Abstract
The relationship between serum total testosterone and prostate cancer (PCa) risk is controversial. The hypothesis that faster age-related reduction in testosterone is linked with increased PCa risk remains untested. We conducted this study at a tertiary-level hospital in southeast of the US, and derived data from the Medical Registry Database of individuals that were diagnosed of any prostate-related disease from 2001-2015. Cases were those diagnosed of PCa and had one or more measurements of testosterone prior to PCa diagnosis. Controls were those without PCa and had one or more testosterone measurements. Multivariable logistic regression models for PCa risk of absolute levels (one-time measure and 5-year average) and annual change in testosterone were respectively constructed. Among a total of 1559 patients, 217 were PCa cases, and neither one-time measure nor 5-year average of testosterone was found to be significantly associated with PCa risk. Among the 379 patients with two or more testosterone measurements, 27 were PCa cases. For every 10 ng/dL increment in annual reduction of testosterone, the risk of PCa would increase by 14% [adjusted odds ratio, 1.14; 95% confidence interval (CI), 1.03-1.25]. Compared to patients with a relatively stable testosterone, patients with an annual testosterone reduction of more than 30 ng/dL had 5.03 [95% CI: 1.53, 16.55] fold increase in PCa risk. This implies a faster age-related reduction in, but not absolute level of serum total testosterone as a risk factor for PCa. Further longitudinal studies are needed to confirm this finding.
Keywords: Prostate cancer, Testosterone, Reduction, Age-related, Biomarker
Introduction
Prostate cancer (PCa) is the most commonly diagnosed solid organ cancer and the second leading cause of cancer death among men in the US 1. PCa represents a predominant threat to the life of men, especially for those aged 50 and older. Despite the recent advancements in both basic and translational research 2, 3, mechanisms, particularly androgen-related mechanisms underpinning prostate carcinogenesis remains poorly understood. As a result, the development and implementation of evidence-based strategies for PCa prevention, early diagnosis, and treatment have been greatly hindered 4.
It has been well established that human prostate gland depends on testosterone for its development, functioning, and homeostasis 5, and most PCa cells also depend on testosterone for growth 6, however, the causal role played by testosterone in prostate carcinogenesis remains just a hypothesis (androgen hypothesis) 7. Although supported by several early studies 8-10, this hypothesis has been questioned more frequently in recent years. Some studies found no association between serum testosterone and PCa risk 11–13; many others showed the association as negative 14, 15; while another group of studies revealed that the relationship between testosterone and PCa risk depends on the grade of PCa phenotypes 16, 17. Collectively, for the association between testosterone and PCa risk, no consensus conclusion has been reached.
Although it has been demonstrated that serum testosterone level in males falls substantially with aging, especially after the age of 40 18, 19, evidence in both human and animal studies suggests that benign prostate tissues, to certain extent are able to maintain the intra-prostatic androgen at functional level, potentially by increasing the expression of androgen receptors and androgen biosynthesis enzymes 20. This adaptation would intensify when the age-related decline in androgen level accelerates after age 40 21. But when the adaptation reaches limit of the compensatory capabilities of prostatic cells, mutation rates and malignant evolution processes may dramatically increase 22. Thus, it is reasonable to hypothesize that it is the age-related decline, rather than absolute level of serum testosterone that contributes to prostate carcinogenesis. Although this hypothesis has been proposed by one previous study 23, it remains untested.
In the present study, we explored the relationship between serum testosterone and risk of PCa using patients’ data from a tertiary hospital. Absolute levels of and age-related changes in serum testosterone were analyzed separately to demonstrate which one is associated with increased risk of PCa.
Materials and Methods
Study Population and Sample
This study targeted patients with any prostatic disease. We derived data from the Medical Registry Database of a tertiary-level hospital in southeast of the US. The records were linked with medical visits, hospitalizations, drug prescriptions, lab results, and medical diagnoses. Patients from 2001 to 2015 were screened for eligibility. A patient was included as case if he was diagnosed of PCa and had at least one measurement of serum total testosterone prior to the first PCa diagnosis. If a patient was not diagnosed of PCa and had at least one measurement of serum total testosterone prior to the most recent diagnosis of benign prostatic diseases (BPD), he was included as a control. With this criterion, 217 PCa patients and 1342 BPD patients were included, yielding a total sample of 1559 patients. To test the association between testosterone levels and PCa, one-time measure (n=1559) and 5-year average of testosterone (n=1159) were analyzed respectively. To test the association between change in testosterone and PCa risk, data of 379 patients were analyzed, including 27 PCa patients and 352 BPD patients.
Outcome Variable
The outcome of interest was newly diagnosed PCa. PCa diagnosis was determined based on medical registry database using the International Classification of Disease, Ninth Revision (ICD-9) with malignant neoplasm of prostate code 185 and carcinoma in situ of prostate code 233.4. For PCa patients, the first diagnosis of PCa was used as the outcome. For non-cancer patients with BPD, the most recent diagnosis of BPD was used as the outcome. BPD diagnosis was determined also using ICD-9 codes (Supplementary Table 1).
Serum Level of Testosterone
To provide robust evidence, two measures were used to assess levels of testosterone (ng/dL): one-time measure was the level measured most recently prior to outcome diagnosis; 5-year average measurement was the arithmetic mean of all the measurements within 5 years prior to outcome diagnosis. The former was analyzed among all the patients, and the latter was analyzed among patients with at least one testosterone measurement within 5 years prior to outcome diagnosis. To provide adequate information, the two testosterone measures were analyzed as both continuous and in quintiles. Preliminary analysis indicated that the mean duration between the most recent testosterone measurement and outcome diagnosis was 3.2 years with standard deviation (SD) of 3.4. To control for potential confounding, this duration was included in multivariable analyses.
Annual Change in Testosterone Level
Annual change in total testosterone (ng/dL per year) was analyzed among patients with two or more testosterone measurements prior to outcome diagnosis. For patients with only two testosterone measurements, change was calculated by subtracting the first measure from the second measure, and then divided by the time interval between the two measures. For patients with more than two measurements, annual change for each patient was computed by first regressing testosterone levels to time durations (in year) from when the testosterones were measured to outcome diagnosis, then taking the beta regression coefficient as the measure of annual change. Likewise, the annual change was analyzed as both continuous and categorical variable. For the categorical measure, three groups were defined: Reduction Group with testosterone decline of more than 30 ng/dL per year (annual change < −30 ng/dL), Relatively Stable Group with changes between −30 to 30 ng/dL per year, and Increase Group with testosterone increase of more than 30 ng/dL per year (annual change > 30 ng/dL). The categorization was determined based on frequency distribution of the estimated annual changes in testosterone. In addition to duration from the most recent testosterone measure to outcome diagnosis (mean = 1.9 years, SD = 2.4), duration between the first and most recent testosterone measures (mean = 5.4 years, SD = 3.4) was also included in multivariable analysis to control for potential confounding.
Covariates
In addition to testosterone, we also assessed other potential influential factors for PCa, including age at outcome diagnosis (< 65 and ≥ 65 years), race (black, white and others), cigarette smoking status at the first hospital admission (current, former, and never), BMI at the first hospital admission (< 25, 25 to < 30, and ≥ 30 kg/m2), PCa family history (yes/no), most recently measured prostate specific antigen (PSA) level (ng/mL), average frequency of PSA testing per year from the first hospital admission to outcome diagnosis, personal disease histories of BPD (yes/no), hypertension (yes/no), and diabetes (yes/no), as well as usage of testosterone supplementary therapy (yes/no) and finasteride (yes/no) by outcome diagnosis.
Statistical Analysis
Characteristics of the study sample were assessed using descriptive statistical methods, including mean, SD, and proportions. Chi-square test (for categorical variables) and Student t-test (for continuous variables) were used to compare sample differences. To assess the associations between testosterone (including levels and changes) and PCa, a bivariate analysis was conducted first to compare testosterone between PCa patients and BPD patients. Results of the bivariate analysis were further verified using two multivariable logistic regression models, one for testosterone being analyzed as a continuous measure and another for testosterone as a categorical measure. Covariates included in the multivariable analysis were those significant in bivariate analysis with p ≤ 0.20 and those although not significant in bivariate analysis but have well-known effect on PCa and/or testosterone level (age, race, and PCa family history, testosterone supplementary therapy, finasteride use). Among the patients in the annual testosterone change analysis, those taking testosterone supplementary therapy were all distributed in BPD group. For the validity of model fit, testosterone supplementary therapy was thus not included in the multivariable model of annual testosterone change. But no association between annual change in testosterone and the therapy (yes/no) was found (p = 0.908) even after adjusting for age, race, and BMI. Adjusted odds ratios (AORs) with 95% confidence intervals (CIs) and p values were reported, with p < 0.05 (two-sided) as statistically significant. All statistical analyses were conducted using SAS 9.4 (SAS Institute, Cary, NC).
Results
Characteristics of study participants
A total of 1559 patients were included in the study, the median age was 67 (range, 20 to 96). Of the total sample, 217 (13.9%) were PCa patients and 1342 (86.1%) were BPD patients; 171 (11.0%) were black and 1016 (65.2%) were white. Chi-square and t-test indicated that patients with PCa differed from patients with BPD in many aspects (Table 1). In addition, correlation analysis indicated weak negative correlations between one-time measure of testosterone and PSA (r = − 0.057, p = 0.027), and between 5-year average testosterone and PSA (r = − 0.090, p = 0.003). No correlation between testosterone annual change and PSA was found (r = − 0.071, p = 0.171). This finding ruled out the possibility of PCa detection being influenced by testosterone-induced PSA fluctuation.
Table 1.
Characteristics of the Study Participants and Comparison between Prostate Cancer Patients and Benign Prostatic Diseases Patients
| Variables | Total (n=1559) | PCa Patients (n=217) |
BPD Patients (n=1342) |
P value * |
|---|---|---|---|---|
| Age at diagnosis, n (%) | 0.156 | |||
| < 65 years | 636 (40.8) | 79 (36.4) | 557 (41.5) | |
| ≥ 65 years | 923 (59.2) | 138 (63.6) | 785 (58.5) | |
| Race, n (%) | 0.243 | |||
| Black | 171 (11.0) | 29 (13.4) | 142 (10.6) | |
| White | 1016 (65.2) | 129 (59.5) | 887 (66.1) | |
| Other | 33 (2.1) | 4 (1.8) | 29 (2.2) | |
| Unknown | 339 (21.7) | 55 (25.4) | 284 (21.2) | |
| Smoking Status, n (%) | 0.351 | |||
| Current smoker | 95 (6.1) | 14 (6.5) | 81 (6.0) | |
| Former smoker | 525 (33.7) | 67 (30.9) | 458 (34.1) | |
| Never smoke | 512 (32.8) | 66 (30.4) | 446 (33.2) | |
| Status unknown | 427 (27.4) | 70 (32.3) | 357 (26.6) | |
| Body mass index (kg/m2) | <0.001 | |||
| < 25.0 | 342 (21.9) | 52 (24.0) | 290 (21.6) | |
| 25.0 to < 30.0 | 182 (11.7) | 15 (6.9) | 167 (12.4) | |
| ≥ 30.0 | 265 (17.0) | 17 (7.8) | 248 (18.5) | |
| Unknown | 770 (49.4) | 133 (61.3) | 637 (47.5) | |
| PCa family history, n (%) | 0.566 § | |||
| Yes | 26 (1.7) | 2 (0.9) | 24 (1.8) | |
| No | 1533 (98.3) | 215 (99.1) | 1318 (98.2) | |
|
The most recent PSA level (ng/mL) |
||||
| Mean (SD) | 3.8 (24.7) | 17.3 (67.4) | 1.9 (5.0) | 0.002 |
| Average frequency of PSA testing per year | ||||
| Median (IQR) | 0.8 (0.5-1.0) | 1.0 (0.3-1.5) | 0.8 (0.5-1.0) | 0.011 |
| Duration from the most recent testosterone measurement to outcome diagnosis (years) | ||||
| Mean (SD) | 3.2 (3.4) | 2.6 (3.1) | 3.3 (3.4) | 0.002 |
| Disease history, n (%) | ||||
| BPD | <0.001 | |||
| Yes | 1293 (82.9) | 150 (69.1) | 1143 (85.2) | |
| No | 266 (17.1) | 67 (30.9) | 199 (14.8) | |
| Hypertension | <0.001 | |||
| Yes | 1157 (74.2) | 129 (59.5) | 1028 (76.6) | |
| No | 402 (25.8) | 88 (40.5) | 314 (23.4) | |
| Diabetes | <0.001 | |||
| Yes | 508 (32.6) | 44 (20.3) | 464 (34.6) | |
| No | 1051 (67.4) | 173 (79.7) | 878 (65.4) | |
| History of prostate related medication, n (%) | ||||
| Testosterone supplementary therapy | 0.038 | |||
| Yes | 39 (2.5) | 1 (0.5) | 38 (2.8) | |
| No | 1520 (97.5) | 216 (99.5) | 1304 (97.2) | |
| Finasteride | 0.381 | |||
| Yes | 76 (4.9) | 8 (3.7) | 68 (5.1) | |
| No | 1483 (95.1) | 209 (96.3) | 1274 (94.9) |
Note: PCa = prostate cancer; BPD = benign prostatic diseases; PSA = prostate specific antigen; SD = standard deviation; IQR = interquartile range.
Chi-square test, or Student t test, or Wilcoxon rank-sum test, and
Fisher’s exact test.
Association between One-Time Measure of Serum Total Testosterone and Risk of PCa
In bivariate analysis among the 1559 patients, Student t-test indicated that one-time measure of testosterone was significantly lower in PCa patients than that in BPD patients (361.9 vs. 386.4 ng/dL, p = 0.039). The difference became non-significant when testosterone was analyzed in quintiles using Chi-square test (p = 0.365) (Table 2). In multivariable logistic regression analysis controlling for covariates, no significant association between one-time measurements of testosterone, whether as continuous or in quintiles, and PCa risk was found (Table 3). In addition, PSA level and average frequency of PSA testing per year were found to be positively associated with PCa risk, and overweight was associated with decreased PCa risk.
Table 2.
Comparisons of Serum Total Testosterone (Levels and Annual Change) between Prostate Cancer Patients and Benign Prostatic Diseases Patients
| Total testosterone in serum | Total | PCa Patients | BPD Patients | P value * |
|---|---|---|---|---|
| One-time measure | ||||
| Sample size, n (%) | 1559 (100.0) | 217 (13.9) | 1342 (86.1) | - |
| Level (ng/dL) | ||||
| Mean (SD) | 383.0 (161.5) | 361.9 (168.0) | 386.4 (160.2) | 0.039 |
| By quintile, n (%) | 0.365 | |||
| Quint 1: 1.0-244.0 ng/dL | 323 (20.7) | 51 (23.5) | 272 (20.3) | |
| Quint 2: 244.1-339.0 ng/dL | 326 (20.9) | 53 (24.4) | 273 (20.3) | |
| Quint 3: 339.1-428.0 ng/dL | 322 (20.7) | 38 (17.5) | 284 (21.2) | |
| Quint 4: 428.1-548.5 ng/dL | 325 (20.9) | 42 (19.4) | 283 (21.1) | |
| Quint 5: 548.6-798.0 ng/dL | 263 (16.9) | 33 (15.2) | 230 (17.1) | |
| Five-year average | ||||
| Sample size, n (%) | 1159 (100.0) | 172 (14.8) | 987 (85.2) | - |
| Level (ng/dL) | ||||
| Mean (SD) | 378.9 (153.8) | 345.2 (162.7) | 384.8 (151.5) | 0.002 |
| By quintile, n (%) | 0.052 | |||
| Quint 1: 1.0-240.5 ng/dL | 232 (20.0) | 45 (26.2) | 187 (19.0) | |
| Quint 2: 240.6-335.0 ng/dL | 235 (20.3) | 41 (23.8) | 194 (19.7) | |
| Quint 3: 335.1-412.0 ng/dL | 229 (19.8) | 31 (18.0) | 198 (20.1) | |
| Quint 4: 412.1-516.5 ng/dL | 232 (20.0) | 31 (18.0) | 201 (20.4) | |
| Quint 5: 516.6-757.0 ng/dL | 231 (19.9) | 24 (14.0) | 207 (21.0) | |
| Annual change | ||||
| Sample size, n (%) | 379 (100.0) | 27 (7.1) | 352 (92.9) | - |
| Annual change (ng/dL) | ||||
| Mean (SD) | 9.3 (53.5) | −16.4 (65.9) | 11.3 (52.0) | 0.009 |
| By group, n (%) | < 0.001 | |||
| Reduction: < −30 ng/dL | 57 (15.0) | 11 (40.7) | 46 (13.1) | |
| Relative stable: −30 to 30 ng/dL | 228 (60.2) | 11 (40.7) | 217 (61.6) | |
| Increase: > 30 ng/dL | 94 (24.8) | 5 (18.6) | 89 (25.3) |
Note: PCa = prostate cancer; BPD = benign prostatic diseases; SD = standard deviation.
Chi-square test or t test.
Table 3.
Associations between One-Time Measure of Serum Total Testosterone and Risk of Prostate Cancer from Multivariable Logistic Regression Analysis
| Predictor variables |
AOR [95% CI] Model I |
AOR [95% CI] Model II |
|---|---|---|
| Serum total testosterone | ||
| Level (every 10 ng/dL) | 1.00 [0.99-1.01] | n/a |
| By quintile | ||
| Quint 1: 1.0-244.0 ng/dL | n/a | 1.13 [0.64-2.03] |
| Quint 2: 244.1-339.0 ng/dL | n/a | 1.57 [0.93-2.64] |
| Quint 3: 339.1-428.0 ng/dL | n/a | 1.00 |
| Quint 4: 428.1-548.5 ng/dL | n/a | 1.10 [0.65-1.87] |
| Quint 5: 548.6-798.0 ng/dL | n/a | 1.20 [0.69-2.10] |
| Age at diagnosis | ||
| < 65 years | 1.00 | 1.00 |
| ≥ 65 years | 1.21 [0.83-1.76] | 1.22 [0.84-1.77] |
| Race | ||
| Black | 4.54 [0.87-23.70] | 4.81 [0.93-24.94] |
| White | 3.46 [0.71-16.79] | 3.62 [0.75-17.44] |
| Others | 1.00 | 1.00 |
| Unknown | 2.17 [0.43-10.92] | 2.28 [0.46-11.35] |
| Body mass index (kg/m2) | ||
| < 25.0 | 1.00 | 1.00 |
| 25.0 to < 30.0 | 0.48 [0.23-0.99] | 0.48 [0.23-0.99] |
| ≥ 30.0 | 0.52 [0.27-1.01] | 0.53 [0.27-1.03] |
| Unknown | 1.02 [0.66-1.60] | 1.01 [0.65-1.58] |
| PCa family history | ||
| Yes | 0.35 [0.05-2.68] | 0.36 [0.05-2.76] |
| No | 1.00 | 1.00 |
| The most recently measured PSA level (ng/mL) | 1.17 [1.11-1.24] | 1.17 [1.11-1.24] |
| Average frequency of PSA testing per year | 1.83 [1.42-2.36] | 1.83 [1.42-2.36] |
| Duration from the most recent testosterone measurement to outcome diagnosis (year) | 0.98 [0.93-1.04] | 0.98 [0.93-1.04] |
| Disease history | ||
| BPD | ||
| Yes | 0.64 [0.40-1.03] | 0.64 [0.40-1.03] |
| No | 1.00 | 1.00 |
| Hypertension | ||
| Yes | 0.75 [0.50-1.11] | 0.74 [0.50-1.11] |
| No | 1.00 | 1.00 |
| Diabetes | ||
| Yes | 0.67 [0.44-1.02] | 0.67 [0.44-1.03] |
| No | 1.00 | 1.00 |
| Prostate-related medication history | ||
| Testosterone supplementary therapy | ||
| Yes | 0.35 [0.05-2.76] | 0.36 [0.05-2.83] |
| No | 1.00 | 1.00 |
| Finasteride | ||
| Yes | 1.82 [0.79-4.21] | 1.93 [0.84-4.46] |
| No | 1.00 | 1.00 |
Note: One-time measure of serum total testosterone was analyzed as a continuous variable in Model I and categorical (quintile) in Model II. Covariates included in the models were those significant in bivariate analysis with p ≤ 0.20 and those although not significant in bivariate analysis but have well-known effect on PCa and/or testosterone level (age, race, PCa family history, testosterone supplementary therapy, and finasteride use). AOR = adjusted odds ratio; PCa = prostate cancer; BPD = benign prostatic diseases; PSA = prostate specific antigen.
Association between 5-Year Average Serum Total Testosterone and Risk of PCa
A total of 1159 patients were included in this analysis, among which 172 (14.8%) were PCa patients and 987 (85.2%) were BPD patients. Student t-test indicated that the 5-year average testosterone level of PCa patients was lower than that of BPD patients (345.2 vs. 384.8 ng/dL, p = 0.002). When testosterone was analyzed in quintiles using Chi-square test, the difference between the two groups became marginally nonsignificant (p = 0.052) (Table 2). After controlling for covariates in multivariable logistic regression analysis, no significant association between 5-year average testosterone and PCa risk was observed, regardless whether the average testosterone was analyzed as continuous or in quintiles. Other factors found to be significantly associated with PCa risk were PSA level and average frequency of PSA testing per year (Table 4).
Table 4.
Associations between 5-Year Average Serum Total Testosterone and Risk of Prostate Cancer from Multivariable Logistic Regression Analysis
| Predictor variables |
AOR [95% CI] Model I |
AOR [95% CI] Model II |
|---|---|---|
| Five-Year average serum total testosterone | ||
| Level (every 10 ng/dL) | 1.00 [0.98-1.01] | n/a |
| By quintile | ||
| Quint 1: 1.0-240.5 ng/dL | n/a | 1.47 [0.75-2.89] |
| Quint 2: 240.6-335.0 ng/dL | n/a | 1.52 [0.81-2.86] |
| Quint 3: 335.1-412.0 ng/dL | n/a | 1.00 |
| Quint 4: 412.1-516.5 ng/dL | n/a | 1.12 [0.58-2.15] |
| Quint 5: 516.6-757.0 ng/dL | n/a | 0.86 [0.44-1.70] |
| Age at diagnosis | ||
| < 65 years | 1.00 | 1.00 |
| ≥ 65 years | 1.37 [0.88-2.13] | 1.39 [0.89-2.16] |
| Race | ||
| Black | 2.89 [0.38-21.95] | 2.85 [0.38-21.61] |
| White | 2.84 [0.42-19.16] | 2.73 [0.40-18.49] |
| Others | 1.00 | 1.00 |
| Unknown | 1.77 [0.25-12.30] | 1.72 [0.25-12.04] |
| Body mass index (kg/m2) | ||
| < 25.0 | 1.00 | 1.00 |
| 25.0 to < 30.0 | 0.64 [0.25-1.67] | 0.63 [0.24-1.63] |
| ≥ 30.0 | 0.74 [0.31-1.73] | 0.74 [0.32-1.74] |
| Unknown | 1.57 [0.90-2.75] | 1.55 [0.88-2.71] |
| PCa family history | ||
| Yes | 0.42 [0.05-3.76] | 0.43 [0.05-3.73] |
| No | 1.00 | 1.00 |
| The most recently measured PSA level (ng/mL) | 1.35 [1.25-1.46] | 1.35 [1.25-1.45] |
| Average frequency of PSA testing per year | 1.67 [1.27-2.21] | 1.66 [1.26-2.20] |
| Disease history | ||
| BPD | ||
| Yes | 0.62 [0.36-1.06] | 0.62 [0.36-1.07] |
| No | 1.00 | 1.00 |
| Hypertension | ||
| Yes | 0.74 [0.46-1.18] | 0.73 [0.46-1.17] |
| No | 1.00 | 1.00 |
| Diabetes | ||
| Yes | 0.61 [0.36-1.03] | 0.61 [0.36-1.03] |
| No | 1.00 | 1.00 |
| Prostate-related medication history | ||
| Testosterone supplementary therapy | ||
| Yes | 0.49 [0.06-3.97] | 0.50 [0.06-4.03] |
| No | 1.00 | 1.00 |
| Finasteride | ||
| Yes | 1.56 [0.46-5.24] | 1.62 [0.48-5.45] |
| No | 1.00 | 1.00 |
Note: Five-Year average of serum total testosterone was analyzed as a continuous variable in Model I and categorical (quintile) in Model II. Covariates included in the models were those significant in bivariate analysis with p ≤ 0.20 and those although not significant in bivariate analysis but have well-known effect on PCa and/or testosterone level (age, race, PCa family history, testosterone supplementary therapy, and finasteride use). AOR = adjusted odds ratio; PCa = prostate cancer; BPD = benign prostatic diseases; PSA = prostate specific antigen.
Association between Annual Change in Serum Total Testosterone and Risk of PCa
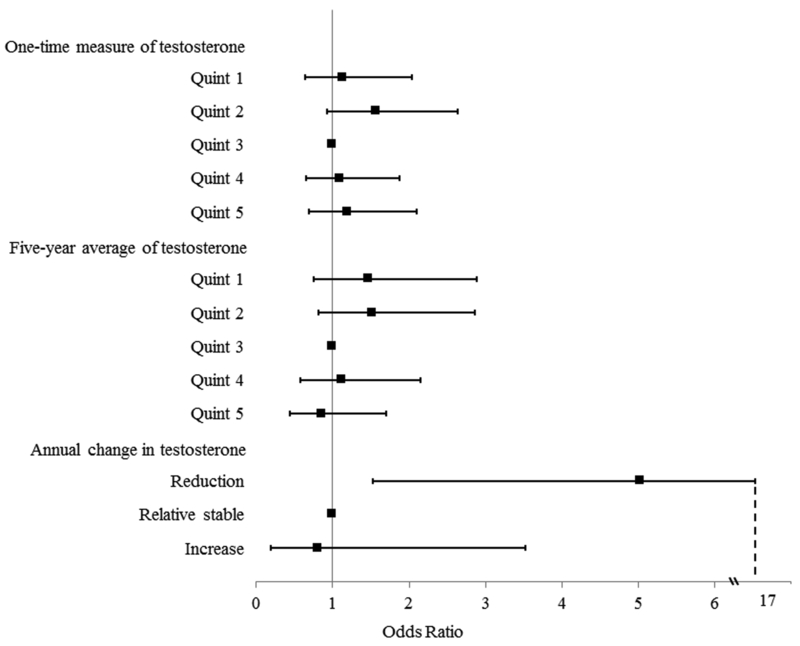
Among the 379 patients with two or more measurements of testosterone, 27 (7.1%) were PCa patients and 352 (92.9%) were BPD patients. On average, PCa patients had an annual decline of 16.4 ng/dL in serum total testosterone, compared to an annual increase of 11.3 ng/dL for BPD patients, and this difference was statistically significant using Student t-test (p = 0.009). When analyzed in the three groups of changes (Reduction, Relative Stable, and Increase group), the difference remained statistically significant (p < 0.001) (Table 2). In addition, several covariates differed between PCa patients and BPD patients, including PSA levels (4.3 vs. 1.6 ng/mL, p < 0.001) and hypertension (63.0 vs. 79.8%, p = 0.039). Results from multivariable logistic regression analysis controlling for covariates indicated that a higher annual reduction in testosterone was significantly associated with increased risk of PCa [AOR = 1.14; 95% CI, 1.03 to 1.25]. When analyzed as a categorical variable with the Relatively Stable group as reference, the Reduction group had a significantly and substantially higher risk of PCa [AOR = 5.03; 95% CI, 1.53 to 16.55], and the Increase group had a lower PCa risk, although no statistical significance was observed. In addition, smoking was found to be independently associated with decreased PCa risk, and higher PSA was associated with increased PCa risk (Table 5). The independent associations between serum total testosterone and risk of PCa from the three perspectives of one-time measure of, 5-year average of, and annual change in testosterone were summarized in Figure 1.
Table 5.
Association between Annual Change in Serum Total Testosterone Level and Risk of Prostate Cancer from Multivariable Logistic Regression Analysis
| Predictor variables |
AOR [95% CI] Model I |
AOR [95% CI] Model II |
|---|---|---|
| Annual change in serum total testosterone | ||
| Amount of reduction (every 10 ng/dL) | 1.14 [1.03-1.25] | n/a |
| By group | ||
| Reduction (< −30 ng/dL) | n/a | 5.03 [1.53-16.55] |
| Relatively stable (−30 to 30 ng/dL) | n/a | 1.00 |
| Increase (> 30 ng/dL) | n/a | 0.81 [0.19-3.52] |
| Age at diagnosis | ||
| < 65 years | 1.00 | 1.00 |
| ≥ 65 years | 1.01 [0.37-2.77] | 1.13 [0.33-3.19] |
| Race | ||
| Black | 0.77 [0.15-3.90] | 0.76 [0.15-3.87] |
| White/other | 1.00 | 1.00 |
| Unknown | 3.97 [0.29-55.13] | 4.32 [0.27-69.97] |
| Smoking status | ||
| Smoker | 0.30 [0.10-0.92] | 0.30 [0.10-0.91] |
| Never smoke | 1.00 | 1.00 |
| Status unknown | 0.10 [0.01-1.40] | 0.09 [0.01-1.50] |
| Body mass index (kg/m2) | ||
| < 25.0 | 1.00 | 1.00 |
| 25.0 to < 30.0 | 0.68 [0.16-2.90] | 0.76 [0.17-3.39] |
| ≥ 30.0 | 0.12 [0.01-1.31] | 0.14 [0.01-1.44] |
| Unknown | 0.33 [0.09-1.21] | 0.40 [0.11-1.46] |
| PCa family history | ||
| Yes | 1.11 [0.11-11.70] | 1.32 [0.13-13.25] |
| No | 1.00 | 1.00 |
| The most recently measured PSA level (ng/mL) | 1.66 [1.33-2.08] | 1.63 [1.31-2.03] |
| Average frequency of PSA testing per year | 0.86 [0.38-1.96] | 0.91 [0.40-2.05] |
| Duration from the most recent testosterone measurement to outcome diagnosis (year) | 0.83 [0.70-1.09] | 0.85 [0.75-1.06] |
| Duration from the first to most recent testosterone measurement (year) | 0.89 [0.76-1.05] | 0.95 [0.79-1.13] |
| Disease history | ||
| Hypertension | ||
| Yes | 0.45 [0.15-1.37] | 0.51 [0.16-1.59] |
| No | 1.00 | 1.00 |
| Medication history, n (%) | ||
| Finasteride | ||
| Yes | 1.90 [0.31-11.90] | 1.76 [0.26-11.87] |
| No | 1.00 | 1.00 |
Note: Annual change in serum total testosterone was analyzed as a continuous variable in Model I and categorical in Model II. Covariates included in the models were those significant in bivariate analysis with p ≤ 0.20 and those although not significant in bivariate analysis but have well-known effect on PCa and/or testosterone level (age, race, PCa family history, and finasteride use). For the validity of model fit, testosterone supplementary therapy was not included in the models as those taking the therapy were all distributed in BPD group. AOR = adjusted odds ratio; PCa = prostate cancer; BPD = benign prostatic diseases; PSA = prostate specific antigen.
Figure 1.
Associations (Adjusted Odds Ratios and 95% Confidence Intervals) between Serum Total Testosterone (One-Time Measure, 5-Year Average, and Annual Change) and Risk of Prostate Cancer
Discussion
The relationship between serum testosterone and PCa risk has been a consistently pursued research question since the pioneering study conducted by Huggins and Hodges 7 in 1941 which suggested a direct correlation between circulating testosterone levels and PCa progression. The present study, for the first time, found direct epidemiological evidence supporting the hypothesis that it is faster age-related reductions, rather than absolute levels in serum testosterone that significantly affects the risk of PCa.
In the current study, one-time measure and 5-year average of serum testosterone levels, whether analyzed as continuous or categorical, were not found to be significantly associated with PCa after adjusting for other covariates (Table 3, Table 4, Figure 1). Despite different study designs (i.e. control group consisted of BPD patients in our study, whereas most prior studies used general population as control group), these results agree with a considerable number of prior studies 11–13. For this observation, various explanations have been suggested, such as lack of prospective study design with sufficient power 12, or serum testosterone is simply unrelated to PCa development 4. However, another potential explanation that PCa development is more influenced by dynamic changes rather than absolute levels in serum testosterone has not been reported previously.
According to the findings of our study, for every 10 ng/dL increment in the annual reduction of testosterone, the risk of PCa would increase by 14% [AOR, 1.14; 95% CI, 1.03–1.25]. Compared to patients with a relatively stable testosterone level, patients in the group with an annual testosterone reduction of more than 30 ng/dL had 5.03 [95% CI: 1.53, 16.55] fold increase in PCa risk (Table 5, Figure 1). This discovery is in line with current endocrinology research in prostate. The homeostasis in normal prostate is maintained by the interaction between stromal and epithelial cells through a paracrine mechanism. In the presence of adequate testosterone, the binding of testosterone to stromal androgen receptor (AR) can produce andromedins, which will promote proliferation of basal epithelial cells by binding to their receptors. On the other hand, binding of testosterone to luminal epithelial AR can counteract the effects of andromedins and promote differentiation of luminal cells 24. When a dramatic age-related decrease in serum testosterone is present, the paracrine mechanism of AR action will be replaced by an emergent autocrine mechanism, leading to selection of pre-neoplastic cells that are less dependent on signals from stromal and basal cells. This could initiate uncontrolled AR-driven proliferation of luminal cells 25. In addition, it is established that a dramatic decline in serum testosterone could increase the expression of AR and androgen biosynthesis genes 20, which will jointly drive chromosomal rearrangements and alter transcriptional programs 26, 27. This can in turn alter the DNA sites to which AR binds and further increase the probability of prostate carcinogenesis. Similar findings have been reported in prostate stem cells in murine model study 28.
This finding is also in accordance with some epidemiological evidence. According to one study 29 based on National Health and Nutritional Examination Survey (NHANES) data, black males, compared to white males, have significantly higher peak levels of testosterone during young age, but the difference diminishes with aging and completely disappears after age 60. It indicated that the testosterone levels in blacks decrease faster with increasing age compared with that in white. This result supports the findings of our study, and may jointly in part explain the observed racial disparities in PCa risk 23.
If confirmed, this finding has important implications for PCa pathogenesis study, PCa prevention, and early diagnosis. First, it will contribute to clarification of the perennial question of relationship between serum testosterone and PCa risk, and thus may help explain the observed association between some proven risk factors (age, race, physical activity, et al) and PCa risk. Based on this knowledge, measures that can slow age-related declines in serum testosterone can potentially be applied to prevent PCa, such as engaging in more physical activity, controlling body weight, supplementing zinc, and preventing vitamin D deficiency, et al 30, 31. These will be of particular importance from the age of 40, when serum testosterone is about to have an obvious decline 29, 32. Second, this will potentially endow positive significance on testosterone replacement therapy (TRT) in preventing PCa, as TRT can effectively smooth the age-related decline in testosterone if given at appropriate time. This is in line with a recent prospective study reporting that TRT among hypogonadal men had preventive effect against PCa 14. However, more confirmatory studies of TRT safety are needed. Third, this finding also has great significance regarding PCa early diagnosis by potentially providing a new biomarker for prostate biopsy. Facing the widely debate on application of PSA for PCa detection 33, 34, adding the age-related testosterone change into the current indicator system of PCa screening would make important clinical significance.
Our study has several strengths. To our knowledge, this is the first empirical study supporting the hypothesis of age-related reductions in testosterone as a risk factor for PCa. Results of our study were robust since we simultaneously analyzed both absolute levels (one-time measure and 5-year average) of and annual changes in total testosterone in one study, and also both continuous and categorical measures of testosterone were considered. Second, the data used in this analysis were extracted from medical registry, thereby avoiding the probability of recall bias, which is inherent to traditional retrospective case-control studies.
Meanwhile, our study has several limitations. First, the intervals between testosterone measurements were not same for all patients, and the sample size of patients having two or more testosterone measurements in the current study was limited. Bias cannot be ruled out without a formally designed longitudinal study. Second, the controls were drawn from BPD patients rather than the general population, this would reduce the generalizability of the study conclusion. Third, although our findings are consistent with much previous evidence, in light of inevitable bias in observational study, more research at multi-scales is needed to elucidate the involved biological mechanisms to provide more convincing evidence. Fourth, findings from previous studies suggested the relationship between testosterone and PCa risk may depend on the grade of PCa phenotypes 16, 17, but in the current study, 47.0% (102/217) of the PCa cases had Gleason score missing, due to that Gleason score was recorded as free-text medical notes in the database and we were only able to retrieve a small portion of the notes through text mining. Among the 115 cancer cases having Gleason score, we respectively constructed logistic regression model for high-grade (Gleason score ≥ 7) vs. low-grade prostate cancer (Gleason score < 7) of one-time measure (high-grade n = 69, low-grade n = 46), 5-year average (high-grade n = 52, low-grade n = 35) of, and annual change (high-grade n = 7, low-grade n = 10) in testosterone level adjusting for age, race, BMI, PCa family history, the most recently measured PSA level, and average frequency of PSA testing per year, no statistical significance of the testosterone measures was found. As the under-power of this analysis, we showed the results in Supplementary Table 2. Besides, some recent studies have shown that in low-intermediate risk PCa, high-risk patterns of tumor upgrading was also associated with increased testosterone levels 35, 36, thus the roles of testosterone change in differing-grade prostate cancer development and progression are all important directions for future research.
Despite these limitations, the present study for the first time provided empirical data supporting the testosterone-reduction hypothesis in explaining the relationship between testosterone and PCa risk. It implies that relevant measures that can smooth age-related reduction in testosterone level have potentials in preventing PCa, but more rigorously designed observational and experimental studies are needed to confirm this finding.
Supplementary Material
Novelty and Impact:
We simultaneously analyzed both absolute levels (one-time measure and 5-year average) of and age-related annual changes in serum total testosterone, and for the first time, found direct epidemiological evidence supporting the hypothesis that it is faster age-related reductions, rather than absolute levels in serum testosterone that significantly increase the risk of prostate cancer. This finding implies that relevant measures that can smooth age-related reduction in testosterone have important potentials in preventing prostate cancer.
Acknowledgements
The authors would like to thank Nickerson Paul, in the Clinical & Translational Science Institute at the University of Florida for data extraction; Jae Min, in the Department of Epidemiology at the University of Florida for editorial contributions to this paper. This study was supported by the UFHCC/IOA Cancer-Aging Collaborative Grant Program, and the Start-Up Package to Dr. Xinguang Chen from the University of Florida.
Footnotes
Conflict of Interest: None declared.
Reference
- 1.CDC. Cancer Among Men, 2016. [Google Scholar]
- 2.Guzel E, Karatas OF, Semercioz A, Ekici S, Aykan S, Yentur S, Creighton CJ, Ittmann M, Ozen M. Identification of microRNAs differentially expressed in prostatic secretions of patients with prostate cancer. Int J Cancer 2015;136: 875–9. [DOI] [PubMed] [Google Scholar]
- 3.Murtola TJ, Karppa EK, Taari K, Talala K, Tammela TL, Auvinen A. 5-Alpha reductase inhibitor use and prostate cancer survival in the Finnish Prostate Cancer Screening Trial. Int J Cancer 2016;138: 2820–8. [DOI] [PubMed] [Google Scholar]
- 4.Nelson WG, Haffner M, Demarzo AM, Yegnasubramanian S: Prostate Cancer: A Comprehensive Perspective Springer-Verlag London Ltd; 2013. [Google Scholar]
- 5.Di Zazzo E, Galasso G, Giovannelli P, Di Donato M, Di Santi A, Cernera G, Rossi V, Abbondanza C, Moncharmont B, Sinisi AA, Castoria G, Migliaccio A. Prostate cancer stem cells: the role of androgen and estrogen receptors. Oncotarget 2016;7: 193–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Michaud JE, Billups KL, Partin AW. Testosterone and prostate cancer: an evidence-based review of pathogenesis and oncologic risk. Therapeutic advances in urology 2015;7: 378–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Higgins C, Hodges C. Studies on prostatic cancer. 1. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Res 1941;1: 293–7. [Google Scholar]
- 8.Guess HA, Friedman GD, Sadler MC, Stanczyk FZ, Vogelman JH, Imperato-McGinley J, Lobo RA, Orentreich N. 5 alpha-reductase activity and prostate cancer: a case-control study using stored sera. Cancer Epidemiol Biomarkers Prev 1997;6: 21–4. [PubMed] [Google Scholar]
- 9.Parsons JK, Carter HB, Platz EA, Wright EJ, Landis P, Metter EJ. Serum testosterone and the risk of prostate cancer: potential implications for testosterone therapy. Cancer Epidemiol Biomarkers Prev 2005;14: 2257–60. [DOI] [PubMed] [Google Scholar]
- 10.Travis RC, Key TJ, Allen NE, Appleby PN, Roddam AW, Rinaldi S, Egevad L, Gann PH, Rohrmann S, Linseisen J, Pischon T, Boeing H, et al. Serum androgens and prostate cancer among 643 cases and 643 controls in the European Prospective Investigation into Cancer and Nutrition. Int J Cancer 2007;121: 1331–8. [DOI] [PubMed] [Google Scholar]
- 11.Schenk JM, Till C, Hsing AW, Stanczyk FZ, Gong Z, Neuhouser ML, Reichardt JK, Hoque AM, Figg WD, Goodman PJ, Tangen CM, Thompson IM. Serum androgens and prostate cancer risk: results from the placebo arm of the Prostate Cancer Prevention Trial. Cancer Causes Control 2016;27: 175–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klap J, Schmid M, Loughlin KR. The relationship between total testosterone levels and prostate cancer: a review of the continuing controversy. J Urol 2015;193: 403–13. [DOI] [PubMed] [Google Scholar]
- 13.Muller RL, Gerber L, Moreira DM, Andriole G, Castro-Santamaria R, Freedland SJ. Serum testosterone and dihydrotestosterone and prostate cancer risk in the placebo arm of the Reduction by Dutasteride of Prostate Cancer Events trial. Eur Urol 2012;62: 757–64. [DOI] [PubMed] [Google Scholar]
- 14.Haider A, Zitzmann M, Doros G, Isbarn H, Hammerer P, Yassin A. Incidence of prostate cancer in hypogonadal men receiving testosterone therapy: observations from 5-year median followup of 3 registries. J Urol 2015;193: 80–6. [DOI] [PubMed] [Google Scholar]
- 15.Mearini L, Zucchi A, Nunzi E, Villirillo T, Bini V, Porena M. Low serum testosterone levels are predictive of prostate cancer. World J Urol 2013;31: 247–52. [DOI] [PubMed] [Google Scholar]
- 16.Pichon A, Neuzillet Y, Botto H, Raynaud JP, Radulescu C, Molinie V, Herve JM, Lebret T. Preoperative low serum testosterone is associated with high-grade prostate cancer and an increased Gleason score upgrading. Prostate Cancer Prostatic Dis 2015;18: 382–7. [DOI] [PubMed] [Google Scholar]
- 17.Baillargeon J, Kuo YF, Fang X, Shahinian VB. Long-term Exposure to Testosterone Therapy and the Risk of High Grade Prostate Cancer. J Urol 2015;194: 1612–6. [DOI] [PubMed] [Google Scholar]
- 18.Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. The Journal of Clinical Endocrinology & Metabolism 2001;86: 724–31. [DOI] [PubMed] [Google Scholar]
- 19.Wu FC, Tajar A, Beynon JM, Pye SR, Silman AJ, Finn JD, O’neill TW, Bartfai G, Casanueva FF, Forti G. Identification of late-onset hypogonadism in middle-aged and elderly men. New England Journal of Medicine 2010;363: 123–35. [DOI] [PubMed] [Google Scholar]
- 20.Zhou Y, Otto-Duessel M, He M, Markel S, Synold T, Jones JO. Low systemic testosterone levels induce androgen maintenance in benign rat prostate tissue. J Mol Endocrinol 2013;51: 143–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou Y, Bolton EC, Jones JO. Androgens and androgen receptor signaling in prostate tumorigenesis. Journal of molecular endocrinology 2015;54: R15–R29. [DOI] [PubMed] [Google Scholar]
- 22.Eikenberry SE, Nagy JD, Kuang Y. The evolutionary impact of androgen levels on prostate cancer in a multi-scale mathematical model. Biol Direct 2010;5: 1745–6150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu X, Chen X, Hu H, Dailey AB, Taylor BD. Current opinion on the role of testosterone in the development of prostate cancer: a dynamic model. BMC cancer 2015;15: 806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu W, Luo Y, Kan M, McKeehan WL. Fibroblast growth factor-10 a second candidate stromal to epithelial cell andromedin in prostate. Journal of Biological Chemistry 1999;274: 12827–34. [DOI] [PubMed] [Google Scholar]
- 25.Gao J, Arnold JT, Isaacs JT. Conversion from a paracrine to an autocrine mechanism of androgen-stimulated growth during malignant transformation of prostatic epithelial cells. Cancer research 2001;61: 5038–44. [PubMed] [Google Scholar]
- 26.Urbanucci A, Sahu B, Seppälä J, Larjo A, Latonen L, Waltering K, Tammela T, Vessella R, Lähdesmäki H, Jänne O. Overexpression of androgen receptor enhances the binding of the receptor to the chromatin in prostate cancer. Oncogene 2012;31: 2153–63. [DOI] [PubMed] [Google Scholar]
- 27.Weischenfeldt J, Simon R, Feuerbach L, Schlangen K, Weichenhan D, Minner S, Wuttig D, Warnatz H-J, Stehr H, Rausch T. Integrative genomic analyses reveal an androgen-driven somatic alteration landscape in early-onset prostate cancer. Cancer cell 2013;23: 159–70. [DOI] [PubMed] [Google Scholar]
- 28.Zhou Y, Jones J. Abstract LB-126: Declining T levels provide increased opportunity for mutagenesis in prostate stem cells: AACR, 2015. [Google Scholar]
- 29.Hu H, Odedina FT, Reams RR, Lissaker CT, Xu X. Racial Differences in Age-Related Variations of Testosterone Levels Among US Males: Potential Implications for Prostate Cancer and Personalized Medication. J Racial Ethn Health Disparities 2015;2: 69–76. [DOI] [PubMed] [Google Scholar]
- 30.Hayes LD, Grace FM, Sculthorpe N, Herbert P, Kilduff LP, Baker JS. Does chronic exercise attenuate age-related physiological decline in males? Research in sports medicine 2013;21: 343–54. [DOI] [PubMed] [Google Scholar]
- 31.Kelly DM, Jones TH. Testosterone: a metabolic hormone in health and disease. Journal of Endocrinology 2013;217: R25–R45. [DOI] [PubMed] [Google Scholar]
- 32.Feldman HA, Longcope C, Derby CA, Johannes CB, Araujo AB, Coviello AD, Bremner WJ, McKinlay JB. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. The Journal of Clinical Endocrinology & Metabolism 2002;87: 589–98. [DOI] [PubMed] [Google Scholar]
- 33.Loughlin KR. PSA velocity: a systematic review of clinical applications. Urol Oncol 2014;32: 1116–25. [DOI] [PubMed] [Google Scholar]
- 34.Force USPST. Final Recommendation Statement: Prostate Cancer: Screening, 2012. [Google Scholar]
- 35.Porcaro AB, Siracusano S, De Luyk N, Corsi P, Sebben M, Tafuri A, Bizzotto L, Tamanini I, Inverardi D, Cerruto MA, Martignoni G, Brunelli M, et al. Low-Risk Prostate Cancer and Tumor Upgrading to Higher Patterns in the Surgical Specimen. Analysis of Clinical Factors Predicting Tumor Upgrading to Higher Gleason Patterns in a Contemporary Series of Patients Who Have Been Evaluated According to the Modified Gleason Score Grading System. Urol Int 2016;97: 32–41. [DOI] [PubMed] [Google Scholar]
- 36.Porcaro AB, De Luyk N, Corsi P, Sebben M, Tafuri A, Processali T, Cerasuolo M, Mattevi D, Cerruto MA, Brunelli M, Siracusano S, Artibani W. Association between Basal Total Testosterone Levels and Tumor Upgrading in Low and Intermediate Risk Prostate Cancer. Urol Int 2017;1: 000459632. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.