Abstract
Objective:
The effect of deliriumon stroke outcome has not been quantified in sub-Saharan Africa. We investigated the prevalence of delirium occurring within one week of stroke in Nigerian survivors and its association with dementia and mortality at 3 months.
Methods:
Delirium was ascertained after repeated assessments within one week of stroke using the Confusion Assessment Method. Demographic and clinical characteristics, stroke severity, current and pre-morbid cognitive functioning were also assessed. Participants were then followed up for 3 months using culturally-validated neuropsychological tools. Probable dementia was ascertained according to the National Institute of Neurological Disorders and Stroke (NINDS-AIREN) criteria. Associations were investigated using both binomial and multinomial logistic regression analyses and presented as odds ratios (O.R) and relative risk ratios (RRR).
Results:
Of 101 consenting stroke survivors, 99 had two assessments for delirium within one week of the stroke. Delirium was present in 33.3% of stroke survivors (65.6% hypoactive, 21.9% hyperactive, and 12.1% mixed type). Having a severe stroke was associated with delirium (O.R=6.2, 95% C.I=1.1–13.8) after adjusting for age, gender, education and economic status, lifestyle factors, multimorbidities and laterality. At follow-up, those with severe stroke had a stronger association between delirium and dementia (RRR=4.3, 95% C.I=1.2–15.6) or death (RRR= 3.7, 95% C.I = 1.1–12.1).
Conclusion:
Delirium, in this sub-Saharan African sample, was already present in about one-third of survivors within one week of stroke. Survivors of severe stroke are at higher risk of delirium and its complications, and could be important target for delirium preventive interventions.
Keywords: Acute confusional state, Risk factors, Prevention, Vascular dementia, Stroke burden
1. Introduction
The neurobehavioural syndrome of delirium is the most common complication of hospital admission in the elderly [1]. It is also an important early marker of poor outcome of in-patient care [2], including long term cognitive impairment or dementia [3]. Among many disorders and interventions that have been associated with delirium [4], having a stroke rank high as an important trigger of its onset. According to some studies conducted in Western Europe and Australia, the prevalence of delirium among stroke survivors may be as high as 48% [5,6]. Currently, very little is known about delirium in sub-Saharan Africa. In a review of 46 studies, drawn from across all 54 countries in the sub-region, where ‘organic brain syndromes’ (including delirium) were investigated [7], only one [8] included a systematic assessment of delirium. That study was conducted using a general psychiatric out-patient sample [8]. Yet, there are no studies of the prevalence and outcome of delirium occurring in the early post stroke period among sub-Saharan African survivors.
Stroke is now among the leading causes of disease, disability, and death in sub-Saharan Africa [9]. A large proportion of the disability and mortality outcomes of stroke in the sub-region may be due to inefficient management in the acute phase after the stroke [10]. Given the paucity of information about post-stroke delirium in sub-Saharan Africa, it is feasible that part of the inefficiency in acute phase management of stroke may include unidentified and untreated delirium [11]. Therefore, epidemiological studies identifying factors associated with poststroke delirium may provide valuable information that will stimulate the development and use of evidence based delirium preventive measures, thereby reducing the risk of short term mortality and longer term disability after stroke.
In the present study, we investigated the prevalence, associated factors and 3-month outcome of delirium occurring within one week after a stroke among 101 survivors admitted for acute phase management in a Nigerian university hospital.
2. Methods
The study is a longitudinal observation of adults Nigerians with delirium occurring in the first week after surviving a stroke. Participants were residents of Ibadan and surrounding communities, and admitted for acute stroke care at the University College Hospital (UCH) Ibadan South-Western Nigeria. Ibadan is inhabited by about 3 million people, who are mostly Yoruba speaking. The UCH is the main referral hospital serving Ibadan and surrounding communities.
Ethical approval was obtained from the University of Ibadan/UCH ethics committee.
2.1. Subjects
Consecutive adult ischaemic or haemorrhagic stroke survivors were recruited after they had been seen by a consultant neurologist primarily responsible for their care. The diagnosis of stroke was confirmed based on neuroimaging and clinical examination criteria [12]. Written consent was obtained from all eligible stroke survivors and/or their spouses or adult children after the procedure of the study was explained to them either in English or the local Yoruba languages. We excluded patients who were unable to communicate reliably, usually because of aphasia, and those with severe co-morbidities (e.g., chronic kidney disease, metastatic cancer, or open tuberculosis).
Following recruitment, subsequent evaluations were conducted during outpatient follow-up visits after discharge from the hospital. In a few cases, usually because of non-attendance to scheduled follow-up visits due to transportation cost, seeking of alternative therapies, or industrial actions in the hospital, follow-up assessments had to be conducted in the homes of the patients.
2.2. Measures
Participants age in years, marital status, and the number of years of completed education were recorded. We ascertained economic status using asset based measures relevant to developing countries [13].
Stroke severity was assessed using the Stroke Levity Scale (SLS) [14]. The SLS uses a stroke severity score of 0–15 calculated by adding up the maximum power in the dextrous upper limb, weaker lower limb, and score for mobility. Lower values represent greater severity. A score of 0–5 = severe stroke, 6–10 = moderate stroke, and 11–15 = mild stroke.
2.2.1. Ascertainment of delirium
Stroke survivors meeting study criteria underwent two assessments for delirium within the first seven days of admission (a maximum of four days between assessments) using both the Confusion Assessment Method (CAM) [15] and Delirium Rating Scale (DRS) [16].
The CAM consists of nine criteria which correlates with the Diagnostic and Statistical Manual (DSM IV) criteria for delirium [17]. The questionnaire takes about 5 min to complete after observation of the patients during a cognitive screening test, usually the Mini-Mental State Examination (MMSE). Additional information is also requested from primary caregivers of patients. The diagnosis of delirium is then made following a standard algorithm which is part of the CAM instrument. According to the scoring convention, a diagnosis of delirium is warranted when a patient displays acute onset and fluctuating course of; 1) inattention, and 2a) disorganised thinking or 2b) altered levels of consciousness [15]. Previous validation of delirium diagnoses made using the CAM compared with clinician diagnoses using DSM IV criteria found a sensitivity of 94–100% and a specificity of 90–95% [18]. In this study, the CAM was administered by a research assistant with experience in epidemiologic research in the older population of south-Western Nigeria. She was trained using the CAM training manual. Inter-rater reliability on a sub-group of 29 stroke survivors independently assessed by the research assistant and a consultant in old age psychiatry (AO) produced Kappa values of between 0.51 and 0.76 for each CAM item, and 0.63 overall.
A psychiatrist administered the DRS which is a 10 item scale that generates a maximum score of 32. A score of 10 or more is compatible with a DSM IV diagnosis of delirium [17]. The DRS demonstrates a high agreement with the CAM.
2.2.2. Outcome
Following participants evaluations in the first week after stroke, outcome assessments were conducted at 3 months follow-up. Mortality data were collected when research supervisors have been reliably informed of the death of a participant usually by a close member of their household.
2.2.2.1. Ascertainment of probable dementia.
Participants were characterised as having probable dementia provided that at 3-months post neurologist-confirmed stroke, there was evidence of decline in memory and two other cognitive domains from the following: orientation, attention, language, visuospatial functions, executive functions, motor control, and praxis. They also had to have additional impairment in activities of daily living (ADL). This corresponds to the ‘National Institute of Neurological Disorders and Stroke and the Association Internationale pour la Recherche et l’Enseignement en Neurosciences (NINDS-AIREN) International Workshop criteria’ [19].
The 10 words list learning and delayed recall tests (10 WDRT) were used for the assessment of memory registration and recall respectively. The 10 WDRT is widely used as a direct cognitive test for memory functions in epidemiological studies conducted in developing countries [20,21]. For the learning phase, participants were asked to repeat 10 words read out to them slowly for a total of three administrations to allow for adequate learning. For each administration, the number of words correctly remembered was noted. The recall phase of the test was assessed after approximately five minutes, during which they were asked other questions from the study. In the local (Nigerian) norms for the 10 WDRT, a score (to the nearest whole number) of 15 or less for the learning phase and 5 for recall warrants a suspicion of dementia in persons with at least one year of formal education. The corresponding scores are 11 and 4 respectively for learning and recall among those with no formal education [21,22].
The animal naming test was used for assessment of executive functions. In this test, participants are required to name as many animals as possible in one minute. In the local (Nigerian) norms for the Animal naming test, a score (to the nearest whole number) of 12 or less warrants a suspicion of executive dysfunction in persons with at least one year of formal education. The score is 9 or less for those with no formal education [22].
For the assessments of the other cognitive domains specified in the NINDS-AIREN criteria, we used the performance of participants in the total and domain scores of the MMSE according to previously published procedure [23]. The MMSE [24] is a 20 item instrument frequently used as a screening tool for global cognitive impairment. It takes about 5–10 min to administer and generates a maximum score of 30 from 10 cognitive domains; orientation (10 points), memory registration (3 points) and recall (3 points), attention (5 points), praxis (3 points), naming (2 points), language repetition (1 point) and reading comprehension (1 point), writing (1 point), and visuoconstructional ability (1 point). According to the local (Nigerian) norms for the MMSE, a total score of 22 or less warrants a suspicion of dementia in persons with at least one year of formal education. The score is 15 or less for those with no formal education [22]. In this study, participants scoring b1 standard deviation below the mean score for individual domain of the MMSE were considered impaired in the relevant domain [23]. For domains with a maximum score of 1, participants scoring zero were considered impaired.
The Informant Questionnaire for Cognitive Decline in the Elderly (IQ-CODE) [25] was used to screen participants for the possibility of cognitive deterioration within 10 years and up to one month before the stroke. In the 16 items version of the IQ-CODE used for the present study, an average score (to the nearest whole number) of 4 or more on each items accurately screens for pre-stroke dementia, the equivalent score for cognitive decline without dementia is between 3 and 4 (Jorm, 1994).
Impairment in ADL was measured using the Barthel index [26]. The version of the Barthel index used for this study consists of 10 items that measure activity of daily living and mobility. The 10 item version of the instrument has a total score of 20, and a difference of 4 from the total score possible is often regarded as evidence of functional impairment [27].
2.3. Statistical analyses
The sample for the present study comprised those who provided data within the first week of surviving a stroke (the baseline) between May 2014 and March 2016. Two of the initial 101 stroke survivors meeting study criteria deteriorated rapidly within a few hours of admission and could not be assessed for delirium. They were thus excluded from the analyses on this ground. Participants were considered to have reached an endpoint if they were available for outcome assessments at 3 months follow-up, or when research supervisors have been reliably informed of their death. Those who could not be traced, had relocated from the study location, or refused further participation after baseline assessments were coded as attritions.
Descriptive statistics such as means and standard deviations were used to summarize quantitative variables while frequencies and percentages were used for categorical variables. Characteristics of the study sample that were recruited but could not be followed up at 3 months were compared with those who were followed up using the chi-squared test or t-test for categorical or continuous variables respectively.
Similar comparisons were carried out between participants with delirium and those not meeting criteria for the syndrome. Subsequently, we conducted logistic regression analyses. We first conducted an unadjusted analysis. Next, we adjusted for age, gender, education and economic status, lifetime use of tobacco or alcohol, diabetes, multimorbidities and side of body affected by stroke. These were factors that might have significantly affected the risk of delirium in our bivariate analyses.
A multinomial regression model was next used to investigate the relationship between having delirium at the baseline and meeting criteria for probable dementia at 3 months follow-up. The entire baseline sample was used for this analysis. Not having probable dementia at 3months follow-up was the reference outcome category in the multinomial model, while death and losses to follow-up were included as other outcome categories. The exponentials of coefficients in the multinomial regression analyses, denoted as the relative risk ratios (RRR) by convention [28,29], represent the risk of the outcomes between those with and without delirium at baseline.
Analyses were conducted using Stata version 13.0 [30] and a level of significance of p b 0.05 two-tailed was set for all analyses.
3. Results
The baseline sample was composed of 47 women and 54 men (Table 1). Their mean age was 61.1 (±12.9) years. Nearly a quarter of the participants had no formal education.
Table 1:
Baseline characteristics of stroke survivors in the study.
| Characteristics | Percent |
|---|---|
| Age ≥ 60 years | 56.4 |
| ≥1-year of formal education | 76.7 |
| Female gender | 46.5 |
| Mild/moderate stroke | 69.9 |
| Previous TIA/stroke | 8.9 |
| Systemic hypertensiona | 77.3 |
| Diabetes | 25.7 |
| Medical comorbiditiesb | 41.0 |
| Medications for comorbidities | 38.6 |
| Regular user of alcohol | 10.2 |
| Regular tobacco smoker | 5.2 |
| Psychosocial stress in the past year | 22.9 |
| Lives alone | 5.3 |
| Low/average economic status | 48.4 |
| DSM IV deliriumc | 33.3 |
| Hypoactive | 65.6 |
| Hyperactive | 21.9 |
| Mixed | 12.1 |
| Characteristics | Mean (SD)/median |
| Age | 61.1 (12.9) |
| Years of formal education | 8.1 (6.4) |
| SLS scoresd | 7.6 (3.4) |
| IQ-CODE scoree | 3.2 (0.5) |
| Blood pressure (mmHg) | |
| Average systolic | 166.6 (26.3) |
| Average diastolic | 97.9 (15.9) |
| Urea (mm/dl) | 37.9 (33.3) |
| Creatinine (mg/dl) | 2.2 (4.6)/1.1 |
| Glucose (mmol/L) | 8.6 (5.6) |
SLS=Stroke Levity Scale, IQ-CODE=Informant Questionnaire for cognitive decline in the Elderly.
Based on self-report of clinician diagnosis.
Self-report of clinician diagnoses endorsed froma list of several commonly diagnosed conditions in the study setting.
Two participants could not be assessed for delirium.
Lower scores indicate greater stroke severity.
Lower scores indicate better pre-stroke cognitive function.
In all, 74.8% of stroke survivors who had baseline assessments (N = 99) provided follow-up information. A total of 24 study participants had died by 3 months follow-up. Eleven had relocated from the Ibadan area, nine could not be traced after their baseline assessments, while four persons refused further participation after the baseline assessments, and one survivor became too ill to be assessed at follow-up. There were no significant differences between participants who were followed up and those who were not. Those who were followed up were 58.1% men. They had a mean age = 60.9 ± 12.6 years and averaged 8.6 ± 6.1 years of formal education.
A total of 33 (33.3%) study participants had delirium within the first week after stroke. Those with severe stroke (p = 0.014) affecting the right side of the body (p = 0.010) were more likely to develop delirium (Fig. 1, Table 2). However, having a severe stroke was the only independent predictor of delirium at baseline (Table 3).
Fig. 1.
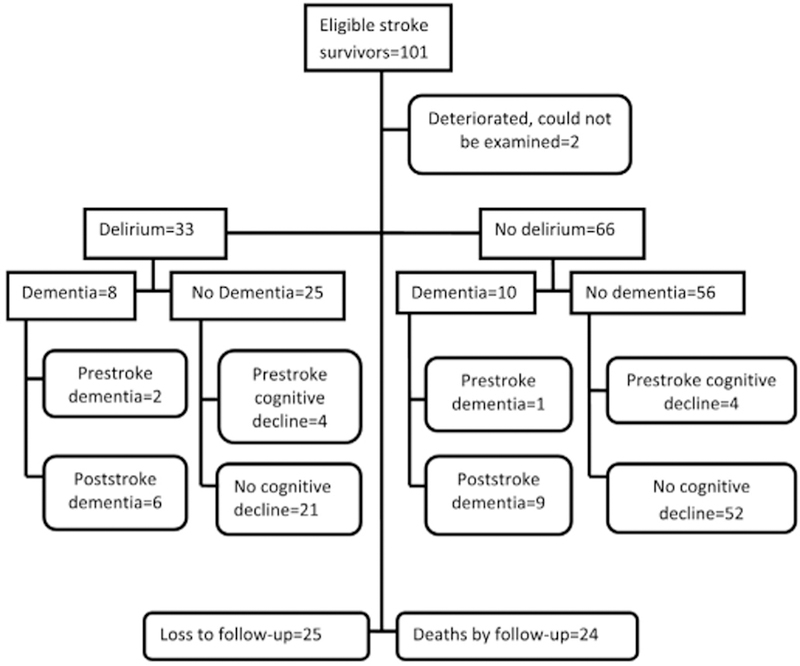
Delirium, dementia and mortality outcomes of stroke survivors.
Table 2.
Unadjusted logistic regression analyses showing baseline risk factors for delirium among stroke survivors.
| Characteristics | Delirium (%) N = 33 |
No delirium (%) N = 66 |
O.R (95% C.I) |
p-Value |
|---|---|---|---|---|
| Demographic factors | ||||
| Age in years | 0.405 | |||
| <60 | 14 (50.0) | 26 (40.6) | 1 | |
| ≥60 | 14 (50.0) | 38 (59.4) | 0.7 (0.3–1.7) | |
| Gender | 0.120 | |||
| Female | 12 (36.4) | 35 (53.0) | 1 | |
| Male | 21 (63.6) | 31 (47.0) | 2.0 (0.8–4.7) | |
| Years of formal | ||||
| education | 0.ԧ | |||
| ≥1 year of education | 22 (73.3) | 46 (79.3) | 1 | |
| No education | 8 (26.7) | 12 (20.7) | 1.4 (0.5–3.9) | |
| Marital status | 0.608 | |||
| Currently married | 23 (69.7) | 47 (74.6) | 1 | |
| Divorced/separated | 10 (30.3) | 16 (25.4) | 1.3 (0.5–3.3) | |
| Economic status | 0.127 | |||
| Higher | 13 (40.6) | 35 (57.4) | 1 | |
| Low/average | 19 (59.4) | 26 (42.6) | 2.0 (0.8–4.7) | |
| Vascular risk factors | ||||
| Alcohol use | 0.885 | |||
| Never drank | 18 (56.3) | 35 (54.7) | 1 | |
| Ever drank | 14 (43.8) | 29 (45.3) | 0.9 (0.4–2.2) | |
| Tobacco smoking | 0.466 | |||
| Never smoked | 24 (80.0) | 55 (85.9) | 1 | |
| Ever smoked | 6 (20.0) | 9 (14.1) | 1.5 (0.5–4.8) | |
| Threatening life-event | 0.801 | |||
| No | 25 (78.1) | 47 (75.8) | 1 | |
| Yes | 7 (21.9) | 15 (24.2) | 0.9 (0.3–2.4) | |
| Previous TIA/stroke | 1.000 | |||
| No | 30 (90.9) | 60 (90.9) | 1 | |
| Yes | 3 (9.1) | 6 (9.1) | 1.0 (0.2–4.3) | |
| Systemic hypertensiona | 0.141 | |||
| No | 4 (12.9) | 17 (26.6) | 1 | |
| Yes | 27 (87.1) | 47 (73.4) | 2.4 (0.7–8.0) | |
| Diabetes | 0.747 | |||
| No | 25 (75.8) | 48 (72.7) | 1 | |
| Yes | 8 (24.4) | 18 (27.3) | 0.9 (0.3–2.2) | |
| Multimorbiditiesb | 0.991 | |||
| Absent | 17 (58.6) | 31 (58.5) | 1 | |
| Present | 12 (41.4) | 22 (41.5) | 1.0 (0.4–2.5) | |
| Medicationsc | 0.384 | |||
| No | 22 (66.7) | 38 (57.6) | 1 | |
| Yes | 11 (33.3) | 28 (42.4) | 0.7 (0.3–1.6) | |
| Pre-stroke dementia | 0.455 | |||
| Absent | 27 (90.0) | 51 (94.4) | 1 | |
| Present | 3 (10.0) | 3 (5.6) | 1.9 (0.4–10.0) | |
| Examination findings | ||||
| Stroke severity | 0.005 | |||
| Mild/moderate stroke | 15 (50.0) | 42 (80.8) | 1 | |
| Severe stroke | 15 (50.0) | 10 (19.2) | 4.2 (1.6–11.3) | |
| Side of body affected | 0.005 | |||
| Left/or both | 9 (34.6) | 34 (69.4) | 1 | |
| Right | 17 (65.4) | 15 (30.6) | 4.3 (1.6–11.8) | |
| Blood pressure (mmHg)d |
||||
| Systolic | 0.609 | |||
| <140 | 4 (13.8) | 10 (18.2) | 1 | |
| ≥140 | 25 (86.2) | 45 (81.8) | 1.4 (0.4–4.9) | |
| Diastolic | 0.739 | |||
| b90 | 10 (34.5) | 17 (30.9) | 1 | |
| ≥90 | 19 (65.5) | 38 (69.1) | 0.9 (0.3–2.2) | |
| Urea (mg/dl) | 0.181 | |||
| ≤20 | 5 (22.7) | 17 (39.5) | 1 | |
| >20 | 17 (77.3) | 26 (60.5) | 2.2 (0.7–7.2) | |
| Glucose (mmol/l) | 0.590 | |||
| ≤6.9 | 6 (31.6) | 11 (25.0) | 1 | |
| N6.9 | 13 (68.4) | 33 (75.0) | 0.7 (0.2–2.4) | |
| Creatinine (mg/dl) | ||||
| Females | 0.796 | |||
| ≤1.2 | 7 (77.8) | 18 (81.8) | 1 | |
| >1.2 | 2 (22.2) | 4 (18.2) | 1.3 (0.2–8.7) | |
| Males | 0.312 | |||
| ≤1.5 | 9 (64.3) | 16 (80.0) | 1 | |
| >1.5 | 5 (35.7) | 4 (20.0) | 2.2 (0.5–10.4) |
O.R = odds ratio, C.I = confidence intervals, SD = standard deviation, mmHg = millimetres of mercury, mg/dl = milligrams per decilitre, mmol/L = millimols/l, TIA = transient ischaemic attack.
Based on self-report of clinician diagnosis.
Self-report of clinician diagnoses of more than one chronic medical condition from a list of several commonly diagnosed conditions in the study setting.
Anticholinergic medications.
Average of two measurements.
Table 3.
Adjusted logistic regression analyses showing baseline risk factors for delirium in stroke survivors.
| Risk factors for delirium | O.Ra (95% C.I) | p-values |
|---|---|---|
| Stroke everity | ||
| Mild/moderate | Reference | 0.005 |
| Severe | Unadjusted: 4.2 (1.6–11.3) | 0.047 |
| Adjusted: 8.5 (1.0–70.6) | ||
| Covariates | ||
| Age ≥ 60 years | 0.6 (0.7–1.9) | 0.232 |
| Male gender | 2.0 (0.3–14.6) | 0.482 |
| No formal education | 1.7 (0.4–10.8) | 0.378 |
| Low/average economic status | 1.1 (0.2–3.9) | 0.806 |
| Lifetime use of alcohol | 0.3 (0.0–1.7) | 0.159 |
| Lifetime Tobacco smoking | 8.3 (0.6–118.0) | 0.120 |
| Diabetes | 0.5 (0.1–4.7) | 0.549 |
| Multimorbidities | 3.3 (0.5–22.1) | 0.223 |
| Right side of body affected | 3.6 (0.6–22.9) | 0.171 |
Adjusted for gender, economic status, lifetime use of alcohol, lifetime tobacco smoking, diabetes, comorbidities, age ≥ 60 years, and education and side of body affected.
At 3 months follow-up, we found that those with severe stroke had a stronger association between delirium and dementia (RRR = 4.3, 95% C.I = 1.2–15.6) or death (RRR = 3.7, 95% C.I = 1.1–12.1).
4. Discussion
We found a prevalence of 33.3% for delirium occurring in the first week after a stroke among survivors in this sub-Saharan African acute stroke care setting. Severe stroke independently predicted delirium and may predict dementia and mortality 3 months later.
Our results are largely in keeping with reports from studies conducted in acute stroke care settings in better resourced parts of the world. In the studies reviewed by McManus and colleagues [11], the prevalence of delirium reported appears dependent on study-specific characteristics such as methods of delirium ascertainment. For example, as delirium is both transient and fluctuant, studies including serial assessments were more likely to report higher prevalence rates. Similarly, rates in studies using diagnostic assessments differed from those using screening measures with varying cut-off points. In all, studies that have been conducted in Western Europe and Australia suggest that the prevalence of post stroke delirium in hospital treated survivors ranges from 13%, when the DRS was used for a one-off screening of survivors at the point of admission [31], to 48% when DSM criteria were used for diagnostic evaluations after repeated assessments within one week of admission [32]. In the present study, we conducted two repeated assessment for delirium within the first week of stroke and applied the diagnostic algorithm in the CAM for the ascertainment of the full syndrome in accordance with DSM IV criteria. In addition, a psychiatrist applied the DRS simultaneously as the CAM assessments, thus providing additional validation of the diagnoses generated using the CAM algorithm. We think that our assessment procedure takes into account the fluctuating nature of delirium and as such likely to produce more accurate results.
The findings in this study suggesting a prospective association between delirium in the first week after a stroke and probable dementia, as well as mortality, in the short to medium term is also in keeping with reports from previous studies of the outcome of post stroke delirium [33,34,35]. We interpret the results of our log multinomial regression analysis with caution. This is because the association found in the present study appear to be moderated by stroke severity, and are therefore not wholly accounted for by delirium. Results of previous prospective longitudinal studies [33,34,35] suggest that the strength of association between delirium and dementia is dependent on the sample size, length of follow-up and attrition rates [36]. In the present study, we have relied on a sample of 99, out of an initial 101 stroke survivors, whom we have followed up for a short period of 3 months. Even though 3 months is the minimum allowable duration for the diagnoses of dementia after a confirmed stroke according to some conventions [19,37,38,39,40], we recognize that this interval may be too short for the impact of delirium to have led to a substantial level of cognitive and functional impairments sufficient for a confident diagnosis of dementia. Also, the possibility exists that the combination of a small sample size and an attrition rate of about 25% may have reduced the power to demonstrate a stronger and independent association. This is regardless of the observation that there were no significant differences between the group that was successfully followed up and those that were not followed up to 3 months in the present study.
There are several important areas of strength in this study. The first is in our method of ascertaining the diagnosis of probable dementia. There is a suggestion in the literature that the NINDS-AIREN criteria, which we have relied on in ascertaining probable dementia in the present study, may have better psychometric indices compared with other contemporary criteria for dementia occurring after a confirmed stroke [40]. In addition, we envisage that our reliance on neuropsychological measures which have been culturally adapted and validated in the study setting [22] will have the effect of increasing the reliability of our diagnoses. Secondly, to the best of our knowledge, this is the first report of the prevalence, associated factors and outcome of post stroke delirium in sub-Saharan Africa. Information about delirium or vascular dementia derived from observational studies in sub-Saharan Africa is still relatively sparse. We note that because of the preliminary nature of our prospective investigation of delirium and dementia in this population, we have not excluded dementia occurring before the stroke event in conducting our outcome analyses.
4.1. Conclusion
As in other contexts, poststroke delirium is common in this sub-Saharan African acute stroke care setting. The full syndrome appears to develop rapidly. According to our findings, it was already present in about one-third of stroke survivors within one week of the stroke event. Survivors of severe stroke may be at greater risk of delirium occurring in this early phase after a stroke, and the syndrome may predict dementia and death in the short to medium term. Therefore, early identification of survivors of severe stroke may offer opportunity for early institution of proven interventions to prevent delirium in the acute phase after stroke thereby preventing dementia and early death, as well as saving health service cost.
Future studies should consider evaluating the impact of delirium on probable vascular dementia over a longer time period. Including following stroke survivors in the community. The methods and measures used for this study means that larger scale observational studies of vascular dementia, including trials of interventions, are feasible in resourcepoor developing countries, such as Nigeria.
Acknowledgement
This study was supported by the Fogarty International Center award number 1R24008878 through the medical education partnership initiative of Nigeria. It was presented in part at the September 2015 Joint meeting of the British Geriatric Society/European Delirium Association special interest group meeting in London, United Kingdom. The content is solely the responsibility of the Authors and does not necessarily represent the official views of the Fogarty International Center or the National Institute of Health.
Footnotes
Funding support: Fogarty International Center award number 1R24008878.
References
- [1].Inouye SK, et al. , Risk factors for delirium at discharge: development and validation of a predictive model, Arch. Intern. Med 167 (13) (2007) 1406–1413. [DOI] [PubMed] [Google Scholar]
- [2].Witlox J, et al. , Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta-analysis, JAMA 304 (4) (2010) 443–451. [DOI] [PubMed] [Google Scholar]
- [3].Pendlebury ST, Rothwell PM, Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: a systematic review and meta-analysis, Lancet Neurol 8 (11) (2009) 1006–1018. [DOI] [PubMed] [Google Scholar]
- [4].Royal college of physicians, The prevention, Diagnosis and Management of Delirium in Older people, National guidelines, 2006.
- [5].Mc Manus J, et al. , The evaluation of delirium post-stroke, Int. J. Geriatr. Psychiatry 24 (11) (2009) 1251–1256. [DOI] [PubMed] [Google Scholar]
- [6].Oldenbeuving AW, et al. , Delirium in acute stroke: a review, Int. J. Stroke 2 (4) (2007) 270–275. [DOI] [PubMed] [Google Scholar]
- [7].Paddick SM, Kalaria RN, Mukaetova-Ladinska EB, The prevalence and clinical manifestations of delirium in sub-Saharan Africa: a systematic review with inferences, J. Neurol. Sci 348 (1–2) (2015) 6–17. [DOI] [PubMed] [Google Scholar]
- [8].Ola BA, et al. , Incidence and correlates of delirium in a West African mental health clinic, Gen. Hosp. Psychiatry 32 (2) (2010) 176–181. [DOI] [PubMed] [Google Scholar]
- [9].Moran A, et al. , The epidemiology of cardiovascular diseases in sub-Saharan Africa: the Global Burden of Diseases, Injuries and Risk Factors 2010 Study, Prog. Cardiovasc. Dis 56 (3) (2013) 234–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ogun SA, et al. , Stroke in south west Nigeria: a 10-year review, Stroke 36 (6) (2005) 1120–1122. [DOI] [PubMed] [Google Scholar]
- [11].McManus J, et al. , Delirium post-stroke, Age Ageing 36 (6) (2007) 613–618. [DOI] [PubMed] [Google Scholar]
- [12].Sacco RL, et al. , An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association, Stroke 44 (7) (2013) 2064–2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ferguson B, Tandon A, Gakidou E, Estimating Permanent income using Indicator variables, in: Murray C, Evans D (Eds.), Health systems Performance Assessment: diabetes, methods and empericism, World Health Organisation, Geneva: 2003, pp. 747–760. [Google Scholar]
- [14].Owolabi MO, Platz T, Proposing the Stroke Levity Scale: a valid, reliable, simple, and time-saving measure of stroke severity, Eur. J. Neurol 15 (6) (2008) 627–633. [DOI] [PubMed] [Google Scholar]
- [15].Inouye SK, et al. , Clarifying confusion: the confusion assessment method. A new method for detection of delirium, Ann. Intern. Med 113 (12) (1990) 941–948. [DOI] [PubMed] [Google Scholar]
- [16].Trzepacz PT, et al. , Is delirium different when it occurs in dementia? A study using the delirium rating scale, J. Neuropsychiatr. Clin. Neurosci 10 (2) (1998) 199–204. [DOI] [PubMed] [Google Scholar]
- [17].American Psychiatric Association, Delirium, dementia and amnestic and other cognitive disorders, Diagnostic and Statistical Manual of Mental disorders, 4 ed.American Psychiatric Association, Washington, DC, 2000. 4th edition (DSM IV) text revision (ed). [Google Scholar]
- [18].Waszynski CM, D.o.N.N.Y., Hartford Institute for Geriatric Nursing, Confusion Assessment Method (CAM), Medsurg Nurs 13 (4) (2004) 269–270. [PubMed] [Google Scholar]
- [19].Roman GC, et al. , Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop, Neurology 43 (2) (1993) 250–260. [DOI] [PubMed] [Google Scholar]
- [20].Prince M, et al. , Dementia diagnosis in developing countries: a cross-cultural validation study, Lancet 361 (9361) (2003) 909–917. [DOI] [PubMed] [Google Scholar]
- [21].Gureje O, et al. , Incidence of and risk factors for dementia in the Ibadan study of aging, J. Am. Geriatr. Soc 59 (5) (2011) 869–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Guruje O, et al. , The CERAD Neuropsychological Test Battery: norms from a Yorubaspeaking Nigerian sample, West Afr. J. Med 14 (1) (1995) 29–33. [PubMed] [Google Scholar]
- [23].Levy R, Aging-associated cognitive decline. Working Party of the International Psychogeriatric Association in collaboration with the World Health Organization, Int. Psychogeriatr 6 (1) (1994) 63–68. [PubMed] [Google Scholar]
- [24].Folstein MF, Folstein SE, McHugh PR, “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician, J. Psychiatr. Res 12 (3) (1975) 189–198. [DOI] [PubMed] [Google Scholar]
- [25].Jorm AF, A short form of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): development and cross-validation, Psychol. Med 24 (1) (1994) 145–153. [DOI] [PubMed] [Google Scholar]
- [26].Mahoney FI, Barthel DW, Functional Evaluation: The Barthel Index, Md. State Med. J 14 (1965) 61–65. [PubMed] [Google Scholar]
- [27].Collin C, et al. , The Barthel ADL Index: a reliability study, Int. Disabil. Stud 10 (2) (1988) 61–63. [DOI] [PubMed] [Google Scholar]
- [28].Train K, Discreet choice methods with simulation, Cambridge University Press, Cambridge United Kingdom, 2003. [Google Scholar]
- [29].Blizzard L, Hosmer DW, The log multinomial regression model for nominal outcomes with more than two attributes, Biom. J 49 (6) (2007) 889–902. [DOI] [PubMed] [Google Scholar]
- [30].Stata Corp, Stata Statistical Software, StataCorp LP, College Station, TX, 2013.
- [31].Caeiro L, et al. , Delirium in acute stroke: a preliminary study of the role of anticholinergic medications, Eur. J. Neurol 11 (10) (2004) 699–704. [DOI] [PubMed] [Google Scholar]
- [32].Gustafson Y, et al. , Acute confusional state (delirium) in stroke patients, Cerebrovasc. Dis 1 (5) (1991) 257–263. [Google Scholar]
- [33].Melkas S, et al. , Post-stroke delirium in relation to dementia and long-term mortality, Int. J. Geriatr. Psychiatry 27 (4) (2012) 401–408. [DOI] [PubMed] [Google Scholar]
- [34].Henon H, et al. , Confusional state in stroke: relation to preexisting dementia, patient characteristics, and outcome, Stroke 30 (4) (1999) 773–779. [DOI] [PubMed] [Google Scholar]
- [35].Sheng AZ, et al. , Delirium within three days of stroke in a cohort of elderly patients, J. Am. Geriatr. Soc 54 (8) (2006) 1192–1198. [DOI] [PubMed] [Google Scholar]
- [36].Ojagbemi A, ffytche DH, Are Stroke survivors with Delirium at higher risk of Poststroke Dementia? Current evidence and future directions, Int. J. Geriatr. Psychiatry 31 (12) (2016) 1289–1294 In press. [DOI] [PubMed] [Google Scholar]
- [37].Wiederkehr S, et al. , Comparability of the clinical diagnostic criteria for vascular dementia: a critical review. Part I, J. Neuropsychiatry Clin. Neurosci 20 (2) (2008) 150–161. [DOI] [PubMed] [Google Scholar]
- [38].Wiederkehr S, et al. , Validity of the clinical diagnostic criteria for vascular dementia: a critical review. Part II, J. Neuropsychiatry Clin. Neurosci 20 (2) (2008) 162–177. [DOI] [PubMed] [Google Scholar]
- [39].Roman GC, Facts, myths, and controversies in vascular dementia, J. Neurol. Sci 226 (1–2) (2004) 49–52. [DOI] [PubMed] [Google Scholar]
- [40].Tang WK, et al. , Impact of applying NINDS-AIREN criteria of probable vascular dementia to clinical and radiological characteristics of a stroke cohort with dementia, Cerebrovasc. Dis 18 (2) (2004) 98–103. [DOI] [PubMed] [Google Scholar]


