SUMMARY
5-aminolevulinic acid-induced protoporphyrin IX fluorescence was authorized in the EU for visualization of tumor tissue during surgery for WHO grade III and IV gliomas in 2007. It facilitates tumor identification and doubles the number of gross total resections that can be achieved in these tumors. The growing acceptance of fluorescence-guided surgery in malignant gliomas brings forward a substantial yield of data on many types of intracranial lesions. The following review summarizes the main findings of these publications and illustrates the limitations, caveats and future perspectives of 5-aminolevulinic acid-induced fluorescence in malignant glioma as well as in other brain neoplasms.
Practice Points.
Protoporphyrin IX (PpIX) is a strong fluorophore and high concentrations of PpIX can be exploited to mark tumor cells.
PpIX accumulation in high-grade gliomas after premedication with 5-aminolevulinic acid has a positive predictive value of 95%.
PpIX fluorescence-guided surgery doubles the number of gross totally resected patients.
Approximately 80% of meningiomas accumulate PpIX regardless of tumor grade.
Dural tail and tumor infiltration into osseus structures can be visualized.
Inadequate tumor marking with 5-aminolevulinic acid is seen in brain metastasis.
Heterogeneous staining and a very high number of false-positive signals prevent diagnostic accuracy in brain metastasis.
Malignant gliomas are neoplasms known for their highly infiltrative growth and invasive potential. The role of extensive resection in the treatment of these tumors has been controversial. In recent years, level 2b evidence in favor of complete resection of enhancing tumor was established [1]. However, published rates for complete resection of malignant gliomas with the help of a microscope with white-light illumination and neuronavigation vary between 31 and 35% [2,3]. Tools to overcome these shortcomings have been investigated by neurosurgeons for decades – fluorescence techniques among them [4].
The first papers detailing the use of 5-aminolevulinic acid (5-ALA) for photodynamic diagnostics go back as far as 1979 [5] and applications to better detect tumors originating in the epidermis, mucosa of oral cavities, bile ducts, urothelium, bronchi and others, also for clinical use, began a mere decade later. In 1998 the first studies on 5-ALA protoporphyrin IX (PpIX) fluorescence with human glioma in vitro and in vivo were published [4,6–8]. In 2002, orphan drug status (EU/3/02/121) was granted by the European Commission for 5-ALA hydrochloride for fluorescence-guided resection of residual glioma. The subsequent Phase III trial demonstrated an almost doubled rate of successful gross total resection without an increase in severe adverse events [9]. Following these positive results, in September 2007, 5-ALA was authorized in the EU for the visualization of tumor tissue during surgery for WHO grade III and IV glioma under the name of Gliolan® (medac, Manchester, UK). Since then, fluorescence-guided resection has been embraced by hundreds of neurosurgeons, not only in Europe, but also in countries ranging from Taiwan to South America. Accordingly, the number of publications on fluorescence-guided surgery in brain tumors has grown from four or five in 2006 to more than 100 in 2012. The following review summarizes the main findings of these publications and illustrates the limitations, caveats and future perspectives of 5-ALA-induced fluorescence in malignant glioma, as well as in other brain neoplasms.
5-aminolevulinic acid
5-ALA is a precursor in the cellular heme biosynthesis regulated by a negative feedback mechanism through the control of ALA synthetase by free heme. The negative feedback mechanism can be overcome by providing the cells with an excess amount of exogenous 5-ALA, resulting in intracellular accumulation of fluorescent porphyrins – mainly PpIX – which cause the cells to become fluorescent and photosensitive. Probably the most relevant factors affecting the potential amount of PpIX accumulation within a cell are increased cellular uptake of 5-ALA, faster PpIX synthesis, reduced PpIX conversion into heme and low availability of ferrous iron [10–12]. As PpIX is a highly fluorescent substance (peak of emitted light at 635 nm) with a maximum excitation at 410 nm, high concentrations of PpIX can be exploited intraoperatively to mark and remove tumor cells by using blue light and a specialized filter that blocks most of the excitation light. 5-ALA itself does not show fluorescence and, as PpIX is a product of cell metabolism and mainly synthesized within the mitochondrium, PpIX does not contaminate the resection cavity during surgery and marks the tumor irrespective of brain shift.
High-grade glioma
A multicenter Phase III trial on 322 high-grade glioma (HGG) patients in Germany with a median follow-up of 35.4 months showed an increase of gross total resection from 36 to 65% in favor of PpIX fluorescence. Patients operated on with fluorescence subsequently demonstrated a progression-free survival of 41.0 (5-ALA), versus 21.1% (control), with no difference in the severity and frequencies of adverse events after 6 months [9].
The advantage of fluorescence-guided surgery over conventional white-light resection in HGG is due to the specific accumulation of PpIX in HGG cells. The selective intratumoral accumulation is a multifactorial process that probably begins with a blood–brain barrier leakage, allowing the 5-ALA molecule to penetrate into the tumor. Gadolinium enhancement (T1 MRI sequences) was shown to correlate with the amount of PpIX within tumor tissue, illustrating the significant role of blood–brain barrier disruption in PpIX accumulation [13]. However, factors such as enhanced microvascular density (MVD) within the tumor tissue, cellular proliferation, shortage of ferrous iron, and slowness and lower availability of ferrochelatase [10–12] in neoplastic cells, all contribute to the accuracy of marking malignant glioma with 5-ALA. Its specific accumulation defines a sharp border of fluorescence between tumor tissue and normal brain within a range of 3–12 h after administration of 5-ALA [6], which can be calculated as a signal intensity ratio between normal brain and tumor as high as 10:1 [7]. The potential to mark HGG cells with 5-ALA-induced fluorescence is further illustrated by a positive intraoperative tumor detection in 84.8% of cases (90% CI: 70.7–93.8%) giving the method a positive predictive value of 95% [14]. When compared with MRI, currently the gold standard in HGG imaging, fluorescence seems to mark tumor tissue more reliably than gadolinium or T2 sequences, and even equaled hematoxylin and eosin staining in an experimental setting with rats [15]. The intensity of the fluorescent signal correlates not only with cell density but also with cell proliferation and malignancy [16]. Signal intensity will therefore be high in areas of vital tumor, lower in areas of infiltrative tumor and lower in grade III than IV gliomas [17]. As vital cells are necessary to convert ALA into PpIX, no accumulation will be found in necrotic areas and hence no signal will mark these specific regions within the tumor. When using PpIX accumulation to guide the resection of HGG, areas of very strong fluorescence and sometimes areas of vague or faint fluorescence can be seen. Areas that display a strong signal in HGG are vital tumor and specificity here again is almost 100% [18]. The specificity decreases with a fading signal intensity as the tumor load within the area of infiltration drops down to the threshold limit of detection. Under optimal conditions with an operation microscope and standard fittings for a 400–440-nm excitation light source, that threshold of detection appears to equate to a concentration of 1 µg/ml of PpIX, which approximately corresponds to a tumor cellularity of 4.5% [19,20]. Thus, the area of detection limit defines a region where infiltration sometimes constitutes solitary glioma cell infiltration, for actively migrating HGG cells also contribute to the overall aspect of the fluorescent signal [21].
Notwithstanding these tumor-specific characteristics, in areas of weak fluorescence in HGGs approximately 3.8% of all biopsies are false positive [14]. To some extent, these false-positive signals originate from gliosis, increased reactive astrocytes [22] and macrophages, but they can also represent extracellular PpIX emitted/leaked from HGG cells [23]. Furthermore, seemingly non-neoplastic PpIX accumulation in HGG has been reported around blood vessels, which might be explained by a high availability of 5-ALA in areas of blood–brain barrier leakage [22], but could also be attributed to dose- and time-dependent increase of PpIX in the intima, media and adventitia of the arterial wall after systemic 5-ALA application [24]. MVD, temperature, pH and hypoxia all seem to play a part in extratumoral PpIX accumulation [25,26]. However, in HGG, false-positive signals seem to occur almost exclusively within areas of vague fluorescence and can thus be avoided when resection is limited to regions of strong fluorescence only.
Owing to the infiltrative characteristics of HGG disease, approximately two-thirds of nonfluorescent biopsies taken from the adjacent brain still contain a small number of tumor cells. We could increase sensitivity by calculating the contribution of these solitary HGG cells to the areal PpIX concentration with specialized optical devices. To lower the detection threshold for PpIX-emitted photons would increase the number of false-positive signals and thus potentially compromise the accuracy of resection and create a safety issue.
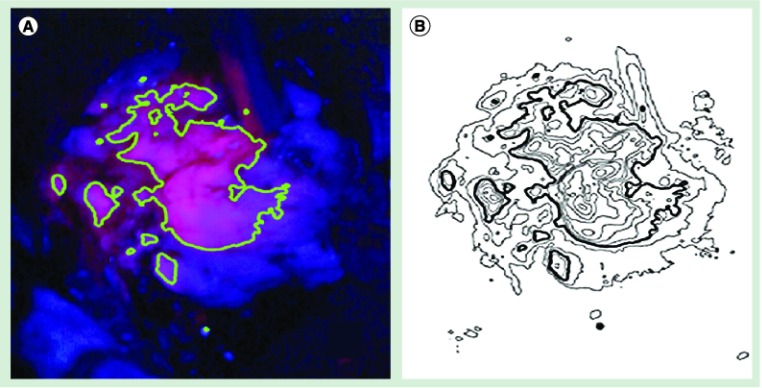
The idea to improve PpIX detection by using optical devices to quantify the emitted wavelength at 635 nm is intriguing and its feasibility has repeatedly been demonstrated [16,18]. However, as we already see a decline in specificity correlating to fading signal intensity in HGG, I personally think that optical devices should be used to reliably define a virtual border between solid tumor and infiltration (solid and vague fluorescence) and not to further increase sensitivity (Figure 1). By omitting the arbitrary and individualized judgment of different fluorescence qualities, fluorescence-guided tumor resection could be made more consistent and safer [18].
Figure 1. Continuous intraoperative protoporphyrin IX fluorescence signal quantification.
(A) The proposed border between solid and vague fluorescence as seen by the surgeon through the microscope [101]. (B) Intraoperative computerized analysis of the signal intensity from the surface of a glioblastoma multiforme at 630–640 nm. Among many other vectors, the computed signal intensity has to be adjusted for distance to light source and final photon detection, tissue optical properties and spatial reflectance (cavities). Based on these analytical calculations and the results from serial biopsies, estimations for the border between solid and infiltrating tumor can be given.
In HGG, PpIX fluorescence positively correlates to MVD, hypoxia, malignancy and proliferation, and thus, not only marks neoplastic cells but also shows the most aggressive areas within the tumor, unchallenged by brain shift or registration failures. Fluorescence can thus be used to detect anaplastic foci in gliomas of estimated lower grades or to confirm biopsy placement within regions of higher metabolism within low-grade gliomas (LGGs) as depicted by PET scans [27,28]. The high diagnostic accuracy of strongly fluorescent neoplastic tissue illustrated by a positive predictive value of 95% actually surpasses the accuracy of cryosections. 5-ALA can therefore be used for positive confirmation of stereotactic biopsy samples to reduce time and morbidity in these procedures. This procedure was initially reported in 2008 and its feasibility was recently confirmed and shown in two further series [18,29,30].
Low-grade glioma
Despite considerable success, 5-ALA-induced fluorescence imaging is currently only useful for tumors with relatively high PpIX accumulation. In tumors without significant blood–brain barrier leakage, without regional hypoxia or with low proliferation, with low enzymatic activities within the porphyrin cascade and low Fe2+ concentrations, accumulation of PpIX is equivalent to that of normal brain or measures only slightly higher. Residual background excitation blue light, used for intraoperative topographic discrimination and safer resection procedures, reduces the ability to detect visual fluorescence even more and hence reduces sensitivity. LGGs therefore are not candidates for conventional PpIX-guided surgery.
Interestingly, otherwise undetectable PpIX accumulation in LGG could be identified with confocal microscopy or fluorescence reflection measurements intraoperatively [31]. It is yet to be determined whether an increase in sensitivity reduces safety and diagnostic accuracy as in HGG, or if continuous reading of different quantitative optical data during the surgical procedure could be a solution for visualizing LGG. The possible impact on patients’ survival of a device that accurately marks LGG independent of brain shift and cumbersome intraoperative MRI can hardly be overestimated. It will certainly be worthwhile to go through the basic scientific process of gathering information on specific characteristics of PpIX metabolism in each of these tumors, for that sort of information will be necessary to interpret the acquired data and translate information into a usable image.
Meningiomas
Several case reports, as well as a number of small illustrative series where PpIX fluorescence was used for resection in meningioma patients have been published [32,33]. A strong, sometimes homogeneous fluorescence, often marked the tumor regardless of its histopathological subtype, tumor grading or proliferation indices [32]. As identification of meningiomas can usually be achieved macroscopically, it took the observation of positive fluorescence within areas of meningeal tumor infiltration and areas of bone invasion to spark interest in 5-ALA in these particular tumors [32,33]. The reason for this enthusiasm lies with the fact that an accurate visualization of tumor infiltration within hyperostotic areas not only could facilitate complete resection and avoid unnecessary openings at the skull base, but would also ultimately improve survival. To date, the extent of bone involvement in meningioma is impossible to delineate reliably [34]. Bone invasion, however, directly affects recurrence rates, morbidity and mortality in meningioma patients [35]. Tumor-specific biomarkers, such as 5-ALA-induced PpIX fluorescence, might not only provide a new visualization modality, but could have a substantial impact on mortality and morbidity in these surgically challenging tumors.
Preliminary data show deviations of fluorescence intensity after 5-ALA application between single meningioma cases [36], as well as differences in intensities on an intratumoral level. Cellular density and areas of densely packed collagenous fibres could be identified to explain some of the intratumoral signal heterogeneity [37]; compartmented vascular density and perfusion, along with regional chromosomal aberration, could be additional reasons. The differences in PpIX accumulation between benign meningioma cell lines were demonstrated to be mainly due to ferrochelatase activity and ferrochelatase synthesis; low ferrochelatase activity yields substantial PpIX accumulation within meningioma cells. Accordingly, substances that directly or indirectly influence ferrochelatase activity can be used to improve detectability of these tumors [38]. Detectability of PpIX in meningiomas can also be improved by optical devices, so that even within regions of nondistinguishable fluorescence, a difference in PpIX concentration can be recognized and possibly correlated to meningioma infiltration [36].
Meningiomas display different patterns of chromosomal and molecular changes that may have an impact on outcome and tumor recurrence [39]. With areas of intense, low and no fluorescent signal in meningiomas after 5-ALA application, the question of whether heterogeneity in fluorescence intensity and molecular heterogeneity correlate, to some degree could prove to be crucial in defining the usefulness of 5-ALA-induced fluorescence for resection control in meningiomas [40].
Other intracranial tumors
Over the years, misinterpretation of preoperative imaging has led to a number of cases where 5-ALA has erroneously been given to patients with a variety of intracranial lesions other than malignant glioma. Often these cases were retrospectively analyzed for clues of PpIX accumulation. Later, prospective studies, usually with a limited number of patients with all sorts of intracranial tumors, were conducted and showed that PpIX accumulation is not specific for malignant glioma. PpIX fluorescence after administration of 5-ALA occurs in lymphoma; melanoma; breast cancer, colon and ovarian carcinoma metastases; vasculitis; chordomas and hemangioblastomas; ependymoma (Figure 2); pituitary adenomas; and many other intracranial lesions [18,41–43]. It seems that, whenever pronounced gadolinium enhancement can be seen on MRI scans and cell proliferation within the tissue is high enough, a fluorescent signal of varying intensity can be detected approximately 4–5 h after administration of 5-ALA. There is, however, next to no information on the specificity and sensitivity in these tumor entities, nor can we be sure about the kinetics of PpIX accumulation within these tissues. However, false-positive staining of the surrounding normal brain is frequently reported, especially in cases of metastasis (Figure 3) [18,42]. Apart from proliferative microglia in the tumor's vicinity and a certain inflammatory reaction that can show intrinsic accumulation of PpIX and, therefore, account for these false positive sightings, neoplastic cells can also actively move PpIX into the extracellular matrix by way of ABCG2 membrane proteins or other porphyrin carriers [44]. Porphyrins are incapable of readily traversing the biological membranes because they carry negative charges and will, therefore, be extruded sooner, more frequently and in higher concentrations from all cells with a high density of ABCG2 carriers. The extracellular PpIX will then be transported by cerebrospinal fluid bulk flow into intact brain areas and cause detectable fluorescence far away from the neoplastic lesion.
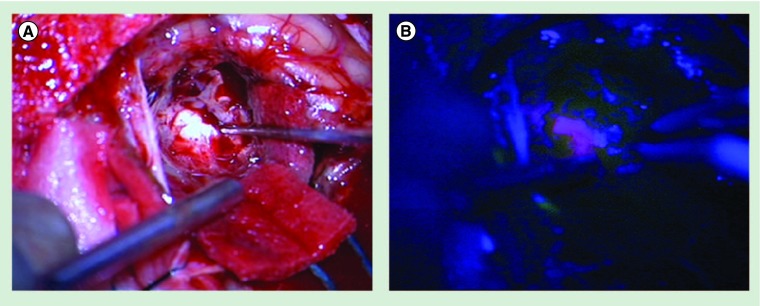
Figure 2. Intraoperative image after complete removal of a cerebellar breast cancer metastasis.
5-aminolevulinic acid was administered to the patient 5 h prior to surgery. (A) Picture taken with the help of the operation microscope under white-light illumination. Suction device and pointer of neuronavigation are visible. (B) Picture taken with the help of the operation microscope under 400-nm blue-light illumination. Suction device and bipolar forceps are visible. A positive protoporphyrin IX staining of the tumor-surrounding tissue can be noticed. The tissue proved to be tumor negative in subsequent histological analysis.
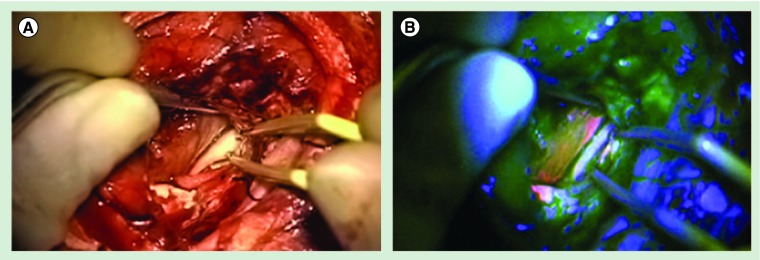
Figure 3. Ependymoma WHO grade III.
(A) Intraoperative view into the ventricle of the left hemisphere of the brain of a patient with ependymoma WHO grade III under white-light. Identical view through the operation microscope with switched-on blue excitation light. (B) The fluorescent surface of the ependymoma is clearly visible and easy to distinguish from the surrounding brain and ventricle's ependyma.
Reuptake through the outer cell membrane of the same porphyrins is then mainly carried out via low-density lipoprotein receptors [44,45]. The amount of extracellular and intracellular PpIX in a given intracranial lesion, therefore, is probably a function of time and the number (densities) of these export and import carriers on the cells’ surfaces. It is not uncommon during surgery to find the actual metastasis without recognizable fluorescence and the surrounding brain structures awash in PpIX. In these cases, to follow the fluorescent signal with the resection would lead to the removal of functional brain and eventually prevent recognition of the lesion itself. Therefore, PpIX fluorescence should not be used in tumors where basic knowledge about PpIX metabolism is not established. In addition, we have to take the sometimes enthusiastic reports of yet another tumor entity that fluoresces [42] with growing caution. Thorough basic science will be needed to establish the exact characteristics of each tumor so that we do not follow fluorescent signals blindly.
Conclusion & future perspective
5-ALA-induced PpIX fluorescence most accurately marks HGGs. By facilitating tumor identification 5-ALA doubles the number of gross total resections that can be achieved. As signal intensity correlates with cell density and malignancy, active fluorescence can also be used to guide biopsy to prevent sample bias and undergrading. For these reasons, PpIX fluorescence is quickly becoming a standard imaging technique in all suspected HGG cases at a growing number of neurosurgical centers. Inadequate tumor marking with 5-ALA is seen in metastasis, where heterogeneous staining of the tumor and a very high number of false-positive signals prevent diagnostic accuracy. In meningiomas, however, dural tail and tumor infiltration into osseus structures can often be visualized using 5-ALA. As these infiltrative parts are often the location of recurrent tumor, improved visualization by fluorescence could change the surgical routine in these challenging tumors. Heterogeneity of the intensity of the fluorescent signal within the tumor, however, is unresolved and larger multicenter studies are needed to clarify this subject. Optical devices to improve the sensitivity of PpIX detection and to quantify the signal in areas of nonrecognizable fluorescence, as well as substances to increase PpIX synthesis in meningioma cells, could either alone or in cooperation be a solution to this problem.
Increasing the sensitivity of detection by lowering the threshold for identification of PpIX could also be a way to identify LGGs. Different systems for quantification have already been tested and preliminary data for their use in LGG exist [16,18,101]. Critical interpretation of the available data, however, suggests that possibly more than one fluorescent tumor marker will have to be calculated to adequately identify and resect LGG.
As digital data acquisition and image interpretation with the available and growing low-cost computing power becomes feasible, it seems reasonable to suggest that the answer to oncological neurosurgery lies with a combination of many fluorescent tumor-specific markers. Different excitation light sources and their emitted spectra could simultaneously be analyzed through digital processing, which is incorporated into the surgeon's microscope, while the operation continues without interruption. The processed data would be used to create a virtual image of the tumor, which could then be transmitted into the surgeon's view as a semi-transparent overlay delivering accurate real-time information on tumor borders, but also instantaneous metabolic information on malignancy, heterogeneity, proliferation, and chemo- or radio-resistance of the tumor and parts thereof.
With 5-ALA we already have an almost perfect instrument to improve resection in HGG. Given these promising results, however, we strive to define additional optical characteristics and fluorescent substances to mark and guide the resection of additional intracranial tumors with equal reliability.
Footnotes
Financial & competing interests disclosure
The author has no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: ▪ of interest ▪▪ of considerable interest
- 1.Stummer W, Reulen HJ, Meinel T, et al. ALA-Glioma Study Group: extent of resection and survival in glioblastoma multiforme: identification of and adjustment for bias. Neurosurgery. 2008;62:564–576. doi: 10.1227/01.neu.0000317304.31579.17. [DOI] [PubMed] [Google Scholar]
- 2.Wirtz CR, Albert FK, Schwaderer M, et al. The benefit of neuronavigation for neurosurgery analyzed by its impact on glioblastoma surgery. Neurol. Res. 2000;22:354–360. doi: 10.1080/01616412.2000.11740684. [DOI] [PubMed] [Google Scholar]
- 3.McGirt MJ, Chaichana KL, Gathinji M, et al. Independent association of extent of resection with survival in patients with malignant brain astrocytoma. J. Neurosurg. 2009;110(1):156–162. doi: 10.3171/2008.4.17536. [DOI] [PubMed] [Google Scholar]
- 4.Hefti M, Mehdorn MH, Albert I, Dörner L. Fluorescence-guided surgery for malignant glioma: a review on aminolevulinic acid induced protoporphyrin IX photodynamic diagnostic in brain tumors. Curr. Med. Imaging Rev. 2010;6(4):254–258. [Google Scholar]
- 5.Malik Z, Djaldetti M. 5-aminolevulinic acid stimulation of porphyrin and hemoglobin synthesis by uninduced friend erythroleukemic cells. Cell Differ. 1979;8:223–233. doi: 10.1016/0045-6039(79)90049-6. [DOI] [PubMed] [Google Scholar]
- 6.Hebeda KM, Saarnak AE, Olivo M, et al. 5-aminolevulinic acid induced endogenous porphyrin fluorescence in 9L and C6 brain tumors and in the normal brain. Acta Neurochir. 1998;140:503–513. doi: 10.1007/s007010050132. [DOI] [PubMed] [Google Scholar]
- 7.Stummer W, Stocker S, Novotny A, et al. In vitro and in vivo porphyrin accumulation by C6 glioma cells after exposure to 5-aminolevulinic acid. J. Photochem. Photobiol. 1998;45:160–169. doi: 10.1016/s1011-1344(98)00176-6. [DOI] [PubMed] [Google Scholar]
- 8.Stummer W, Stocker S, Wagner S, et al. Intraoperative detection of malignant gliomas by 5-aminolevulinic acid-induced porphyrin fluorescence. Neurosurgery. 1998;42:518–526. doi: 10.1097/00006123-199803000-00017. [DOI] [PubMed] [Google Scholar]
- 9.Stummer W, Pichelmeyer U, Meinel T, et al. Fluorescence guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicenter Phase III trial. Lancet Oncol. 2006;7:392–401. doi: 10.1016/S1470-2045(06)70665-9. [DOI] [PubMed] [Google Scholar]; ▪▪ Phase III study for 5-aminolevulinic acid (5-ALA)-induced protoporphyrin IX (PpIX) fluorescence-guided resection of high-grade glioma. The study shows a significant benefit in terms of progression-free survival for patients with fluorescence-guided resection.
- 10.Peng Q, Warloe T, Berg K, et al. 5-aminolevulinic acid-based photodynamic therapy. Clinical research and future challenges. Cancer. 1997;79(12):2282–2308. doi: 10.1002/(sici)1097-0142(19970615)79:12<2282::aid-cncr2>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 11.Kondo M, Hirota N, Takaoka T, Kajiwara M. Heme-biosynthetic enzyme activities and porphyrin accumulation in normal liver and hepatoma cell lines of rat. Cell Biol. Toxicol. 1993;9(1):95–105. doi: 10.1007/BF00755143. [DOI] [PubMed] [Google Scholar]
- 12.Chang YZ, Qian ZM, Du JR, et al. Ceruloplasmin expression and its role in iron transport in C6 cells. Neurochem. Int. 2007;50(5):726–733. doi: 10.1016/j.neuint.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 13.Valdés PA, Moses ZB, Kim A, et al. Gadolinium- and 5-aminolevulinic acid-induced protoporphyrin IX levels in human gliomas: an ex vivo quantitative study to correlate protoporphyrin IX levels and blood–brain barrier breakdown. J. Neuropathol. Exp. Neurol. 2012;71(9):806–813. doi: 10.1097/NEN.0b013e31826775a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roberts DW, Valdes PA, Harris BT, et al. Coregistered fluorescence-enhanced tumor resection of malignant glioma: relationships between δ-aminolevulinic acid-induced protoporphyrin IX fluorescence, magnetic resonance imaging enhancement, and neuropathological parameters. J. Neurosurg. 2011;114(3):595–603. doi: 10.3171/2010.2.JNS091322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Samkoe KS, Gibbs-Strauss SL, Sexton KJ, et al. Protoporphyrin IX fluorescence contrast in invasive glioblastomas is linearly correlated with GD enhanced magnetic resonance imaging contrast but has higher diagnostic accuracy. J. Biomed. Opt. 2011;16(9):096008. doi: 10.1117/1.3622754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Valdes PA, Kim A, Brantsch M, et al. δ-aminolevulinic acid-induced protoporphyrin IX concentration correlates with histopathological markers of malignancy in human gliomas: the need for quantitative fluorescence-guided resection to identify regions of malignancy. Neuro Oncol. 2011;13(8):846–856. doi: 10.1093/neuonc/nor086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johansson A, Palte G, Schnell O, Tonn JC, Herms J, Stepp H. 5-aminolevulinic acid-induced protoporphyrin IX levels in tissue of human malignant brain tumors. Photochem. Photobiol. 2010;86(6):1373–1378. doi: 10.1111/j.1751-1097.2010.00799.x. [DOI] [PubMed] [Google Scholar]
- 18.Hefti M, von Campe G, Moschoplus M, et al. 5-aminolevulinic acid-induced protoporphyrin IX fluorescence in high grade glioma surgery. Swiss Med. Wkly. 2008;138:180–185. doi: 10.4414/smw.2008.12077. [DOI] [PubMed] [Google Scholar]; ▪ Study illustrating the use of PpIX fluorescence to confirm samples taken by stereotactic biopsies. The objective was to extend the indication of 5-ALA use beyond resection control.
- 19.Leblond F, Fontaine KM, Valdes P, et al. Brain tumor resection guided by fluorescence imaging. In: Kessel DH, editor. Optical Methods for Tumor Treatment and Detection: Mechanisms and Techniques in Photodynamic Therapy XVIII (Proceedings of SPIE Volume 7164) SPIE Press; WA, USA: 2009. [Google Scholar]
- 20.Chatillon CE, Petrecca K. Emerging imaging and operative techniques for glioma surgery. In: Mohan R, editor. Advances in Cancer Management. InTech; Rijeka, Croatia: 2012. [Google Scholar]
- 21.Albert I, Luginbuehl V, Looser H, Hefti M. 5-aminolevulinic acid induced photodynamic therapy in migratory glioma cells. Photodiagnosis Photodyn. Ther. 2011;8(2):166. [Google Scholar]
- 22.Ando T, Kobayashi E, Liao H, et al. Precise comparison of protoporphyrin IX fluorescence spectra with pathological results for brain tumor tissue identification. Brain Tumor Pathol. 2011;28(1):43–51. doi: 10.1007/s10014-010-0002-4. [DOI] [PubMed] [Google Scholar]
- 23.Utsuki S, Oka H, Sato S, et al. Histological examination of false positive tissue resection using 5-aminolevulinic acid-induced fluorescence guidance. Neurol. Med. Chir. 2007;47(5):210–214. doi: 10.2176/nmc.47.210. [DOI] [PubMed] [Google Scholar]
- 24.Webber J, Kessel D, Fromm D. Side effects and photosensitation of human tissues after aminolevulinic acid. J. Surg. Res. 1997;68:31–37. doi: 10.1006/jsre.1997.5004. [DOI] [PubMed] [Google Scholar]
- 25.Moan J, Berg K, Gadmar OB, Iani V, Ma L, Juzenas P. The temperature dependence of protoporphyrin IX production in cells and tissues. Photochem. Photobiol. 1999;70(4):669–673. [PubMed] [Google Scholar]
- 26.Friberg EG, Cunderlíková B, Pettersen EO, Moan J. pH effects on the cellular uptake of four photosensitizing drugs evaluated for the use of in photodynamic therapy in cancer. Cancer Lett. 2003;195:73–80. doi: 10.1016/s0304-3835(03)00150-2. [DOI] [PubMed] [Google Scholar]
- 27.Widhalm G, Wolfsberger S, Minchev G, et al. 5-aminolevulinic acid is a promising marker for detection of anaplastic foci in diffusely infiltrating gliomas with non-significant contrast enhancement. Cancer. 2010;116(6):1545–1552. doi: 10.1002/cncr.24903. [DOI] [PubMed] [Google Scholar]
- 28.Ewelt C, Floeth FW, Felsberg J, et al. Finding the anaplastic focus in diffuse glioma: the value of Gd-DTPA enhanced MRI, FET-PET, and intraoperative, ALA-derived tissue fluorescence. Clin. Neurol. Neurosurg. 2011;113(7):541–547. doi: 10.1016/j.clineuro.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 29.Von Campe G, Moschopulos M, Hefti M. 5-aminolevulinic acid-induced protoporphyrin IX fluorescence as immediate intraoperative indicator to improve the safety of malignant high grade brain tumor diagnosis in frameless stereotactic biopsy. Acta Neurochir. 2012;154(4):585–588. doi: 10.1007/s00701-012-1290-8. [DOI] [PMC free article] [PubMed] [Google Scholar]; ▪ Study illustrating the use of PpIX fluorescence to confirm samples taken by stereotactic biopsies. The objective was to extend the indication of 5-ALA use beyond resection control.
- 30.Widhalm G, Minchev G, Woehrer A, et al. Strong 5-aminolevulinic acid-induced fluorescence is a novel intraoperative marker for representative tissue samples in stereotactic brain tumor biopsies. Neurosurg. Rev. 2012;35(3):381–391. doi: 10.1007/s10143-012-0374-5. [DOI] [PubMed] [Google Scholar]; ▪ Study illustrating the use of PpIX fluorescence to confirm samples taken by stereotactic biopsies. The objective was to extend the indication of 5-ALA use beyond resection control.
- 31.Sanai N, Snyder LA, Honea NJ, et al. Intraoperative confocal microscopy in the visualization of 5-aminolevulinic acid fluorescence in low-grade gliomas. J. Neurosurg. 2011;115(4):740–748. doi: 10.3171/2011.6.JNS11252. [DOI] [PubMed] [Google Scholar]
- 32.Kajimoto Y, Kuriowa T, Miyatake S, et al. Use of 5-aminolevulinic acid in fluorescence-guided resection of meningioma with high risk of recurrence. Case report. J. Neurosurg. 2007;106:1070–1074. doi: 10.3171/jns.2007.106.6.1070. [DOI] [PubMed] [Google Scholar]
- 33.Morofuji Y, Matsuo T, Hayashi Y, Suyama K, Nagata I. Usefulness of intraoperative photodynamic diagnosis using 5-aminolevulinic acid for meningiomas with cranial invasion: technical case report. Neurosurgery. 2008;62(3 Suppl. 1):102–103. doi: 10.1227/01.neu.0000317378.22820.46. [DOI] [PubMed] [Google Scholar]
- 34.Pieper DR, Al-Mefty O, Hanada Y, Buechner D. Hyperostosis associated with meningioma of the cranial base: secondary changes of tumor invasion. Neurosurgery. 1999;44(4):742–747. doi: 10.1097/00006123-199904000-00028. [DOI] [PubMed] [Google Scholar]
- 35.Gabeau-Lacet D, Aghi M, Betensky RA, Barker FG, Loeffler JS, Louis DN. Bone involvement predicts outcome in atypical meningioma. J. Neurosurg. 2009;111(3):464–471. doi: 10.3171/2009.2.JNS08877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bekelis K, Valdes PA, Erkmen K, et al. Quantitative and qualitative 5 aminolevulinic acid-induced protoporphyrin IX fluorescence in skull base meningiomas. Neurosurg. Focus. 2011;30(5):E8. doi: 10.3171/2011.2.FOCUS1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hefti M. Comment concerning: intraoperative 5-aminolevulinic acid-induced fluorescence in meningiomas, Acta Neurochir DOI 10.1007/s00701–010–0708–0704, intratumoral heterogeneity and fluorescence intensity in meningioma after 5-ALA pretreatment. Acta Neurochir. 2011;153(4):959–960. doi: 10.1007/s00701-011-0950-4. [DOI] [PubMed] [Google Scholar]
- 38.Hefti M, Holenstein F, Albert I, Looser H, Luginbuehl V. Susceptibility to 5-aminolevulinic acid based photodynamic therapy in WHO I meningioma cells corresponds to ferrochelatase activity. Photochem. Photobiol. 2011;87(1):235–241. doi: 10.1111/j.1751-1097.2010.00821.x. [DOI] [PubMed] [Google Scholar]
- 39.Scheck AC, Shapiro JR, Coons SW, Norman SA, Johnson PC. Biological and molecular analysis of a low grade recurrence of glioblastoma multiforme. Clin. Cancer Res. 1996;2:187–199. [PubMed] [Google Scholar]
- 40.Whitson WJ, Valdes PA, Harris BT, Paulsen KD, Roberts DW. Confocal microscopy for the histologic fluorescence pattern of a recurrent atypical meningioma. Neurosurgery. 2011;68(6):1768–1773. doi: 10.1227/NEU.0b013e318217163c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eljamel MS, Leese G, Moseley HJ. Intraoperative optical identification of pituitary adenomas. Neurooncology. 2009;92(3):417–421. doi: 10.1007/s11060-009-9820-9. [DOI] [PubMed] [Google Scholar]
- 42.Kamp MA, Grosser P, Felsberg J, et al. 5-aminolevulinic acid (5-ALA)-induced fluorescence in intracerebral metastases: a retrospective study. Acta Neurochir. 2012;154(2):223–228. doi: 10.1007/s00701-011-1200-5. [DOI] [PubMed] [Google Scholar]
- 43.Utsuki S, Oka H, Kijima C, Miyajima Y, Hagiwara H, Fujii K. Utility of intraoperative fluorescent diagnosis of residual hemangioblastoma in using 5-aminolevulinic acid. Neurol. India. 2011;59(4):612–615. doi: 10.4103/0028-3886.84349. [DOI] [PubMed] [Google Scholar]
- 44.Krishnamurthy P, Xie T, Schuetz JD. The role of transporters in cellular heme and porphyrin homeostasis. Pharmacol. Ther. 2007;114(3):345–358. doi: 10.1016/j.pharmthera.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 45.Shibata Y, Matsumura A, Yoshida F, et al. Competitive uptake of porphyrin in LDL via LDL receptor in glioma cell lines: flow cytometric analysis. Cancer Lett. 2001;166(1):79–87. doi: 10.1016/s0304-3835(00)00717-5. [DOI] [PubMed] [Google Scholar]
▪ Patent
- 101.Hefti M, Looser H, Landolt H. 2009. US20090202119.