Abstract
Several prokaryotic and eukaryotic expression systems have been used for in vitro production of viruses’ proteins. However eukaryotic expression system was always the first choice for production of proteins that undergo post-translational modification such as glycosylation. Recombinant baculoviruses have been widely used as safe vectors to express heterologous genes in the culture of insect cells, but the manipulation involved in creating, titrating, and amplifying viral stocks make it time consuming and laborious. Therefore, to facilitate rapid expression in insect cell, a plasmid based expression system was used to express herpes simplex type 1 glycoprotein D (HSV-1 gD) and varicella zoster glycoprotein E (VZV gE). Recombinant plasmids were generated, transfected into insect cells (SF9), and both glycoproteins were expressed 48 h post-infection. A protein with approximately molecular weight of 64-kDa and 98-kDa for HSV-1 gD and VZV gE respectively was expressed and confirmed by SDS. Proteins were detected in insect cells cytoplasm and outer membrane by immunofluorescence. The antigenicity and immunoreactivity of each protein were confirmed by immunoblot and ELISA. Results suggest that this system can be an alternative to the traditional baculovirus expression for small scale expression system in insect cells.
Keywords: HSV, VZV, Baculovirus expression system, InsectDirect system
1. Introduction
Herpes simplex viruses (HSV) and varicella-zoster virus (VZV) are responsible for a wide range of human diseases with overlapping degree in clinical presentations and the human pathogen that can produce a wide range of mucocutaneous manifestations. The infections extending from skin and genital lesions to the central nervous system, can be caused by bacteria, viruses, fungi, or parasites (Wong et al., 2016). The biological and scientific nature of the disorders caused by these viruses has been elucidated, at least in part, during the past 150 years. The researchers are proving the infectious nature of the pathogens, understanding the basic concepts of latency and reactivation, devising specific antiviral agents, and sequencing the entire viral genomes were all products of the biological and molecular revolution of biomedical research of the 20th century. Although herpes infections do not attract the level of public attention that they did before the era of HIV/AIDS, they are still of major medical importance in the realms of dermatology, paediatrics, infectious diseases, obstetrics and gynaecology, and neurology (Steiner et al., 2007). Herpes simplex type 1 is responsible for 90–95% of cases while herpes simplex type 2 (HSV-2) contributes the remaining 5–10% of herpes simplex encephalitis (HSE) cases (Stone and Hawkins, 2007). Meningitis, encephalitis, and myelitis are caused by HSV viruses in the CNS disease. HSV-2 has more often been linked to recurrent aseptic meningitis than HSV-1 and causes neurological complications more often than most other viruses. However, HSV-2 meningitis has also been recognised as a significant cause of morbidity and mortality in immunocompromised patients (Akya et al., 2015).
VZV a member of herpesvirus family causes chickenpox (varicella), generally in infants, may reactivate decades later to produce shingles (zoster). The neurological complications of VZV are rarely seen during the primary infection (varicella cerebellitis) and more often during the reactivation phase. However, most severe neurological complications occur in immunocompromised patients and include aseptic meningitis, encephalitis with vasculitis, ventriculitis, severe necrotising myelitis, post-herpetic neuralgia and leukoencephalopathy (Corti et al., 2015).
Acute disseminated encephalomyelitis (ADEM) is an uncommon disorder of the central nervous system that occurs after viral illness or vaccination. ADEM resulting from VZV vaccination is estimated to occur in 1 in every 1000 cases of ADEM (Idrissova et al., 2003). Symptoms usually occur after days (2–30 days) of neurological illness and are characterised by an acute onset of focal neurological signs and encephalopathy (Sonneville et al., 2009). Symptoms include; neck stiffness, seizures and fever, and although the latter is not a constant feature, it is present in 75% of severe cases (Sonneville et al., 2009). Clinical diagnosis of these infections is mostly based on the presence of characteristic vascular eruption; however, it can be similar to other viral or nonviral skin infections (Crum-Cianflone, 2008) Therefore, confirmatory test is needed, this can be done by a number of laboratory tests such as virus isolation, direct or indirect immunofluorescence, detecting viral DNA by polymerase chain reaction (PCR), and detecting antibody responses to the viruses using enzyme-linked immunosorbant assay (ELISA). Serological assays using viral antigen play a role in diagnosis and epidemiological studies, however these assays are varying in sensitivity, specificity and in the methodology for detecting HSV and VZV antibody.
Over the past 20 years, the baculovirus expression system has become one of the most popular vehicles for the production of large quantities of recombinant protein. Baculovirus protein expression is a eukaryotic based expression system and thus offers protein modification and a processing pattern similar to those of higher eukaryotic cells. Although recent advances in baculovirus technology include the development of a wide variety of transfer vectors and cloning methods, simplified recombinant virus isolation, quantification methods, and development of cell culture technology have improved. However, the manipulation involved in creating, titrating, and amplifying viral stocks makes it time consuming and laborious. Therefore, in this study a plasmid based expression system InsectDirect (Novagen, Merck, USA) was used to express the full length of HSV-1 gD and VZV gE recombinant glycoproteins in insect cells. The present study demonstrates that the InsectDirect system employs an optimal expression vector with high efficiency for transfection and it seems to be an ideal system for rapid expression of virus glycoproteins in insect cells.
2. Materials and methods
2.1. Genotyping of HSV-1 gD and VZV gE DNA fragment
Genotyping was performed with specific primers used to amplify the full open reading frame of each DNA region encodes HSV-1 gD and VZV gE proteins, for HSV-1 gD a sense primer HSVgDF (5′-CAGGGACCCGGTATGGGGGGGGCT-3′) and reverse primer HSVgDR (5′-GGCACCAGAGCGTTCTAGTAAAACAAGGGCTGG-3′), for VZV gE, forward primer VZVgEF (5′-CAGGGACCCGGTATGGGGACAGTTAATAAA-3′) and reverse primer VZVgER (5′-GGCACCAGAGCGTTTCACCGGGTCTTATCTAT-3′). To generate vector specific compatible overhangs, the forward and reverse primers were incorporated with victor specific sequence (under lined). Master mix reaction were consisting of 45 μl [5 μl 10 × buffer (50 mM KCl, 1.5 mM MgCl2 and10 mM Tris–HCl; pH 8.3), 1 μl (10 mM) of dNTP, 1 μl of each oligonucleotide primer, 36.5 μl SDW and 0.5 U of AmpliTaq Gold polymerase]. 5 μl of each sample were added including a negative control to a final volume of 50 μl. The cycling programme used included a single initial denaturation at 94 °C for 6 min followed by 40 cycles of denaturation at 94 °C for 1 min, annealing at 58 °C for 1 min, extension at 72 °C for 1 min and a final step of 72 °C for 5 min. Amplification of VZV gE was carried out as above except the extension time was extended to 1.30 min at 72 °C. 5 μl of each sample were added including a negative control to a final volume of 50 μl. PCR products were then purified using a modified Microcon centrifuge method (YM-100 Centrifugal F Micron YM-100 Kit) and analysed by 2% agarose gel electrophoresis (Heaton et al., 2015).
2.2. Recombinant plasmid construction
To construct the recombinant plasmid, the ligation-independent cloning (LIC) strategy was used. Briefly, the insert with overhangs prepared by PCR was treated with T4 DNA polymerase in the presence of dATP, and then annealed with the pIEx/Bac-3 3C/LIC vector (Novagen, Merck, USA) containing the hr5 (homologous region 5) enhancer and the ie1 (immediate early 1) promoter from Autographa californica nuclear polyhedrosis virus (AcNPV). Then, the annealed complex was transformed into E. coli host (NovaBlue GigaSingles™ Competent Cells, K-12 strain (Novagen, Merck, USA). Screening for the recombinant plasmid was carried out using PCR, in one stage, vector-specific primers (IE1 Promoter and IE1 Terminator) were used at the both ends, and in other stages one vector-specific primer was used in combination with HSV-1 gD or VZV gE gene primers. Recombinant plasmid was then purified using Mobius™ 200 kit (Novagen, Merck, USA), and then analysed using 1% agarose gel electrophoresis in Tris–phosphate buffer and then stained with ethidium bromide solution for 10 min and viewed in an ultraviolet transilluminator.
2.3. Insect cell transfection
Protein expression of each virus was carried out using a 10 ml suspension culture of Spodoptera frugiperda (SF9) cells (Novagen, Merck, USA). Briefly 8 ml of fresh dilution of SF9 cells (1 × 107 viable cells/ml) were prepared from an exponentially growing shake culture. In a 1.5 ml tube 20 μl of plasmid DNA (20 μg) was diluted in 1 ml of BacVector® Insect Cell Medium (Novagen, Merck, USA). At the same time a 100 μl of GeneJuice Transfection reagent (Novagen property) was diluted in 1 ml of the same media in a 1.5 ml tube. The diluted plasmid DNA was then added dropwise to the diluted GeneJuice Transfection reagent and was mixed gently to avoid precipitation, and then left at room temperature for 15 min. Finally, the entire volume was added to the cells and incubated for 48 h at 150 rpm on orbital shakers at 28 °C with caps containing 0.2 μm filter until maximum target protein expression was achieved (2–3 days).
2.4. Proteins solubilisation
Since a large amount of the full glycosylated form of HSV-1 gD and VZV gE was integrated into SF9 outer membrane, therefore extracting the proteins from cells membrane, was an essential step in protein purification. Earlier study by Wang et al. (2006) was performed in which the protein purification carried out using the non-ionic detergent (Pentaethyleneglycolmonodecylether (C10E5)) and this study was performed with some minor modifications. Briefly, a suspension culture of SF9 cells (2.5 × 108) was transfected with the recombinant plasmid and then incubated for 2 days on orbital shakers at 28 °C. After incubation cells were harvested by low speed centrifugation (2000 g × 10 min), and washed 3 times with ice-cold phosphate buffer solution (PBS, pH 7.3). Cells pellet (5 × 107/ml) was then resuspended in an ice-could solubilising buffer (20 mM Tris–HC, pH 7.8, 2 mM Phenylmethylsulfonyl fluoride (PMSF), 1 mM Tosyl-Lys-chloromethylketone (TLCK)) and then the same volume of buffer containing 2% (v/v) of C10E5 was added (final detergent concentration 1%). Cells suspension was then incubated on ice for 2 h. After incubation cells debris was removed by low speed centrifugation (2000 g × 10 min) and supernatant was then ultracentrifugated (70000 g × 1 h, 4 °C). 50 μl of the final supernatant (containing solubilised protein) were stored at +4 °C for protein analysis and the remaining was used in the purification step.
2.5. Protein purification
Protein purification was carried out using his-bind purification kit (Novagen, Merck, USA). Briefly, in a 20 ml tube 500 μl of Ni–NTA his-binding resin was mixed with the remaining of the solubilised protein (∼10 ml). Tube was then placed on orbital shakers (150 rpm) at room temperature for 2 h. Purification column was assembled (chromatography column + 15 ml collecting tube), and then the entire volume was added into the reservoir of the column. Column was left to flow by gravity (∼5 to 10 min) and then transferred to a new collecting 15 ml tube and 4 ml of washing buffer was added to the column and left to flow. The washing step was repeated once and the column was transferred to a new collecting tube fallowed by the addition of 1 ml of elution buffer (Novagen property) was added. 50 μl of each tube were stored at +4 °C for protein analysis. The eluted protein was then stored in −70 C at 100 μl aliquot for protein characterisation.
2.6. Recombinant proteins characterisation [indirect immunofluorescence (IF)]
Indirect immunofluorescence analysis was performed using fluorescein-conjugated rabbit anti mouse IgG (cell surface, cytoplasmic). Briefly, to examine cell surface immunofluorescence, cells were incubated with mouse anti HSV gD or VZV gE monoclonal antibody for 1 h at 37 °C with gentle agitation, washed with PBS, fixed on Lab-Tek chamber slides with 100% cold acetone for 10 minutes, stained with fluorescein-conjugated rabbit anti-mouse IgG antibody for 1 h at 37 °C, and then rewashed. To examine intracellular (total cell) immunofluorescence, cells were washed with PBS, fixed on Lab-Tek chamber slides with acetone for 20 min and incubated with mouse anti gD or gE mouse monoclonal antibody for 1 h at 37 °C. Cells were then washed 3 times with PBS, stained with fluorescein-conjugated rabbit anti-mouse IgG antibody for 1 h at 37 °C washed again and left to air dry for 10 min. A negative control (non infected cells) was included in each slide for comparison. Slides were then examined with an incident light fluoresce microscope (Dialux 20 EB, Leitz Wetzlar, Germany).
2.7. Sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS–PAGE)
Proteins were separated by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS–PAGE) under denaturing reducing conditions. Briefly, 14 μl of sample (transfected cells (5 × 103/ml), solubilised protein, purification flow, first wash, second wash, and eluted protein) was mixed with sample loading buffer (4 μl of NuPAGE LDS sample buffer, 6 μl deionised water and 1 μl reducing agent). Samples were heated at 70 °C for 10 min and a 16 μl of each sample was analysed using NuPAGE 4–12% Bis–Tris gel (Invitrogen, Carlsbad, CA) for 45 min at 200V. Gel was then stained with SimpleyBlue™ SafeStain (Invitrogen), and then removed from the tank and placed into a plastic container, rinsed three times for 5 min with 100 ml deionised water to remove SDS and buffer salts. Excess water was drained and 20 ml of Coomassie Blue stain was added and kept on a shaker for 1 h at room temperature. For maximum staining sensitivity 20 ml of 20% NaCl was added to the same water and left on the shaker for another 2 h.
2.8. Immunoblot (western blot, dot blot)
Western blot analysis was carried out under denaturing reducing condition. After electrophoresis, proteins were transferred to a polyvinylidene difluoride (PVDF, Invitrogen, Carlsbad, CA). For dot blot 35 μl of each sample (transfected cells (5 × 103/ml), solubilised protein, purification flow, first wash, second wash, first elute and second elute) was transferred to dot blot manifold I (Schleicher & Schuell, Germany) and vacuum-absorbed onto a 0.45 μ nitrocellulose membrane. Each sample was washed three times with 100 μl of washing buffer (10 mM PBS, 0.05% Tween 20) and then air-dried. Both membranes were then blocked in blocking buffer (2.5 mg skimmed milk, 50 ml PBS Ph 7.3, 0.1% Tween 20) overnight at 4 °C. Membranes were then incubated with anti-HSV gD or VZV gE monoclonal antibody (Abcam) diluted in blocking buffer for 1 h at 37 °C with gentle agitation, followed by 3 washing steps each of 5 mentis and incubated with peroxidase-labelled antibody diluted in diluent buffer (10 mM PBS + 0.1% Tween) for 1 h at 37 °C. Each membrane was washed and then incubated with one tablet of SIGMA FASTTM (4-chloro-2-methylbenzenediazonium/3-hydroxy-2-naphthoic acid 2,4-dimethylanilide phosphate) dissolved in 10 ml of deionised water on a rotor at room temperature until the bands appeared. The membrane was rinsed with distiled water and left to dry on filter paper.
3. Results
3.1. Determining the sequence of gD of HSV-1 and VSV gE
Nine complete sequences for the full HSV-1 gD gene and 12 sequences for the VZV gE gene obtained from Gene Bank were analysed and compared with each other. Alignment analyses used the Bio Edit Sequence Alignment Editor Programme. Both HSV-1 gD and VZV gE sequences were found to be identical for all isolates. The full length of HSV-1 was 1185 bp spanning the region 138,419–139,603 bp of HSV-1 genome, and the full length of VZV gE was 1872 bp spanning the region 115808 to 117679 of the VZV genome. The sequence of HSV-1 gD (accession NC001348) and VZV gE (accession NC001348) gene was selected for this study.
3.2. Construction of recombinant plasmid
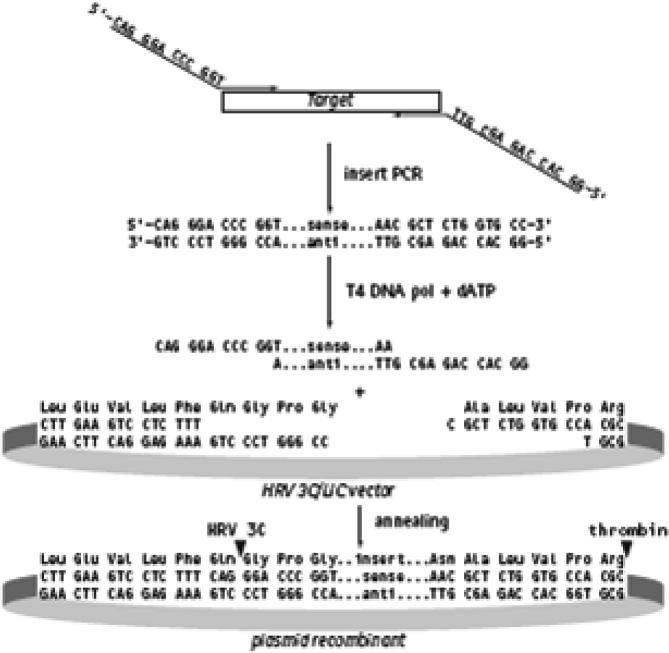
The strategy used for construction of the recombinant plasmids containing the entire length of HSV-1 gD (1185 bp) and VZV gE (1875 bp) DNA fragment is shown in Fig. 1. A starting codon (ATG) was incorporated in 5′-end after the overhang sequence of each forward primer and termination codon (TAG) at the 3′ end of each reverse primer for each virus and there were no HSV-1 or VZV noncoding sequences present after the termination codon. The complete DNA fragments of each virus plus the overhang sequence were then generated by PCR and annealed within the 5LIC cloning site of the pIEx/Bac-3 3C/LIC vector. PCR secreting for the recombinant plasmid using victor-specific primer or in combination with insert-specific primer has confirmed that PCR product was inserted into the cloning site in the right orientation. The plasmid vector is incorporated with 10-His. Tag sequence at the N-terminal to allow protein
Figure 1.
This figure represents the details of the 3C/LIC cloning strategy, PCR product containing the 5LIC extension were treated with LIC-qualified T4 DNA polymerase in the presence of dATB, and then annealed to the 3C/LIC vector.
3.3. Purification by affinity chromatography (Plasmid DNA Purity and Concentration)
Plasmid concentration was determined using UV spectrophotometry. The plasmid DNA yield with A260/A280 absorbance >1.7 was found to be 25 μg/ml and 30 μg/ml for HSV-1 gD and VZV gE respectively. The plasmid copy number for each virus insert was calculated using the formula: Plasmid molecular weight = Number of base pairs × molecular mass of deoxynucleotide base pair For HSV-1 gD plasmid the molecular weight of the pIEx/Bac-3 3C/LIC (6763 bp) plus HSV-1 gD (1242) = (6.763 + 1.242) × 103 × 660 = 5.28 × 106 Dalton. Each 1 Mole of plasmid contains 5.28 × 106 g and with Avogadro’s number = 6.023 × 1023 then each plasmid contains 6.023 × 1023 molecules Using the UV spectrophotometer the concentration of HSV-1 gD plasmid was found to be 25 μg/ml, therefore: 25 μg of recombinant plasmid contains: 6.023 × 1023 × 20/5.28 × 106 × 106 = 22.81 × 1011 copy/ml (11.4 × 109 copy/5 μl). For VZV gE plasmid the molecular weight the pIEx/Bac-3 3C/LIC (6763 bp) plus VZV gE (1934) = (6.763 + 1.934) × 103 × 660 = 5.74 × 106 Dalton; 30 μg of recombinant plasmid contains: 6.023 × 1023 × 20/5.74 × 106 × 106 = 20.99 × 1011 copy/ml (10.5 × 109 copy/5 μl).
3.4. InsectDirect™ system
In the InsectDirect™ expression system HSV-1 gD and VZV gE were expressed using 6 well plates and 10 ml suspension cultures. Cells were examined daily (24, 48 and 72 h p.i) no clear signs of infection were observed in comparison with uninfected cells. Transfected cells continued to grow normally and beyond confluency, as they were not subjected to contact inhibition. However, after 72 h p.i signs resembling those of an unhealthy culture, such as floating cells, appearance of granules and cells lysis were observed in both transfected and non-transfected cells. Protein expression was observed by 48 h (50–65% of cells) and maximum protein expression was found at 72 h p.i (95% of cells) as assessed by immunofluorescence assay.
3.5. Protein purification and quantification
Since the large amount of the fully glycosylated form of HSV-1 gD and VZV gE was found to be integrated into the outer membrane of SF9 cells, extraction of the proteins from the cell membrane was considered an essential step in protein purification. A number of methods were evaluated such as disruption of cell membrane with glass beads or solubilising membrane protein with detergents. Use of a non-ionic detergent (C10E5) was found to be the most suitable method. Protein quantification results using the BCA assay showed that yields of purified protein from the small scale suspension cultures of the InsectDirect™ expression system ranged from 25 to 60 μg/10 ml, whereas 130 to 210 μg/10 ml was obtained with the BacMagic expression system. For large scale protein expression; the purified protein yield of the BacMagic expression system ranged from 0.5 to 1.5 mg/100 ml. An example of the protein quantification method and the mechanism of action of detergent on integral and peripheral membrane protein.
3.6. Recombinant proteins characterisation (analysis of immunofluorescence)
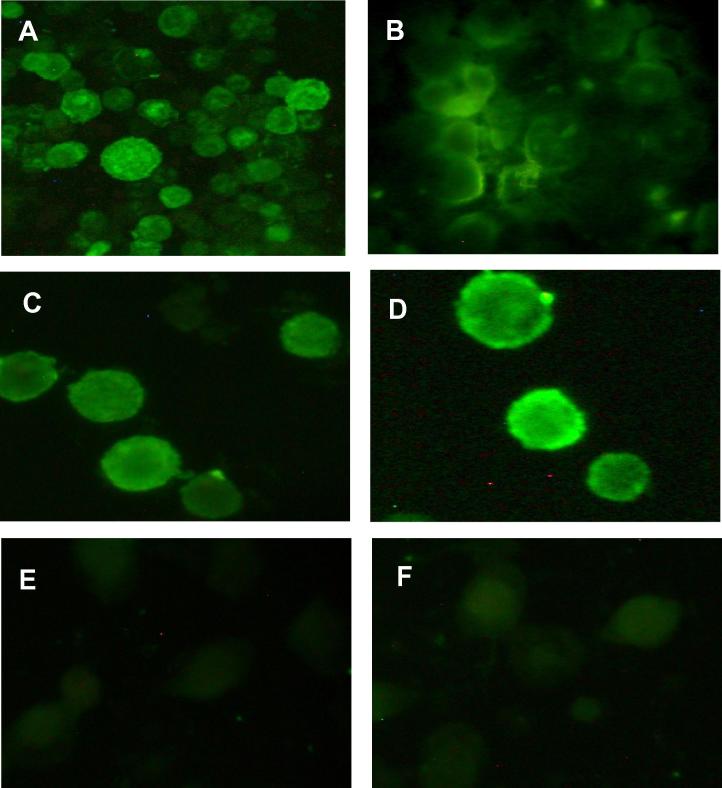
To determine the efficiency of protein expression and the localisation of the expressed proteins an indirect immunofluorescence staining was carried out using anti HSV gD and VZV gE monoclonal antibody (Fig. 3). Both expressed glycoproteins were detected in the cytoplasms of the acetone-fixed transfected cells (Fig.2A and C) and at cells membrane of the unfixed cells (Fig.2B and D). To determine the specify of the staining in the assay, a transfected cell with recombinant plasmid containing HSV gD were reacted with VZV gE monoclonal antibody and vice versa, however no staining was observed (Fig.2E) in comparison with negative control (Fig.2F). Expressed proteins were observed at 48 h p.i with a percentage of positive cells that ranged from 50% to 75%. However, it was best observed at 72 h (95%), therefore this time were selected for protein expression experiments.
Figure 3.
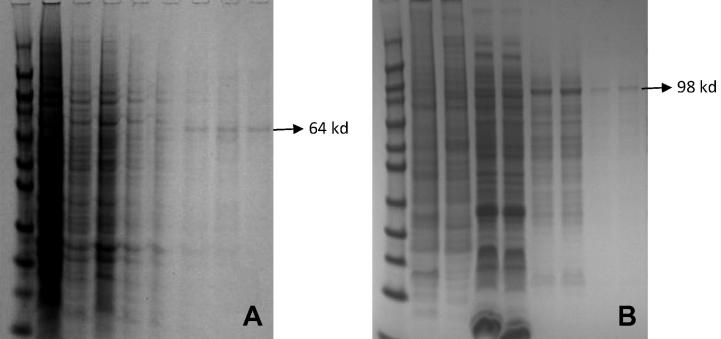
(A) Time course of HSV-1 gD expression analysed by SDS–PAGE. Lane 1: molecular weight marker; Lane 2–5: 2–5 day p.i.
Figure 2.
Expressions of HSV-1 gD and VZV gE in insect cells, cells were infected with recombinant plasmid. At 72 h p.i, transfected cells were analysed for synthesis and localisation of the viral glycoproteins within the cell and at the cell surface. Expressed glycoproteins gD and gE were detected in the cytoplasms of the acetone-fixed transfected cells (A and C) and on cells membrane of the unfixed cells (B and D). Neither antibody was reacted with the negative control (E and F).
3.7. SDS–PAGE and immunoblot analysis
The effects of various harvesting time duration for the production of recombinant proteins were examined by SDS–PAGE analysis. SF9 cells were transfected with each recombinant plasmids and the synthesis of each protein was analysed using SDS–PAGE at different harvesting time points (48, 72 and 96 h). Results showed that both recombinant proteins were expressed in SF9 cells at 48 h; however, the peak of expression was obtained at 72 and 96 h p.i. Coomassie blue-stained SDS–PAGE showed that recombinant HSV-1 gD and VZV gE appeared as single bands with a molecular weight of 64-kDa and 98-kDa respectively. Western blot and dot blot analysis of the expressed proteins using monoclonal anti HSV-1 gD and VZV gE has also showed that each protein was expressed in SF9 cell. In comparison with the molecular weight markers, HSV-1 gD and VZV gE appeared as 64-kDa and 98-kDa respectively. SDS–PAGE and immune blot analysis of recombinant infected cell medium did not detect any of the expressed proteins. This suggests that the expressed proteins are retained in the cell or in the cell membrane and it is not secreted into the medium (Fig. 4).
Figure 4.
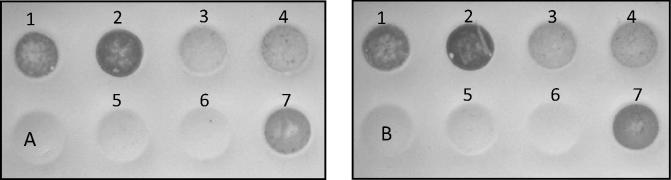
Fig. 3 dot blot analyses of HSV-1 gD (A) and VZV gE (B) recombinant protein, samples were transferred to dot blot manifold and vacuum-absorbed onto a 0.45 μ nitrocellulose membrane, incubated with the selected antibody and then incubated with peroxidase-labelled antibody. Lane 1 transfected cells; Lane 2 solubilised protein; Lane 3 purification flow; Lane 4 first wash; Lane 5 negative control (non-infected cells were subjected to the same procedure of the purification process); Lane 6 second wash; Lane 7 eluted recombinant protein.
4. Discussion
Conventional methods for detecting CNS infection with alpha herpesviruses (HSV-1, HSV-2, VZV) and determining intrathecal IgG antibody productions are time and sample consuming. They can exhaust up to 1 ml of sample and the assays may take more than 24 h to produce results. A more rapid assay requiring minimal sample volume and time to produce results would therefore be an important diagnostic tool for use in patients with CNS infection. The overarching aim of the present study was to improve the diagnosis of viral CNS infections.
In this current study, we assisted a novel way for protein expression system termed as InsectDirect. A number of prokaryotic and eukaryotic expression systems have been used for in vitro production of HSV gD and VZV gE including bacteria (Watson et al., 1982), yeast (Stanberry et al., 1987, van Kooij et al., 2002), Vaccinia (Paoletti et al., 1984), mammalian cells (Stanberry and Myers, 1988, Haumont et al., 1996) and baculovirus (Krishna et al., 1989, Ghiasi et al., 1991, Landolfi et al., 1993, Sisk et al., 1994, Kimura et al., 1997, Ikoma et al., 2002). Although E. coli and yeast expression systems have been used for the last 20 years as an invaluable resource for production of large quantities of proteins, expressed by these systems lack many of the post translational modifications associated with proteins produced by eukaryotic cells (glycosylation and cleavage). Other systems are also limited in terms of safety and the quantity of recombinant protein produced. However, baculovirus expression systems have been found to be the most useful system to produce fully functional glycosylated glycoproteins similar to their authentic counterparts from mammalian cells (Fotouhi et al., 2008).
This study showed that HSV-1 gD was expressed in insect cells, and appeared as 48 kd, however it was slightly smaller than virion HSV-1 gD made in baby hamster kidney (58 kd). The study found that antibody taken from mice infected with HSV-1 reacted with both proteins. Immunisation of mice with an extract of the expressed gD induced a high titre of complement and independent neutralising antibody (Krishna et al., 1989). In a further study to investigate the possibility of using baculovirus to express a large quantity of HSV-1 gD, Ghiasi et al. (1991), constructed a recombinant baculovirus containing the full-length of HSV-1 gD using HSV-1 strain KOS as the template. In the study, SF9 cells were transfected and the recombinant protein was purified using immunoaffinity selection. Characterisation of the recombinant protein was carried out using SDS, immunofluorescence and western blot. The study showed that recombinant protein was expressed, transported to the membrane of SF9 cells and reacted with gD specific antibody. Although the expressed protein was slightly bigger than that reported previously (52 kd), it still appeared to be slightly smaller than HSV-1 gD made in Vero cells. Landolfi et al. (1993) also expressed the full-length of HSV-2 gD, aiming to study the viability of using gD2 as a vaccine subunit.
Although the baculovirus system has been widely used for the production and study of structural and non-structural proteins of a number of viruses including, human polyomavirus, poliovirus, influenza virus, human papillomavirus and herpesvirus (Possee, 1986, Neufeld et al., 1991, Ghiasi et al., 1991, Sisk et al., 1994 Landolfi et al., 1993, Ghiasi et al., 1994, Kimura et al., 1997, Kimura et al., 1998, Ikoma et al., 2002, Giavedoni, 2005, Hsu et al., 2009) traditional baculovirus expression methods have several disadvantages in terms of low frequency of recombination, protein production and the requirement for several rounds of plaque purification (Fotouhi et al., 2008).
The InsectDirect System is a plasmid-based expression system that enables rapid small-scale expression in insect cells. It can be scaled up to isolate milligram quantities of target protein without the need to generate a recombinant baculovirus and this expression system employ a combination of expression vector, high efficiency transfection reagent, lysis reagents and Ni–NTA His-Bind Resin for affinity purification of his-tag fusion proteins (Loomis et al., 2005). Immunoreactivity of the recombinant proteins was also studied by immunoblot (western blot and dot blot) using monoclonal antibody targeted against each protein. In immunoblot, samples (cell lysate), solubilised protein, purification flow and wash, transfection media and negative control (solubilised protein from uninfected cells) were analysed using the appropriate monoclonal antibody. Results showed that cell lysate, solubilised proteins and purified proteins samples reacted with the monoclonal antibody, whereas the negative control as well as the media of the transfected cells did not. This confirmed that both proteins were expressed and either retained in the cell or cell membrane and not secreted into the medium. Such results are in accordance with previous studies (Ghiasi et al., 1991, Wu and Forghani, 1997, Fotouhi et al., 2008). Western blot results showed that gD and gE were expressed and present as proteins of relative molecular weight of 64 kd and 98 kd respectively. In the case of HSV-1 gD the observed size (64 kd) was larger than any previously reported recombinant protein expressed in insect cells using other baculovirus expression systems (Krishna et al., 1989, Ghiasi et al., 1991, Fotouhi et al., 2008). Such results suggest that in this project the expressed gD underwent more extensive post-translation addition of carbohydrate than other reported proteins using this system. In the case of VZV gE the observed size (98 kd) was similar to that reported by Wu and Forghani (1997) and bigger than that reported by Olson et al. (1997). In both studies VZV gE was expressed using traditional baculovirus systems under the control of the polyhedron promoter. The results obtained from immunoblot and IF assays confirmed that these recombinant proteins were antigenically and functionally similar to their authentic counterparts, therefore production and purification of these proteins was carried out. As stated previously, one of the unique features of using this expression system is the simplicity of purification of the recombinant protein. The plasmid contains a 10 polyhistidine tag sequence upstream of the cloning site (3C/LIC). In this case the fused protein will be expressed with an N-terminal his-tag, which can be used later to purify the expressed protein using the nickel-nitrilotriacetic acid (Ni–NTA) system. In addition, expression of the target protein immediately downstream of the HRV 3C protease cleavage site helps the removal of the encoded fused sequences after purification of the recombinant protein if it is needed. In general, HSV-1 gD and VZV gE glycoproteins were expressed as early as 2 days p.i, yields the same protein with no differences in size or immunoreactivity. The only differences found between the two systems were the protein yields. Such results were expected because each system was originally designed for different purposes.
5. Conclusion
Although the InsectDirect system protein yield can be scaled up by using several culture bottles, it is not cost effective for production of recombinant protein in large quantities, since reagents such as plasmid purification kits and insect GeneJuice are required constantly for protein expression. Results suggest that this system can be an alternative to the traditional baculovirus expression for small scale expression screening system in insect cells.
Footnotes
Peer review under responsibility of King Saud University.
References
- Akya A., Ahmadi K., Zehtabian S., Salimi A., Elahi A., Madani S.H. Study of the frequency of herpesvirus infections among patients suspected aseptic meningitis in the west of Iran. Jundishapur J. Microbiol. 2015;8(10):e22639. doi: 10.5812/jjm.22639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corti M., Villafañe M.F., Vittar N., Banco M.C., Priarone M., Mammana L., Gilardi L. Meningoencephalitis due to varicella zoster virus in aids patients. Report of eleven cases and review of the literature. Rev. Inst. Med. Trop. Sao Paulo. 2015;57(6):505–508. doi: 10.1590/S0036-46652015000600007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crum-Cianflone N.F. Bacterial, fungal, parasitic, and viral myositis. Clin. Microbiol. Rev. 2008;21(3):473–494. doi: 10.1128/CMR.00001-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotouhi F., Soleimanjahi H., Roostaee M.H., Dalimi Asl.A. Expression of the herpes simplex virus type 2 glycoprotein D in baculovirus expression system and evaluation of its immunogenicity in guinea pigs. Iran. Biomed. J. 2008;12(2):59–66. [PubMed] [Google Scholar]
- Ghiasi H., Kaiwar R., Slanina S., Nesburn A.B., Wechsler S.L. Expression and characterization of baculovirus expressed herpes simplex virus type 1 glycoprotein L. Arch. Virol. 1994;138(3–4):199–212. doi: 10.1007/BF01379126. [DOI] [PubMed] [Google Scholar]
- Ghiasi H., Nesburn A.B., Wechsler S.L. Cell surface expression of herpes simplex virus type 1 glycoprotein H in recombinant baculovirus-infected cells. Virology. 1991;185(1):187–194. doi: 10.1016/0042-6822(91)90766-5. [DOI] [PubMed] [Google Scholar]
- Giavedoni L.D. Simultaneous detection of multiple cytokines and chemokines from nonhuman primates using luminex technology. J. Immunol. Methods. 2005;301(1–2):89–101. doi: 10.1016/j.jim.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Haumont M., Jacquet A., Massaer M., Deleersnyder V., Mazzu P., Bollen A., Jacobs P. Purification, characterization and immunogenicity of recombinant varicella-zoster virus glycoprotein gE secreted by Chinese hamster ovary cells. Virus Res. 1996;40(2):199–204. doi: 10.1016/0168-1702(95)01270-2. 29. [DOI] [PubMed] [Google Scholar]
- Heaton P.R., Espy M.J., Binnicker M.J. Evaluation of 2 multiplex real-time PCR assays for the detection of HSV-1/2 and Varicella zoster virus directly from clinical samples. Diagn. Microbiol. Infect. Dis. 2015;81(3):169–170. doi: 10.1016/j.diagmicrobio.2014.11.012. [DOI] [PubMed] [Google Scholar]
- Hsu H.Y., Joos T.O., Koga H. Multiplex microsphere-based flow cytometric platforms for protein analysis and their application in clinical proteomics – from assays to results. Electrophoresis. 2009;30(23):4008–4019. doi: 10.1002/elps.200900211. [DOI] [PubMed] [Google Scholar]
- Idrissova Zh.R., Boldyreva M.N., Dekonenko E.P., Malishev N.A., Leontyeva I.Y., Martinenko I.N., Petrukhin A.S. Acute disseminated encephalomyelitis in children: clinical features and HLA-DR linkage. Eur. J. Neurol. 2003;10(5):537–546. doi: 10.1046/j.1468-1331.2003.00639.x. [DOI] [PubMed] [Google Scholar]
- Ikoma M., Liljeqvist J.A., Groen J., Glazenburg K.L., The T.H., Welling-Wester S. Use of a fragment of glycoprotein G-2 produced in the baculovirus expression system for detecting herpes simplex virus type 2-specific antibodies. J. Clin. Microbiol. 2002;40(7):2526–2532. doi: 10.1128/JCM.40.7.2526-2532.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura H., Straus S.E., Williams R.K. Baculovirus expression, purification, and properties of varicella-zoster virus gE, gI, and the complex they form. J. Infect. Dis. 1998;178(Suppl. 1):S13–S15. doi: 10.1086/514256. [DOI] [PubMed] [Google Scholar]
- Kimura H., Straus S.E., Williams R.K. Varicella-zoster virus glycoproteins E and I expressed in insect cells form a heterodimer that requires the N-terminal domain of glycoprotein I. Virology. 1997;233(2):382–391. doi: 10.1006/viro.1997.8625. [DOI] [PubMed] [Google Scholar]
- Krishna S., Blacklaws B.A., Overton H.A., Bishop D.H., Nash A.A. Expression of glycoprotein D of herpes simplex virus type 1 in a recombinant baculovirus: protective responses and T cell recognition of the recombinant-infected cell extracts. J. Gen. Virol. 1989;70(7):1805–1814. doi: 10.1099/0022-1317-70-7-1805. 1. [DOI] [PubMed] [Google Scholar]
- Landolfi V., Zarley C.D., Abramovitz A.S., Figueroa N., Wu S.L., Blasiak M., Ishizaka S.T., Mishkin E.M. Baculovirus-expressed herpes simplex virus type 2 glycoprotein D is immunogenic and protective against lethal HSV challenge. Vaccine. 1993;11(4):407–414. doi: 10.1016/0264-410x(93)90280-b. [DOI] [PubMed] [Google Scholar]
- Loomis K.H., Yaeger K.W., Batenjany M.M., Mehler M.M., Grabski A.C., Wong S.C., Novy R.E. InsectDirect™ System: rapid, high-level protein expression and purification from insect cells. J. Struct. Funct. Genomics. 2005;6(2–3):189–194. doi: 10.1007/s10969-005-5241-y. [DOI] [PubMed] [Google Scholar]
- Neufeld K.L., Richards O.C., Ehrenfeld E. Expression and characterization of poliovirus proteins 3B VPg, 3C pro, and 3D pol in recombinant baculovirus-infected Spodoptera frugiperda cells. Virus Res. 1991;19(2):173–188. doi: 10.1016/0168-1702(91)90044-v. [DOI] [PubMed] [Google Scholar]
- Olson J.K., Bishop G.A., Grose C. Varicella-zoster virus Fc receptor gE glycoprotein: serine/threonine and tyrosine phosphorylation of monomeric and dimeric forms. J. Virol. 1997;71(1):110–119. doi: 10.1128/jvi.71.1.110-119.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti E., Lipinskas B.R., Samsonoff C., Mercer S., Panicali D. Construction of live vaccines using genetically engineered poxviruses: biological activity of vaccinia virus recombinants expressing the hepatitis B virus surface antigen and the herpes simplex virus glycoprotein D. Proc. Natl. Acad. Sci. U.S.A. 1984;81(1):193–197. doi: 10.1073/pnas.81.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Possee R.D. Cell-surface expression of influenza virus haemagglutinin in insect cells using a baculovirus vector. Virus Res. 1986;5(1):43–59. doi: 10.1016/0168-1702(86)90064-x. [DOI] [PubMed] [Google Scholar]
- Sisk W.P., Bradley J.D., Leipold R.J., Stoltzfus A.M., Ponce de Leon M., Hilf M., Peng C., Cohen G.H., Eisenberg R.J. High-level expression and purification of secreted forms of herpes simplex virus type 1 glycoprotein gD synthesized by baculovirus-infected insect cells. J. Virol. 1994;68(2):766–775. doi: 10.1128/jvi.68.2.766-775.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonneville R., Klein I., de Broucker T., Wolff M. Post-infectious encephalitis in adults: diagnosis and management. J. Infect. 2009;58(5):321–328. doi: 10.1016/j.jinf.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanberry L.R., Bernstein D.I., Burke R.L., Pachl C., Myers M.G. Vaccination with recombinant herpes simplex virus glycoproteins: protection against initial and recurrent genital herpes. J. Infect. Dis. 1987;155(5):914–920. doi: 10.1093/infdis/155.5.914. [DOI] [PubMed] [Google Scholar]
- Stanberry L.R., Myers M.G. Evaluation of varicella-zoster antiviral drugs by a nucleic acid hybridization assay. Antiviral Res. 1988;9(6):367–377. doi: 10.1016/0166-3542(88)90038-1. [DOI] [PubMed] [Google Scholar]
- Steiner I., Kennedy P.G., Pachner A.R. The neurotropic herpes viruses: herpes simplex and varicella-zoster. Lancet Neurol. 2007;6(11):1015–1028. doi: 10.1016/S1474-4422(07)70267-3. [DOI] [PubMed] [Google Scholar]
- Stone M.J., Hawkins C.P. A medical overview of encephalitis. Neuropsychol. Rehabil. 2007;17(4–5):429–449. doi: 10.1080/09602010601069430. [DOI] [PubMed] [Google Scholar]
- van Kooij A., Middel J., Jakab F., Elfferich P., Koedijk D.G., Feijlbrief M., Scheffer A.J., Degener J.E., The T.H., Scheek R.M., Welling G.W., Welling-Wester S. High level expression and secretion of truncated forms of herpes simplex virus type 1 and type 2 glycoprotein D by the methylotrophic yeast Pichia pastoris. Protein Expr. Purif. 2002;25(3):400–408. doi: 10.1016/s1046-5928(02)00034-7. [DOI] [PubMed] [Google Scholar]
- Wang Z., Stalcup L.D., Harvey B.J., Weber J., Chloupkova M., Dumont M.E., Dean M., Urbatsch I.L. Purification and ATP hydrolysis of the putative cholesterol transporters ABCG5 and ABCG8. Biochemistry. 2006;45(32):9929–9939. doi: 10.1021/bi0608055. [DOI] [PubMed] [Google Scholar]
- Watson R.J., Weis J.H., Salstrom J.S., Enquist L.W. Herpes simplex virus type-1 glycoprotein D gene: nucleotide sequence and expression in Escherichia coli. Science. 1982;218(4570):381–384. doi: 10.1126/science.6289440. [DOI] [PubMed] [Google Scholar]
- Wong A.A., Pabbaraju K., Wong S., Tellier R. Development of a multiplex real-time PCR for the simultaneous detection of herpes simplex and varicella zoster viruses in cerebrospinal fluid and lesion swab specimens. J. Virol. Methods. 2016;229:16–23. doi: 10.1016/j.jviromet.2015.12.009. [DOI] [PubMed] [Google Scholar]
- Wu L., Forghani B. Characterization of neutralizing domains on varicella-zoster virus glycoprotein E defined by monoclonal antibodies. Arch. Virol. 1997;142(2):349–362. doi: 10.1007/s007050050081. [DOI] [PubMed] [Google Scholar]