Abstract
Isobaric tagging reagents have become an invaluable tool for multiplexed quantitative proteomic analysis. These reagents can label multiple, distinct peptide samples from virtually any source material (e.g., tissue, cell line, purified proteins), allowing users the opportunity to assess changes in peptide abundances across many different time points or experimental conditions. Here, we describe the application of isobaric peptide labeling, specifically 8plex isobaric tags for relative and absolute quantitation (8plex iTRAQ), for quantitative phosphoproteomic analysis of cultured cells or tissue suspensions. For this particular protocol, labeled samples are pooled, fractionated by strong cation exchange chromatography, enriched for phosphopeptides, and analyzed by tandem mass spectrometry (LC-MS/MS) for both peptide identification and quantitation.
Keywords: IMAC, Isobaric tags, Isotopic labeling, iTRAQ, LC-MS/MS, Mass spectrometry, Multiplexing, Phosphopeptide, Phosphoproteomics, Reporter ion, TMT
1 Introduction
Reversible protein phosphorylation is a key post-translational modification responsible for various cellular regulatory mechanisms. Protein phosphorylation studies are challenging since phosphorylated proteins are often low in abundance and of low stoichiometry. Moreover, phosphorylated peptides from a mixture often exhibit low ionization efficiencies during LC-MS/MS analysis due to ion suppression effects. Thus, careful sample preparation, adequate sample amount, and efficient phosphopeptide enrichment steps are basic requirements for any successful phosphoproteomic analysis.
Phosphopeptide enrichment methods are widely adapted to the “bottom up” proteomics approach which is characterized by proteolytic digestion of proteins into peptide fragments prior to analysis by mass spectrometry. Immobilized metal affinity chromatography (IMAC) is based on the affinity of the negatively charged phosphate groups on phosphopeptides for a positively charged metal ion column matrix, and it remains the most widely used method for affinity enrichment [1–3]. However, metal oxides, especially titanium dioxide (TiO2), are common alternatives to IMAC and often can isolate unique subsets of phosphopeptides not enriched by other methods [4, 5].
Novel MS acquisition techniques have also spurred growth in the field of phosphoproteomics. Techniques such as neutral loss scanning, precursor ion scanning, and multi-stage activation (MSA) have been successfully applied to the routine identification of protein phosphorylation from complex biological samples [6–8]. New fragmentation methods including HCD, ECD, and ETD have also been utilized for protein phosphorylation analysis, which has allowed better fragmentation of the phosphorylated peptides, improved assignment of phosphorylation sites, and increased the sensitivity of MS-based protein phosphorylation analysis [9–11].
One of the breakthroughs in the field of proteomics has been the development of a vast array of quantitative methods. These include various label-free methods, stable isotope labeling, and targeted quantification techniques. All methods are applicable to phosphoproteomics, and quantitative phosphoproteomics has become an important method for measuring changes in protein phosphorylation on a global scale. Stable isotope labeling approaches generally produce more reliable quantification results compared to label-free quantification. Stable isotope labeling strategies include stable isotope labeling by amino acids in cell culture (SILAC), dimethyl labeling, and the use of isobaric tagging reagents such as isobaric tags for relative and absolute quantitation (iTRAQ) and the tandem mass tagging (TMT) approach. Although all three methods have their strengths and weaknesses, a recent study indicates that all three can reach a similar level of sensitivity based on the number of identified proteins using a classical (MS2-based) shotgun approach [12]. SILAC and dimethyl labeling strategies quantify peptides at the MS1 level. The more differential labels are used, the more complex the MS1 spectra will be. Thus, normally only two or three differential labels are used. The isobaric tagging strategy, on the other hand, quantifies peptides at the MS2 level. Differentially labeled peptides will have the same m/z (at the MS1 level) and will be selected for MS2 analysis at the same time. Therefore isobaric labeling can allow quantitative comparison of up to ten different peptide samples, e.g., using the commercially available TMT 10plex kit. It is worth noting reports of the use of hyperplexing (i.e., 18-plex), as well as a more recent 54-plex technique, which have greatly enhanced the capacity for sample multiplexing with isobaric reagents [13, 14]. In addition to the advantage provided by multiplexing, isobaric tagging approaches are relatively easy to perform. Furthermore, these approaches can be adapted to label virtually any sample type (e.g., cell line, tissue, or purified proteins).
The isobaric tagging approach is based on the covalent labeling of the N-terminus and side-chain primary amines of peptides with tags of varying masses through NHS-ester chemistry, followed by MS analysis [15, 16]. The structure of each reagent consists of three distinct regions: (1) a cleavable reporter group of a specific mass for peptide quantitation (113, 114, 115, 116, 117, 118, 119, and 121 Da in the case of 8plex iTRAQ), (2) a mass normalizer or “balancer” region that makes each tag isobaric, and (3) an amine reactive group that will covalently attach the tag to the peptide (see Fig. 1). Relative quantification of a peptide is based on different reporter ions generated in the low mass area of its MS2 spectra (see Fig. 2). Due to the small size of the reporter ions, iTRAQ is compatible only with wider mass range instruments such as triple quadrupole and the Orbitrap generation of mass spectrometers, not with traditional ion traps. The signals of these reporter ions normally do not interfere with b and y ions used for peptide identification. Peptide samples to be labeled with isobaric tagging reagents should be free of the following: thiols, high concentrations of detergents or denaturants, and chemicals/buffers with primary amines other than the analyte of interest. Primary amines can react with the isobaric tagging reagents resulting in insufficient labeling of sample peptides. Equal amounts of labeled samples are then pooled, fractionated and enriched for phosphopeptides, and followed by LC-MS/MS analysis. The same peptide from differentially labeled samples will still possess the same mass, i.e., the original mass plus the mass of the isobaric tag less one proton due to conjugation (+304 Da in the case of 8plex iTRAQ). Thus, the isobaric tagging approach does not lead to more complex MS1 spectra as the differentially labeled peptides co-elute from the HPLC prior to MS analysis. During LC-MS analysis, these peptides are co-isolated for MS/MS fragmentation, where they generate the same b and y ion series for peptide identification while the relative quantification information is retained in the ratios of the reporter ion series. The fact that isobaric tagging reagents allow multiplexing is advantageous for research projects involving a time course design, e.g., monitoring changes in protein expression or changes in the level of various post-translational modifications following hormone stimulation across different time points or biological conditions. The labeling step for the isobaric tagging approach is performed after protein digestion, thus any variability in sample handling prior to sample pooling will increase the quantification biases. A normalization procedure can be adapted to correct for these quantification errors (see protocol below). Once the labeled peptides are pooled, further experimental biases will be minimized. For phosphoproteomics workflows in particular, the fractionation step as well as the phosphopeptide enrichment step should not introduce additional quantification errors since the samples should have already been pooled before these steps. There are currently two types of iTRAQ reagents available: 4plex and 8plex. With the 4plex reagent, up to four different biological conditions can be investigated at the same time; and with the 8plex reagent, up to eight. The 4plex and 8plex reagents have different structures in the balancer group region; however, they show only slight differences in sensitivity. There was an initial report that showed that 4plex kits may generate higher numbers of protein identifications compared to 8-plex kits [17]. However, it was later shown that 8plex iTRAQ provides more consistent quantification ratios compared to 4plex, and provides comparable total identifications while allowing more experimental conditions to be investigated in a large scale proteomics study [18].
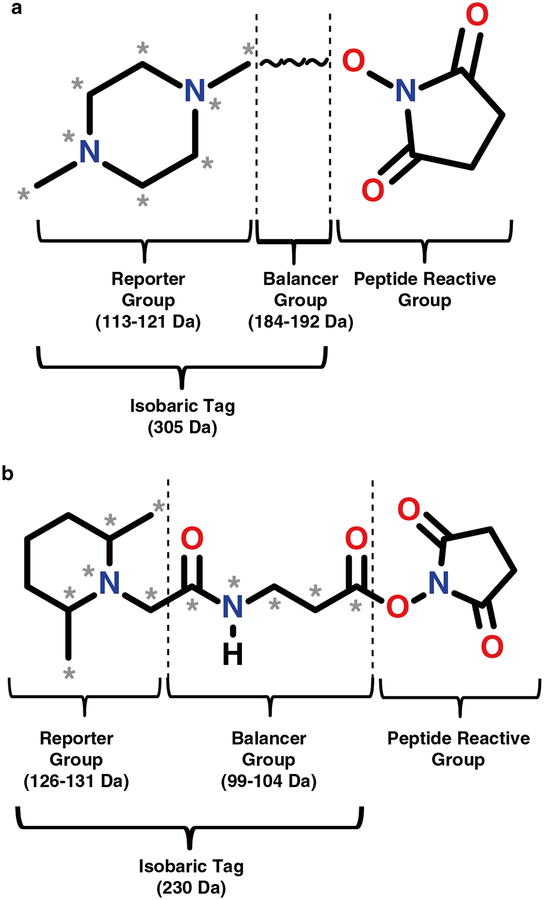
Fig. 1.
Chemical structures of isobaric tagging reagents. The general structure for both 8plex iTRAQ (a) and 6plex TMT (b) tags consists of an MS-cleavable reporter group, a balancer group of variable sizes to make the tag isobaric, and a peptide reactive group for labeling. Asterisks indicate positions of 13C and 15N heavy isotope substitutions which are used to generate reporter ions of various sizes. Vertical dashed lines indicate bonds that break during labeling (right-hand lines) and bonds that break during MS fragmentation (left-hand lines). Note: the structure of the 8plex iTRAQ balancer group is not yet published
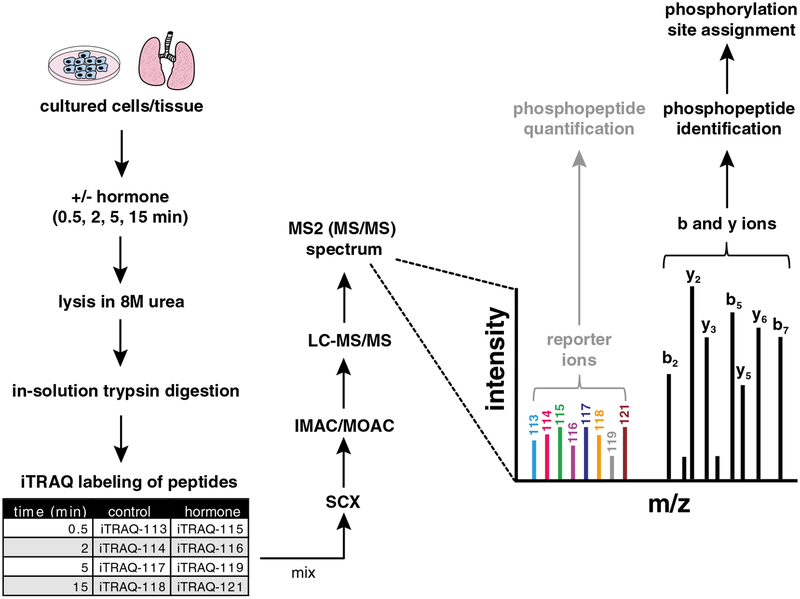
Fig. 2.
Experimental workflow for iTRAQ-based quantitative phosphoproteomic analysis. Cultured cells/tissue suspensions are treated with or without hormone for the indicated times followed by lysis in 8 M urea. Protein lysates are then digested with trypsin, desalted, and labeled with 8plex iTRAQ reagents. Strong cation exchange chromatography (SCX) stratifies the sample into 20 fractions followed by either immobilized metal affinity chromatography (IMAC) or metal oxide affinity chromatography (MOAC), which will enrich each fraction for phosphopeptides. Phosphopeptides are analyzed by tandem mass spectrometry (LC-MS/MS) in which the fragmentation is performed by higher energy collision induced dissociation (HCD), and the mass-to-charge ratio (m/z) and intensity of corresponding peptide ions are measured by an orbitrap-based mass spectrometer. In the MS2 spectrum, the pattern of b and y ions allows for phosphopeptide identification through database searching (black peaks), while the intensities of the iTRAQ reporter ions allow for relative quantification of phosphopeptide abundances across the eight different experimental conditions (colored peaks)
One common problem encountered during LC-MS/MS analysis of iTRAQ or TMT labeled complex samples is the co-isolation of contaminant ions with similar m/z values and elution times. This means that for a given peptide, the reporter ion ratios in its corresponding MS2 spectrum do not reflect the true quantification ratios for that peptide, but instead reflect the sum of all reporter ion intensities produced by that peptide and from all other contaminating peptides co-isolated with the peptide of interest. This phenomenon is often referred to as “isolation interference” or “ratio compression,” as it tends to compress peptide quantitation ratios toward unity (i.e., 1). The problem can be partially alleviated by performing fractionation at the peptide level using techniques such as SCX or HILIC chromatography. Fractionation reduces the complexity of the original sample and is usually based on an alternative peptide separation strategy other than C18 (normally the method of choice for HPLC separation coupled to MS analysis). At the data analysis level, software such as Proteome Discoverer (Thermo Scientific) can calculate isolation interference scores based on the unassigned peaks and their intensities presented in MS2 spectra. Using an appropriate isolation interference score cutoff, users can filter large-scale iTRAQ quantification data with more reliable results. At the MS acquisition level, two MS techniques have been adopted for overcoming the ratio compression problem: gas phase fractionation [19] and MS3 acquisition [20]. Gas phase fractionation uses the proton-transfer ion-ion reactions (PTR) to reduce the precursor ion charge state and gets rid of contaminating ions with different charge states. MS3 acquisition provides an additional isolation and fragmentation event that helps minimize the interference problem. However, it was noted that the MS3 method suffered from reduced sensitivity. To overcome issues with sensitivity, a relatively recent approach was developed called Synchronous Precursor Selection (MultiNotch) MS3 which allows isolation of multiple MS2 product ions simultaneously, helping to increase the intensity of reporter ions in MS3 spectra and improving sensitivity, precision, and accuracy in MS quantification [21].
In this chapter we will introduce a standard workflow for 8plex iTRAQ labeling of peptides isolated from mammalian cells or tissue suspensions for multiplexed quantitative phosphoproteomic analysis. A similar workflow was recently used to successfully probe the phosphorylation dynamics of the vasopressin V2 receptor signaling pathway in mammalian kidney [22].
2 Materials
Note: All reagents including water, acetonitrile, and isopropanol should be HPLC-grade or higher.
2.1 Preparation of Cell Lysates
Cells.
Hormone for stimulation (For example: vasopressin).
Cell Lysis Buffer, 8 M urea, 50 mM Tris–HCl, 75 mM NaCl, 1× Halt Protease and Phosphatase Inhibitor Cocktail.
Benchtop Centrifuge.
Probe sonicator (Misonix 3000 or equivalent).
Reagents for protein assay (e.g., BCA assay).
1.5 ml microcentrifuge tubes.
2.2 In-Solution Protease Digestion
50 mM Ammonium Bicarbonate (AmBic) Buffer, 0.2 g ammonium bicarbonate in 50 ml HPLC-grade water.
250 mM Dithiothreitol (DTT) stock, 7.7 mg DTT in 200 μl AmBic.
250 mM Iodoacetamide stock, 9.3 mg iodoacetamide in 200 μl AmBic.
1 μg/μl Trypsin stock, 100 μg Trypsin Gold in 100 μl of 50 mM acetic acid. Keep on ice until ready to use, then freeze the unused portion at −20 °C.
100 % Formic Acid.
Benchtop Centrifuge.
pH meter or pH paper.
Waters Oasis HLB 1 cc Desalting Cartridges (WAT094225 or equivalent).
100 % Acetonitrile (ACN).
Water (LC/MS grade).
Savant SC100 SpeedVac with RT490 Refrigerated Condensation Trap.
2.3 iTRAQ Labeling
iTRAQ 8plex Multi-plex Kit (AB SCIEX): 5× 1-U vials of each iTRAQ 8plex reagent (i.e., 113, 114, 115, 116, 117, 118, 119, and 121), Dissolution Buffer pH 8.5 (0.5 M triethylammonium bicarbonate, TEAB), and isopropanol. Important note: The denaturant, reducing reagent, and cysteine-blocking reagent vials provided with this kit are not used in this protocol.
100 % Formic Acid.
Savant SC100 SpeedVac with RT490 Refrigerated Condensation Trap.
pH meter or pH paper.
15 ml conical tubes.
2.4 Sample Fractionation by Strong Cation Exchange Chromatography
PolySulfoethyl A SCX column (4.6 mm ID × 20 cm length, 5-μm particle size, 300-Å pore size; PolyLC).
SCX Buffer A, 5 mM KH2PO4/25 % ACN, pH 2.67. Dissolve 0.68 g KH2PO4 in 747 ml LC-MS/MS grade water. Monitoring with a pH meter and with constant mixing, add ~2.5–3 ml of 1 N HCl to bring pH to 2.67. Add 250 ml 100 % ACN and mix.
SCX Buffer B, 5 mM KH2PO4/500 mM KCl/25 %ACN, pH 2.67. Dissolve 0.68 g KH2PO4 and 37.29 g KCl in 747 ml LC-MS/MS grade water. Monitoring with a pH meter and with constant mixing, add ~2.5–3 ml of 1 N HCl to bring pH to 2.67. Add 250 ml 100 % ACN and mix.
HPLC system (Agilent HP1100 System or equivalent).
Waters Oasis HLB cartridge.
2.5 Phosphopeptide Enrichment
Pierce Fe-NTA Phosphopeptide Enrichment Kit (Pierce/Thermo).
Pierce Graphite Spin Columns (Pierce/Thermo).
2.6 LC-MS/MS Analysis
Eksigent Nanoflow LC system connected to an LTQ Orbitrap Velos mass spectrometer or an equivalent LC-MS/MS system.
MS Buffer A: 0.1 % formic acid in water.
MS Buffer B: 0.1 % formic acid in acetonitrile.
2.7 Phosphopeptide Identification (ProteinDatabase Searching)
Proteome Discoverer Software (or equivalent)
3 Methods
3.1 Preparation of Cell Lysates
Incubate cell line/tissue suspensions with hormone/reagent of choice for the appropriate amounts of time. The amount of protein for each sample should be at least 100 μg (optimally 500 μg) for each desired experimental condition. A typical 8 plex iTRAQ time course experimental design is provided in Fig. 2 (see Note 1).
Following incubation, briefly spin samples at 10,000 × g for 30 s to pellet the cells and remove the supernatant.
Resuspend cell pellets in 150 μl of Cell Lysis Buffer in a 1.5 ml microcentrifuge tube.
Place samples in a small container of wet ice. Sonicate immediately using a Misonix probe sonicator or equivalent for 1 min, setting 1, with 0.5 s bursts.
Spin at >10,000 × g for 10 min in a benchtop centrifuge to pellet cellular debris. Transfer the supernatants to new micro-centrifuge tubes.
Perform a protein assay (e.g., BCA assay). The samples should contain at least 100 μg (optimally 500 μg) of protein and the concentration should be approximately 4 μg/μl (see Note 2).
3.2 In-Solution Protease Digestion
Reduce the samples by adding DTT to a final concentration of 10 mM. Incubate 1 h at 37 °C.
Alkylate the samples by adding iodoacetamide to a final concentration of 40 mM. Incubate 1 h, no longer. (Protect sample from light.)
Quench the excess iodoacetamide by adding another 40 mM DTT. Incubate for at least 15 min at room temperature.
Dilute the samples to <1 M urea with 50 mM AmBic.
Add trypsin at a trypsin-to-protein ratio of 1:20 to 1:100 (weight: weight). Ideally, the final trypsin concentration in the sample should be ≥12 ng/μl. Incubate at 37 °C for 16 h.
Terminate the reaction by adding 100 % formic acid to a final concentration of 0.5 %.
Spin the samples at ≥16,000 × g for 20 min at 4 °C in a bench-top centrifuge to pellet any insoluble material. Transfer the supernatants to fresh tubes. Check that the pH is <4.0.
- Desalt the samples using a Waters Oasis HLB cartridge (see Note 3).
-
(a)Condition the cartridge with 1 ml of 100 % ACN.
-
(b)Equilibrate with 1 ml of 0.1 % formic acid.
-
(c)Slowly apply the peptide sample to the cartridge (1 drop every 3 s).
-
(d)Wash the cartridge three times with 1 ml of 0.1 % formic acid.
-
(e)Elute the desalted peptides slowly using 1 ml of 0.1 % formic acid/50 % ACN.
-
(f)Vacuum-concentrate the samples down to <10 μl using a SpeedVac.
-
(a)
At any step in the protocol that includes vacuum concentration of peptides, samples can be stored at ≤ −20 °C.
Vacuum concentration using a SpeedVac is often a slow process, especially for larger volumes or less volatile liquids. For convenience, samples can be safely left overnight in the SpeedVac without compromising the integrity of the peptide sample.
3.3 iTRAQ Labeling
(Note: The following protocol is for labeling 500 μg of peptide per iTRAQ channel. At least 100 μg of peptide per iTRAQ channel should be used. Please scale the amount of each reagent accordingly.)
Bring iTRAQ reagent vials, Dissolution Buffer, and isopropanol to room temperature (see Note 4).
- Preparation of iTRAQ reagents.
-
(a)Briefly spin iTRAQ reagent vials to bring the liquid to the bottom of tube.
-
(b)Add 70 μl of isopropanol to each vial. Vortex and spin.
-
(c)Combine the contents of the five duplicate iTRAQ reagent vials into a single vial for each reagent. Each iTRAQ reagent vial should now contain approximately 350–370 μl of reagent.
-
(d)Vortex the tubes and spin again.
-
(a)
Resuspend the peptide samples in 150 μl of iTRAQ Dissolution Buffer.
Add the total contents of each iTRAQ reagent vial to each sample according to your particular experimental design (An example is provided in Fig. 2). Vortex briefly to mix (see Note 5).
Incubate for 2 h at room temperature.
Quench the reaction by adding formic acid to a final concentration of 0.5 %. Samples can be stored at −80 °C if necessary before proceeding with the rest of the protocol.
Vacuum-concentrate the samples to <50 μl to remove the majority of isopropanol. Important: Avoid letting samples dry completely or they will be difficult to resuspend during the next step.
Resuspend each sample in 500 μl of 0.5 % formic acid.
Combine all 8 iTRAQ-labeled samples into a single 15 ml conical tube. Check that the pH is <4.0.
Divide the sample equally across four desalting cartridges. Desalt the sample as in step 8, Subheading 3.2. Vacuum-concentrate the sample to a volume <10 μl.
3.4 Sample Fractionation by Strong Cation Exchange Chromatography
Resuspend the sample in 300 μl of SCX Buffer A. Check that the pH is 2.6–3.0.
Load the sample onto a conditioned PolySulfoethyl A SCX column attached to an HPLC system (Agilent HP1100 System or equivalent).
Run at a flow rate of 1 ml/min using the following gradient: 100 % buffer A and 0 % buffer B for 2 min; 0–14 % buffer B for 33 min; 14–100 % buffer B for 1 min; 100 % buffer B held for 4 min.
Collect fractions every 1.5 min. Based on the chromatographic profile at 214 nm, pool the samples down to 20 fractions (see Note 6).
Vacuum-concentrate the samples to a volume <10 μl. Resuspend samples in 0.1 % formic acid (see Note 7). Check that the pH is <4.0.
Desalt each fraction using a Waters Oasis HLB cartridge and reduce volume to <10 μl by vacuum-concentration (see step 8, Subheading 3.2).
3.5 Phosphopeptide Enrichment
Process all 20 SCX fractions by immobilized metal affinity chromatography (IMAC) or metal oxide affinity chromatography (MOAC) to enrich for phosphopeptides (see Note 8).
For IMAC, resuspend the labeled peptide samples in 200 μl of Binding Buffer (Pierce Fe-NTA Phosphopeptide Enrichment Kit).
Add sample to a Fe-NTA spin column and incubate for 20 min at room temperature with end-over-end rotation. Centrifuge the column at 1000 × g for 1 min. Discard the flow-through. Transfer column to a new tube.
Add 100 μl of Wash Buffer A to the spin column and gently mix the contents by tapping the side of the column. Do not pipette up and down.
Centrifuge the column at 1000 × g for 1 min. Discard the flow-through.
Repeat steps 4 and 5 once.
Add 100 μl of Wash Buffer B to the spin column and gently mix the contents as before.
Centrifuge the column at 1000 × g for 1 min. Discard the flow-through.
Repeat steps 7 and 8 once.
Add 100 μl of ultrapure water to the column and gently mix. Centrifuge the column at 1000 × g for 1 min. Discard the flow-through.
Transfer the column to a new collection tube and add 50 μl of Elution Buffer directly to the resin. Incubate for 5 min at room temperature.
Centrifuge the column at 1000 × g for 1 min. Retain eluate for analysis.
Repeat steps 11 and 12, Subheading 3.5, two additional times and pool the elution fractions.
Acidify the pooled elution by adding 200 μl of 2.5 % TFA.
Desalt samples using Pierce Graphite Spin Columns prior to analysis by mass spectrometry.
3.6 LC-MS/MS Analysis
Resuspend the desalted, phosphopeptide-enriched samples in 20 μl of 0.1 % formic acid.
Inject 10 μl of each sample onto an Eksigent Nanoflow LC system connected to an LTQ Orbitrap Velos mass spectrometer or an equivalent LC-MS/MS system (see Note 9). Save the other half of each sample for a subsequent LC-MS/MS run.
The following MS instrument parameters should be used: peptides ionized via a nano-spray ion source; MS run time of 65 min; spectra recorded in data-dependent acquisition mode with the dynamic exclusion option enabled; each survey MS scan followed by Higher Energy Collision Induced Dissociation (HCD) fragmentation of the top six most abundant precursor ions; both survey MS as well as MS2 scans acquired by the Orbitrap mass analyzer with a resolution of 30,000 and 7500 at m/z of 400 for MS and MS2 scans, respectively. For more effective fragmentation of iTRAQ-labeled peptides, set the normalized collision energy to 45 % (i.e., 10–15 % higher than for native peptides) or use a stepped normalized collisional energy scheme during HCD [23]. To minimize isolation interference, the precursor isolation window should be set to as narrow a width as possible (given that the sensitivity is not compromised). We recommend an isolation window of 3 m/z (i.e., ±1.5 m/z).
3.7 Phosphopeptide Identification (Protein Database Searching)
Search MS2 spectra (RAW files) using Proteome Discoverer Software running the Sequest search algorithm on a concate-nated database containing both forward and reversed complement sequences from the latest version of the NCBI Refseq Protein Database from the appropriate species. Append a list of common contaminating proteins (e.g., porcine trypsin and human keratin) (http://www.thegpm.org/crap/) (see Note 10).
The following MS search parameters are recommended: precursor ion tolerance set to 25 ppm; fragment ion tolerance set to 0.05 Da; three missed trypsin cleavages; static modifications are carbamidomethylation of cysteine (+57.021 Da) and iTRAQ 8plex modification of lysine and peptide N-termini (+304.205 Da); variable modifications are oxidation of methionine (+15.995 Da), phosphorylation of serine, threonine, and tyrosine (+79.966 Da), and iTRAQ 8plex modification of tyrosine (+304.205 Da); target-decoy filter set to a 1 % false discovery rate (FDR) at the peptide level; known contaminant ions should be excluded. In addition, each batch of iTRAQ reagents contains trace levels of isotopic impurities. Thus, users should also set the isotope correction factors based on the values provided in the certificate of analysis that comes with each iTRAQ kit.
Phosphorylation sites should be assigned using a phosphorylation site assignment algorithm such as PhosphoRS (provided with Proteome Discoverer Software), PhosSA [24], or Ascore [25] (see Note 11).
Phosphopeptides that match to more than one protein iso-form should be identified using programs such as MassSieve [26] and ProMatch [27]. Although it is not necessary to eliminate these peptide IDs from further analysis, iTRAQ quantification values obtained from these “ambiguous” peptides may reflect average peptide abundances from multiple protein isoforms that may be present in the sample.
3.8 Phosphopeptide Quantification
MS2 iTRAQ reporter ion intensities for phosphopeptides that possess the same linear amino acid sequence as well as the same site(s) of modification (including all types of modifications, not just phosphorylation) should be summed for each individual iTRAQ channel (see Note 12).
-
The desired relative abundance ratios are then calculated for each phosphopeptide (see the experimental design in Fig. 2).
For an arbitrary peptide X:
then: -
These values are then normalized using a global correction factor based on the ratio of the summed reporter ion intensities of all peptides in each corresponding iTRAQ channel.
If the summation of all reporter ion intensities for all peptides in each channel are:
then the normalization factor is:
and the normalized abundance ratio for peptide X is: -
The final step is to take the log2 of this normalized ratio.
The log2 normalized ratio is then used to calculate the mean and standard deviation of the relative abundance of each peptide among all biological replicates (see Note 13).
A one-sample t-test can be used to calculate a p-value for each peptide. Specifically, all log2 normalized ratios for a given peptide are compared to a hypothetical mean of 0 [i.e., log2(1) = 0 is equivalent to a fold change of 1, or no change].
- To correct for the higher number of false positive hits produced by multiple testing (i.e., thousands of peptides are routinely analyzed in a single data set), we recommend the use of a multiple testing correction method. The Benjamini and Hochberg (BH) False Discovery Rate [28] is relatively easy to calculate and represents an acceptable tradeoff between sensitivity and specificity (see Note 14). To calculate:
-
(a)Rank the p-value of each peptide from smallest to largest. The smallest p-value has a rank of r = 1, the next has a rank of r = 2, etc.
-
(b)Compare each peptide’s p-value to (r/n) Q, where n is the total number of peptides and Q is the chosen FDR (usually 0.05 or less).
-
(c)A p-value is considered significant (i.e., passed the FDR filter) if p < (r/n)Q.
-
(d)For a list of ten peptides that will be filtered for a FDR (Q) value of 0.05 or 5 %, see the example below. In this case, only the top three peptides will pass the BH 5 % filter [p < (r/n)Q].
-
(a)
| Peptide | Rank (r) | p-value | (r/n)Q |
|---|---|---|---|
| Peptide 1 | 1 | 0.001 | 0.005 |
| Peptide 2 | 2 | 0.002 | 0.010 |
| Peptide 3 | 3 | 0.011 | 0.015 |
| Peptide 4 | 4 | 0.077 | 0.020 |
| Peptide 5 | 5 | 0.210 | 0.025 |
| Peptide 6 | 6 | 0.350 | 0.030 |
| Peptide 7 | 7 | 0.410 | 0.035 |
| Peptide 8 | 8 | 0.650 | 0.040 |
| Peptide 9 | 9 | 0.740 | 0.045 |
| Peptide 10 | 10 | 0.920 | 0.050 |
Acknowledgments
This work was supported by the Intramural Programs of the National Heart, Lung, and Blood Institute (Project Z01-HL-001285) and by the National Research University Project, Office of Higher Education Commission (WCU-006-HR-57).
Footnotes
The experimental design in Fig. 2 describes a generic time course analysis of the effects of a hormone on global protein phosphorylation. The time points can be altered depending on the choice of hormone/drug as well as the system being studied. It is recommended that the length of each hormone treatment has its own time-matched control to account for fluctuations in basal phosphorylation levels with time. An alternative use of the 8plex iTRAQ methodology would be a dose-response assay to determine the effects of different concentrations of a hormone/drug on global protein phosphorylation.
If the sample is too diluted, you will need to concentrate the sample. We recommend a centrifugal filtration unit such as a Microcon YM-10 from Millipore.
We recommend using an HLB 1 cc/30 mg cartridge (WAT094225 or equivalent) which has a 1–5 mg peptide binding capacity. As gravity elution is not practical, a 5 cc syringe mounted on a luer adaptor (WAT054260) is recommended for controlled positive displacement of buffers and sample.
The iTRAQ 8plex Multi-plex Kit provides five 1-U tubes of each of eight different iTRAQ reagents (i.e., 113, 114, 115, 116, 117, 118, 119, and 121). Each unit can label up to 100 μg of peptide sample. Therefore, you will need all five vials of each reagent to label 500 μg of peptide sample for each experimental condition.
After adding the iTRAQ reagent to your sample, check that the pH is between 8.0 and 8.5 to ensure efficient labeling. Other requirements for efficient labeling include avoiding buffers with primary amines (e.g., ammonium bicarbonate and Tris), a Dissolution Buffer concentration of 120–150 mM, an organic concentration >65 %, an iTRAQ reagent concentration of 40 mM ± 5 %, and a peptide concentration of 0.5–1 mg/ml.
Due to the presence of negatively charged phosphate groups, phosphopeptides will not bind as strongly as unphosphorylated peptides to the negatively charged SCX resin. Thus, the majority of phosphopeptides will elute in earlier SCX fractions, while unphosphorylated peptides will tend to elute later. However, due to the presence of missed trypsin cleavages and other factors, phosphopeptides can be distributed across all SCX fractions.
iTRAQ-labeled peptides are larger and more hydrophobic than their unlabeled peptide counterparts. Adding 3–5 % ACN to resuspend dried peptides following the labeling reaction may increase recovery.
We use the Fe-NTA Phosphopeptide Enrichment Kit, although Ga+3-based IMAC or TiO2-based enrichment are both viable alternatives. If you choose the Fe-NTA method, we recommend that the final desalting step is done using Pierce Graphite Spin Columns.
The LC portion of this particular LC-MS/MS setup uses a C18 pre-column for desalting. The captured peptides are then directed to a PicoFrit reversed-phase analytical column.
Besides Sequest, other algorithms that can be used to search phosphoproteomic data include Mascot, InsPecT, and X!Tandem. Also, besides searching the RefSeq protein database, other protein databases (e.g., Swiss-Prot) can be used.
As phosphopeptides often contain multiple serine, threonine, and tyrosine residues, it is of critical importance to verify that the site(s) of phosphorylation reported by the initial search algorithm are correct or if an alternative phosphorylation configuration is more likely. Search engines such as Sequest are not designed for this purpose and often report incorrect phosphorylation sites.
This method ensures that the more intense spectra (i.e., the ones that often have more accurate reporter ion intensities) contribute more to the final calculated ratio.
We recommend replicating each experimental condition at least three times (biological replicates are preferable to technical replicates) to obtain the most accurate quantitation values and for proper statistical analyses.
Other multiple testing correction methods include Bonferroni, Bonferroni Step-Down, and Westfall and Young Permutation. These methods are more stringent (i.e., they will produce a lower number of false positives and a higher number of false negatives) which will reduce the sensitivity of the analysis.
References
- 1.Nuwaysir LM, Stults JT (1993) Electrospray ionization mass spectrometry of phosphopeptides isolated by on-line immobilized metal-ion affinity chromatography. J Am Soc Mass Spectrom 4(8):662–669 [DOI] [PubMed] [Google Scholar]
- 2.Kange R, Selditz U, Granberg M, Lindberg U, Ekstrand G, Ek B, Gustafsson M (2005) Comparison of different IMAC techniques used for enrichment of phosphorylated peptides. J Biomol Tech 16(2):91–103 [PMC free article] [PubMed] [Google Scholar]
- 3.Thingholm TE, Jensen ON (2009) Enrichment and characterization of phosphopeptides by immobilized metal affinity chromatography (IMAC) and mass spectrometry. Methods Mol Biol 527:47–56, Xi [DOI] [PubMed] [Google Scholar]
- 4.Larsen MR, Thingholm TE, Jensen ON, Roepstorff P, Jorgensen TJ (2005) Highly selective enrichment of phosphorylated peptides from peptide mixtures using titanium dioxide microcolumns. Mol Cell Proteomics 4(7):873–886 [DOI] [PubMed] [Google Scholar]
- 5.Klemm C, Otto S, Wolf C, Haseloff RF, Beyermann M, Krause E (2006) Evaluation of the titanium dioxide approach for MS analysis of phosphopeptides. J Mass Spectrom 41(12):1623–1632 [DOI] [PubMed] [Google Scholar]
- 6.Schlosser A, Pipkorn R, Bossemeyer D, Lehmann WD (2001) Analysis of protein phosphorylation by a combination of elastase digestion and neutral loss tandem mass spectrometry. Anal Chem 73(2):170–176 [DOI] [PubMed] [Google Scholar]
- 7.Bateman RH, Carruthers R, Hoyes JB, Jones C, Langridge JI, Millar A, Vissers JP (2002) A novel precursor ion discovery method on a hybrid quadrupole orthogonal acceleration time-of-flight (Q-TOF) mass spectrometer for studying protein phosphorylation. J Am Soc Mass Spectrom 13(7):792–803 [DOI] [PubMed] [Google Scholar]
- 8.Schroeder MJ, Shabanowitz J, Schwartz JC, Hunt DF, Coon JJ (2004) A neutral loss activation method for improved phosphopeptide sequence analysis by quadrupole ion trap mass spectrometry. Anal Chem 76(13):3590–3598 [DOI] [PubMed] [Google Scholar]
- 9.Stensballe A, Jensen ON, Olsen JV, Haselmann KF, Zubarev RA (2000) Electron capture dissociation of singly and multiply phosphorylated peptides. Rapid Commun Mass Spectrom 14(19):1793–1800 [DOI] [PubMed] [Google Scholar]
- 10.Molina H, Horn DM, Tang N, Mathivanan S, Pandey A (2007) Global proteomic profiling of phosphopeptides using electron transfer dissociation tandem mass spectrometry. Proc Natl Acad Sci U S A 104(7):2199–2204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jedrychowski MP, Huttlin EL, Haas W, Sowa ME, Rad R, Gygi SP (2011) Evaluation of HCD- and CID-type fragmentation within their respective detection platforms for murine phosphoproteomics. Mol Cell Proteomics 10(12):M111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Altelaar AF, Frese CK, Preisinger C, Hennrich ML, Schram AW, Timmers HT, Heck AJ, Mohammed S (2013) Benchmarking stable isotope labeling based quantitative proteomics. J Proteomics 88:14–26 [DOI] [PubMed] [Google Scholar]
- 13.Dephoure N, Gygi SP (2012) Hyperplexing: a method for higher-order multiplexed quantitative proteomics provides a map of the dynamic response to rapamycin in yeast. Sci Signal 5(217):rs2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Everley RA, Kunz RC, McAllister FE, Gygi SP (2013) Increasing throughput in targeted proteomics assays: 54-plex quantitation in a single mass spectrometry run. Anal Chem 85(11):5340–5346 [DOI] [PubMed] [Google Scholar]
- 15.Thompson A, Schafer J, Kuhn K, Kienle S, Schwarz J, Schmidt G, Neumann T, Johnstone R, Mohammed AK, Hamon C (2003) Tandem mass tags: a novel quantification strategy for comparative analysis of complex protein mixtures by MS/MS. Anal Chem 75(8):1895–1904 [DOI] [PubMed] [Google Scholar]
- 16.Ross PL, Huang YN, Marchese JN, Williamson B, Parker K, Hattan S, Khainovski N, Pillai S, Dey S, Daniels S, Purkayastha S, Juhasz P, Martin S, Bartlet-Jones M, He F, Jacobson A, Pappin DJ (2004) Multiplexed protein quantitation in Saccharomyces cerevisiae using aminereactive isobaric tagging reagents. Mol Cell Proteomics 3(12):1154–1169 [DOI] [PubMed] [Google Scholar]
- 17.Pichler P, Kocher T, Holzmann J, Mazanek M, Taus T, Ammerer G, Mechtler K (2010) Peptide labeling with isobaric tags yields higher identification rates using iTRAQ 4-plex compared to TMT 6-plex and iTRAQ 8-plex on LTQ Orbitrap. Anal Chem 82(15):6549–6558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pottiez G, Wiederin J, Fox HS, Ciborowski P (2012) Comparison of 4-plex to 8-plex iTRAQ quantitative measurements of proteins in human plasma samples. J Proteome Res 11(7):3774–3781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wenger CD, Lee MV, Hebert AS, McAlister GC, Phanstiel DH, Westphall MS, Coon JJ (2011) Gas-phase purification enables accurate, multiplexed proteome quantification with isobaric tagging. Nat Methods 8(11):933–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ting L, Rad R, Gygi SP, Haas W (2011) MS3 eliminates ratio distortion in isobaric multiplexed quantitative proteomics. Nat Methods 3(11):937–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Viner R, Bomgarden R, Blank M, Rogers J (2013) Increasing the Multiplexing of Protein Quantitation from 6- to 10-Plex with Reporter Ion Isotopologues. Thermo Scientific Poster Note PN ASMS13, W617 [Google Scholar]
- 22.Hoffert JD, Pisitkun T, Saeed F, Song JH, Chou CL, Knepper MA (2012) Dynamics of the G protein-coupled vasopressin V2 receptor signaling network revealed by quantitative phosphoproteomics. Mol Cell Proteomics 11(2):M111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diedrich JK, Pinto AF, Yates JR III (2013) Energy dependence of HCD on peptide fragmentation: stepped collisional energy finds the sweet spot. J Am Soc Mass Spectrom 24(11):1690–1699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saeed F, Pisitkun T, Hoffert JD, Wang G, Gucek M, Knepper MA (2012) An efficient dynamic programming algorithm for phosphorylation site assignment of large-scale mass spectrometry data. Proceedings (IEEE Int Conf Bioinformatics Biomed), pp 618–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beausoleil SA, Villen J, Gerber SA, Rush J, Gygi SP (2006) A probability-based approach for high-throughput protein phosphorylation analysis and site localization. Nat Biotechnol 24(10):1285–1292 [DOI] [PubMed] [Google Scholar]
- 26.Slotta DJ, McFarland MA, Markey SP (2010) MassSieve: panning MS/MS peptide data for proteins. Proteomics 10(16):3035–3039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tchapyjnikov D, Li Y, Pisitkun T, Hoffert JD, Yu MJ, Knepper MA (2010) Proteomic profiling of nuclei from native renal inner medullary collecting duct cells using LC-MS/MS. Physiol Genomics 40(3):167–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Statist Soc Ser B 57:289–300 [Google Scholar]