Abstract
A frequent cross-sectional study was conducted to determine the patterns of antimicrobial resistance in 296 bacterial strains isolated from in-patient faecal samples of Government Vellore Medical College and Hospital, Vellore. Isolation and identification of bacterial strains were done using enrichment media, selective media, and biochemical tests. Antimicrobial susceptibility testing by the disc diffusion method and minimal inhibitory concentration method was conducted and the strains were subjected to extended spectrum beta-lactamases screening. Antibiotic sensitivity pattern of Staphylococcus spp. showed oxacillin resistance. Almost all the strains were sensitive to linezolid, vancomycin, gentamycin and chloramphenicol. In gram negative isolates ciprofloxacin and tobramycin showed better sensitivity and ceftazidime showed a higher percentage of resistance by MIC. Out of 250 isolates, Enterobacteriaceae showed positive for 86/250, 82/250 and 94/250 isolates and 3/10, 4/10 and 4/10 non-Enterobacteriaceae isolates were found to be positive for CTX-M gene, TEM gene and SHV gene, respectively. This study helps to assess/analyse the relation between the spectrum of microorganisms present in various grades of faecal carriage and their susceptibility pattern in this part of the Vellore town.
Keywords: Faecal carriage, Bacteria, ESBL, Vellore
1. Introduction
Antibacterial resistance has been recognized as an up-and-coming worldwide problem in both human and animals, and antibacterial agent use is considered the most important factor for the emergence, selection, and dissemination of antimicrobial agent-resistant bacteria (Prescott et al., 2000). The principle behind the development of resistance is that bacteria in the guts of humans are subjected to different types, concentrations, and frequencies of antimicrobial agents. Over time, selective pressure selects resistant bacteria that have specific fingerprints for resistance to the antimicrobial agents that have been used. On other hand, contamination of the hospital environment leads to the development of the antimicrobial agent resistance.
Worldwide, there are increasing problems with multiresistant bacteria. The most important drug resistance today is caused by methicillin-resistant Staphylococcus aureus (MRSA) (Udo et al., 2001, Borg, 2003), vancomycin resistant enterococci (VRE) (Calfee et al., 2003, Kuriyama et al., 2003) and Enterobacteriaceae with extended-spectrum beta-lactamases (ESBL). The common aerobic pathogens isolated from the chronic wounds are S. aureus, coagulase negative Staphylococcus (CONS), S. epidermidis and Streptococcus spp. Recent clinical attention has focused on the increasing frequency of non-lactose fermenting gram-negative pathogens responsible for hospital-acquired infections, Pseudomonas aeruginosa and coliform bacteria (Armstrong et al., 1995). Multidrug resistant organisms (MDROs) in the foot and hand ulcer infection may increase the duration of hospital stay, an increase in cost of management and might lead to additional morbidity and mortality (Gadepalli et al., 2006). The first ESBL isolates were discovered in Western Europe in the mid 1960’s. They were found in a variety of Enterobacteriaceae spp. Most beta lactamases function by a serine ester hydrolysis mechanism, rendering the antibiotics inactive. Beta-lactamases of gram negative species are periplasmic and protect the cell (Thomson and Sanders, 1992).
Resistance to broad-spectrum cephalosporins has emerged in strains of members of the family Enterobacteriaceae following frequent use of these drugs in the hospital setting. Endemic and epidemic nosocomial infections caused by ESBL-producing Escherichia coli and Klebsiella pneumoniae cells represent a persistent problem in many parts of the world (Komatsu et al., 2003). Epidemic strains of cephalosporin-resistant E. coli and K. pneumoniae have been associated with increased morbidity and mortality in hospitalized patients. Appropriate isolation measures need to be taken, hand hygiene procedures may need reinforcement, possible environmental reservoirs need elimination, and antibiotics policies may need reconsideration (Gruteke et al., 2003, Muzaheed Doi et al., 2008).
To test this hypothesis, the two objectives of this study were (i) to identify patterns of antimicrobial agent resistance of bacterial strains obtained from the faecal carriage of in-patients of Government Vellore Medical College and Hospital, Vellore (ii) to subject Enterobacteriaceae and non-Enterobacteriaceae bacteria to ESBL screening.
2. Materials and methods
2.1. Sample collection
Specimens (faecal samples) for microbiological studies were obtained from in-patients admitted in different wards of the Government Vellore Medical College and Hospital (GVMCH), Vellore. Faecal samples were collected in plastic universal containers that were immediately put on ice. Clinical details of the patients were also collected. The transport media (Muller Hinton broth) was used for specimen transport to our laboratory.
2.2. Sample processing
A total of 187 faecal samples were collected. Direct examinations of the specimen provided immediate semi-quantitative information about the types of organisms present, and other distinguishing characteristics of the specimens were also noted.
A direct smear was made from the specimen, and inoculated into appropriate aerobic plating media, such as Blood agar and MacConkey agar. The inoculated plates were immediately incubated under aerobic condition at 37 °C for 24 h, respectively. The direct smear was dried, fixed, and gram stained. The gram-stained smear was examined for information about the types of organism present. The aerobic plates were examined after 24 h of incubation. The isolated microorganisms were identified using standard methods. Identification of isolates were done based on colony morphology, gram staining, motility, catalase test, oxidase test, coagulase test, standard biochemical tests and oxidation-fermentation test and other tests (Collee et al., 1996).
Each isolate was grown overnight (18–20 h) on Blood agar or MacConkey agar. A single colony was made in the stock medium i.e., 5% trypticase soy broth plus 20% v/v. All the isolates were kept frozen at −70 °C until further use.
2.3. Antimicrobial susceptibility test by disc diffusion method
All the isolates tested for antimicrobial susceptibility against antimicrobial agents. Gram-positive bacteria were tested against the various antibiotics such as ampicillin (A) – 10 μg, chloramphenicol (C) – 30 μg, ciprofloxacin (Cf) – 5 μg, linezolid (Lz) – 10 μg, penicillin G (P) – 10 units, streptomycin (S) – 10 μg, vancomycin (Va) – 30 μg, oxacillin (Oxa) – 30 μg, gentamycin (G) – 10 μg and co-trimoxazole (Co) – 25 μg disc. Gram-negative bacteria isolated from samples were tested against various antibiotics such as amikacin (Ak) – 30 μg, ampicillin (A) – 10 μg, aztreonam (Ao) – 30 μg, cefepime (Cpm) – 30 μg, ceftazidime (Ca) – 30 μg, cephotaxime (Ce) – 30 μg, chloramphenicol (C) – 30 μg, ciprofloxacin (Cf) – 5 μg, gentamycin (G) – 120 μg, imipenem (I) – 10 μg, nalidixic acid (Na) – 30 μg, norfloxacin (Nx) – 10 μg, tetracycline (T) – 30 μg, trimethoprim (Tr) – 5 μg, ticarcillin (Tc) – 7.5 μg and piperacillin/tazobactam (Pt) – 30 μg disc. S. aureus ATCC 25923, Enterococcus faecalis ATCC 29212, K. pneumoniae ATCC 70063, P. aeruginosa ATCC 27853 and E. coli ATCC11775 were used as control strains.
2.4. Antimicrobial susceptibility test by micro-broth dilution method
The stock solution of oxacillin, cephotaxime, ceftazidime and imipenem drugs were prepared dilution range of 1–64, 1–256, 1–256 and 1–256 respectively, according to the CLSI (2006) recommendation. Inoculum of the test organisms were prepared from colonies grown on Nutrient agar (Hi-media), which had been incubated overnight (18–20 h) at 37 °C in the incubator. Colonies were suspended in Mueller Hinton broth (Himedia) and adjusted to a turbidity of a 0.5 McFarland standard (1 × 108 CFU/ml).
Each well of the microtitre plate, containing 100 μl of the respective antimicrobial solution followed was added with test bacterial suspension (5 μl; 1 × 108 CFU/ml). The quality control strains, S. aureus ATCC 25923, E. faecalis ATCC29212, K. pneumoniae ATCC70063 and P. aeruginosa ATCC 27853 were used in each run of daily testing. Each batch included a growth control well (no antimicrobial agent) and a negative control well (un-inoculated). After inoculation, each tray was covered with a lid to prevent evaporation during incubation. The micro dilution trays were incubated at 37 °C for 16–20 h in ambient incubator prior to reading. Care was taken to ensure that no more than four trays were stacked in an array.
2.5. Phenotypic ESBL detection
The antimicrobial discs and combination discs (cephotaxime 30 μg, ceftazidime 30 μg, cefepime 30 μg, cephotaxime 30 μg/clavulanate 10 μg, ceftazidime 30 μg/clavulanate 10 μg and cefepime 30 μg/clavulanate 10 μg) were placed on each plate; the plates were incubated at 37 °C and were incubated for 16–18 h. After incubation, each plate was examined. The diameters of the inhibition zones were measured. An organism was interpreted as the ESBL producer (positive) if there was an increase of ⩾5 mm in the inhibition zone of the combination disc when compared to the corresponding cephalosporin disc (CLSI, 2006).
2.6. Molecular detection of ESBL genes
β-lactamase genes (blaTEM, blaSHV, blaCTX-M) were detected by polymerase chain reaction (PCR) using reverse and forward primer pairs (Lim et al., 2009) with boiled suspension of bacterial cells, which were used as DNA template. All the PCR mixes were made in sterile 0.2 ml PCR tubes and subjected to thermal cycles created in the Eppendorf-Mastercycler personnel PCR Machine.
2.7. Agarose gel electrophoresis
Agarose gel (1%) with ethidium bromide (50 μg/ml) was made in 0.5× TAE buffer. A 10 μl of the PCR product mixed with 2 μl of gel loading dye and PCR products was resolved at 100 V for 20 min. 5 μl of 200 bp DNA ladder (Himedia) was used to detect the size of the fragment in the gel. After the electrophoresis was run, the gel was documented using Bio-rad gel documentation system; the image was processed and represented in the results section.
3. Results
3.1. Collection and processing of faecal samples
Totally 187 faecal samples were collected. Since 12 samples were inadequate for further processing due to poor consistency (i.e., too watery) or inadequate quantity and were not used in the study, totally 175 samples were studied, 102 (58.28%) were from men and 73 (41.71%) were from women. In this study it was noted that single isolates were present in 62.5% of the collected samples and multiple isolates were noted in 37.5% of the collected samples.
3.2. Characterization of bacterial isolates
Out of 296 isolates, 36 (12.16%) were gram positive organisms and 260 (87.83%) were gram negative. A standard microbial identification procedure (Collee et al., 1996) was used to characterize the isolated bacterial strains and the results were compared with Bergey’s manual of systematic bacteriology and the identification chart was prepared for the isolates. From the identification the following number of bacterial isolate were obtained, Coagulase negative Staphylococcus – 07, S. aureus – 11, E. faecalis – 18, K. pneumonia – 15, E. coli – 178, Citrobacter freundii – 26, Enterobacter aerogenes – 19, Proteus mirabilis – 12 and P. aeruginosa – 10.
3.3. Antimicrobial sensitivity pattern by disc diffusion test
The results of the antimicrobial sensitivity pattern by disc diffusion method for bacterial isolates are represented in Table 1. Antibiotic sensitivity pattern of Staphylococcus spp. (includes S. aureus (11) and CONS (7)) showed that oxacillin resistance, i.e., methicillin resistant Staphylococcus (MRS), was 61.11%. Almost all the strains were sensitive to linezolid, vancomycin, gentamycin and chloramphenicol. Enterococci spp. showed uniform susceptibility to vancomycin, linezolid and tetracycline, which showed intermediate sensitivity to linezolid and vancomycin by disc diffusion.
Table 1.
Antibiotic resistance pattern by disc diffusion method.
| Antibiotic |
Staphylococcus spp. (n = 18) |
Enterococcus faecalis (n = 18) |
Enterobacteriaceae (n = 250) |
Pseudomonas aeruginosa (n = 10) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R (%) | I (%) | S (%) | R (%) | I (%) | S (%) | R (%) | I (%) | S (%) | R (%) | I (%) | S (%) | |
| Oxacillin | 61.11 | 11.11 | 27.77 | – | – | – | – | – | – | – | – | – |
| Vancomycin | 0 | 0 | 100 | 0 | 0 | 100 | – | – | – | – | – | – |
| Gentamycin | 44.4 | 5.55 | 49.99 | 16.6 | 16.6 | 66.6 | 52 | 8 | 40 | – | – | – |
| Ciprofloxacin | 33.3 | 38.85 | 27.75 | 0 | 27.7 | 72.2 | 48 | 44 | 8 | 30 | 20 | 50 |
| Erythromycin | 11.11 | 55.5 | 33.33 | – | – | – | – | – | – | – | – | – |
| Chloramphenicol | 22.22 | 11.11 | 66.66 | 0 | 66.6 | 33.3 | – | – | – | – | – | – |
| Co-trimoxazole | 8.85 | 11.11 | 38.85 | – | – | – | – | – | – | – | – | – |
| Tetracycline | 27.75 | 38.85 | 33.33 | 0 | 22.2 | 77.7 | 82 | 6 | 8 | – | – | – |
| Linezolid | 0 | 0 | 100 | 0 | 0 | 100 | – | – | – | – | – | – |
| Clindamycin | 44.44 | 5.55 | 49.99 | – | – | – | – | – | – | – | – | – |
| Streptomycin | – | – | – | 27.7 | 27.7 | 44.4 | – | – | – | – | – | – |
| Penicillin | – | – | – | 50 | 27.7 | 22.2 | – | – | – | – | – | – |
| Ampicillin | – | – | – | 33.3 | 0 | 66.6 | 58.4 | 4 | 38.4 | – | – | – |
| Cefepime | – | – | – | – | – | – | 68.8 | 11.2 | 20 | 50 | 20 | 30 |
| Cephotaxime | – | – | – | – | – | – | 56 | 24 | 20 | 60 | 10 | 30 |
| Ceftazidime | – | – | – | – | – | – | 48 | 22.4 | 29.6 | 70 | 20 | 10 |
| Imipenem | – | – | – | – | – | – | 12 | 16 | 72 | 40 | 20 | 40 |
| Trimethoprim | – | – | – | – | – | – | 78.4 | 8 | 10.4 | – | – | – |
| Aztreonam | – | – | – | – | – | – | 72 | 8 | 20 | – | – | – |
| Amoxicillin | – | – | – | – | – | – | 44 | 12 | 44 | – | – | – |
| Tobramycin | – | – | – | – | – | – | – | – | – | 20 | 10 | 70 |
| Piperacillin/tazobactam | – | – | – | – | – | – | – | – | – | 90 | 10 | 0 |
| Amikacin | – | – | – | – | – | – | – | – | – | 80 | 0 | 20 |
| Ticarcillin | – | – | – | – | – | – | – | – | – | 70 | 20 | 10 |
| Aztreonam | – | – | – | – | – | – | – | – | – | 90 | 0 | 10 |
Enterobacteriaceae showed a wide range of resistance to many of the drugs tested. Imipenem, cephotaxime, ceftazidime and cefepime showed 12% (30/250), 56% (140/250), 48% (120/250) and 68.8% (172/250) resistance, respectively. Whereas, aztreonam, trimethoprim and tetracycline showing a higher percentage of resistance 72%, 78.4% and 82%, respectively, was observed.
Antibiotic sensitivity pattern of Non-Enterobacteriaceae include P. aeruginosa (10). From 10 isolates of P. aeruginosa tested the following resistant pattern was observed: cephotaxime 60% (6/10), ceftazidime 70% (7/10) and cefepime 50% (5/50). Whereas other beta-lactams like imipenem, aztreonam, ticarcillin and piperacillin/tazobactam showed resistant patterns of 40% (4/10), 80% (8/10), 70% (7/10), and 90% (9/10), respectively. Aminoglycosides like tobramycin and amikacin showed 2/10 (20%) and 8/10 (80%) of resistance, respectively.
3.4. MIC by micro-broth dilution method
The micro-broth dilution method was done for gram positive (oxacillin) and gram negative (i.e., cephotaxime, ceftazidime and Imipenem) bacterial pathogen; the results are shown in Table 2. Dilution ranges of 1 to >32 μg/ml for oxacillin and 1–256 μg/ml for cephotaxime, ceftazidime and imipenem were used. According to the micro-broth dilution method, 9.09% (1/11) of MSSA and 90.90% (10/11) are MRSA. This is comparatively much higher than coagulase negative staphylococci which showed 57.14% (4/7) resistance and 42.85% (3/7) sensitivity to methicillin.
Table 2.
Minimal inhibitory concentration by micro-broth dilution method.
| S. no | Drug concentration (μg/ml) |
P. aeruginosa |
Enterobacteriaceae^ |
S. aureus | CONS | ||||
|---|---|---|---|---|---|---|---|---|---|
| CTX | CAZ | I | CTX | CAZ | I | Oxa | Oxa | ||
| 1 | 256 | 4 | 3 | 2 | 70 | 84 | 6 | – | – |
| 2 | 128 | 2 | 1 | 1 | 40 | 76 | 0 | – | – |
| 3 | 64 | 0 | 4 | 0 | 11 | 0 | 0 | – | – |
| 4 | 32 | 0 | 0 | 0 | 09 | 30 | 62 | 3 | 0 |
| 5 | 16 | 1 | 1 | 2 | 20 | 25 | 48 | 1 | 0 |
| 6 | 8 | 1 | 0 | 2 | 60 | 15 | 68 | 4 | 1 |
| 7 | 4 | 0 | 1 | 1 | 25 | 20 | 26 | 2 | 3 |
| 8 | 2 | 1 | 0 | 2 | 5 | 0 | 40 | 1 | 2 |
| 9 | 1 | 1 | 0 | 0 | 10 | 0 | 0 | 0 | 1 |
| Total | 10 | 10 | 10 | 250 | 250 | 250 | 11 | 07 | |
CTX – cephotaxime, CAZ – ceftazidime, I – imipenem, Oxa – oxacillin, Enterobacteriaceae^-include E. coli, P. mirabilis, K. pneumoniae, E. aerogenes, and C. freundii.
From 250 Enterobacteriaceae isolates, 40% (100/250), 11.6% (29/250) and 48.4% (121/250) were susceptible, intermediately resistant and resistant to cephotaxime, respectively. For ceftazidime, 14% (35/250), 10% (25/250) and 76% (190/250) were susceptible, intermediately resistant and resistant, respectively. For imipenem, 26.4% (66/250), 27.2% (68/250) and 46.4% (116/25) were susceptible, intermediately resistant and resistant, respectively. Comparisons of MIC and disc diffusion method for antibiotic sensitivity of Enterobacteriaceae against 3 drugs cephotaxime, ceftazidime and imipenem were carried out. Totally, 121 out of 250 resistant in MIC and 29/250 isolates showed intermediately resistant in MIC among these 140/250 resistant, 60/250 intermediate resistant and 50/25 were sensitive in disc diffusion method of cephotaxime. The second drug ceftazidime MIC showed an increased number of resistant isolates (190/250) compared to disc diffusion results (120/25). In the third antibiotic imipenem, 116 out of 250 were resistant in MIC and 30/250 resistant in disc diffusion.
In P. aeruginosa, 60% (6/10) of the isolates tested were resistant, 10% (1/10) isolates were intermediately resistant and 30% (3/10) isolates were sensitive to cephotaxime, 80% (8/10) isolates were resistant, 10% (1/10) were intermediately resistant while remaining isolates 10% (1/10) were sensitive to ceftazidime. In case of imipenem, 5/10 isolates tested was resistant, another 2/10 isolates were intermediately resistant, while 3/10 isolates were found to be sensitive. Totally, 220/296 (74.32%) multidrug resistance isolates found out of 275 specimens were found in this study, including both gram-positive and gram-negative pathogens.
3.5. Phenotypic extended-spectrum beta-lactamase screening for gram-negative bacterial isolates
In the present study, all 260 gram-negative isolates were subjected to ESBL screening using a single disc synergy test (Table 3). The antibiotic combination of cephotaxime + clavulanate, ceftazidime + clavulanate and cefepime + clavulanate showed ESBL positivity of 103/260, 78/260 and 61/260, respectively. Although in in vitro tests, ESBLs are inhibited by beta-lactamase inhibitors such as clavulanic acid, the activity of beta-lactam/beta-lactamase inhibitor combination agents was influenced by the bacterial inoculum, dose administration regimen and specific type of ESBL present.
Table 3.
Phenotypic extended-spectrum beta-lactamase screening for gram-negative bacteria.
| Gram negative bacteria | Isolates (n) | Cephotaxime + clavulanic acid |
Ceftazidime + clavulanic acid |
Cefepime + clavulanic acid |
|||
|---|---|---|---|---|---|---|---|
| P | N | P | N | P | N | ||
| Enterobacteriaceae | 250 | 97 | 153 | 72 | 178 | 58 | 192 |
| Pseudomonas aeruginosa | 10 | 6 | 4 | 6 | 4 | 3 | 7 |
| Total | 260 | 103 | 157 | 78 | 182 | 61 | 199 |
P – positive, N – negative, Enterobacteriaceae^-include E. coli, P. mirabilis, K. pneumoniae, E. aerogenes and C. freundii.
3.6. Genotypic extended-spectrum beta-lactamase screening for gram-negative bacterial isolates
Today over 150 different ESBLs have been described. Most of them are derivatives of TEM or SHV or CTX-M enzymes. There are now more than 90 TEM type β lactamases and more than 25 SHV type enzymes described.
3.7. CTX-M
In recent years a new family of plasmid mediated ESBLs called CTX-M that preferentially hydrolyze cefotaxime has arisen. In this study, ESBL gene detection by PCR using universal primers showed that out of 260 isolates, Enterobacteriaceae tested positive for 86 isolates and 3 P. aeruginosa isolates were found to be positive for CTX-M gene (Amplicon size is 593 bp; Fig. 1). The preponderance of CTX-M type ESBLs was found in strains of E. coli (69/178) and K. pneumoniae (7/15) (Table 4).
Figure 1.
Agarose gel showing products of CTX-M PCR amplification.
Table 4.
Genotypic ESBL screening for gram-negative bacteria.
| Gram negative bacteria | Isolates (n) | CTXM |
TEM |
SHV |
CTXM + TEM |
CTXM + SHV |
TEM + SHV |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P | N | P | N | P | N | P | N | P | N | P | N | ||
| Enterobacteriaceae^ | 250 | 86 | 164 | 82 | 168 | 94 | 156 | 42 | 208 | 57 | 193 | 66 | 184 |
| Pseudomonas aeruginosa | 10 | 3 | 7 | 4 | 6 | 4 | 6 | 2 | 8 | 1 | 9 | 1 | 9 |
| Total | 260 | 89 | 171 | 86 | 174 | 98 | 162 | 46 | 216 | 58 | 202 | 67 | 193 |
P – positive, N – negative, Enterobacteriaceae^-include E. coli, P. mirabilis, K. pneumoniae, E. aerogenes and C. freundii.
3.8. TEM
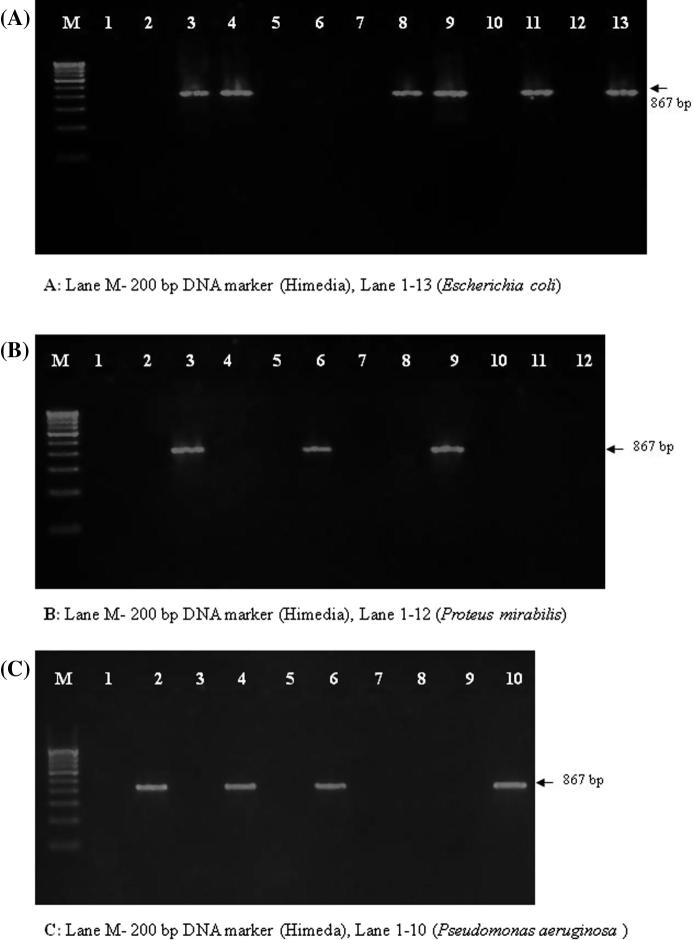
In this study, likewise when the same set of isolates were subjected to TEM gene detection Enterobacteriaceae showed 82/250 positive for TEM and 4/10 isolates of P. aeruginosa were found to be positive for the TEM gene (Amplicon size: 867 bp; Fig. 2). The greater part of TEM type ESBLs is found in strains of E. coli (71/178) and P. aeruginosa (4/10) and followed by P. mirabilis (3/12) (Table 4).
Figure 2.
Agarose gel showing products of TEM PCR amplification.
3.9. SHV
In this study, SHV 94/250 and 4/10 SHV isolates were positive for Enterobacteriaceae and P. aeruginosa, respectively (Fig. 3). The majority of SHV type ESBLs was found in strains of E. coli (66/178). However, these enzymes have also been found in K. pneumonia, P. mirabilis and P. aeruginosa. Several organisms yielded more than one ESBL gene which is compared in Table 4.
Figure 3.
Agarose gel showing products of SHV PCR amplification.
4. Discussion
Worldwide, there are increasing problems with multi-resistant bacteria. The development of resistance is that bacteria in the guts of humans are subjected to different types, concentrations, and frequencies of antimicrobial agents. Poly-microbial nature of faecal samples was observed, 175 faecal samples in triplicate were analysed and 296 bacterial pathogens were isolated. S. aureus is an important cause of healthcare associated infections (Lowy, 1998). The mechanism by which intestinal colonization by S. aureus might lead to an increased risk of staphylococcal infections is not known. Squier et al. (2002) indicate that intestinal colonization by S. aureus could be associated with increased frequency of colonization or contamination of skin sites, thereby increasing the risk for contamination of devices, wounds, and mucous membranes. Though it was observed to have a higher percentage of MRSA in this study, the isolates were uniformly susceptible to vancomycin, the incidence of vancomycin-intermediate S. aureus (VISA) and vancomycin-resistant S. aureus (VRSA) has been increasing in various parts of the world. The first clinical isolate of VRSA reported from the United States in 2002 (CDC, 2002).
E. faecalis was the common isolate than E. faecium as seen in all other clinical specimens. A faecal indicator bacterium with a host range limited to humans and a few other warm-blooded animal species would also simplify microbial source tracking because only a few animal species would be required for any host origin database. All the strains showed intermediate sensitivity to linezolid and vancomycin by disc diffusion, the result is similar to that of studies reported by Abdulrazak et al. (2005). E. faecalis is the most prevalent species cultured from humans, accounting for more than 90% of clinical isolates (De Perio et al., 2006).
Enterobacteria in faecal flora are often reported to be highly resistant. E. coli are the main species; resistance data on other species are rare. Gram negative organisms showed a varied pattern of resistance to the disc diffusion method. Gadepalli et al. (2006) showed a different pattern for P. aeruginosa where both cephotaxime and ceftazidime having same resistance of 61.1%, piperacillin/tazobactam (72.2%). Whereas, this study found different resistance pattern of cephotaxime and ceftazidime showing 60% and 70%, respectively. The piperacillin/tazobactam showed 100% resistance pattern almost in concordance with that of showing resistant pattern of (90%). Apart from these various resistant patterns towards commonly used antibiotics a different observation was reported by Bansal et al. (2008) showing sensitivity to amikacin, ceftazidime and piperacillin. In MIC analysis showing the resistance pattern of the isolates, it was found that Pseudomonas which is the commonest isolate, was highly resistant to ceftazidime (80%) followed by cefotaxime. This is in contrast, however, to some other study which reported that Pseudomonas was highly sensitive to ceftazidime (Revathi et al., 1998).
The erosion of effective antimicrobials continues as witnessed increased frequency of resistance to all drugs in particular, the fluoroquinolones, vancomycin and carbapenems, which are often the drugs of last choice. New drugs that can combat multiple drug resistant gram-positive bacteria have averted forthcoming crises. With the relative absence of new antimicrobials coming to market and with new threats arising from the gram-negative bacteria, however, the number of drug options leaves us perilously close to none or only a single effective agent for some life-threatening infections. Hundreds of beta-lactam-degrading enzymes are rapidly undermining the mainstay penicillins and late generation cephalosporin agents. The increase in metallo-beta-lactamases, which are active against carbapenems and most other beta-lactams, is an alarming new development (Livermore and Woodford, 2000).
With the emergence of antimicrobial resistance in bacteria as a global problem, national and international surveillance programmes have been developed to monitor resistance (Felmingham and Gruneberg, 1996, Pfaller et al., 1997, Turner et al., 1999, Jones and Masterton, 2001, Felmingham, 2002). The emergence of plasmid mediated extended spectrum β-lactamases (ESBLs) in members of family Enterobacteriaceae has become a worldwide problem (Paterson and Bonomo, 2005). Many clinical microbiology laboratories make no effort to detect ESBL production by gram negative bacilli and have a problem in detecting ESBL mediated resistance. A few point mutations at selected loci within the gene give rise to extended spectrum phenotype (Levison et al., 2002, Cagatay et al., 2003, Komatsu et al., 2003). These enzymes are not very closely related to TEM or SHV β lactamases and exhibit approximately 40% identity with these two commonly isolated β lactamases (Glupczynski et al., 2007, Kim et al., 2007, Muzaheed Doi et al., 2008).
TEM-1 is the most commonly encountered β lactamases in gram negative bacteria. Up to 90% of ampicillin resistance in E. coli is due to the production of TEM-1. This enzyme is also responsible for ampicillin and penicillin resistance. TEM-1 is able to hydrolyze penicillins and early cephalosporins such as cephalothin and cephaloridine. TEM-2, the first derivative of TEM-1, has a single aminoacid substitution from the original β lactamase. TEM-3 originally reported in 1989, was the first TEM type β-lactamase that displayed the ESBL phenotype. In the years since that first report, over 90 additional TEM derivatives have been described. Some of these β lactamases are inhibitor resistant enzymes, but the majority of the new derivatives are ESBLs (Vourli et al., 2004, Glupczynski et al., 2007).
The SHV-1 β lactamase is most commonly found in K. pneumonia and E. coli and is responsible for up to 20% of plasmid mediated ampicillin resistance. Unlike the TEM type β lactamase, there are relatively a few derivatives of SHV-1. Furthermore, the changes that have been observed in blaSHV to give rise to the SHV variants occur in fewer positions within the structural gene. The majority of SHV variants possessing an ESBL phenotype were characterized by the substitution of lysine for glutamate at position 238 (Levison et al., 2002, Paterson and Bonomo, 2005). This study helps to assess/analyse the relation between the spectrum of microorganisms present in various grades of faecal samples and their susceptibility pattern in this part of the Vellore town.
5. Conclusions
Out of 296 isolates, 36 (12.16%) were gram positive organisms and 260 (87.83%) were gram negative. The antimicrobial sensitivity pattern by disc diffusion method and the antimicrobial sensitivity pattern by disc diffusion method were performed. Phenotypic extended-spectrum beta-lactamase screening for gram-negative bacterial isolates was studied. The antibiotic combination of cephotaxime + clavulanate, ceftazidime + clavulanate and cefepime + clavulanate showed ESBL positivity of 103/260, 78/260 and 61/260, respectively. Although in in vitro tests, ESBLs are inhibited by beta-lactamase inhibitors such as clavulanic acid, the activity of beta-lactam/beta-lactamase inhibitor combination agents was influenced by the bacterial inoculum, dose administration regimen and specific type of ESBL present. Genotypic extended-spectrum beta-lactamase screening for gram-negative bacterial isolates was confirmed by TEM or SHV or CTX-M enzymes. The preponderance of CTX-M type ESBLs was found in strains of E. coli (69/178) and K. pneumoniae (7/15). The greater part of TEM type ESBLs is found in strains of E. coli (71/178) and P. aeruginosa (4/10) and followed by P. mirabilis (3/12). The majority of SHV type ESBLs was found in strains of E. coli (66/178).
Acknowledgements
Authors are thankful to Dr. Chidamabram Ramalingam, Dean, School of Biosciences and Technology (SBST), Vellore Institute of Technology (VIT) for providing facilities and encouragement.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abdulrazak A., Bitar Z.I., Al-Shamali A.A., Mobasher L.A. Bacteriological study of diabetic foot infections. J. Diabetes Complications. 2005;19:138–141. doi: 10.1016/j.jdiacomp.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Armstrong D.G., Liswood P.J., Todd W.F., William J. Prevalence of mixed infections in the diabetic pedal wound. A retrospective review of 112 infections. J. Am. Podiatr. Med. Assoc. 1995;85:533–537. doi: 10.7547/87507315-85-10-533. [DOI] [PubMed] [Google Scholar]
- Bansal K., Connelly R., Traber D., Enkhbaatar P. Methicillin-resistant Staphylococcus aureus enhances alveolar epithelial cell permeability through vascular endothelial growth factor and cytoskeletal disruption. Crit. Care. 2008;12:38. [Google Scholar]
- Borg M.A. Bed occupancy and overcrowding as determinant factors in the incidence of MRSA infections within general ward settings. J. Hosp. Infect. 2003;54:316–318. doi: 10.1016/s0195-6701(03)00153-1. [DOI] [PubMed] [Google Scholar]
- Cagatay, A.A., Kocagoz, T., Eraksoy, H., 2003. Dio-sensimedia: a novel culture medium for rapid detection of extended spectrum β-lactamases. BMC Infect. Dis., 3, 22. Available from <http://www.biomedcentral.com/1471-2334/22>. [DOI] [PMC free article] [PubMed]
- Calfee D.P., Giannetta E.T., Durbin L.J., Germanson T.P., Farr B.M. Control of endemic vancomycin-resistant Enterococcus among inpatients at a University Hospital. Clin. Infect. Dis. 2003;37:326–332. doi: 10.1086/376624. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) Staphylococcus aureus resistant to vancomycin – United States. MMWR Morb. Mortal. Wkly. Rep. 2002;51:565–567. [PubMed] [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI), 2006. Quality control and minimal inhibitory concentration (MIC) limits for broth microdilution and MIC interpretive breakpoints. Supplement M27–S2. Clinical and Laboratory Standards Institute, Wayne, PA.
- Collee J.G., Marmion B.P., Fraser A.G., Simmons A. 14th ed. Churchill-Livingstone; New York: 1996. Mackie and McCartney Practical Medical Microbiology. [Google Scholar]
- De Perio M.A., Yarnold P.R., Warren J., Noskin G.A. Risk factors and outcomes associated with non-Enterococcus faecalis, non-Enterococcus faecium enterococcal bacteremia. Infect. Control Hosp. Epidemiol. 2006;27:28–33. doi: 10.1086/500000. [DOI] [PubMed] [Google Scholar]
- Felmingham D. The need for antimicrobial resistance surveillance. J. Antimicrob. Chemother. 2002;50:1–7. doi: 10.1093/jac/dkf807. [DOI] [PubMed] [Google Scholar]
- Felmingham D., Gruneberg R.N. A multicenter collaborative study of the antimicrobial susceptibility of community-acquired, lower respiratory tract pathogens 1992–1993: the Alexander Project. J. Antimicrob. Chemother. 1996;38:1–57. doi: 10.1093/jac/38.suppl_a.1. [DOI] [PubMed] [Google Scholar]
- Gadepalli R., Dhawan B., Sreenivas V., Kapil A., Ammini A.C., Chaudhry R. A clinico-microbiological study of diabetic foot ulcers in an Indian tertiary care hospital. Diabetes Care. 2006;29:1727–1732. doi: 10.2337/dc06-0116. [DOI] [PubMed] [Google Scholar]
- Glupczynski Y., Berhin C., Bauraing C., Bogaerts P. Evaluation of a new selective chromogenic agar medium for detection of extended-spectrum β-lactamase producing Enterobacteriaceae. J. Clin. Microbiol. 2007;45:501–505. doi: 10.1128/JCM.02221-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruteke P., Goessens W., Van Gils J., Peerbooms P., Den Toom N.L., Van Santen-Verheuvel M., Van Belkum A., Verbrugh H. Patterns of resistance associated with integrons, the extended-spectrum β-lactamase SHV-5 Gene, and a multidrug efflux pump of Klebsiella pneumoniae causing a nosocomial outbreak. J. Clin. Microbiol. 2003;41:1661–1666. doi: 10.1128/JCM.41.3.1161-1166.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R.N., Masterton R. Determining the value of antimicrobial surveillance programs. Diagn. Microbiol. Infect. Dis. 2001;41:171–175. doi: 10.1016/s0732-8893(01)00318-2. [DOI] [PubMed] [Google Scholar]
- Kim S., Hu J., Gautom R., Kem J., Lee B., Boyle D.S. CTX-M extended-spectrum beta-lactamases, Washington State. Emerg. Infect. Dis. 2007;13:513–514. doi: 10.3201/eid1303.060479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu M., Aihara M., Shimakawa K., Iwasaki M., Nagasaka Y., Fukuda S., Matsuo S., Iwatani Y. Evaluation of Micro Scan ESBL confirmation panel for Enterobacteriaceae-producing, extended-spectrum β-lactamases isolated in Japan. Diagn. Microbiol. Infect. Dis. 2003;46:125–130. doi: 10.1016/s0732-8893(03)00041-5. [DOI] [PubMed] [Google Scholar]
- Kuriyama T., Williams D.W., Patel M., Lewis M.A., Jenkins L.E., Hill D.W., Hosein I.K. Molecular characterization of clinical and environmental isolates of vancomycin resistant Enterococcus faecium and Enterococcus faecalis from a teaching hospital in Wales. J. Med. Microbiol. 2003;52:821–827. doi: 10.1099/jmm.0.05123-0. [DOI] [PubMed] [Google Scholar]
- Levison M.E., Mailapur Y.V., Pradhan S.K., Jacoby G.A., Adams P., Emery C.L., May P.L., Pitsakis P.G. Regional occurrence of plasmid-mediated SHV-7, an extended-spectrum blactamase, in Enterobacter cloacae in Philadelphia teaching hospitals. Clin. Infect. Dis. 2002;35:1551–1554. doi: 10.1086/344178. [DOI] [PubMed] [Google Scholar]
- Lim K.T., Yeo C.C., Yasin R.M., Balan G., Thong K.L. Characterization of multidrug-resistant and extended-spectrum b-lactamase-producing Klebsiella pneumoniae strains from Malaysian hospitals. J. Med. Microbiol. 2009;58:1463–1469. doi: 10.1099/jmm.0.011114-0. [DOI] [PubMed] [Google Scholar]
- Livermore D.M., Woodford N. Carbapenemases: a problem in waiting? Curr. Opin. Microbiol. 2000;3:489–495. doi: 10.1016/s1369-5274(00)00128-4. [DOI] [PubMed] [Google Scholar]
- Lowy F.D. Staphylococcus aureus infections. N. Engl. J. Med. 1998;339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- Muzaheed Doi Y., Adams-Haduch J.M., Endimiani A., Sidjabat H.E., Gaddad S.M., Paterson D.L. High prevalence of CTX-M-15-producing Klebsiella pneumoniae among inpatients and outpatients with urinary tract infection in Southern India. J. Antimicrob. Chemother. 2008;61:1393–1394. doi: 10.1093/jac/dkn109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson D.L., Bonomo R.A. Extended spectrum β-lactamases: a clinical update. Clin. Microbiol. Rev. 2005;18:657–686. doi: 10.1128/CMR.18.4.657-686.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaller M.A., Jones R.N., Doern G.V., Kugler K. Bacterial pathogens isolated from patients with bloodstream infection: frequencies of occurrence and antimicrobial susceptibility patterns from the SENTRY antimicrobial surveillance program (United States and Canada, 1997) Antimicrob. Chemother. 1997;42:1762–1770. doi: 10.1128/aac.42.7.1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott J.F., Baggot J.D., Walker R.D. 3rd ed. Iowa State University Press; Ames: 2000. Antimicrobial Therapy in Veterinary Epidemiology. [Google Scholar]
- Revathi G., Puri J., Jain B.K. Bacteriology of burns. Burns. 1998;24:347–349. doi: 10.1016/s0305-4179(98)00009-6. [DOI] [PubMed] [Google Scholar]
- Squier C., Rihs J.D., Risa K.J., Sagnimeni A., Wagener M.M., Stout J., Muder R.R., Singh N. Staphylococcus aureus rectal carriage and its association with infections in patients in a surgical intensive care unit and a liver transplant unit. Infect. Control Hosp. Epidemiol. 2002;23:495–501. doi: 10.1086/502095. [DOI] [PubMed] [Google Scholar]
- Thomson K.S., Sanders C.C. Detection of ESBLs in members of the family Enterobacteriaceae. Comparison of the double disk and three-dimensional test. Antimicrob. Agents Chemother. 1992;36:1877–1882. doi: 10.1128/aac.36.9.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner P.J., Greenhalgh J.M., Edwards J.R., McKellar J. The MYSTIC (meropenem yearly susceptibility test information collection) programme. Int. J. Antimicrob. Agents. 1999;13:117–125. doi: 10.1016/s0924-8579(99)00115-6. [DOI] [PubMed] [Google Scholar]
- Udo E.E., Jacob L.E., Mathew B. Genetic analysis of methicillin-resistant Staphylococcus aureus expressing high- and low-level mupirocin resistance. J. Med. Microbiol. 2001;50:909–915. doi: 10.1099/0022-1317-50-10-909. [DOI] [PubMed] [Google Scholar]
- Vourli S., Giakkoupi P., Miriagou V., Tzelepi E., Vatopoulos A.C., Tzouvelekis L.S. Novel GES/IBC extended spectrum β-lactamase variants with carbapenemase activity in clinical enterobacteria. FEMS Microbiol. Lett. 2004;234:209–213. doi: 10.1016/j.femsle.2004.03.028. [DOI] [PubMed] [Google Scholar]