI] [PubMed] [Google Scholar]
- Maddox WT , Ashby FG , Ing AD , Pickering AD . 2004. . Disrupting feedback processing interferes with rule-based but not information-integration category learning . Mem Cognit . 32(4) : 582 – 591 . [DOI] [PubMed] [Google Scholar]
- Maddox WT , Ashby FG , Waldron EM . 2002. . Multiple attention systems in perceptual categorization . Mem Cognit . 30(3) : 325 – 339 . [DOI] [PubMed] [Google Scholar]
- Maddox WT , Bohil CJ , Ing AD . 2004. . Evidence for a procedural-learning-based system in perceptual category learning . Psychon Bull Rev . 11(5) : 945 – 952 . [DOI] [PubMed] [Google Scholar]
- Maddox WT , Chandrasekaran B , Smayda K , Yi HG . 2013. . Dual systems of speech category learning across the lifespan . Psychol Aging . 28(4) : 1042 – 1056 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddox WT , Filoteo JV , Hejl KD , Ing AD . 2004. . Category number impacts rule-based but not information-integration category learning: further evidence for dissociable category-learning systems . J Exp Psychol Learn Mem Cogn . 30(1) : 227 – 245 . [DOI] [PubMed] [Google Scholar]
- Maddox WT , Filoteo JV , Lauritzen JS . 2007. . Within-category discontinuity interacts with verbal rule complexity in perceptual category learning . J Exp Psychol Learn Mem Cogn . 33(1) : 197 – 218 . [DOI] [PubMed] [Google Scholar]
- Maddox WT , Love BC , Glass BD , Filoteo JV . 2008. . When more is less: feedback effects in perceptual category learning . Cognition . 108(2) : 578 – 589 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura EM , Maddox WT , Filoteo JV , Ing AD , Gitelman DR , Parrish TB , Mesulam M-M , Reber PJ . 2007. . Neural correlates of rule-based and information-integration visual category learning . Cereb Cortex . 17(1) : 37 – 43 . [DOI] [PubMed] [Google Scholar]
- Pacheco J , Beevers CG , McGeary JE , Schnyer DM . 2012. . Memory monitoring performance and PFC activity are associated with 5-HTTLPR genotype in older adults . Neuropsychologia . 50(9) : 2257 – 2270 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul EJ , Ashby FG . 2014. . A neurocomputational theory of how explicit learning bootstraps early procedural learning . Front Comput Neurosci . 7 : 177 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson R , McGeary JE , Maddox WT , Beevers CG . 2015. . Serotonin promoter polymorphism (5-HTTLPR) predicts biased attention for emotion stimuli Preliminary evidence of moderation by the social environment . Clin Psychol Sci . 1 – 7 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezawas L , Meyer-Lindenberg A , Drabant EM , Verchinski BA , Munoz KE , Kolachana BS , Egan MF , Mattay VS , Hariri AR , Weinberger DR . 2005. . 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression . Nat Neurosci . 8(6) : 828 – 834 . [DOI] [PubMed] [Google Scholar]
- Poldrack RA , Clark J , Pare-Blagoev EJ , Shohamy D , Creso Moyano J , Myers C , Gluck MA . 2001. . Interactive memory systems in the human brain . Nature . 414(6863) : 546 – 550 . [DOI] [PubMed] [Google Scholar]
- Poldrack RA , Foerde K . 2008. . Category learning and the memory systems debate . Neurosci Biobehav Rev . 32(2) : 197 – 205 . [DOI] [PubMed] [Google Scholar]
- Reber PJ , Gitelman DR , Parrish TB , Mesulam MM . 2003. . Dissociating explicit and implicit category knowledge with fMRI . J Cogn Neurosci . 15(4) : 574 – 583 . [DOI] [PubMed] [Google Scholar]
- Seger CA . 2008. . How do the basal ganglia contribute to categorization? Their roles in generalization, response selection, and learning via feedback . Neurosci Biobehav Rev . 32(2) : 265 – 278 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seger CA , Cincotta CM . 2006. . Dynamics of frontal, striatal, and hippocampal systems during rule learning . Cereb Cortex . 16(11) : 1546 – 1555 . [DOI] [PubMed] [Google Scholar]
- Seger CA , Cincotta CM . 2002. . Striatal activity in concept learning . Cogn Affect Behav Neurosci . 2(2) : 149 – 161 . [DOI] [PubMed] [Google Scholar]
- Seger CA , Cincotta CM . 2005. . The roles of the caudate nucleus in human classification learning . J Neurosci . 25(11) : 2941 – 2951 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvaraj S , Godlewska BR , Norbury R , Bose S , Turkheimer F , Stokes P , Rhodes R , Howes O , Cowen PJ . 2011. . Decreased regional gray matter volume in S’ allele carriers of the 5-HTTLPR triallelic polymorphism . Mol Psychiatry . 16(5) : 471 – 473 . [DOI] [PubMed] [Google Scholar]
- Seymour B , Daw ND , Roiser JP , Dayan P , Dolan R . 2012. . Serotonin selectively modulates reward value in human decision-making . J Neurosci . 32(17) : 5833 – 5842 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sham P . 1998. . Statistics in Human Genetics . New York, New York: : Arnold; . [Google Scholar]
- Sheehan DV , Lecrubier Y , Sheehan KH , Amorim P , Janavs J , Weiller E , Hergueta T , Baker R , Dunbar GC . 1998. . The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10 . J Clin Psychiatry . 59(Suppl 20) : 22 – 33 ; quiz 34–57 . [PubMed] [Google Scholar]
- Shohamy D , Myers CE , Grossman S , Sage J , Gluck MA , Poldrack RA . 2004. . Cortico-striatal contributions to feedback-based learning: converging data from neuroimaging and neuropsychology . Brain . 127(4) : 851 – 859 . [DOI] [PubMed] [Google Scholar]
- Smith JD , Berg ME , Cook RG , Murphy MS , Crossley MJ , Boomer J , Spiering B , Beran MJ , Church BA , Ashby FG et al. . 2012. . Implicit and explicit categorization: a tale of four species . Neurosci Biobehav Rev . 36(10) : 2355 – 2369 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JD , Boomer J , Zakrzewski AC , Roeder JL , Church BA , Ashby FG . 2013. . Deferred feedback sharply dissociates implicit and explicit category learning . Psychol Sci . 252 : 447 – 457 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiering BJ , Ashby FG . 2008. . Response processes in information-integration category learning . Neurobiol Learn Mem . 90(2) : 330 – 338 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam H , Maddox WT , Huang-Pollock CL . 2013. . Posterror slowing predicts rule-based but not information-integration category learning . Psychon Bull Rev . 20(6) : 1343 – 1349 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Team RC . 2014. . R: a language and environment for statistical computing. Vienna, Austria: R foundation for Statistical Computing, 2012: Open access Available from: URL http://cran.r-project.org .
- Waldron EM , Ashby FG . 2001. . The effects of concurrent task interference on category learning: Evidence for multiple category learning systems . Psychon Bull Rev . 8(1) : 168 – 176 . [DOI] [PubMed] [Google Scholar]
- Weiss EM , Schulter G , Fink A , Reiser EM , Mittenecker E , Niederstatter H , Nagl S , Parson W , Papousek I . 2014. . Influences of COMT and 5-HTTLPR polymorphisms on cognitive flexibility in healthy women: inhibition of prepotent responses and memory updating . PLoS One . 9(1) : e85506 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendland JR , Martin BJ , Kruse MR , Lesch KP , Murphy DL . 2006. . Simultaneous genotyping of four functional loci of human SLC6A4, with a reappraisal of 5-HTTLPR and rs25531 . Mol Psychiatry . 11(3) : 224 – 226 . [DOI] [PubMed] [Google Scholar]
- Worbe Y , Savulich G , de Wit S , Fernandez-Egea E , Robbins TW . 2015. . Tryptophan depletion promotes habitual over goal-directed control of appetitive responding in humans . Int J Neuropsychopharmacol . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi HG , Maddox WT , Mumford JA , Chandrasekaran B . 2014. . The role of corticostriatal systems in speech category learning . Cereb Cortex . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalsman G , Huang YY , Oquendo MA , Burke AK , Hu XZ , Brent DA , Ellis SP , Goldman D , Mann JJ . 2006. . Association of a triallelic serotonin transporter gene promoter region (5-HTTLPR) polymorphism with stressful life events and severity of depression . Am J Psychiatry . 163(9) : 1588 – 1593 . [DOI] [PubMed] [Google Scholar]
- Zeithamova D , Maddox WT . 2006. . Dual task interference in perceptual category learning . Mem Cogn . 34 : 387 – 398 . [DOI] [PubMed] [Google Scholar]
- Zeithamova D , Maddox WT . 2007. . The role of visuo-spatial and verbal working memory in perceptual category learning . Mem Cognit . 35(6) : 1380 – 1398 . [DOI] [PubMed] [Google Scholar]