Abstract
Microorganisms based biosynthesis of nanomaterials has triggered significant attention, due to their great potential as vast source of the production of biocompatible nanoparticles (NPs). Such biosynthesized functional nanomaterials can be used for various biomedical applications. The present study investigates the green synthesis of silver nanoparticles (Ag NPs) using the fungus Curvularia pallescens (C. pallescens) which is isolated from cereals. The C. pallescens cell filtrate was used for the reduction of AgNO3 to Ag NPs. To the best of our knowledge C. pallescens is utilized first time for the preparation of Ag NPs. Several alkaloids and proteins present in the phytopathogenic fungus C. pallescens were mainly responsible for the formation of highly crystalline Ag NPs. The as-synthesized Ag NPs were characterized by using UV–Visible spectroscopy, X-ray diffraction and transmission electron microscopy (TEM). The TEM micrographs have revealed that spherical shaped Ag NPs with polydisperse in size were obtained. These results have clearly suggested that the biomolecules secreted by C. pallescens are mainly responsible for the formation and stabilization of nanoparticles. Furthermore, the antifungal activity of the as-prepared Ag NPs was tested against Cladosporium fulvum, which is the major cause of a serious plant disease, known as tomato leaf mold. The synthesized Ag NPs displayed excellent fungicidal activity against the tested fungal pathogen. The extreme zone of reduction occurred at 50 μL, whereas, an increase in the reduction activity is observed with increasing the concentration of Ag NPs. These encouraging results can be further exploited by employing the as synthesized Ag NPs against various pathogenic fungi in order to ascertain their spectrum of fungicidal activity.
Keywords: Curvularia pallescens, Biosynthesis, Silver nanoparticles, Transmission electron microscope, Antifungal activity, Cladosporum fulvum
1. Introduction
Nanomaterials represent an important class of functional materials due to their extraordinary physicochemical properties. These properties have been extensively utilized for several technological and biomedical purposes including, in the fabrication of various nano-devices, biosensors, bio implants etc (Cook et al., 2008, Bunker et al., 2010, Akbarzadeh et al., 2012, Ashajyothi et al., 2014, Prasai et al., 2015). Particularly, the recent advancements in the field of nanotechnology have led to the development of a variety of new nano-structured materials with desired design and properties (Feng et al., 2000, Elgorban et al., 2015, Shi et al., 2015). Despite tremendous progress in the design and preparation of various nanomaterials for different applications, several challenges exist in many fields including, biomedical sector. For instance, the efficient production of nano drugs with customized size, shape and other physicochemical properties is highly desirable to develop important new types of drugs for various life threatening diseases (Feng et al., 2000, Elgorban et al., 2015, Shi et al., 2015). In other cases, the recent surge in the spreading of different types strain resistant bacteria and fungi requires the development of several new kinds of nanoparticle based antibacterial and antifungal materials. Therefore, the advancement in the design of latest nanomaterials and the development of new techniques for the preparation of nanoparticles to be utilized in the fabrication of efficient nano devices is highly imminent.
Among various nanomaterials, silver nanoparticles (Ag NPs) have gained considerable attention of scientists and biologists, due to their remarkable physicochemical and biological properties (Li et al., 2014). These properties have led to their wide applications in various fields, including, energy sector, and electronics, sensing and health care (Rycenga et al., 2011, Konop et al., 2016). Predominantly, the outstanding antimicrobial properties of Ag NPs have led to the development of a wide variety of nanosilver products, such as, nanosilver-coated wound dressings, contraceptive devices, surgical instruments, and implants (Alarcon et al., 2016, GhavamiNejad et al., 2016, Pazos et al., 2016). Ag NPs have been prepared by using various physical and chemical methods based on the accessibility and feasibility of protocols to attain the required applications (Samal et al., 2013, Sun, 2013) The physical methods include, ball milling, flame pyrolysis, electric arc discharge, laser ablation etc (James et al., 2012), which often require expensive instruments, high temperature and pressure (Xu et al., 2013). Whereas, the chemical methods, involve the concepts of wet chemistry (Polavarapu and Liz-Marzán, 2013). In this process, the formation of NPs is carried out via the reduction/decomposition of metal complexes in solutions using various chemical reductants, such as sodium borohydride, hydrazine or at elevated temperatures (Tahir et al., 2013, Tan and Cheong, 2013) Moreover, to achieve stable dispersions of Ag NPs to make them compatible for various applications, different types of additional stabilizers are often required to prevent aggregation (Mourdikoudis and Liz-Marzán, 2013). Although, these methods have been extensively applied but the reactants, reductants, stabilizers and various organic solvents used in these methods are toxic and potentially hazardous for the environment (Farooq Adil and Siddiqui, 2015).
Therefore, considering the extensive biological applications of Ag NPs, the development of facile approaches for their preparation under ambient conditions using non-toxic reagents and solvents is highly desirable (Marambio-Jones and Hoek, 2010). So far, considerable progress has been made in the preparation of Ag NPs under physiological and eco-friendly conditions, and several green methods have been developed in this regard (Hebbalalu et al., 2013). These methods include electrochemical, microwave, sonochemical, supercritical liquids, ionic liquids etc (Kalathil et al., 2011, Zaarour et al., 2014). Recently, great interest has been generated toward various biological systems for inspiration and using biomolecules as a tool for the synthesis of functional nanomaterials. Therefore, the trend of applying biomaterials, such as, microorganisms, marine organisms, proteins and plant extracts in the green synthesis of Ag NPs has gained enormous popularity in the scientific community (Akhtar et al., 2013, Singh et al., 2016). Among these methods, the utilization of different microorganisms in the preparation of Ag NPs has gained significant attention. The microorganisms not only facilitate the synthesis of the Ag NPs by acting as reducing agents, but also functionalize the surface of NPs (Anand and Mandal, 2015). These multifunctional microorganisms greatly reduce the number of steps in the reaction and also eliminate the use of external stabilizers. Therefore, several unicellular and multicellular microorganisms, including bacteria and different genera of fungi, have been successfully tested for the preparation of Ag NPs. For instance, in different studies, Pseudomonas stutzeri AG256 from Ag mines and Lactobacillus found in buttermilk have been applied for the preparation of Ag NPs (Klaus et al., 1999, Nair and Pradeep, 2002).
Apart from the bacterial mediated synthesis, various eukaryotic organisms, such as, different types of fungal based approaches have been found to be more popular for the preparation of Ag NPs, since, these organisms are easy to handle, secrete more enzymes and usually grow on simple media. Till date, several pathogenic fungi such as Aspergillus fumigatus (Bhainsa and D’souza, 2006), A. terreus (Kora and Sashidhar, 2015) and the bioagent fungi like Trichoderma harzianum (Ahluwalia et al., 2014), and Trichoderma viride (Chitra and Annadurai, 2013) have been successfully used for the synthesis of Ag NPs. In another study, the biosynthesis of Ag NPs by the thermophilic fungus Humicola sp. is reported (Syed et al., 2013). The fungus reduces Ag+ ions and leads to the formation of spherical shaped, stable extracellular Ag NPs. Detailed analysis of the sample has suggested that various proteins, which were secreted by the fungus were mainly responsible for the formation and stabilization of NPs.
In this study, Curvularia pallescens, isolated from cereal is used for the synthesis of Ag NPs. Curvularia is a relatively large genus of fungi belonging to some of the most common saprophytes or phytopathogenic organisms (Freire et al., 1998). Currently, there are ∼133 species of Curvularia, which occurs mostly in tropical and subtropical areas (Manamgoda et al., 2015). Several species of Curvularia are commonly isolated from soil, air, organic matter, plants and animals, including humans. Apart from this a number of fungi belonging to the species Curvularia are also identified as rice pathogens, including Curvularia pallescens (Almaguer et al., 2013). The phytopathogenic fungus Curvularia pallescens consists of several alkaloids and proteins, such as curvupallides A, B and C, which possess an unusual α,β-unsaturated ene-amide γ-lactam, which might have played a vital role in the reduction of Ag+ ions (Abraham et al., 1995, Mukherjee et al., 2001, Dey et al., 2016).
Apart from the useful applications of fungus in the preparation of various nanomaterials, different fungal strains are also responsible for a variety of problems. Among the many funguses known, Cladosporium fulvum is a biotrophic fungal pathogen that causes serious diseases in different plants (Joosten et al., 1997). Cladosporium is a relatively large genus of fungi belonging to some of the most common indoor and outdoor molds. Several species of Cladosporium, including fungi, are commonly found on plants (both living and dead plants), and produces olive-green to brown or black colonies (Joosten et al., 1997). The genus Cladosporium contained around 800 plant-pathogenic and saprotrophic species, which are often highly osmotolerant, and grow easily on common media. Among various species of Cladosporium, Cladosporium fulvum which causes leaf mold of tomato is an important genetic model, which facilitated the understanding of the genetics of host resistance (Rivas and Thomas, 2005). The fungus causes serious economic losses to commercially grown tomato produced in open field, high tunnels and greenhouses in all the worlds (Rivas and Thomas, 2005).
Herein, we report on the green synthesis of Ag NPs via the reduction of Ag ions using the fungus Curvularia pallescens, which is isolated from cereal. (Scheme 1) The as-prepared Ag NPs were characterized using various microscopic and analytical techniques including X-ray powder diffraction (XRD), Fourier-transform infrared spectroscopy (FT-IR), ultraviolet–visible absorption (UV-vis) spectroscopy, and transmission electron microscopy (TEM). Furthermore, the antifungal activity of the as-prepared Ag NPs was tested against Cladosporium fulvum which causes several serious plant diseases, including tomato leaf mold infection.
Scheme 1.
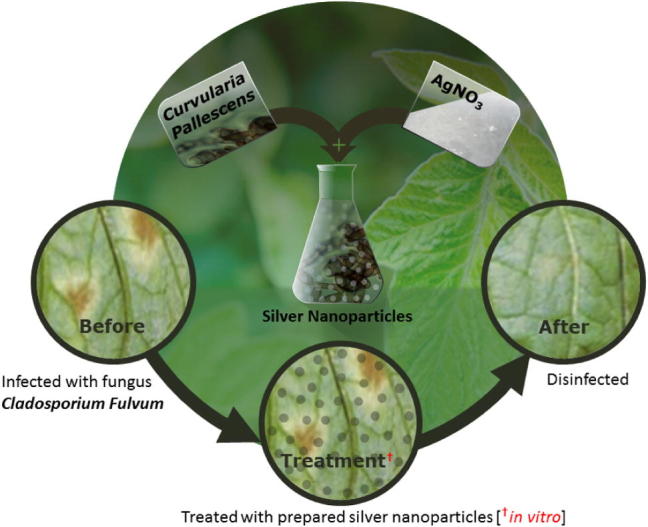
Schematic illustration of the bioengineered silver nanoparticles (Ag NPs) using Curvularia pallescens and their activity (in vitro) against Cladosporum fulvum which is usually found on tomato leaf mold.
2. Materials and methods
The organisms used for the green synthesis was Cladosporum fulvum, which was isolated from tomato leaves dropped untimely. Colonies grown on potato sucrose agar as described in Joosten and De Wit (1999). While, the fungus Curvularia pallescens was obtained from Botany and Microbiology Department, College of Science, King Saud University, Saudi Arabia. Chemicals were purchased from various firms such as Alfa Aesar, Sigma Aldrich etc…
2.1. Nanoparticles biosynthesis
The biosynthesis of Ag NPs was performed using freshly grown C. pallescens. For this purpose, C. pallescens was grown in the liquid medium (KH2PO4 7.0 g; K2HPO4 2.0 g; MgSO4.7H2O 0.1 g; (NH4)2SO4 1.0 g; glucose 10.0 g and yeast extract 0.6 g). Initially, the Erlenmeyer flask was inoculated with C. pallescens, and incubated on an incubator shaker at 27 °C and at 150 rpm. The fungus biomass was collected after 7 days by filtering with Whatman filter paper No. 1. Subsequently, the fungus biomass was rinsed three times with distilled water to separate any component of culture medium. Twenty gram mycelial mats were blended with 100 mL of sterilized distilled water for 48 h at 27 °C in the flasks and agitated at 150 rpm. After 48 h, the fungus cell filtrate was detached from the biomass through Whatman filter paper No. 1. In order to prepare the Ag NPs using freshly isolated fungus, 50 mL of silver nitrate (1 mM) was mixed with 50 mL of culture filtrate in a 250-mL flask. The cell filtrate without AgNO3 served as control.
2.2. Characterizations of NPs
Optical absorption measurements were performed using UV Shimadzu 3101 PC spectrophotometer. The incident photon flux was normal to the surface, and the investigated wave-length range was 300–900 nm. Surface morphology examined using scanning electron microscope (SEM) (EDX, JEOL model JSM-6380). EDS combined with SEM used to quantify the compositional analysis of nanoparticle. The formation of nanoparticles was confirmed using TEM (JEOL-1010). X-ray powder diffraction (XRD) analysis was performed on X Pert Pro diffractometer by using Cu Kα radiation at 40 kV and 40 mA. The scans were typically performed over a 2α range from 10° to 85° at a speed of 0.02/s, with an aperture slit, an anti-scatter slit, and a receiving slit of 2 mm, 6 mm, and 0.2 mm, respectively. X-rays Cu-Kα wavelength was (1.54056 Å).
2.3. Antifungal activity of Ag NPs against Cladosporum fulvum
The antifungal activity of Ag NPs against Cladosporum fulvum (Syn. Fulvia fulva) causing tomato leaf mold was studied on Potato dextrose agar (PDA). The Ag NP solutions were added to Erlenmeyer flasks at 0, 25, 50, 75, 100, 150 and 200 ppm and autoclaved. Flasks were poured into Petri plates. The Petri plates containing Ag NPs were incubated at 25 ± 2 °C. After 2 days, disks from mycelial (3 mm) were taken from the edge of 7-day-old fungal cultures, put in the center of each plate containing Ag NPs, plates without Ag+ served as control. The petri dishes incubated at 28 ± 2 °C for 5 days. The inhibition zones in radial growth were measured after 5 days according to this formula
where R is the radial growth of fungal mycelia on the control and r the mycelial growth of fungal mycelia on the plate treated with Ag NPs.
3. Results and discussion
3.1. Characterization
3.1.1. Optical absorption measurements
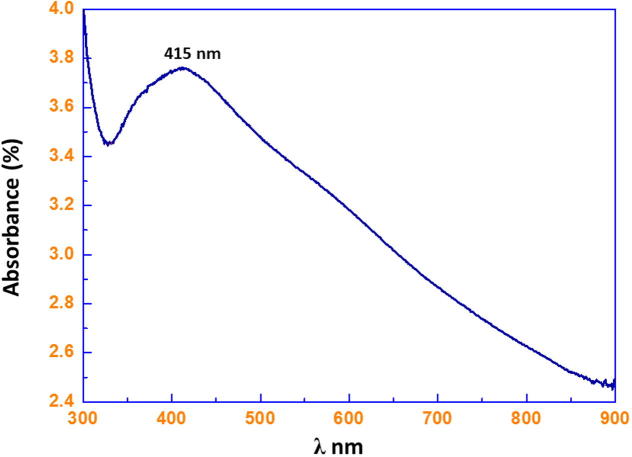
C. pallescens fungal extract was used for the biosynthesis of Ag NPs under facile conditions. It was observed that on the addition of fungal extract into the aqueous solution of AgNO3, the color of the solution gradually changed from light yellow to dark brown, indicating the formation of Ag NPs. Under a similar set of conditions, no change in the color of AgNO3 solution was observed even after several hours. The formation of the as-prepared Ag NPs was initially monitored by UV–vis analysis. Typically, Ag NPs exhibit absorption under a visible range of 380–450 nm, depending on the shape and size of the NPs. The UV–vis spectrometry is a spectroscopic technique involving the use of light in the visible, near ultra-violet and near infrared regions to cause electronic transitions in the target material. The absorption spectrum of metal NPs is sensitive to several factors, including particle size, shape, and particle–particle interaction (agglomeration) with the medium. Therefore, the aqueous bioreduction of Ag+ ions can be effectively monitored by a UV–vis spectrophotometer. Optical absorption of Ag NPs is illustrated in Fig. 1, which clearly demonstrated the appearance of absorption peak at 415 nm. Generally, a broad peak at a higher wavelength indicates an increase in particle size, whereas a narrow line at a shorter wavelength represents smaller particle size.
Figure 1.
Ultraviolet–visible (UV–vis) absorption spectra of the corresponding solution depicting the formation of Ag NPs.
In the case of Pulicaria glutinosa plant extract mediated biosynthesis of Ag NPs reported in our previous study (Khan et al., 2013), the absorption peaks appeared in the range of 420–460 nm (depending on the concentration of plant extract), where Ag NPs with a particle size between 20 and 60 nm were obtained. However, in this case, the narrow absorption peak at lower wavelength (415 nm) clearly demonstrated the smaller size of NPs in the range of 10–25 nm, which is also confirmed by TEM analysis.
3.1.2. Transmission electron microscope
The as-prepared nanoparticles were subjected to transmission electron microscopy (TEM) to understand the morphology of Ag NPs formed and the particle size distribution graph is illustrated in Fig. 2. From the micrograph obtained (Fig.2A) it can be clearly observed that the Ag NPs obtained are spherical in nature and are well-dispersed, with meager agglomerations. In order to obtain the mean particle size the images obtained were analyzed using image processing software ImageJ. The values obtained were plotted as a histogram and it was found that the mean particle size of the Ag NPs obtained was found to be 16.74 ± 0.62 nm it is clear that the variation of particle size from 3 to 13 nm, good gaussian variation is obtained as shown as in Fig.2B.
Figure 2.
(A) TEM image of the biosynthesized Ag NPs obtained by using C. pallescens (B) Particle size distribution graph of the nanoparticles obtained.
3.1.3. Energy dispersive spectrometry
The sample was subjected to EDS in order to ascertain the presence of elemental Ag in the sample. Measurement of energy dispersive spectrometry (EDS) of Ag NPs is illustrated in Fig. 3. From the spectrum obtained it can be concluded that the silver nanoparticles are formed and the mass % obtained is ∼20% from all samples.
Figure 3.
Energy dispersive X-ray spectrum (EDX) of as-synthesized Ag NPs confirming the composition of product.
3.1.4. X-ray analysis
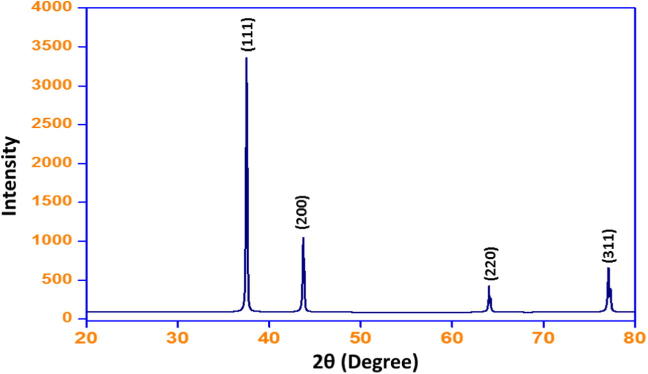
X-ray diffraction studies of green synthesized Ag NPs were carried out to ascertain the presence of crystalline Ag NPs and to correlate the data obtained with data from the crystallographic database. The XRD spectrum is illustrated in Fig. 4. As shown in the figure there are four distinct reflections in the diffractogram at 2θ 37.9469 (1 1 1), 44.1015 (2 0 0), 64.1373 (2 2 0), 77.0091 (3 1 1). These characteristic reflections can be indexed to the face centered cubic (fcc) structure of Ag (PDF File No. 040783, space group: Fm3 m). The intense reflection at (1 1 1), in comparison with the other three, may indicate the preferred growth direction of the nanocrystals (Khan et al., 2014). On the basis of the half width of the (1 1 1) reflection, the average crystallite size (∼20 nm) of the Ag NPs was determined using the Scherrer equation (Scherrer, 1912).
Figure 4.
XRD patterns of the silver nanoparticles obtained.
The observed and calculated lattice parameters, unit cell volume, and space group for PDF File No. 040783, and differences between them are compiled in Table 1. Table 2 depicts the observed and calculated crystallographic data as well as the Miller indices (h k l), where d –spacing is the inter-planar spacing, and 2θ is the diffraction angle.
Table 1.
Observed and calculated lattice parameters, unit cell volume, and space group for PDF file No. 040783.
| a | B | c | α | β | γ | Volume | Space Group | |
|---|---|---|---|---|---|---|---|---|
| Ag NPs | 4.107 | 4.107 | 4.107 | 90 | 90 | 90 | 69.277 | Fm3 m |
| 4.086 | 4.086 | 4.086 | 90 | 90 | 90 | 68.23 | Fm3 m |
Table 2.
Observed and calculated crystallographic data as well as the Miller indices.
| h | k | l | 2θ Calc. | 2θ Obs. | Difference | d. Obs. | d. Calc. |
|---|---|---|---|---|---|---|---|
| 1 | 1 | 1 | 37.5000 | 37.9469 | −0.4469 | 2.3984 | 2.3712 |
| 2 | 0 | 0 | 43.7200 | 44.1015 | −0.3815 | 2.0706 | 2.0535 |
| 2 | 2 | 0 | 64.0400 | 64.1373 | −0.0973 | 1.4540 | 1.4521 |
| 3 | 1 | 1 | 77.1200 | 77.0091 | 0.1109 | 1.2368 | 1.2383 |
The Average grain size (D) for nano particles was calculated using the following equations:
where β is the full–width at half–maximum of peaks, λ is the X-ray wavelength, and  is the diffraction angle.
is the diffraction angle.
3.2. Biological Evaluation of Ag NPs
3.2.1. Antifungal activity
The inhibition effect of Ag NPs at various concentrations was analyzed in PDA (Table 3). The higher suppression of C. fulvum growth was recorded at 200 ppm concentration. The lowest level of reduction was noticed against C. fulvum on PDA treated with a 50 ppm concentration of Ag NPs. It was observed that increased inhibition by increasing the concentration of silver nanoparticles. This high antimicrobial activity of Ag NPs could be related to the high intensity at which the solution was able to saturate and adhere to hyphe of fungi and to disrupt phytopathogenic fungi. Studies on the inhibitory action mechanism of Ag+ on microorganisms have shown that onto treatment with silver ions, DNA collapse its capability to replicate (Bidnenko et al., 2002), which inactivated expression of ribosomal subunit proteins, in addition to some other enzymes and cellular proteins essential to adenosine triphosphate production (Feng et al., 2000). As it has been the assumption that Ag+ affects primarily the function of membrane-associated enzymes, such as those found in the respiratory chain (McDonnell and Russell, 1999). Therefore, it was concluded that silver nanoparticles have significant antifungal activity, and further examination for field applications is needed.
Table 3.
Effect of Ag NPs against Cladosporum fulvum.
| 0 | 50 ppm |
100 ppm |
150 ppm |
200 ppm |
ED50 | ED95 | Slope ± SE | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| R.G.a | Inh.%b | R.G.a | Inh.% b | R.G.a | Inh.% b | R.G.a | Inh.% b | ||||
| 0.00 | 65.00 | 27.78 | 51.75 | 42.50 | 49.75 | 44.72 | 39.00 | 55.56 | 164.3 | 4782.6 | 1.12 ± 6.2 |
R.G. = Radial growth.
Inh.% = Inhibition%.
4. Conclusion
We have demonstrated a green approach for the synthesis of Ag NPs using fungal strain Curvularia pallescens as a bioreductant. Applying this methodology yielded highly crystalline, spherical-shaped Ag NPs without the usage of any harmful reducing or capping agents. The as prepared Ag NPs were characterized and confirmed using the spectroscopic as well as microscopic techniques. These Ag NPs were evaluated for their fungicidal activity against Cladosporum fulvum and were found to display excellent activity against the fungal strain tested. These encouraging results can easily be exploited for the large-scale synthesis of efficient and low-cost Ag NPs and can be tested for various applications such as catalysts and biomedical applications including, biosensors.
Acknowledgments
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for its funding this Research group No (RG-1436-009).
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Abdallah M. Elgorban, Email: elgorban@yahoo.com.
Syed Farooq Adil, Email: sfadil@ksu.edu.sa.
References
- Abraham W.-R., Meyer H. Curvupallides, a new class of alkaloids from the fungus Curvularia pallescens. Tetrahedron. 1995;51(17):4947–4952. [Google Scholar]
- Ahluwalia V., Kumar J. Green synthesis of silver nanoparticles by Trichoderma harzianum and their bio-efficacy evaluation against Staphylococcus aureus and Klebsiella pneumonia. Ind. Crops Prod. 2014;55:202–206. [Google Scholar]
- Akbarzadeh A., Samiei M. Magnetic nanoparticles: preparation, physical properties, and applications in biomedicine. Nanoscale Res. Lett. 2012;7(1):1–13. doi: 10.1186/1556-276X-7-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhtar M.S., Panwar J. Biogenic synthesis of metallic nanoparticles by plant extracts. ACS Sustainable Chem. Eng. 2013;1(6):591–602. [Google Scholar]
- Alarcon E., Vulesevic B. Coloured cornea replacements with anti-infective properties: expanding the safe use of silver nanoparticles in regenerative medicine. Nanoscale. 2016;8(12):6484–6489. doi: 10.1039/c6nr01339b. [DOI] [PubMed] [Google Scholar]
- Almaguer M., Rojas T.I. Effect of temperature on growth and germination of conidia in Curvularia and Bipolaris species isolated from the air. Aerobiologia. 2013;29(1):13–20. [Google Scholar]
- Anand K.K.H., Mandal B.K. Activity study of biogenic spherical silver nanoparticles towards microbes and oxidants. Spectrochim. Acta Part A. 2015;135:639–645. doi: 10.1016/j.saa.2014.07.013. [DOI] [PubMed] [Google Scholar]
- Ashajyothi C., Oli A.K. Potential bactericidal effect of silver nanoparticles synthesised from enterococcus species. Orient. J. Chem. 2014;30(3):1253–1262. [Google Scholar]
- Bhainsa K.C., D’souza S. Extracellular biosynthesis of silver nanoparticles using the fungus Aspergillus fumigatus. Colloids Surf. B. 2006;47(2):160–164. doi: 10.1016/j.colsurfb.2005.11.026. [DOI] [PubMed] [Google Scholar]
- Bidnenko V., Ehrlich S.D. Replication fork collapse at replication terminator sequences. EMBO J. 2002;21(14):3898–3907. doi: 10.1093/emboj/cdf369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunker K., Sturgeon J. Nanoparticle characterization in pharmaceutical applications using electron microscopy. Micros. Microanal. 2010;16(S2):668–669. [Google Scholar]
- Chitra K., Annadurai G. Bioengineered silver nanobowls using Trichoderma viride and its antibacterial activity against gram-positive and gram-negative bacteria. J. Nanostruct. Chem. 2013;3(1):1–7. [Google Scholar]
- Cook S., Padmos J. Surface structural characteristics and tunable electronic properties of wet-chemically prepared Pd nanoparticles. J. Chem. Phys. 2008;128(15):154705. doi: 10.1063/1.2901034. [DOI] [PubMed] [Google Scholar]
- Dey D., Saha B. Mass spectrometry-based identification of allergens from Curvularia pallescens, a prevalent aerospore in India. Biochim. Biophys. Acta. 2016;1864(7):869–879. doi: 10.1016/j.bbapap.2016.03.012. [DOI] [PubMed] [Google Scholar]
- Elgorban A.M., El-Samawaty A.E.-R.M. Antifungal silver nanoparticles: synthesis, characterization and biological evaluation. Biotechnol. Biotechnol. Equip. 2015:1–7. [Google Scholar]
- Farooq Adil S., Siddiqui M. Rafiq H. Biogenic synthesis of metallic nanoparticles and prospects toward green chemistry. Dalton Trans. 2015;44(21):9709–9717. doi: 10.1039/c4dt03222e. [DOI] [PubMed] [Google Scholar]
- Feng Q., Wu J. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J. Biomed. Mater. Res. 2000;52(4):662–668. doi: 10.1002/1097-4636(20001215)52:4<662::aid-jbm10>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Freire S.V.P., Paiva L.M. Morphological, cytological, and cultural aspects of Curvularia pallescens. Revista de Microbiol. 1998;29(3) [Google Scholar]
- GhavamiNejad A., Park C.H. In situ synthesis of antimicrobial silver nanoparticles within antifouling zwitterionic hydrogels by catecholic redox chemistry for wound healing application. Biomacromolecules. 2016;17(3):1213–1223. doi: 10.1021/acs.biomac.6b00039. [DOI] [PubMed] [Google Scholar]
- Hebbalalu D., Lalley J. Greener techniques for the synthesis of silver nanoparticles using plant extracts, enzymes, bacteria, biodegradable polymers, and microwaves. ACS Sustainable Chem. Eng. 2013;1(7):703–712. [Google Scholar]
- James S.L., Adams C.J. Mechanochemistry: opportunities for new and cleaner synthesis. Chem. Soc. Rev. 2012;41(1):413–447. doi: 10.1039/c1cs15171a. [DOI] [PubMed] [Google Scholar]
- Joosten M., De Wit P. The Tomato-Cladosporium fulvum interaction: a versatile experimental system to study plant-pathogen interactions. Ann. Rev. Phytopathol. 1999;37(1):335–367. doi: 10.1146/annurev.phyto.37.1.335. [DOI] [PubMed] [Google Scholar]
- Joosten M., Vogelsang R. The biotrophic fungus Cladosporium fulvum circumvents Cf-4-mediated resistance by producing unstable AVR4 elicitors. Plant Cell. 1997;9(3):367–379. doi: 10.1105/tpc.9.3.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalathil S., Lee J. Electrochemically active biofilm-mediated synthesis of silver nanoparticles in water. Green Chem. 2011;13(6):1482–1485. [Google Scholar]
- Khan M., Khan M. Green synthesis of silver nanoparticles mediated by Pulicaria glutinosa extract. Int. J. Nanomed. 2013;8:1507–1516. doi: 10.2147/IJN.S43309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M., Khan M. Biogenic synthesis of palladium nanoparticles using Pulicaria glutinosa extract and their catalytic activity towards the Suzuki coupling reaction. Dalton Trans. 2014;43(24):9026–9031. doi: 10.1039/c3dt53554a. [DOI] [PubMed] [Google Scholar]
- Klaus T., Joerger R. Silver-based crystalline nanoparticles, microbially fabricated. Proc. Natl. Acad. Sci. 1999;96(24):13611–13614. doi: 10.1073/pnas.96.24.13611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konop M., Damps T. Certain aspects of silver and silver nanoparticles in wound care: a minireview. J. Nanomat. 2016 [Google Scholar]
- Kora A.J., Sashidhar R.B. Antibacterial activity of biogenic silver nanoparticles synthesized with gum ghatti and gum olibanum: a comparative study. J. Antibiot. 2015;68(2):88–97. doi: 10.1038/ja.2014.114. [DOI] [PubMed] [Google Scholar]
- Li H., Xia H. Synthesis of monodisperse, quasi-spherical silver nanoparticles with sizes defined by the nature of silver precursors. Langmuir. 2014;30(9):2498–2504. doi: 10.1021/la4047148. [DOI] [PubMed] [Google Scholar]
- Manamgoda D.S., Rossman A.Y. A taxonomic and phylogenetic re-appraisal of the genus Curvularia (Pleosporaceae): human and plant pathogens. Phytotaxa. 2015;212(3):175–198. [Google Scholar]
- Marambio-Jones C., Hoek E.M. A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. J. Nanopart. Res. 2010;12(5):1531–1551. [Google Scholar]
- McDonnell G., Russell A.D. Antiseptics and disinfectants: activity, action, and resistance. Clin. Microbiol. Rev. 1999;12(1):147–179. doi: 10.1128/cmr.12.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourdikoudis S., Liz-Marzán L.M. Oleylamine in nanoparticle synthesis. Chem. Mater. 2013;25(9):1465–1476. [Google Scholar]
- Mukherjee P., Ahmad A. Fungus-mediated synthesis of silver nanoparticles and their immobilization in the mycelial matrix: a novel biological approach to nanoparticle synthesis. Nano Lett. 2001;1(10):515–519. [Google Scholar]
- Nair B., Pradeep T. Coalescence of nanoclusters and formation of submicron crystallites assisted by Lactobacillus strains. Cryst. Growth Des. 2002;2(4):293–298. [Google Scholar]
- Pazos E., Sleep E. Nucleation and growth of ordered arrays of silver nanoparticles on peptide nanofibers: hybrid nanostructures with antimicrobial properties. J. Am. Chem. Soc. 2016 doi: 10.1021/jacs.6b01570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polavarapu L., Liz-Marzán L.M. Growth and galvanic replacement of silver nanocubes in organic media. Nanoscale. 2013;5(10):4355–4361. doi: 10.1039/c3nr01244a. [DOI] [PubMed] [Google Scholar]
- Prasai B., Ren Y. Synthesis-atomic structure-properties relationships in metallic nanoparticles by total scattering experiments and 3D computer simulations: case of Pt–Ru nanoalloy catalysts. Nanoscale. 2015;7(17):8122–8134. doi: 10.1039/c5nr00800j. [DOI] [PubMed] [Google Scholar]
- Rivas S., Thomas C.M. Molecular interactions between tomato and the leaf mold pathogen Cladosporium fulvum. Annu. Rev. Phytopathol. 2005;43:395–436. doi: 10.1146/annurev.phyto.43.040204.140224. [DOI] [PubMed] [Google Scholar]
- Rycenga M., Cobley C.M. Controlling the synthesis and assembly of silver nanostructures for plasmonic applications. Chem. Rev. 2011;111(6):3669–3712. doi: 10.1021/cr100275d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samal A.K., Polavarapu L. Size Tunable Au@ Ag core–shell nanoparticles: synthesis and surface-enhanced Raman scattering properties. Langmuir. 2013;29(48):15076–15082. doi: 10.1021/la403707j. [DOI] [PubMed] [Google Scholar]
- Scherrer P. Springer; 1912. Bestimmung der inneren Struktur und der Größe von Kolloidteilchen mittels Röntgenstrahlen. [Google Scholar]
- Shi D., Sadat M. Photo-fluorescent and magnetic properties of iron oxide nanoparticles for biomedical applications. Nanoscale. 2015;7(18):8209–8232. doi: 10.1039/c5nr01538c. [DOI] [PubMed] [Google Scholar]
- Singh P., Kim Y.-J. Biological synthesis of nanoparticles from plants and microorganisms. Trends Biotechnol. 2016 doi: 10.1016/j.tibtech.2016.02.006. [DOI] [PubMed] [Google Scholar]
- Sun Y. Controlled synthesis of colloidal silver nanoparticles in organic solutions: empirical rules for nucleation engineering. Chem. Soc. Rev. 2013;42(7):2497–2511. doi: 10.1039/c2cs35289c. [DOI] [PubMed] [Google Scholar]
- Syed A., Saraswati S. Biological synthesis of silver nanoparticles using the fungus Humicola sp. and evaluation of their cytoxicity using normal and cancer cell lines. Spectrochim. Acta Part A. 2013;114:144–147. doi: 10.1016/j.saa.2013.05.030. [DOI] [PubMed] [Google Scholar]
- Tahir M.N., Natalio F. Controlled synthesis of linear and branched Au@ ZnO hybrid nanocrystals and their photocatalytic properties. Nanoscale. 2013;5(20):9944–9949. doi: 10.1039/c3nr02817h. [DOI] [PubMed] [Google Scholar]
- Tan K.S., Cheong K.Y. Advances of Ag, Cu, and Ag–Cu alloy nanoparticles synthesized via chemical reduction route. J. Nanopart. Res. 2013;15(4):1–29. [Google Scholar]
- Xu H., Zeiger B.W. Sonochemical synthesis of nanomaterials. Chem. Soc. Rev. 2013;42(7):2555–2567. doi: 10.1039/c2cs35282f. [DOI] [PubMed] [Google Scholar]
- Zaarour M., El Roz M. Photochemical preparation of silver nanoparticles supported on zeolite crystals. Langmuir. 2014;30(21):6250–6256. doi: 10.1021/la5006743. [DOI] [PubMed] [Google Scholar]