Abstract
A recent study reported that Panax ginseng (P. ginseng) has a protective effect on the development of benign prostatic hyperplasia (BPH). KH053 is used as a new herbal prescription consisting of P. ginseng and bee-pollen. The present study aimed to investigate whether the KH053 has inhibition effects on the development of benign prostatic hyperplasia (BPH) using an animal model with testosterone induced BPH. The experiment was carried out in forty male Wistar 7 week old rats that were divided into four groups (control group, BPH group, positive group, and KH053 group). One group was used as the control and the three groups received subcutaneous injections of testosterone 20 mg/kg for 4 weeks to induce BPH. One of them received KH053 by oral gavage daily at doses of 200 mg/kg concurrently with the testosterone. The positive group received finasteride at a dose of 1 mg/kg with testosterone. After 4 weeks, all rats were sacrificed and analyzed for prostate weight, and growth factors. Results revealed that, compared to rats in the BPH group, KH053 showed that the prostate weight and dihydrotestosterone (DHT) levels in serum were significantly decreased and the decreases in hyperplasia in prostate were also observed. In addition, immunohistochemistry (IHC) also revealed that the protein expressions of growth factors [transforming growth factor β1 (TGF-β1) and vascular endothelial growth factor (VEGF)] in prostate tissue were decreased in the KH053 group. In conclusion, these results suggest that KH053, comprising P. ginseng and bee-pollen, inhibits the development of BPH in Wistar rat model and might be used as functional food for BPH.
Keywords: KH053, Panax ginseng, Benign prostatic hyperplasia, BPH
1. Introduction
Benign prostatic hyperplasia (BPH) is one of the urological diseases in old men. The incidence of BPH shows about 50–60% in men at ages 40–60 and greater than 90% in men over 80 (Isaacs and Coffey, 1989). BPH is characterized by an enlargement of the prostate surrounding the urethra leading to constriction of the urethra (Yono et al., 2008). And also BPH is characterized by hyper-proliferation of the epithelium and stromal region (McNeal, 1978). These characters of BPH result in voiding problems manifested by difficulty of urination and irritative symptoms respectively (Andersson and Russell, 1990). Although the etiology of BPH has been exactly unknown, it was suggested that sex hormones namely androgen, testosterone, and dihydrotestosterone (DHT) might contribute to prostatic enlargement in BPH. The α1-adrenergic receptor (α1-AR) antagonists are the initial drugs for treating BPH (O’Leary, 2003). And the 5-alpha-reductase inhibitors block the action of 5-alpha-reductase, the enzyme that converts from testosterone into DHT (Carson and Rittmaster, 2003).
The activity of 5-alpha-reductase inhibitors results in an increased testosterone level and decreased DHT level. 5-alpha-reductase inhibitors are used to treat baldness, BPH and prostate cancer. However, the drug has limitations because of its side effects, such as decreased libido, erectile dysfunction, and nasal congestion (DuBeau and Resnick, 1992). Thus, alternative herbal-based therapy has been investigated (Bailey et al., 2007). Medicinal plants have various applications in the treatment of diseases (Balamurugan, 2015). Recent studies showed that a commonly used medicinal plant has an effective effect on the treatment of BPH or an inhibited effect on the development of BPH. Kim et al. (2014) showed that Panax ginseng has an inhibited effect on BPH in the animal BPH model. Another research also reported that Red ginseng and 20(S)-Rg3 regulated testosterone-induced prostate hyperplasia by deregulating androgen receptor signaling (Bae et al., 2012). Ginseng has been known as the name of the root of P. ginseng. It is variously used to treat specific diseases, as traditional medicine in East Asia. And numerous studies showed that P. ginseng has protective effects against degenerative and aging diseases, such as neurodegenerative diseases (Cho, 2012), anti lipid accumulation (Yang et al., 2014), diabetic nephropathy (Quan et al., 2013), osteoporosis (Siddiqi et al., 2013), regulating the inflammatory response (Kim et al., 2015), ischemia, oxidative stress (Lim et al., 2013), and potential chemopreventive effects (Kim et al., 2013, Sharma and Goyal, 2015).
Bee-collected pollen is an apicultural product. It includes nutritionally valuable substances and considerable amounts of biologically active substances (Cheng et al., 2013). It is also rich in carbohydrates, crude fibers, lipids, vitamins, and phenolic compounds (Feas et al., 2012). However, the specific chemical composition of bee pollen depends strongly on the bees’ species, botanical and geographic origin (Pascoal et al., 2014). Several studies reported that it has various effects to strengthen the resistance of the body to diseases. Elberry et al. (2011) showed that date palm pollen treatment reduced the number of prostatic acini in the BPH rat model and decreased the production of pro-inflammatory cytokines. In the present study, the mixture of P. ginseng and bee pollen is designed as formula KH053. The effect of KH053 on a testosterone-induced BPH rat model was determined.
2. Materials and methods
2.1. Plant material
The dried roots of P. ginseng and bee pollen were used (Dongsung Pharmaceuticals, Seoul, Republic of Korea). They were identified by Dr. Sang-Won Lee in National Institute of Horticultural and Herbal Science (NIHHS), and voucher specimens (numbers KH13 and H14) were deposited with Department of Medicinal Crop Research Institute, NIHHS, Eumsung. We selected P. ginseng for this research. P. ginseng is sensitive to high heat. As P. ginseng grows the precious plants are taken great care daily to insure the highest quality. The P. ginseng garden is covered with straw to control weeds and protect the sensitive young plants from the long cold winter.
2.2. Preparation of KH053
The dried roots of P. ginseng and bee pollen were extracted separately with 100% water for 6 h in a reflux apparatus. After refluxing, the extracts were filtered, the filtrates were evaporated in a rotary evaporator, and the samples were lyophilized in a freeze dryer (Operon, Gyeonggi, Republic of Korea). The extract yields of P. ginseng, was 21%. Each herb of KH053 was mixed in the following ratio: P. ginseng:A. japonica = 12:28. The KH053 was dissolved in distilled water and sequentially passed through 0.22-μm filters for sterilization.
2.3. Animals
Seven-week-old male Wistar rats with an average body weight of 250 ± 10 g were brought from animal company (Central Lab Animal Inc., Seoul, Korea). The animal room was always regulated at 22 ± 2 °C and at 40–70% relative humidity with a 12-h light/dark cycle. All experiments were performed according to the protocols approved by the Animal Care Committee of the Animal Center at Kyung Hee University [KHUASP(SE)-13-024].
2.4. Induction of BPH and treatments
Firstly, the testis organ of the rats was removed to exclude the influence of intrinsic testosterone except for the rats in the control group. The spermatic cord and blood vessels were ligated with Silkam sutures 3/8–16 mm (B. Braun Surgical SA, Rubi, Spain) and the testis organ was resected. In order to induce BPH in rats, a subcutaneous injection of testosterone (20 mg/kg; Wako chemicals, Tokyo, Japan) for 4 weeks after castration was carried out. And, next day, rats were divided into 4 groups (each group, n = 10): (1) control group, (2) testosterone-induced (subcutaneous) BPH group, and (3) KH053 treated group (200 mg/kg oral administration). (4) Finasteride (Sigma–Aldrich, St. Louis, MO, USA) treat group as positive group. Based on a previous study, we treated rats with 200 mg/kg of KH053 (Hong et al., 2013, Kim et al., 2014). All materials were administered to the animals in the morning for 30 days.
2.5. Blood collection and biochemical analysis
After 4 weeks, all rats were fasted overnight. Blood was obtained from the heart of the rat. Blood was collected in a serum separating tube (SST tube). Blood samples were centrifuged at 3000g for 15 min. The serum was separated from blood and stored at −20 °C. The total protein, glutamic oxaloacetic transaminase (GOT, AST), glutamic pyruvic transaminase (GPT, ALT), and DHT levels were analyzed by Green lab (Seoul, Korea). After the animals were sacrificed, the tissue of prostate was stored in formaldehyde solution in order to evaluate pathological change and expression of growth factors.
2.6. Histopathological examination
Prostate tissues embedded in paraffin wax were cut into 10-μm-thick sections using a microsection machine. Staining with hematoxylin and eosin was firstly performed. The stained prostate tissues were mounted and cover slipped using mounting solution and then examined under a microscope.
2.7. Immunohistochemistry (IHC)
After deparaffinization in prostate tissues, immune histochemistry (IHC) was performed on 10-μm-thick sections. Antigen retrieval was performed using citrate buffer, pH 6.0, for 10 min prior to peroxide quenching with 3% H2O2 in phosphate buffered saline (PBS) for 15 min. The prostate tissues were then washed in PBS and preblocked with normal goat or rabbit serum for 10 min. For reaction with primary antibody, slides were incubated with anti-transforming growth factor – b1 (TGF-B1) (Santa Cruz Biotechnology, Santa Cruz, CA, USA) in a 1:200 dilution and then anti-vascular endothelial growth factor (VEGF) (Santa Cruz Biotechnology) in a 1:500 dilution overnight at 4 °C. The sections were then incubated with biotinylated secondary antibodies (1:1000) for 1 h. Following a washing step with PBS, streptavidin–horseradish peroxidase was applied. Finally, the sections were rinsed in PBS and developed with diaminobenzidine tetrahydrochloride substrate for 10 min. At least three random fields of each section were examined at ×100 (Kim et al., 2014).
2.8. Statistical analyses
All the values are presented as mean avera ± standard error. The differences of values between the groups were statistically analyzed by a one-way analysis of variance (ANOVA), followed by a nonparametric post hoc Tukey test. All P-values are two-tailed and significance was considered as P < 0.05. All statistical analyses were performed using PASW Statistics ver. 22.0 (SPSS Inc., Chicago, IL, USA).
3. Results
3.1. Effect of KH053 on weight change
Body weight gains in each group are presented in Table 1. The final body weight was measured after 4 weeks. Each group showed an increase compared to that of the initial body weight (control group, 111.00 ± 8.59; BPH group, 105.57 ± 5.04; KH053 group, 107.86 ± 19.75; finasteride group; 106.33 ± 11.84). The differences in weight change among the groups did not show any significant (P > 0.05).
Table 1.
Body weight gains in each group.
| Group | Initial weight (g) | Final weight (g) | Total body weight gains for 30 days (g) |
|---|---|---|---|
| Control | 250 | 361.00 ± 8.58 | 111.00 ± 8.59 |
| BPH | 250 | 355.57 ± 5.03 | 105.57 ± 5.04 |
| KH053 | 250 | 357.86 ± 19.74 | 107.86 ± 19.75 |
| Finasteride | 250 | 356.33 ± 11.83 | 106.33 ± 11.84 |
Values are presented as mean ± standard error (n = 10).
Control, not treated group; BPH, testosterone induced BPH group; KH053, testosterone induced BPH with KH053 (200 mg/kg) group; finasteride, testosterone induced BPH with finasteride (1 mg/kg. p.o.) group.
3.2. Effect of KH053 on total protein, AST, and ALT levels in serum
In order to evaluate toxicity in the liver, AST and ALT were tested. The AST and ALT levels in serum were not significantly different among groups (Table 2). KH053 did not promote the activity of the serum toxicity marker enzymes, such as AST and ALT. It is indicated that rats of each group had a normal function of livers. Total protein levels in the serum of the BPH group were slightly increased compared to that of the control group. However, the differences among the groups did not show any significance (P > 0.05). The total protein levels of the KH053 group were slightly elevated compared to those of the control (P > 0.05).
Table 2.
Total protein, AST and ALT serum level in each group.
| Group | Total protein (g/dL) | AST (U/L) | ALT (U/L) |
|---|---|---|---|
| Control | 3.06 ± 0.19 | 57.12 ± 5.20 | 25.75 ± 1.53 |
| BPH | 4.02 ± 0.35 | 82.29 ± 6.36 | 36.00 ± 4.58 |
| KH053 | 3.60 ± 0.15 | 59.00 ± 6.87 | 29.50 ± 2.50 |
| Finasteride | 3.26 ± 0.13 | 66.38 ± 6.51 | 27.5 ± 1.95 |
Values are presented as mean ± standard error (n = 10).
Control, not treated group; BPH, testosterone induced BPH group; KH053, testosterone induced BPH with KH053 (200 mg/kg) group; finasteride, testosterone induced BPH with finasteride (1 mg/kg. p.o.) group.
3.3. Effect of KH053 on the prostate
Prostate weights of rats in the BPH group (1.94 ± 0.07 g) showed a significant increase compared to those of rats in the control group (0.84 ± 0.03 g; P < 0.001) (Table 3). KH053 treated groups (1.63 ± 0.06 g, P < 0.01) showed significant decreases in prostate weights compared with those of the BPH group. Prostate weights in the finasteride group were also decreased markedly compared with the BPH group. These results were similar to those for the KH053-treated group.
Table 3.
Prostate weight in each group.
| Group | Prostate (g) |
|---|---|
| Control | 0.84 ± 0.03 |
| BPH | 1.94 ± 0.07### |
| KH053 | 1.63 ± 0.06⁎⁎ |
| Finasteride | 1.66 ± 0.07⁎⁎ |
Values are presented as mean ± standard error (n = 10).
Control, not treated group; BPH, testosterone induced BPH group; KH053, testosterone induced BPH with KH053 (200 mg/kg) group; finasteride, testosterone induced BPH with finasteride (1 mg/kg. p.o.) group.
P < 0.001 compared with the control group.
P < 0.01 compared with the BPH group.
3.4. Measurement of DHT levels in the serum
The BPH group showed a significant increase in serum DHT level (345 ± 8.17 pg/mL, P < 0.01) compared with the control group (159 ± 8.34 pg/mL; Fig. 1). In contrast, the finasteride-treated group showed a significantly reduced serum DHT level (250.57 ± 25.68 pg/mL, P < 0.01) compared with the BPH group. Similarly to finasteride-treated group, the KH053-treated groups showed significant reduction in DHT levels (249.86 ± 35.07 pg/mL in the 200 mg/kg group, P < 0.05) compared with the BPH group.
Figure 1.
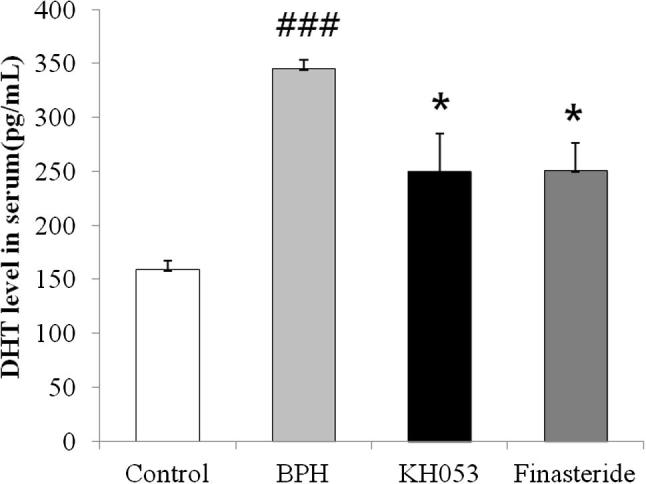
Effects of KH053 on the DHT levels in serum. Control, not treated group; BPH, testosterone induced BPH group; KH053, testosterone induced BPH with KH053 (200 mg/kg) group; finasteride, testosterone induced BPH with finasteride (1 mg/kg. p.o.) group. ###P < 0.001 compared with the control group. *P < 0.05 compared with the BPH group.
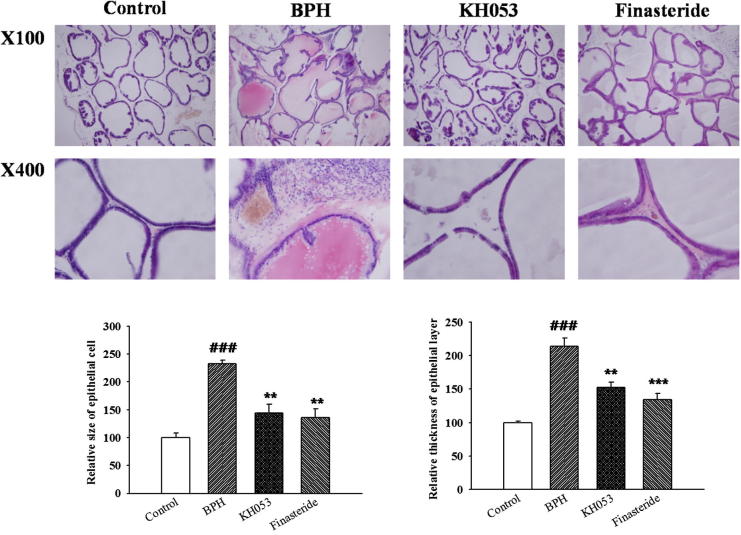
3.5. Effect of KH053 on the histopathology of the prostate tissue
As shown in Fig. 2, there was no change in the histoarchitecture of prostate gland in the control group. The prostate tissue was tightly packed; epithelium was cuboidal and regular in size. However, the prostate of the BPH group showed prostatic epithelial hyperplasia (P < 0.001). The epithelial cell layer and stromal cell space in the BPH group were larger. KH053-treated rats significantly showed a reduction in both epithelial size and thickness of epithelial layer compared with that of the BPH group, which was similar to the reduction in finasteride group.
Figure 2.
Histological examination. Control, not treated group; BPH, testosterone induced BPH group; KH053, testosterone induced BPH with KH053 (200 mg/kg) group; finasteride, testosterone induced BPH with finasteride (1 mg/kg. p.o.) group. ###P < 0.001 compared with the control group. **P < 0.01 and ***P < 0.01 compared with the BPH group.
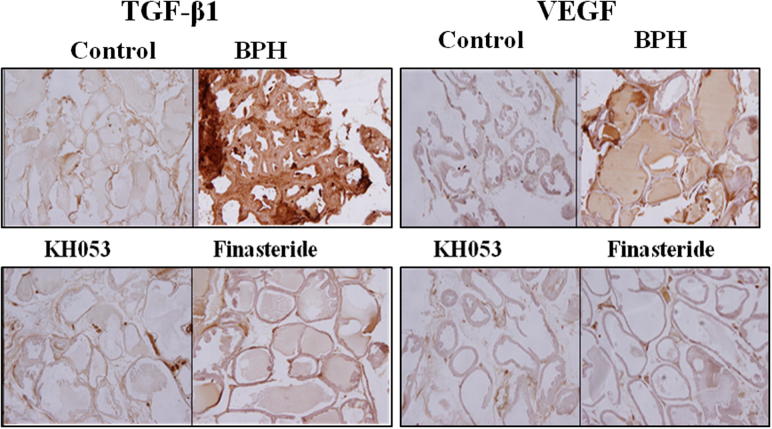
3.6. Expression of VEGF and TGF-β1 in prostate tissue
In order to examine the effects of the administration of KH053 on the expression of growth factors in prostate tissue, In Fig. 3, the protein expressions of growth factors (VEGF and TGF-β1) in the BPH group were increased than those of the control group (P < 0.05). These protein expressions of VEGF and TGF-β1 in both the KH053 group and the finasteride group observed a decrease compared with those of the BPH group (P < 0.05).
Figure 3.
The expressions of TGF-β1 and VEGF immunohistochemistry of prostate tissue from the rats (×100). Control, not treated group; BPH, testosterone induced BPH group; KH053, testosterone induced BPH with KH053 (200 mg/kg) group; finasteride, testosterone induced BPH with finasteride (1 mg/kg. p.o.) group. To determinate a mechanism which could explain the effects of KH053 on BPH, the expression of TGF-β1 and VEGF in the rat prostates were examined. As shown in Fig. 2, immunohistochemical results showed TGF-β1 and VEGF are rarely expressed in normal prostate, weak labeling observed in prostatic tissues in control group. Immunohistochemical detection also revealed an increase in TGF-β1 and VEGF expression in prostatic epithelial cells in the BPH model group. A moderate expression of TGF-β1 and VEGF was also observed in the BPH model group. Immunohistochemical result shows that the level of VEGF was significantly higher in the BPH group, while no significant difference was seen in the KH053 group as compared with the finasteride group.
4. Discussion
The main objective of this study was to evaluate whether a KH053 could prevent the development of BPH in an animal model. BPH showed enlargement of the prostate through a proliferative process of the stromal and epithelial elements. KH053 significantly reduced the pathology in inhibiting prostate growth in our results. KH053 treated animals showed significant reductions in prostate weights, DHT, and decreased prostatic epithelial hyperplasia with decreased TGF-β1 and VEGF expression.
There are two drugs used for BPH therapy. They are 5α-reductase inhibitors (5-ARIs) and α-adrenergic receptor blockers (Carson and Rittmaster, 2003, O’Leary, 2003). The α1-adrenergic receptor (α1-ARs) antagonists are the initial drugs for treating BPH (O’Leary, 2003). 5-ARIs are related to metabolic transformations of a variety of endogenous steroids. 5-ARIs generally prevent conversion of testosterone, DHT in androgen-associated disorders. So, 5-ARIs are used to the treatment of BPH and androgenetic alopecia (Rosen et al., 2005, Miller and Tarter, 2009). However, the drug has unfavorable side effects, such as orthostatic hypotension, decreased libido, and erectile dysfunction (Roehrborn, 2001, Roehrborn et al., 2002, MacDonald and Wilt, 2005). DHT connects to nuclear androgen receptors (ARs) on the surface of stromal and epithelial cells. It activates the transcription of various growth factors that are mitogenic for the epithelial and stromal cells of the prostate (Carson and Rittmaster, 2003, Rittmaster et al., 2008). Thus, an increased DHT level could contribute to the development of BPH. In our results, KH053 significantly showed a decreased DHT level.
TGF-β1 has been known as multifunctional cytokine. It plays an important role in numerous pathways, such as extracellular matrix production and degradation, cell differentiation, proliferation, and apoptosis (Fleisch et al., 2006). VEGF plays a major role in metastasis and angiogenesis. VEGF is a major factor in angiogenesis as it influences endothelial cell growth (Carson and Rittmaster, 2003) and acts in prostate stimulating the secretion of extracellular matrix elements, proliferation and angiogenesis, and also contributing to the glandular hyperplasia. A previous study reported that DHT could increase the transcription of VEGF and the secretion of biologically active VEGF from human prostatic stroma (Levine et al., 1998). In our results, KH053 significantly showed decreased expressions of TGF-β1 and VEGF.
In this study, KH053, comprising P. ginseng and bee-pollen, treatment in rats induced BPH effectively reduced prostate weight, both epithelial size and thickness of epithelial layer, DHT level compared to those of rats induced BPH. In addition, we observed that KH053 contributed to decreased expressions of TGF-β1 and VEGF in prostate tissues. These results indicate that KH053 has inhibited effects on the development of BPH in an animal model and may be effective strategies for clinical application of KH053 to BPH. In further studies, clinical trials need to investigate whether KH053 is safe, effective for humans and/or BPH patients.
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Acknowledgement
This study was supported by grants from the “Cooperative Research Program for Agriculture Science & Technology Development (Project No. PJ0095582015)” Rural Development Administration, Republic of Korea.
Footnotes
Peer review under responsibility of King Saud University.
References
- Andersson S., Russell D.W. Structural and biochemical properties of cloned and expressed human and rat steroid 5 alpha-reductases. Proc Natl Acad Sci U S A. 1990;87:3640–3644. doi: 10.1073/pnas.87.10.3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae J.S., Park H.S., Park J.W., Li S.H., Chun Y.S. Red ginseng and 20(S)-Rg3 control testosterone-induced prostate hyperplasia by deregulating androgen receptor signaling. J. Nat. Med. 2012;66:476–485. doi: 10.1007/s11418-011-0609-8. [DOI] [PubMed] [Google Scholar]
- Bailey D.T., Dalton C., Joseph D.F., Tempesta M.S. Can a concentrated cranberry extract prevent recurrent urinary tract infections in women? A pilot study. Phytomedicine. 2007;14:237–241. doi: 10.1016/j.phymed.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Balamurugan R. Smilax chinensis Linn. (Liliaceae) root attenuates insulin resistance and ameliorate obesity in high diet induced obese rat. South Indian J. Biol. Sci. 2015;1(1):47–51. [Google Scholar]
- Carson C., Rittmaster R. The role of dihydrotestosterone in benign prostatic hyperplasia. Urology. 2003;61:2–7. doi: 10.1016/s0090-4295(03)00045-1. [DOI] [PubMed] [Google Scholar]
- Cheng N., Ren N., Gao H., Lei X., Zheng J., Cao W. Antioxidant and hepatoprotective effects of Schisandra chinensis pollen extract on CCl4-induced acute liver damage in mice. Food Chem. Toxicol. 2013;55:234–240. doi: 10.1016/j.fct.2012.11.022. [DOI] [PubMed] [Google Scholar]
- Cho I.H. Effects of Panax ginseng in neurodegenerative diseases. J. Ginseng Res. 2012;36:342–353. doi: 10.5142/jgr.2012.36.4.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuBeau C.E., Resnick N.M. Controversies in the diagnosis and management of benign prostatic hypertrophy. Adv. Intern. Med. 1992;37:55–83. [PubMed] [Google Scholar]
- Elberry A.A., Mufti S.T., Al-Maghrabi J.A., Abdel-Sattar E.A., Ashour O.M., Ghareib S.A., Mosli H.A. Anti-inflammatory and antiproliferative activities of date palm pollen (Phoenix dactylifera) on experimentally-induced atypical prostatic hyperplasia in rats. J. Inflamm. 2011;8:40. doi: 10.1186/1476-9255-8-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feas X., Vazquez-Tato M.P., Estevinho L., Seijas J.A., Iglesias A. Organic bee pollen: botanical origin, nutritional value, bioactive compounds, antioxidant activity and microbiological quality. Molecules. 2012;17:8359–8377. doi: 10.3390/molecules17078359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleisch M.C., Maxwell C.A., Barcellos-Hoff M.H. The pleiotropic roles of transforming growth factor beta in homeostasis and carcinogenesis of endocrine organs. Endocr. Relat. Cancer. 2006;13:379–400. doi: 10.1677/erc.1.01112. [DOI] [PubMed] [Google Scholar]
- Hong S.H., Suk K.T., Choi S.H., Lee J.W., Sung H.T., Kim C.H., Kim E.J., Kim M.J., Han S.H., Kim M.Y. Anti-oxidant and natural killer cell activity of Korean red ginseng (Panax ginseng) and urushiol (Rhus vernicifera Stokes) on non-alcoholic fatty liver disease of rat. Food Chem. Toxicol. 2013;55:586–591. doi: 10.1016/j.fct.2013.01.022. [DOI] [PubMed] [Google Scholar]
- Isaacs J.T., Coffey D.S. Etiology and disease process of benign prostatic hyperplasia. Prostate Suppl. 1989;2:33–50. doi: 10.1002/pros.2990150506. [DOI] [PubMed] [Google Scholar]
- Kim H., Lee H.J., Kim D.J., Kim T.M., Moon H.S., Choi H. Panax ginseng exerts antiproliferative effects on rat hepatocarcinogenesis. Nutr. Res. 2013;33:753–760. doi: 10.1016/j.nutres.2013.07.005. [DOI] [PubMed] [Google Scholar]
- Kim S.K., Chung J.H., Lee B.C., Lee S.W., Lee K.H., Kim Y.O. Influence of Panax ginseng on alpha-adrenergic receptor of benign prostatic hyperplasia. Int. Neurourol. J. 2014;18:179–186. doi: 10.5213/inj.2014.18.4.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y.O., Kim Y., Lee G.E., Na S.W., Hong S.P., Arasu M.V., Yoon Y.W., Kim J. Panax ginseng improves functional recovery after contusive spinal cord injury by regulating the inflammatory response in rats an in-vivo study. Evid. Based Complement. Altern. Med. 2015 doi: 10.1155/2015/817096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine A.C., Liu X.H., Greenberg P.D., Eliashvili M., Schiff J.D., Aaronson S.A., Holland J.F., Kirschenbaum A. Androgens induce the expression of vascular endothelial growth factor in human fetal prostatic fibroblasts. Endocrinology. 1998;139:4672–4678. doi: 10.1210/endo.139.11.6303. [DOI] [PubMed] [Google Scholar]
- Lim K.H., Kang C.W., Choi J.Y., Kim J.H. Korean red ginseng induced cardioprotection against myocardial ischemia in guinea pig. Korean J. Physiol. Pharmacol. 2013;17:283–289. doi: 10.4196/kjpp.2013.17.4.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald R., Wilt T.J. Alfuzosin for treatment of lower urinary tract symptoms compatible with benign prostatic hyperplasia: a systematic review of efficacy and adverse effects. Urology. 2005;66:780–788. doi: 10.1016/j.urology.2005.05.001. [DOI] [PubMed] [Google Scholar]
- McNeal J.E. Origin and evolution of benign prostatic enlargement. Invest. Urol. 1978;15:340–345. [PubMed] [Google Scholar]
- Miller J., Tarter T.H. Combination therapy with dutasteride and tamsulosin for the treatment of symptomatic enlarged prostate. Clin. Interv. Aging. 2009;4:251–258. doi: 10.2147/cia.s4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Leary M.P. Lower urinary tract symptoms/benign prostatic hyperplasia: maintaining symptom control and reducing complications. Urology. 2003;62:15–23. doi: 10.1016/s0090-4295(03)00480-1. [DOI] [PubMed] [Google Scholar]
- Pascoal A., Rodrigues S., Teixeira A., Feas X., Estevinho L.M. Biological activities of commercial bee pollens: antimicrobial, antimutagenic, antioxidant and anti-inflammatory. Food Chem. Toxicol. 2014;63:233–239. doi: 10.1016/j.fct.2013.11.010. [DOI] [PubMed] [Google Scholar]
- Quan H.Y., Kim D.Y., Chung S.H. Korean red ginseng extract alleviates advanced glycation end product-mediated renal injury. J. Ginseng Res. 2013;37:187–193. doi: 10.5142/jgr.2013.37.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittmaster R., Hahn R.G., Ray P., Shannon J.B., Wurzel R. Effect of dutasteride on intraprostatic androgen levels in men with benign prostatic hyperplasia or prostate cancer. Urology. 2008;72:808–812. doi: 10.1016/j.urology.2008.06.032. [DOI] [PubMed] [Google Scholar]
- Roehrborn C.G. Efficacy and safety of once-daily alfuzosin in the treatment of lower urinary tract symptoms and clinical benign prostatic hyperplasia: a randomized, placebo-controlled trial. Urology. 2001;58:953–959. doi: 10.1016/s0090-4295(01)01448-0. [DOI] [PubMed] [Google Scholar]
- Roehrborn C.G., Boyle P., Nickel J.C., Hoefner K., Andriole G., Aria A., Investigators A.S. Efficacy and safety of a dual inhibitor of 5-alpha-reductase types 1 and 2 (dutasteride) in men with benign prostatic hyperplasia. Urology. 2002;60:434–441. doi: 10.1016/s0090-4295(02)01905-2. [DOI] [PubMed] [Google Scholar]
- Rosen R.C., Giuliano F., Carson C.C. Sexual dysfunction and lower urinary tract symptoms (LUTS) associated with benign prostatic hyperplasia (BPH) Eur. Urol. 2005;47:824–837. doi: 10.1016/j.eururo.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Sharma J., Goyal P.K. Chemoprevention of chemical-induced skin cancer by Panax ginseng root extract. J. Ginseng Res. 2015;39:265–273. doi: 10.1016/j.jgr.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqi M.H., Siddiqi M.Z., Ahn S., Kang S., Kim Y.J., Sathishkumar N., Yang D.U., Yang D.C. Ginseng saponins and the treatment of osteoporosis: mini literature review. J. Ginseng Res. 2013;37:261–268. doi: 10.5142/jgr.2013.37.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S.O., Park H.R., Sohn E.S., Lee S.W., Kim H.D., Kim Y.C., Kim K.H., Na S.W., Choi H.-K., Arasu M.V., Kim Y.O. Classification of ginseng berry (Panax ginseng C.A. MEYER) extract using 1H NMR spectroscopy and its inhibition of lipid accumulation in 3 T3-L1 cells. BMC Complement. Altern. Med. 2014;14:455. doi: 10.1186/1472-6882-14-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yono M., Yamamoto Y., Imanishi A., Yoshida M., Ueda S., Latifpour J. Differential effects of prazosin and naftopidil on pelvic blood flow and nitric oxide synthase levels in spontaneously hypertensive rats. J. Recept. Signal Transduct. Res. 2008;28:403–412. doi: 10.1080/10799890802176626. [DOI] [PubMed] [Google Scholar]